Figure 1.
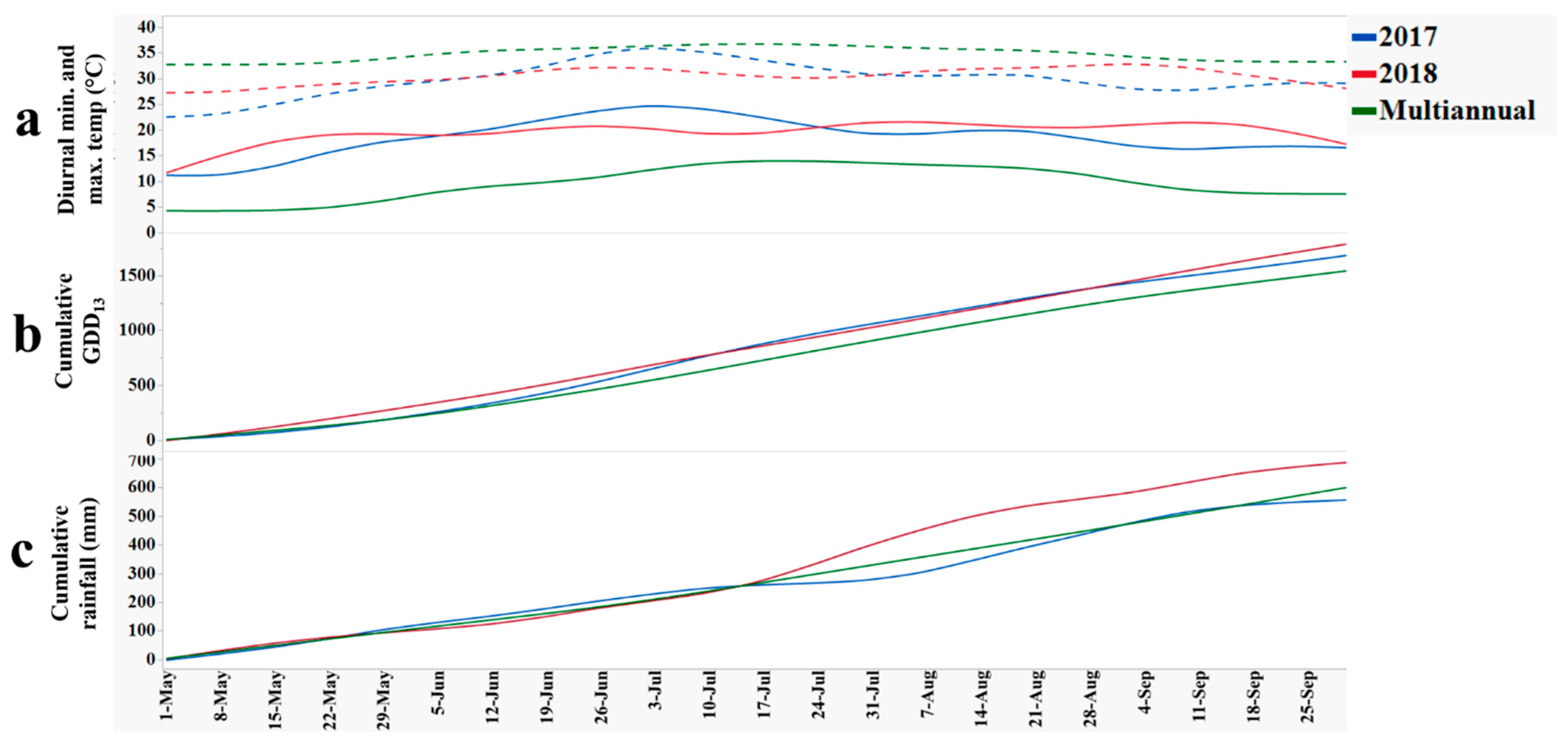
Weather data at Suffolk, VA, including: (a) diurnal minimum (solid line) and maximum (dashed line) temperatures (°C); (b) cumulative growing degree days calculated from daily min and max temperatures with 13 °C as base temperature (GDD13); and (c) cumulative rainfall (mm) for 2017, 2018, and multiannual (1984–2019) average.
Figure 1.
Weather data at Suffolk, VA, including: (a) diurnal minimum (solid line) and maximum (dashed line) temperatures (°C); (b) cumulative growing degree days calculated from daily min and max temperatures with 13 °C as base temperature (GDD13); and (c) cumulative rainfall (mm) for 2017, 2018, and multiannual (1984–2019) average.
Figure 2.
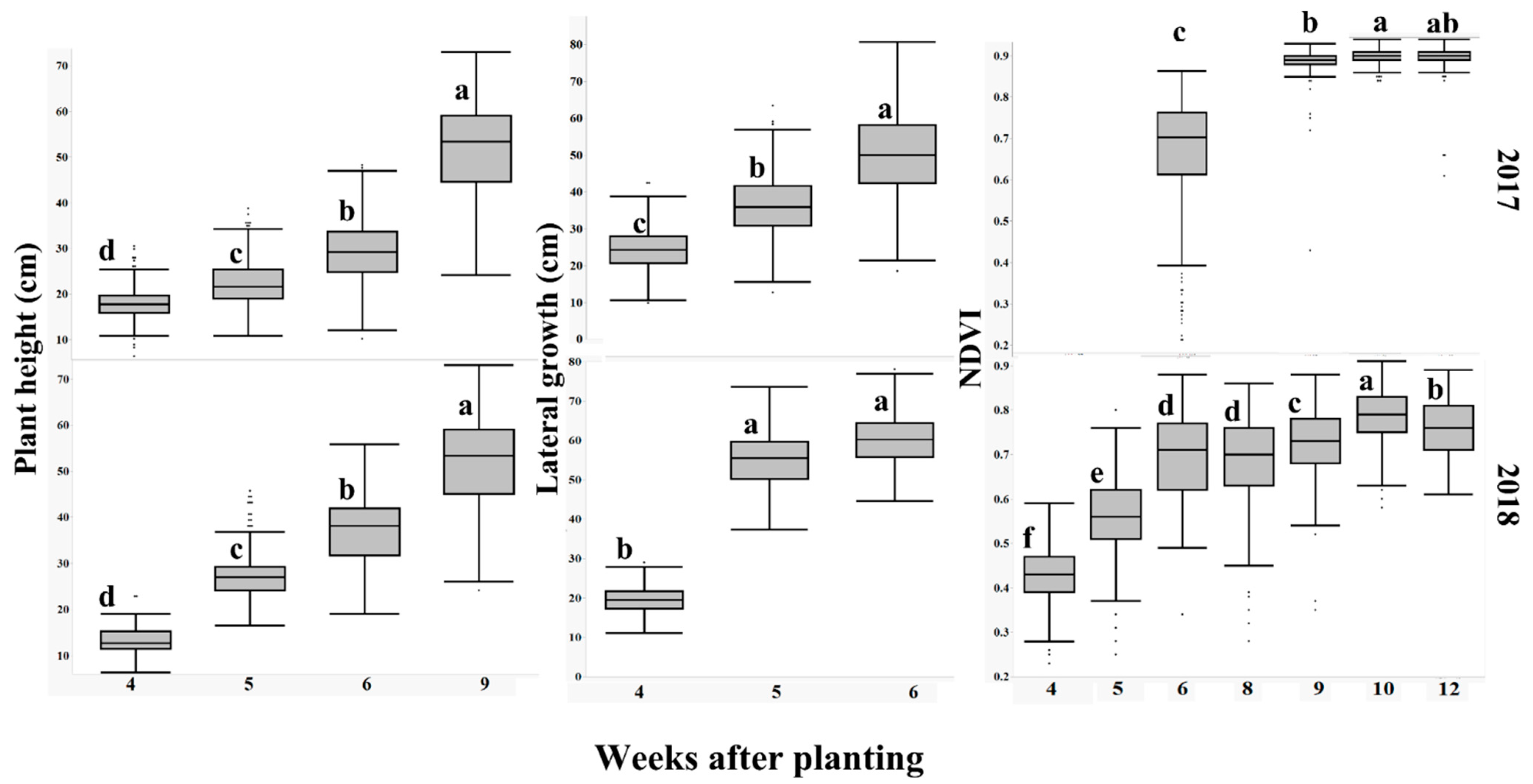
The x-axis shows progression of measured growth traits (plant height, lateral growth, and NDVI) for 104 mini-core peanut genotypes in 2017, and 88 in 2018, with weeks after planting on the y-axis. Each box and whisker plot represents the measured trait, including all genotypes on that day. Plots with the same letters are not significantly different across weeks after planting using LS means at α = 0.05.
Figure 2.
The x-axis shows progression of measured growth traits (plant height, lateral growth, and NDVI) for 104 mini-core peanut genotypes in 2017, and 88 in 2018, with weeks after planting on the y-axis. Each box and whisker plot represents the measured trait, including all genotypes on that day. Plots with the same letters are not significantly different across weeks after planting using LS means at α = 0.05.
Figure 3.
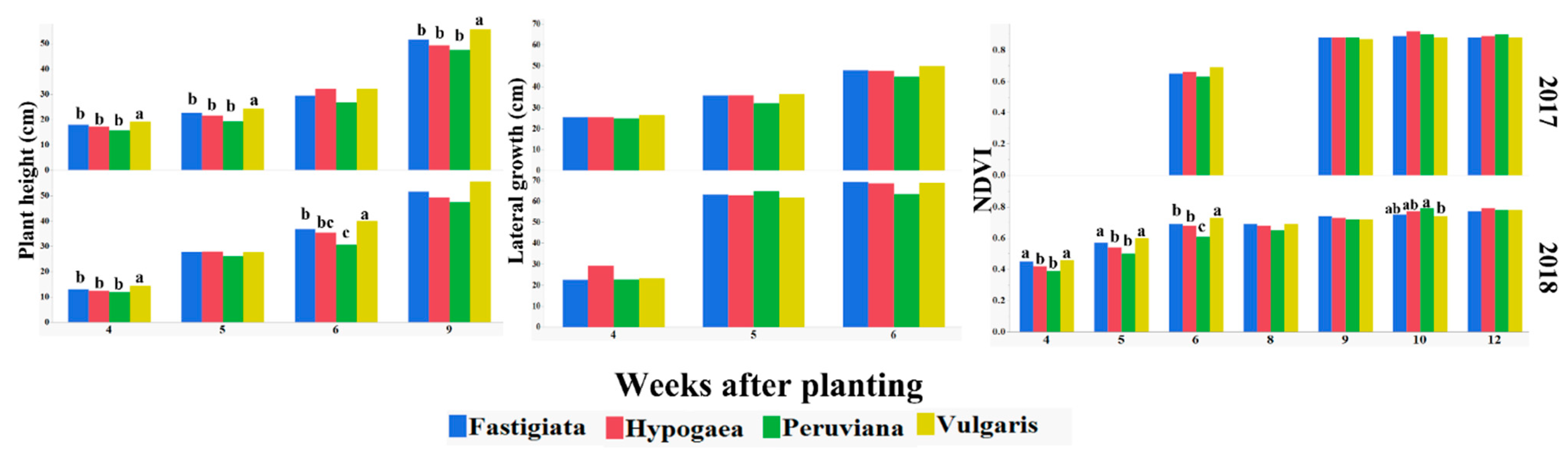
The y-axis shows progression of measured growth traits (plant height, lateral growth, and NDVI) for four peanut varieties (fastigiata, hypogaea, peruviana, and vulgaris) of the U.S. mini-core peanut collection in 2017 and 2018, with weeks after planting on the x-axis. The bars with no or similar letters are not significantly different within individual weeks after planting using LS means at α = 0.05.
Figure 3.
The y-axis shows progression of measured growth traits (plant height, lateral growth, and NDVI) for four peanut varieties (fastigiata, hypogaea, peruviana, and vulgaris) of the U.S. mini-core peanut collection in 2017 and 2018, with weeks after planting on the x-axis. The bars with no or similar letters are not significantly different within individual weeks after planting using LS means at α = 0.05.
Figure 4.
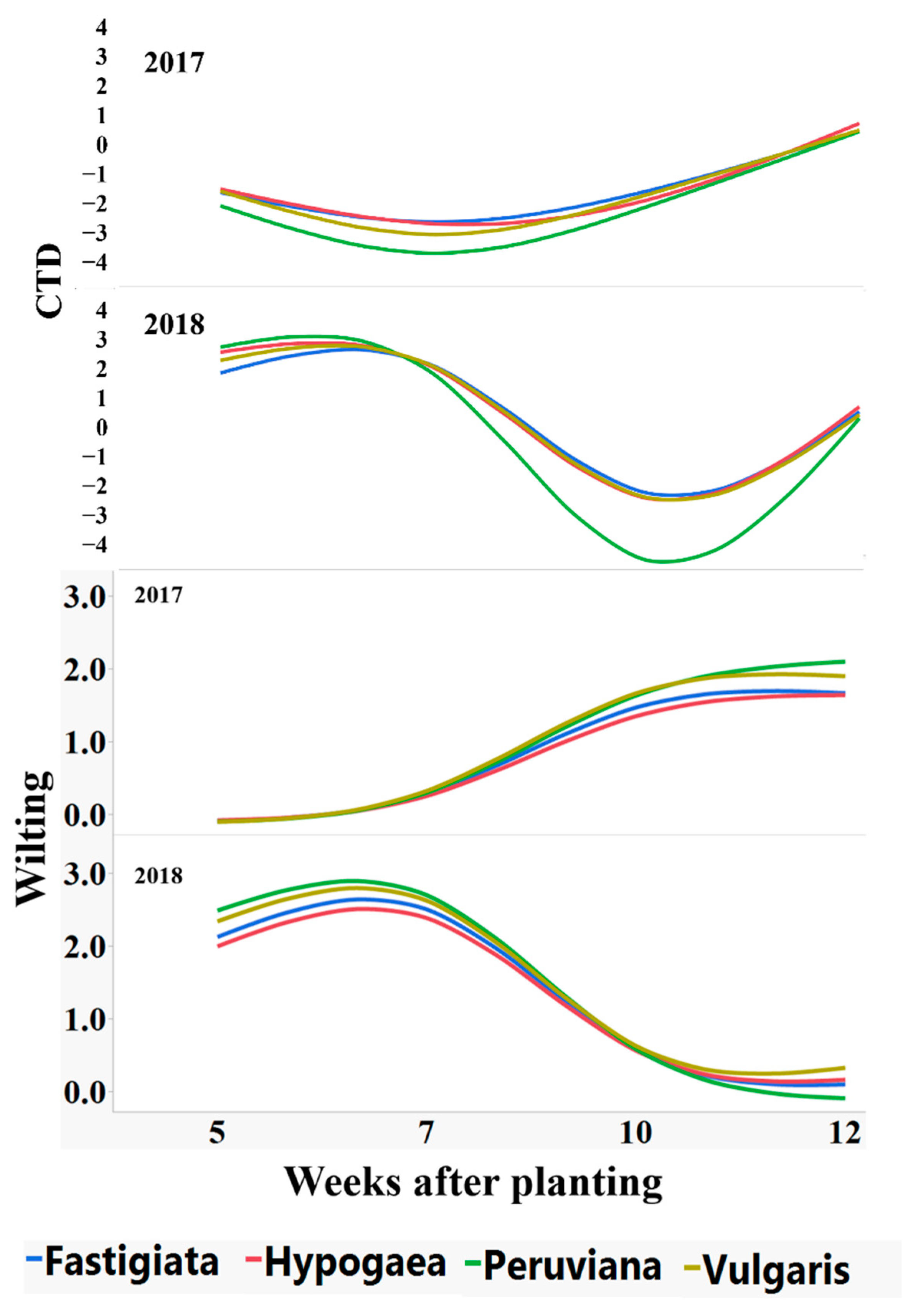
Progression of canopy temperature depression (CTD, canopy minus air temperature), and leaf wilting of peanut varieties over the growing seasons during 2017 and 2018.
Figure 4.
Progression of canopy temperature depression (CTD, canopy minus air temperature), and leaf wilting of peanut varieties over the growing seasons during 2017 and 2018.
Figure 5.
Correlation of wilting and tomato spotted wilt (TSW) with plant height for the U.S. peanut mini-core collection genotypes. Each data point for the upper graph is average plant height vs. maximum wilting at 10 weeks after planting (WAP), averaged across replications; each data point for the lower graph is average plant height vs. TSW at 12 WAP, averaged across replications in 2017.
Figure 5.
Correlation of wilting and tomato spotted wilt (TSW) with plant height for the U.S. peanut mini-core collection genotypes. Each data point for the upper graph is average plant height vs. maximum wilting at 10 weeks after planting (WAP), averaged across replications; each data point for the lower graph is average plant height vs. TSW at 12 WAP, averaged across replications in 2017.
Table 1.
List of the 112 accessions of the U.S. mini-core peanut germplasm collection, including PI numbers from the GRIN database. The table includes market type, variety, pod type, pod shape, and 100-seed weight, compiled from different sources (in the footnote). Kernel color (CIE Lab) is also included, derived from seed pictures available on the GRIN database. The underlined PI and CC numbers were planted for this study.
Table 1.
List of the 112 accessions of the U.S. mini-core peanut germplasm collection, including PI numbers from the GRIN database. The table includes market type, variety, pod type, pod shape, and 100-seed weight, compiled from different sources (in the footnote). Kernel color (CIE Lab) is also included, derived from seed pictures available on the GRIN database. The underlined PI and CC numbers were planted for this study.
| S. No. | PI Number | CC | Market Type 1 | Market Type 2 | Variety 3 | Pod Type 4 | Pod Shape 4 | 100 Seed wt. (g) 4 | Kernel Color 5 |
|---|
| Hue | L | a | b |
|---|
| 1 | PI 152146 | 406 | Spanish | Spanish | . | Spanish | hypogaea | 48.5 | 28.4 | 67.2 | 3.96 | 26.0 |
| 2 | PI 155107 | 384 | . | Valencia | vulgaris | Spanish | vulgaris | 38.3 | 28.9 | 67.3 | 4.18 | 27.1 |
| 3 | PI 157542 | 553 | . | Runner | vulgaris | Virginia | hypogaea | 60.8 | 26.6 | 64.2 | 3.79 | 23.1 |
| 4 | PI 158854 | 559 | Valencia | Valencia | fastigiata | . | vulgaris | 60.8 | 10.6 | 57.0 | 11.73 | 18.6 |
| 5 | PI 159786 | 334 | Virginia | Virginia | hypogaea | Virginia | . | 34.8 | 27.2 | 64.2 | 4.03 | 24.1 |
| 6 | PI 162655 | 388 | Spanish | Spanish | hypogaea | Virginia | . | 39.5 | 28.1 | 67.3 | 4.73 | 27.1 |
| 7 | PI 162857 | 731 | Virginia | Virginia | hypogaea | Virginia | hypogaea | 87.2 | 24.4 | 61.3 | 7.25 | 25.7 |
| 8 | PI 196622 | 802 | Virginia | Virginia | hypogaea | Virginia | . | 50.9 | 26.1 | 61.8 | 5.28 | 24.6 |
| 9 | PI 196635 | 270 | Runner | Runner | hypogaea | Virginia | . | 32.8 | 26.9 | 64.4 | 4.57 | 24.9 |
| 10 | PI 200441 | 266 | Spanish | Spanish | fastigiata | Spanish | vulgaris | 42.4 | 28.7 | 69.0 | 3.18 | 25.3 |
| 11 | PI 240560 | 725 | . | Runner | hypogaea | Spanish | . | 38.2 | 29.4 | 67.5 | 4.00 | 27.4 |
| 12 | PI 259617 | 508 | . | Mixed | fastigiata | Valencia | fastigiata | . | 11.4 | 57.2 | 9.60 | 17.6 |
| 13 | PI 259658 | 506 | Runner | Runner | hypogaea | Virginia | . | . | 24.7 | 64.8 | 6.77 | 26.0 |
| 14 | PI 259836 | 546 | Spanish | Spanish | fastigiata | Valencia | . | 30.8 | 13.0 | 58.8 | 8.58 | 17.9 |
| 15 | PI 259851 | 277 | Virginia | Virginia | hypogaea | Virginia | hypogaea | 59.8 | 26.2 | 65.4 | 5.14 | 25.2 |
| 16 | PI 262038 | 408 | Valencia | Valencia | fastigiata | Valencia | . | 34.3 | 10.3 | 57.4 | 10.00 | 17.3 |
| 17 | PI 268586 | 580 | Valencia | Valencia | hypogaea | Virginia | . | 48.1 | 18.6 | 57.3 | 0.99 | 13.2 |
| 18 | PI 268696 | 338 | Spanish | Spanish | hypogaea | Spanish | . | 38.5 | 30.1 | 69.0 | 3.04 | 26.7 |
| 19 | PI 268755 | 481 | . | Runner | hypogaea | Spanish | . | 49.5 | 28.6 | 66.8 | 3.22 | 24.8 |
| 20 | PI 268806 | 477 | Spanish | Spanish | hypogaea | Spanish | . | 49.0 | 29.7 | 67.7 | 3.27 | 26.5 |
| 21 | PI 268868 | 367 | Virginia | Virginia | hypogaea | Virginia | fastigiata | 46.2 | 29.3 | 67.9 | 1.91 | 23.2 |
| 22 | PI 268996 | 458 | . | Runner | hypogaea | Virginia | . | 38.7 | 26.7 | 64.0 | 4.75 | 24.8 |
| 23 | PI 270786 | 485 | . | Mixed | hypogaea | Spanish | . | 38.5 | 25.9 | 51.0 | −0.89 | 11.9 |
| 24 | PI 270905 | 446 | . | Mixed | hypogaea | Virginia | . | 48.8 | 25.3 | 64.0 | 5.17 | 24.0 |
| 25 | PI 270907 | 433 | . | Mixed | hypogaea | Virginia | hypogaea | 47.1 | 27.1 | 65.4 | 4.06 | 24.4 |
| 26 | PI 270998 | 468 | . | Mixed | vulgaris | Spanish | . | . | 16.6 | 61.2 | 5.68 | 17.7 |
| 27 | PI 271019 | 579 | . | Mixed | vulgaris | Spanish | . | 35.0 | 28.2 | 68.0 | 4.55 | 27.1 |
| 28 | PI 274193 | 208 | Virginia | Virginia | hypogaea | Spanish | vulgaris | 52.3 | 6.6 | 54.0 | 9.50 | 14.7 |
| 29 | PI 288146 | 516 | Virginia | Virginia | vulgaris | Spanish | . | 36.8 | 28.7 | 66.8 | 3.87 | 26.2 |
| 30 | PI 288210 | 526 | . | Runner | vulgaris | Virginia | hypogaea | 31.7 | 31.1 | 65.6 | −0.58 | 18.7 |
| 31 | PI 290536 | 233 | Virginia | . | hypogaea | Virginia | hypogaea | 40.1 | 26.4 | 65.2 | 4.42 | 24.2 |
| 32 | PI 290560 | 221 | . | Spanish | vulgaris | Spanish | vulgaris | 36.0 | 31.1 | 68.4 | 2.49 | 26.8 |
| 33 | PI 290566 | 227 | Runner | Runner | fastigiata | Valencia | fastigiata | 43.2 | 26.6 | 64.0 | 3.78 | 23.0 |
| 34 | PI 290594 | 230 | Runner | Runner | hypogaea | Valencia | fastigiata | 48.8 | 26.4 | 65.9 | 5.83 | 26.6 |
| 35 | PI 290620 | 223 | Virginia | Virginia | fastigiata | Spanish | vulgaris | 44.8 | 28.8 | 63.5 | 2.34 | 22.6 |
| 36 | PI 292950 | 728 | Runner | Mixed | hypogaea | Virginia | hypogaea | 67.8 | 45.6 | 71.8 | −4.63 | 27.1 |
| 37 | PI 295250 | 540 | Virginia | Virginia | hypogaea | Virginia | hypogaea | 44.8 | 9.8 | 58.6 | 11.37 | 18.1 |
| 38 | PI 295309 | 541 | . | Mixed | hypogaea | Virginia | hypogaea | 56.9 | 26.0 | 64.0 | 4.30 | 23.3 |
| 39 | PI 295730 | 8 | Virginia | Virginia | fastigiata | Valencia | vulgaris | 41.2 | 27.2 | 65.4 | 4.16 | 24.7 |
| 40 | PI 296550 | 534 | . | Runner | hypogaea | Virginia | hypogaea | 78.7 | 29.3 | 67.2 | 2.99 | 25.2 |
| 41 | PI 296558 | 535 | . | Runner | hypogaea | Virginia | hypogaea | 56.2 | 27.1 | 67.7 | 4.23 | 25.2 |
| 42 | PI 298854 | 342 | . | Runner | hypogaea | Virginia | hypogaea | 80.9 | 24.0 | 62.5 | 6.55 | 24.5 |
| 43 | PI 313129 | 381 | . | Mixed | fastigiata | Valencia | . | 46.7 | 31.1 | 67.9 | 2.61 | 26.8 |
| 44 | PI 319768 | 529 | Virginia | Virginia | hypogaea | Virginia | hypogaea | 45.2 | 28.4 | 61.5 | 4.99 | 26.7 |
| 45 | PI 319770 | . | . | . | . | . | vulgaris | 44.2 | 34.0 | 66.1 | 0.82 | 26.0 |
| 46 | PI 323268 | 812 | Virginia | Virginia | hypogaea | Virginia | . | 72.0 | 25.6 | 63.1 | 5.01 | 24.0 |
| 47 | PI 325943 | 548 | Valencia | Valencia | hypogaea | Valencia | fastigiata | 42.9 | 8.9 | 56.9 | 11.09 | 17.2 |
| 48 | PI 331297 | 202 | . | Mixed | hypogaea | Virginia | hypogaea | . | 10.4 | 53.9 | 9.74 | 16.6 |
| 49 | PI 331314 | 187 | . | Mixed | hypogaea | Valencia | vulgaris | 33.4 | 17.2 | 51.6 | 9.80 | 20.6 |
| 50 | PI 337293 | 431 | Valencia | . | hypogaea | Spanish | . | 44.0 | 25.0 | 57.5 | 2.04 | 17.6 |
| 51 | PI 337399 | 808 | Spanish | . | hypogaea | Spanish | . | 40.8 | 27.3 | 66.9 | 4.57 | 25.8 |
| 52 | PI 337406 | 310 | Runner | Runner | fastigiata | Spanish | vulgaris | 39.9 | 30.7 | 51.5 | −1.75 | 12.0 |
| 53 | PI 338338 | 552 | . | Valencia | peruviana | . | . | 34.1 | 14.5 | 54.7 | 1.70 | 12.2 |
| 54 | PI 339960 | 189 | Valencia | Valencia | fastigiata | Valencia | fastigiata | 54.3 | 9.3 | 58.1 | 11.75 | 18.0 |
| 55 | PI 343384 | 249 | Intermediate | Mixed | hypogaea | Virginia | hypogaea | 57.3 | 13.3 | 56.2 | 9.59 | 18.5 |
| 56 | PI 343398 | 246 | . | Virginia | fastigiata | Virginia | . | 63.0 | 25.9 | 62.9 | 5.14 | 24.4 |
| 57 | PI 355268 | 805 | Virginia | . | hypogaea | Virginia | . | 45.4 | 25.4 | 62.8 | 5.45 | 24.3 |
| 58 | PI 355271 | 287 | . | Runner | hypogaea | Virginia | . | 58.1 | 24.4 | 65.8 | 5.25 | 23.6 |
| 59 | PI 356004 | 488 | . | Mixed | fastigiata | Valencia | . | 38.2 | 11.1 | 57.3 | 9.86 | 17.6 |
| 60 | PI 370331 | 542 | Virginia | Virginia | hypogaea | Virginia | hypogaea | . | 9.6 | 54.9 | 9.00 | 15.9 |
| 61 | PI 371521 | 255 | . | . | hypogaea | Virginia | hypogaea | . | 25.8 | 65.6 | 4.81 | 24.3 |
| 62 | PI 372271 | 294 | Virginia | Virginia | hypogaea | Valencia | fastigiata | 45.8 | 25.7 | 64.4 | 4.94 | 24.1 |
| 63 | PI 372305 | 698 | Virginia | Virginia | hypogaea | Virginia | . | 43.7 | 28.7 | 66.4 | 3.10 | 24.5 |
| 64 | PI 399581 | 296 | Virginia | Virginia | hypogaea | Virginia | hypogaea | 52.6 | 28.0 | 61.9 | 4.82 | 26.0 |
| 65 | PI 403813 | 588 | Spanish | Valencia | vulgaris | Valencia | vulgaris | 36.0 | 10.2 | 55.5 | 10.73 | 17.5 |
| 66 | PI 407667 | 740 | Spanish | Spanish | vulgaris | Spanish | . | 59.0 | 26.2 | 66.2 | 4.13 | 23.7 |
| 67 | PI 408743 | 631 | Intermediate | Mixed | . | Spanish | vulgaris | . | 46.3 | 71.5 | −4.93 | 23.7 |
| 68 | PI 429420 | 787 | Valencia | Valencia | fastigiata | Valencia | . | 44.3 | 9.0 | 55.4 | 10.89 | 16.9 |
| 69 | PI 433347 | 643 | Spanish | . | . | Virginia | hypogaea | . | 33.0 | 62.3 | 0.54 | 22.8 |
| 70 | PI 442768 | 763 | Virginia | Virginia | hypogaea | Virginia | hypogaea | 43.0 | 29.0 | 64.2 | 4.01 | 26.2 |
| 71 | PI 461427 | 647 | . | . | hypogaea | Valencia | fastigiata | 47.3 | 11.3 | 57.3 | 10.02 | 17.8 |
| 72 | PI 461434 | 798 | . | Runner | hypogaea | runner | vulgaris | 47.3 | 29.8 | 69.1 | 3.24 | 26.8 |
| 73 | PI 468271 | . | . | . | . | Virginia | hypogaea | . | 47.2 | 59.9 | −4.40 | 16.4 |
| 74 | PI 471952 | 760 | Spanish | Spanish | hypogaea | Virginia | hypogaea | 73.5 | 24.1 | 63.1 | 5.82 | 23.7 |
| 75 | PI 471954 | 781 | Valencia | Valencia | fastigiata | Valencia | fastigiata | 41.0 | 28.4 | 64.2 | 5.16 | 27.6 |
| 76 | PI 475863 | 87 | Valencia | Valencia | fastigiata | Valencia | fastigiata | 38.5 | 18.5 | 61.5 | 8.26 | 21.6 |
| 77 | PI 475918 | 605 | . | . | fastigiata | Valencia | . | 37.3 | 12.0 | 58.9 | 9.71 | 18.2 |
| 78 | PI 475931 | 610 | Virginia | Virginia | . | Valencia | fastigiata | . | 29.7 | 57.6 | −1.14 | 14.5 |
| 79 | PI 476025 | 711 | . | . | fastigiata | Valencia | . | 56.7 | 20.3 | 54.6 | 0.23 | 12.4 |
| 80 | PI 476432 | 703 | Intermediate | Mixed | hypogaea | Spanish | . | . | 7.2 | 58.1 | 10.68 | 16.2 |
| 81 | PI 476596 | . | . | Runner | . | . | . | . | . | . | . | . |
| 82 | PI 476636 | 678 | Virginia | Virginia | hypogaea | Virginia | . | 50.5 | 29.6 | 62.9 | 2.77 | 24.2 |
| 83 | PI 478819 | 650 | Valencia | Valencia | vulgaris | Virginia | . | 55.4 | 28.1 | 65.3 | 3.47 | 24.4 |
| 84 | PI 478850 | 747 | . | Valencia | fastigiata | Valencia | peruviana | 33.4 | 8.2 | 56.5 | 12.64 | 17.8 |
| 85 | PI 481795 | 673 | Spanish | Spanish | hypogaea | Spanish | . | 34.8 | 27.2 | 66.5 | 4.43 | 25.4 |
| 86 | PI 482120 | 775 | . | Spanish | hypogaea | . | . | 36.6 | 27.0 | 67.3 | 5.44 | 27.0 |
| 87 | PI 482189 | 755 | Spanish | Spanish | fastigiata | Valencia | fastigiata | 41.0 | 28.6 | 67.0 | 3.82 | 26.0 |
| 88 | PI 493329 | 12 | Valencia | Valencia | fastigiata | Valencia | fastigiata | 40.9 | 26.0 | 61.3 | 3.68 | 21.8 |
| 89 | PI 493356 | 16 | Virginia | Virginia | fastigiata | Valencia | fastigiata | 34.2 | 9.7 | 57.6 | 10.57 | 17.4 |
| 90 | PI 493547 | 33 | Valencia | Valencia | fastigiata | . | . | 37.6 | 11.1 | 55.8 | 9.92 | 17.4 |
| 91 | PI 493581 | 38 | Valencia | Valencia | fastigiata | Valencia | fastigiata | 39.7 | 12.2 | 58.0 | 9.27 | 17.8 |
| 92 | PI 493631 | 41 | Valencia | Valencia | fastigiata | . | . | 40.6 | 9.2 | 53.6 | 12.25 | 17.7 |
| 93 | PI 493693 | 47 | Virginia | Virginia | fastigiata | Valencia | fastigiata | 53.5 | 28.1 | 67.8 | 4.34 | 26.6 |
| 94 | PI 493717 | 50 | Valencia | Valencia | fastigiata | . | . | 52.0 | 26.9 | 66.7 | 5.85 | 27.4 |
| 95 | PI 493729 | 53 | . | . | fastigiata | . | . | 40.1 | 29.4 | 68.1 | 3.30 | 26.2 |
| 96 | PI 493880 | 68 | Valencia | Valencia | fastigiata | . | . | 50.7 | 5.1 | 54.5 | 15.37 | 17.4 |
| 97 | PI 493938 | 75 | . | . | fastigiata | . | . | 33.5 | 27.1 | 55.7 | −1.34 | 12.5 |
| 98 | PI 494018 | 80 | . | . | vulgaris | . | . | 35.2 | 29.4 | 50.8 | −1.73 | 11.4 |
| 99 | PI 494034 | 82 | Spanish | Spanish | vulgaris | . | . | 33.0 | 29.1 | 54.7 | −1.39 | 12.9 |
| 100 | PI 494795 | 166 | Runner | Runner | hypogaea | . | . | . | 31.2 | 67.1 | −0.85 | 18.5 |
| 101 | PI 496401 | 115 | Virginia | Virginia | hypogaea | . | . | 45.7 | 27.0 | 62.3 | 4.49 | 24.3 |
| 102 | PI 496448 | 119 | Virginia | Virginia | hypogaea | . | . | 47.5 | 26.2 | 63.3 | 5.67 | 25.6 |
| 103 | PI 497318 | 92 | . | . | hypogaea | Valencia | . | 42.9 | 22.4 | 59.3 | 3.03 | 17.7 |
| 104 | PI 497395 | 97 | Virginia | Virginia | hypogaea | . | . | . | 6.3 | 53.5 | 9.09 | 14.3 |
| 105 | PI 497517 | 112 | Valencia | Valencia | fastigiata | . | . | 37.5 | 9.8 | 56.6 | 11.11 | 17.7 |
| 106 | PI 497639 | 132 | Valencia | Valencia | fastigiata | Valencia | fastigiata | . | 32.3 | 69.2 | 2.46 | 28.7 |
| 107 | PI 497668 | . | . | . | . | . | . | . | . | . | . | . |
| 108 | PI 502037 | . | . | . | . | Valencia | peruviana | . | 35.2 | 65.4 | −1.11 | 21.3 |
| 109 | PI 502040 | 149 | Spanish | Spanish | fastigiata | . | . | 24.9 | 31.6 | 68.0 | 2.17 | 26.7 |
| 110 | PI 502111 | 155 | . | Valencia | peruviana | Valencia | peruviana | . | 23.5 | 55.4 | 5.05 | 20.5 |
| 111 | PI 502120 | 157 | . | Virginia | peruviana | . | . | 53.8 | 29.1 | 66.1 | 3.76 | 26.2 |
| 112 | PI 504614 | 125 | . | Mixed | hypogaea | Virginia | hypogaea | 53.7 | 28.5 | 67.3 | 3.79 | 25.8 |
Table 2.
Peanut crop growth stages, with respect to weeks after planting (WAP), when the measurements were taken.
Table 2.
Peanut crop growth stages, with respect to weeks after planting (WAP), when the measurements were taken.
| WAP | Crop Growth Stages | Measurements Taken |
|---|
| | | 2017 | 2018 |
|---|
| 0 | Planting (15 May 2017 and 13 May 2018) | | |
| 2 | Emergence | Stand count | Stand count |
| 3 | ![Agronomy 12 01945 i001 Agronomy 12 01945 i001]() | | Thrips damage |
| 4 | Plant height, lateral growth, aerial measurements | Plant height, lateral growth, NDVI, aerial measurements |
| 5 | Plant height, lateral growth, CTD, wilting | Plant height, lateral growth, NDVI, CTD, wilting |
| 6 | Beginning bloom | Plant height, lateral growth, NDVI, aerial measurements | Plant height, lateral growth, NDVI, aerial measurements |
| 7 | Beginning peg | Wilting, CTD | Wilting, CTD |
| 8 | Beginning pod | Aerial measurements | NDVI, aerial measurements |
| 9 | Pod development | Plant height, NDVI | Plant height, NDVI |
| 10 | Full pod | NDVI, CTD, wilting, disease rating, aerial measurements | NDVI, CTD, wilting, disease rating, aerial measurements |
| 11 | Beginning seed | | |
| 12 | | NDVI, CTD, disease rating, wilting, aerial measurements | NDVI, CTD, disease rating, wilting, aerial measurements |
| 13 | Full seed | | |
| 14 | Beginning maturity | Aerial measurements | Aerial measurements |
| 15 | | | |
| 16 | Digging (15 September 2017 and 17 September 2018) | | |
| 17 | Post-digging | Pod yield measurements, post-harvest sprouting | Pod yield measurements, post-harvest sprouting |
Table 3.
Spectral reflectance (red, green, blue, and near-infrared) derived using aerial images, and vegetation indices derived using reflectance (S. No. 1–24); and red-green-blue (RGB) color space indices derived from the same images using Breedpix software and indices derived using arithmetic combinations of color indices (S. no. 24–48).
Table 3.
Spectral reflectance (red, green, blue, and near-infrared) derived using aerial images, and vegetation indices derived using reflectance (S. No. 1–24); and red-green-blue (RGB) color space indices derived from the same images using Breedpix software and indices derived using arithmetic combinations of color indices (S. no. 24–48).
| S. No. | Indices | Full Name | Formula | Reference |
|---|
| 1 | | Red | Aerial leaf reflectance |
| 2 | | Green |
| 3 | | Blue |
| 4 | | Near-Infrared (NIR) |
| 5 | BGI | Blue green pigment index | | [66] |
| 6 | RGR | Red-Green ratio | | [67] |
| 7 | NPPR | Normalized Plant Pigment ratio | | [68] |
| 8 | NGRDI | Normalized Green Red Difference Index | | [69] |
| 9 | PPR | Plant Pigment Ratio | | [70] |
| 10 | NCPI | Normalized Pigment Chlorophyll Index | | [71] |
| 11 | NDVI | Normalized difference vegetation index | | [72] |
| 12 | SRI | Simple ratio index | | [73] |
| 13 | GRVI | Green Ratio Vegetation Index | | [74] |
| 14 | IO | Simple Ratio Red/Blue Iron Oxide | | [75] |
| 15 | GNDVI | Green Normalized difference vegetation index | | [76] |
| 16 | BNDVI | Blue Normalized difference vegetation index | | [77] |
| 17 | CIG | Chlorophyll index green | | [78] |
| 18 | CVI | Coloration index | | [79] |
| 19 | GLI | Green leaf index | | [80] |
| 20 | GBNDVI | Green-Blue NDVI | | [81] |
| 21 | GRNDVI | Green-Red NDVI | | [81] |
| 22 | RBNDVI | Red-Blue NDVI | | [81] |
| 23 | mSR | Modified Simple Ratio | | [82] |
| 24 | GARI | Green atmospherically resistant vegetation index | | [76] |
| 25 | | Intensity | Measures greyness in 0 (black) to 1 (white) scale in HSI color space | [83] |
| 26 | | Hue | Color judgement (in °) based on position in HSI color space | [83] |
| 27 | | Saturation | Measures dilution of pure color (hue) with white light within 0 to 1 | [83] |
| 28 | | Lightness | Light reflected by a non-luminous body [0 (black) to 100 (white)] | [84] |
| 29 | | a* | color shift from green (−a) to red (+a) in CIE-Lab color space | [84] |
| 30 | | b* | color shift from blue (−b) to yellow (+b) in CIE-Lab color space | [84] |
| 31 | | u* | color shift from green (−a) to red (+a) in CIE-Luv color space | [84] |
| 32 | | v* | color shift from blue (−b) to yellow (+b) in CIE-Luv color space | [84] |
| 33 | GA | Green area | Percentage of pixels in 60°–120° hue angle in CIE-Lab | [85] |
| 34 | GGA | Greener area | Percentage of pixels in 80°–120° hue angle in CIE-Lab | [85] |
| 35 | CSI | Crop senescence index | | [86] |
| 36 | ab | | a* × b* | [28] |
| 37 | uv | | u* × u* | [28] |
| 38 | abI | ab Index | | |
| 39 | uvI | uv Index | | |
| 40 | auI | au Index | | |
| 41 | bvI | bv Index | | |
| 42 | NDabI | Normalized difference ab Index | | |
| 43 | NDuvI | Normalized difference uv Index | | |
| 44 | NDLab | Normalized difference CIELab Index | | [87] |
| 45 | NDLuv | Normalized difference CIELuv Index | | [87] |
| 46 | GI | Greenness Index | | |
| 47 | GPI | Greenness product index | GA × GGA | |
| 48 | NDGI | Normalized difference greenness Index | | |
Table 4.
a: Analysis of variance for the effect of genotype, year, and their interaction on morphological, physiological, and agronomic characteristics, measured in 2017 and 2018 on the U.S. peanut germplasm mini-core collection. b: Analysis of variance for the effect of variety, year, and their interaction on morphological, physiological, and agronomic characteristics, measured in 2017 and 2018 on the U.S. peanut germplasm mini-core collection.
Table 4.
a: Analysis of variance for the effect of genotype, year, and their interaction on morphological, physiological, and agronomic characteristics, measured in 2017 and 2018 on the U.S. peanut germplasm mini-core collection. b: Analysis of variance for the effect of variety, year, and their interaction on morphological, physiological, and agronomic characteristics, measured in 2017 and 2018 on the U.S. peanut germplasm mini-core collection.
| Source of Variation | | Stand Count | Plant Height | Lateral Growth | NDVI | CTD | Wilting | TSW | SSR | SB | CBR | Thrips Damage | Pod Yield | Sprouting |
|---|
| | DF | p-Value |
| year | 1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| block | 2 | 0.001 | 0.110 | <0.0001 | 0.703 | <0.0001 | <0.0001 | 0.0003 | <0.0001 | 0.619 | 0.009 | 0.248 | 0.211 | <0.0001 |
| genotype | 102 | <0.0001 | <0.0001 | <0.0001 | 0.074 | 0.114 | <0.0001 | <0.0001 | 0.039 | 0.232 | 0.329 | 0.678 | <0.0001 | 0.005 |
| year*genotype | 87 | <0.0001 | <0.0001 | 0.0041 | 0.461 | 0.003 | 0.366 | 0.409 | 0.011 | 0.049 | 0.658 | 0.303 | <0.0001 | 0.766 |
| Error | 381 | | | | | | | | | | | | | |
| year | 1 | 0.164 | 0.061 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.005 |
| block | 2 | 0.193 | 0.236 | <0.0001 | 0.701 | <0.0001 | 0.0016 | 0.001 | <0.0001 | 0.619 | 0.01 | 0.249 | 0.425 | <0.0001 |
| variety | 3 | 0.090 | 0.0003 | 0.925 | 0.2159 | 0.527 | <0.0001 | 0.406 | 0.454 | 0.092 | 0.839 | 0.512 | 0.096 | 0.11 |
| year*variety | 3 | 0.092 | 0.013 | 0.129 | 0.6538 | 0.315 | 0.9781 | 0.262 | 0.427 | 0.097 | 0.668 | 0.512 | 0.283 | 0.976 |
| Error | 566 | | | | | | | | | | | | | |
Table 5.
Plant growth and yield parameters (stand count, plant height, lateral growth, normalized ifference vegetation index (NDVI), canopy temperature depression (CTD), leaf wilting, pod yield, and post-harvest sprouting) of 104 mini-core genotypes of peanut in 2017, and 88 in 2018. The plant height, lateral growth, and NDVI, are measured at maximum vegetative growth [6 weeks after planting (WAP). Leaf wilting and canopy temperature depression (CTD) are the average of two dates (10 and 12 WAP in 2017 and 5 to 7 WAP in 2018), with highest values corresponding to sudden droughts. The values followed by the same letters are not significantly different using Fisher’s protected LSD at α = 0.05.
Table 5.
Plant growth and yield parameters (stand count, plant height, lateral growth, normalized ifference vegetation index (NDVI), canopy temperature depression (CTD), leaf wilting, pod yield, and post-harvest sprouting) of 104 mini-core genotypes of peanut in 2017, and 88 in 2018. The plant height, lateral growth, and NDVI, are measured at maximum vegetative growth [6 weeks after planting (WAP). Leaf wilting and canopy temperature depression (CTD) are the average of two dates (10 and 12 WAP in 2017 and 5 to 7 WAP in 2018), with highest values corresponding to sudden droughts. The values followed by the same letters are not significantly different using Fisher’s protected LSD at α = 0.05.
| Genotypes | Stand Count (Plants/Plot) | Plant Height (cm) | Lateral Growth (cm) | NDVI (0–1) | CTD (°C) | Leaf Wilting (0–5) | Pod Yield
(kg ha−1) | Sprouting
(#/Plot) |
|---|
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 |
|---|
| Wynne | 33 | k-s | 16 | j | 24 | h-t | 26 | a | 49 | a-k | 67 | a | 0.90 | a-d | 0.69 | a | −0.8 | e-u | 3.2 | a-d | 1.2 | f-j | 1.5 | h | 8253 | a-d | 6276 | a | 0 | a | 0 | h |
| Walton | 40 | c-q | 36 | c-i | 24 | f-t | 28 | a | 53 | a-j | 68 | a | 0.89 | a-d | 0.74 | a | −0.4 | b-q | 1.8 | d-r | 1.2 | f-j | 2.0 | b-h | 8459 | a | 6915 | a | 2 | a | 0 | h |
| TVOL14 | 47 | a-m | 50 | a-g | 32 | a-p | 31 | a | 51 | a-k | 64 | a | 0.89 | a-d | 0.69 | a | −0.1 | a-k | 1.0 | qr | 1.6 | c-j | 2.7 | a-h | 5915 | a-j | 2424 | f-s | 3 | a | 4 | gh |
| TS90 | 49 | a-j | 52 | a-e | 30 | a-r | 29 | a | 51 | a-k | 72 | a | 0.88 | a-d | 0.75 | a | −0.6 | b-t | 2.5 | b-o | 2.1 | a-i | 3.0 | a-h | 7056 | a-h | 2702 | c-r | 6 | a | 5 | gh |
| TROL11 | 32 | l-s | 43 | a-i | 22 | j-t | 23 | a | 46 | a-l | 58 | a | 0.85 | cd | 0.67 | a | 0.5 | a-c | 3.6 | a-c | 1.1 | g-j | 2.9 | a-h | 5999 | a-j | 3260 | c-m | 5 | a | 8 | e-h |
| NMVAL | 53 | a-e | 52 | a-c | 34 | a-l | 28 | a | 52 | a-j | 59 | a | 0.87 | a-d | 0.75 | a | −0.4 | b-q | 1.4 | k-r | 2.1 | a-j | 3.3 | a-e | 4667 | a-j | 1627 | rs | 1 | a | 28 | b-d |
| CC812 | 47 | a-m | 39 | b-i | 28 | b-s | 27 | a | 61 | a-e | 58 | a | 0.88 | a-d | 0.76 | a | 0.2 | a-f | 2.2 | c-q | 1.1 | g-j | 1.7 | f-h | 6214 | a-j | 3766 | b-g | 1 | a | 0 | h |
| CC808 | 53 | a-e | 53 | a-c | 33 | a-m | 28 | a | 51 | a-k | 62 | a | 0.87 | a-d | 0.78 | a | 0.8 | a | 1.8 | f-r | 2.4 | a-e | 2.8 | a-h | 4829 | a-j | 2874 | c-r | 3 | a | 13 | c-h |
| CC805 | 49 | a-j | 37 | c-i | 24 | h-t | 31 | a | 52 | a-j | 64 | a | 0.88 | a-d | 0.77 | a | −0.8 | f-u | 2.6 | b-n | 1.1 | g-j | 2.1 | b-h | 5481 | a-j | 2860 | c-r | 1 | a | 0 | h |
| CC802 | 52 | a-g | 51 | a-f | 28 | b-s | 26 | a | 48 | a-k | 63 | a | 0.89 | a-d | 0.79 | a | −0.8 | e-u | 2.4 | c-p | 1.3 | e-j | 2.7 | a-h | 4768 | a-j | 2702 | c-r | 3 | a | 7 | f-h |
| CC798 | 51 | a-h | 50 | a-f | 31 | a-r | 28 | a | 50 | a-k | 55 | a | 0.89 | a-d | 0.80 | a | −1.7 | tu | 2.8 | a-k | 1.5 | c-j | 2.8 | a-h | 4911 | a-j | 4004 | bc | 3 | a | 9 | e-h |
| CC787 | 47 | a-m | 43 | a-i | 38 | a-g | 28 | a | 59 | a-f | 64 | a | 0.87 | a-d | 0.75 | a | −0.2 | a-n | 1.7 | h-r | 2.0 | a-j | 3.0 | a-h | 4640 | b-j | 2311 | i-s | 4 | a | 2 | gh |
| CC781 | 50 | a-i | 53 | a-c | 37 | a-h | 28 | a | 61 | a-d | 64 | a | 0.88 | a-d | 0.71 | a | −0.3 | a-q | 2.5 | b-o | 2.4 | a-e | 3.3 | a-d | 5047 | a-j | 2602 | d-s | 1 | a | 28 | b-d |
| CC775 | 37 | f-r | 38 | c-i | 32 | a-o | 28 | a | 52 | a-j | 66 | a | 0.88 | a-d | 0.71 | a | −0.4 | b-q | 3.8 | ab | 1.5 | c-j | 2.2 | a-h | 4476 | d-j | 1747 | p-s | 2 | a | 4 | gh |
| CC760 | 49 | a-j | 42 | a-i | 43 | a | 27 | a | 65 | a | 71 | a | 0.86 | a-d | 0.66 | a | −0.2 | a-m | 1.1 | p-r | 2.7 | a-c | 3.6 | a | 5902 | a-j | 3147 | c-o | 5 | a | 30 | bc |
| CC755 | 52 | a-g | 47 | a-i | 36 | a-i | 25 | a | 60 | a-e | 62 | a | 0.86 | b-d | 0.75 | a | 0.6 | ab | 2.8 | a-k | 2.3 | a-g | 3.3 | a-d | 4550 | c-j | 2425 | f-s | 1 | a | 4 | gh |
| CC740 | 50 | a-h | 43 | a-i | 27 | d-s | 25 | a | 42 | a-l | 58 | a | 0.88 | a-d | 0.71 | a | −1.4 | p-u | 1.4 | l-r | 2.0 | a-j | 2.4 | a-h | 6423 | a-i | 2964 | c-r | 6 | a | 27 | b-e |
| CC725 | 50 | a-h | 51 | a-e | 38 | a-f | 26 | a | 49 | a-k | 62 | a | 0.88 | a-d | 0.74 | a | −0.8 | f-u | 2.3 | c-q | 1.3 | e-j | 2.6 | a-h | 5176 | a-j | 2776 | c-r | 8 | a | 28 | b-d |
| CC711 | 38 | e-r | 32 | h-j | 27 | b-s | 28 | a | 46 | a-l | 68 | a | 0.88 | a-d | 0.74 | a | −0.2 | a-n | 2.9 | a-j | 1.7 | b-j | 2.3 | a-h | 4094 | f-j | 2284 | i-s | 2 | a | 0 | h |
| CC703B | 39 | d-q | 50 | a-h | 23 | i-t | 26 | a | 52 | a-j | 57 | a | 0.90 | a-d | 0.77 | a | −1.2 | k-u | 1.7 | i-r | 1.0 | h-j | 1.8 | e-h | 6593 | a-i | 3711 | b-h | 1 | a | 0 | h |
| CC703A | 48 | a-j | 50 | a-g | 28 | b-s | 27 | a | 40 | b-l | 59 | a | 0.87 | a-d | 0.77 | a | −1.4 | n-u | 1.7 | g-r | 2.1 | a-i | 2.7 | a-h | 5210 | a-j | 3386 | b-m | 1 | a | 0 | h |
| CC698 | 23 | r-u | 40 | a-i | 19 | n-t | 28 | a | 31 | j-l | 57 | a | 0.89 | a-d | 0.73 | a | −0.7 | e-u | 2.7 | a-m | 0.8 | j | 1.8 | e-h | 3522 | g-j | 3360 | c-m | 1 | a | 0 | h |
| CC678 | 15 | t-v | 41 | a-i | 18 | o-t | 28 | a | 36 | f-l | 55 | a | 0.89 | a-d | 0.76 | a | −1.0 | g-u | 2.4 | b-p | 1.0 | h-j | 2.0 | b-h | 5327 | a-j | 3475 | b-k | 5 | a | 0 | h |
| CC673 | 50 | a-i | 51 | a-e | 32 | a-o | 24 | a | 48 | a-k | 55 | a | 0.88 | a-d | 0.71 | a | −0.7 | c-t | 2.7 | a-l | 1.4 | d-j | 2.3 | a-h | 4465 | d-j | 3141 | c-o | 4 | a | 8 | e-h |
| CC650 | 48 | a-k | 42 | a-i | 29 | b-s | 29 | a | 50 | a-k | 56 | a | 0.88 | a-d | 0.73 | a | −1.1 | i-u | 2.3 | c-q | 1.3 | e-j | 2.3 | a-h | 8351 | a-c | 1772 | n-s | 1 | a | 5 | gh |
| CC643 | 28 | p-t | 43 | a-i | 28 | b-s | 27 | a | 52 | a-j | 66 | a | 0.89 | a-d | 0.69 | a | −0.3 | a-q | 2.2 | d-r | 2.1 | a-i | 3.3 | a-e | 5373 | a-j | 2382 | g-s | 3 | a | 2 | gh |
| CC605 | 56 | ab | 52 | a-d | 41 | a-c | 27 | a | 64 | ab | 59 | a | 0.89 | a-d | 0.71 | a | −0.7 | e-u | 2.2 | c-q | 2.8 | ab | 3.0 | a-h | 5650 | a-j | 2824 | c-r | 3 | a | 13 | c-h |
| CC588 | 58 | a | 47 | a-i | 40 | a-d | 31 | a | 54 | a-j | 63 | a | 0.88 | a-d | 0.66 | a | −0.3 | a-q | 2.2 | c-q | 3.0 | a | 3.0 | a-h | 4247 | f-j | 2327 | h-s | 6 | a | 30 | bc |
| CC580 | 52 | a-f | 53 | a-c | 35 | a-k | 25 | a | 53 | a-j | 67 | a | 0.86 | a-d | 0.72 | a | −0.6 | b-s | 2.9 | a-j | 1.8 | a-j | 2.9 | a-h | 4925 | a-j | 2918 | c-r | 1 | a | 6 | gh |
| CC579 | 49 | a-j | 49 | a-h | 32 | a-p | 29 | a | 43 | a-l | 66 | a | 0.87 | a-d | 0.67 | a | −0.5 | b-q | 1.9 | d-r | 1.5 | c-j | 2.2 | a-h | 4815 | a-j | 2702 | c-r | 4 | a | 7 | f-h |
| CC559 | 48 | a-j | 36 | c-i | 24 | h-t | 27 | a | 37 | e-l | 55 | a | 0.90 | a-d | 0.68 | a | −0.6 | b-r | 1.8 | f-r | 1.3 | e-j | 1.8 | d-h | 3743 | g-j | 2101 | k-s | 2 | a | 0 | h |
| CC553 | 53 | a-d | 50 | a-g | 33 | a-m | 26 | a | 47 | a-l | 61 | a | 0.88 | a-d | 0.74 | a | −1.7 | s-u | 1.8 | e-r | 1.1 | g-j | 2.1 | b-h | 6291 | a-j | 2425 | f-s | 1 | a | 10 | d-h |
| CC548 | 36 | h-s | 44 | a-i | 26 | d-s | 28 | a | 56 | a-i | 61 | a | 0.88 | a-d | 0.71 | a | −0.3 | a-q | 2.3 | c-q | 1.0 | h-j | 1.5 | h | 6446 | a-i | 3523 | b-j | 0 | a | 0 | h |
| CC546 | 52 | a-e | 51 | a-e | 35 | a-l | 27 | a | 49 | a-k | 59 | a | 0.87 | a-d | 0.71 | a | −1.9 | u | 2.6 | b-n | 2.2 | a-h | 2.7 | a-h | 4700 | a-j | 2354 | h-s | 2 | a | 9 | e-h |
| CC535 | 51 | a-h | 33 | f-j | 32 | a-q | 25 | a | 45 | a-l | 61 | a | 0.90 | a-c | 0.70 | a | −0.7 | d-t | 3.1 | a-g | 1.0 | h-j | 1.5 | h | 6792 | a-i | 3161 | c-n | 0 | a | 0 | h |
| CC529 | 47 | a-l | 47 | a-i | 24 | g-t | 26 | a | 38 | d-l | 63 | a | 0.91 | a | 0.66 | a | −0.2 | a-n | 2.7 | a-m | 1.3 | e-j | 2.1 | b-h | 5032 | a-j | 2977 | c-r | 1 | a | 4 | gh |
| CC526 | 31 | n-s | 56 | ab | 26 | f-t | 30 | a | 44 | a-l | 65 | a | 0.88 | a-d | 0.77 | a | 0.2 | a-g | 0.8 | r | 1.9 | a-j | 3.1 | a-g | 5480 | a-j | 3186 | c-m | 1 | a | 17 | b-h |
| CC508 | 52 | a-e | 50 | a-g | 38 | a-g | 24 | a | 54 | a-j | 65 | a | 0.87 | a-d | 0.71 | a | −0.7 | e-u | 2.4 | c-p | 1.8 | b-j | 2.8 | a-h | 5653 | a-j | 2989 | c-r | 0 | a | 2 | gh |
| CC488 | 55 | ab | 52 | a-d | 40 | a-e | 24 | a | 53 | a-j | 65 | a | 0.89 | a-d | 0.79 | a | −0.2 | a-o | 2.3 | c-q | 2.3 | a-f | 3.3 | a-e | 5507 | a-j | 2523 | e-s | 3 | a | 20 | b-g |
| CC485 | 39 | d-q | 44 | a-i | 31 | a-r | 29 | a | 49 | a-k | 58 | a | 0.88 | a-d | 0.70 | a | 0.4 | a-e | 1.7 | i-r | 2.3 | a-g | 2.6 | a-h | 4152 | f-j | 1628 | rs | 2 | a | 8 | e-h |
| CC481 | 46 | a-n | 47 | a-i | 31 | a-r | 27 | a | 51 | a-k | 69 | a | 0.89 | a-d | 0.72 | a | 0.1 | a-h | 1.4 | k-r | 1.6 | c-j | 2.7 | a-h | 5050 | a-j | 3414 | b-l | 7 | a | 18 | b-h |
| CC477 | 50 | a-i | 52 | a-d | 30 | a-r | 29 | a | 45 | a-l | 65 | a | 0.86 | a-d | 0.73 | a | 0.0 | a-i | 2.1 | d-r | 1.4 | d-j | 2.5 | a-h | 4427 | e-j | 3857 | b-e | 8 | a | 17 | b-h |
| CC458 | 35 | i-s | 47 | a-i | 18 | p-t | 24 | a | 43 | a-l | 58 | a | 0.90 | a-c | 0.74 | a | −0.2 | a-n | 2.0 | d-r | 1.0 | h-j | 1.5 | h | 4340 | e-j | 2931 | c-r | 3 | a | 0 | h |
| CC446 | 50 | a-i | 53 | a-c | 34 | a-l | 25 | a | 42 | a-l | 61 | a | 0.85 | d | 0.72 | a | −1.2 | k-u | 2.5 | b-p | 1.8 | a-j | 2.3 | a-h | 4139 | f-j | 2079 | l-s | 6 | a | 11 | d-h |
| CC431 | 50 | a-i | 46 | a-i | 30 | a-s | 27 | a | 46 | a-l | 58 | a | 0.88 | a-d | 0.70 | a | −0.6 | c-t | 3.1 | a-i | 1.8 | a-j | 2.8 | a-h | 4982 | a-j | 1758 | o-s | 0 | a | 14 | c-h |
| CC408 | 49 | a-j | 49 | a-h | 36 | a-i | 27 | a | 56 | a-h | 59 | a | 0.88 | a-d | 0.73 | a | 0.0 | a-j | 1.3 | m-r | 2.6 | a-d | 3.2 | a-f | 5881 | a-j | 2496 | e-s | 5 | a | 30 | bc |
| CC406 | 51 | a-h | 48 | a-h | 30 | a-s | 28 | a | 42 | a-l | 66 | a | 0.89 | a-d | 0.74 | a | −0.4 | b-q | 2.1 | d-r | 1.4 | d-j | 2.5 | a-h | 5806 | a-j | 3600 | b-i | 3 | a | 3 | gh |
| CC388 | 49 | a-j | 44 | a-i | 29 | b-s | 25 | a | 48 | a-k | 63 | a | 0.87 | a-d | 0.93 | a | 0.3 | a-f | 2.7 | b-m | 1.9 | a-j | 2.8 | a-h | 5263 | a-j | 2867 | c-r | 1 | a | 30 | bc |
| CC384 | 54 | a-c | 48 | a-i | 34 | a-l | 24 | a | 46 | a-l | 64 | a | 0.88 | a-d | 0.60 | a | 0.2 | a-g | 2.5 | b-o | 1.3 | e-j | 2.0 | b-h | 4341 | e-j | 3022 | c-q | 5 | a | 5 | gh |
| CC381 | 46 | a-n | 34 | e-j | 24 | h-t | 29 | a | 49 | a-k | 60 | a | 0.88 | a-d | 0.64 | a | −1.3 | l-u | 3.1 | a-h | 1.0 | h-j | 1.8 | e-h | 6070 | a-j | 2563 | d-s | 0 | a | 0 | h |
| CC342 | 46 | a-n | 34 | e-j | 26 | e-t | 31 | a | 63 | a-c | 70 | a | 0.89 | a-d | 0.75 | a | −0.2 | a-o | 2.4 | c-p | 1.1 | g-j | 1.6 | gh | 7030 | a-h | 2456 | f-s | 1 | a | 0 | h |
| CC338 | 52 | a-f | 46 | a-i | 33 | a-m | 28 | a | 42 | b-l | 61 | a | 0.88 | a-d | 0.76 | a | 0.2 | a-g | 2.3 | c-q | 1.9 | a-j | 2.3 | a-h | 4770 | a-j | 3121 | c-p | 1 | a | 25 | b-f |
| CC310 | 57 | a | 49 | a-h | 30 | a-r | 29 | a | 50 | a-k | 63 | a | 0.87 | a-d | 0.72 | a | 0.2 | a-g | 1.2 | o-r | 2.2 | a-h | 2.8 | a-h | 5483 | a-j | 3170 | c-m | 2 | a | 35 | ab |
| CC296 | 47 | a-k | 43 | a-i | 29 | a-s | 26 | a | 50 | a-k | 60 | a | 0.89 | a-d | 0.75 | a | −0.2 | a-o | 2.2 | d-r | 2.1 | a-i | 2.6 | a-h | 6463 | a-i | 2425 | f-s | 0 | a | 4 | gh |
| CC287 | 49 | a-j | 40 | a-i | 28 | b-s | 29 | a | 53 | a-j | 64 | a | 0.88 | a-d | 0.75 | a | −0.3 | a-p | 3.2 | a-e | 1.1 | g-j | 1.9 | c-h | 5697 | a-j | 2441 | f-s | 1 | a | 3 | gh |
| CC277 | 49 | a-j | 33 | e-j | 30 | a-r | 30 | a | 47 | a-l | 63 | a | 0.90 | ab | 0.68 | a | −1.4 | o-u | 2.3 | c-q | 1.2 | f-j | 1.8 | e-h | 5439 | a-j | 2707 | c-r | 1 | a | 0 | h |
| CC266 | 29 | o-t | 44 | a-i | 25 | f-t | 33 | a | 42 | a-l | 74 | a | 0.87 | a-d | 0.65 | a | −0.3 | a-q | 1.8 | f-r | 1.8 | b-j | 2.2 | a-h | 4076 | f-j | 3323 | c-m | 1 | a | 15 | c-h |
| CC249 | 48 | a-k | 44 | a-i | 34 | a-l | 28 | a | 47 | a-l | 72 | a | 0.90 | a-c | 0.78 | a | −0.6 | c-t | 1.9 | d-r | 1.1 | g-j | 1.9 | c-h | 6295 | a-j | 3154 | c-n | 1 | a | 1 | h |
| CC246 | 51 | a-g | 36 | c-i | 25 | f-t | 28 | a | 40 | c-l | 69 | a | 0.88 | a-d | 0.78 | a | −1.7 | r-u | 2.4 | b-p | 1.2 | f-j | 2.1 | b-h | 8095 | a-e | 2538 | e-s | 3 | a | 0 | h |
| CC233 | 47 | a-l | 45 | a-i | 21 | k-t | 29 | a | 46 | a-l | 61 | a | 0.89 | a-d | 0.73 | a | −0.5 | b-q | 1.3 | n-r | 1.0 | h-j | 1.8 | e-h | 6400 | a-j | 3198 | c-m | 0 | a | 0 | h |
| CC230 | 49 | a-j | 49 | a-h | 27 | b-s | 25 | a | 58 | a-h | 65 | a | 0.90 | a-c | 0.74 | a | −1.3 | k-u | 2.6 | b-o | 1.0 | h-j | 2.4 | a-h | 6660 | a-i | 3324 | c-m | 0 | a | 1 | h |
| CC227 | 47 | a-l | 38 | b-i | 25 | f-t | 32 | a | 48 | a-k | 70 | a | 0.89 | a-d | 0.69 | a | −1.2 | j-u | 2.5 | b-p | 1.3 | e-j | 2.5 | a-h | 6504 | a-i | 2626 | c-s | 1 | a | 18 | b-h |
| CC223 | 49 | a-j | 33 | e-j | 27 | c-s | 30 | a | 58 | a-g | 64 | a | 0.90 | a-d | 0.74 | a | −0.3 | a-q | 3.2 | a-f | 1.4 | d-j | 2.5 | a-h | 7222 | a-g | 2087 | k-s | 0 | a | 2 | gh |
| CC221 | 46 | a-n | 43 | a-i | 32 | a-p | 28 | a | 42 | a-l | 58 | a | 0.87 | a-d | 0.76 | a | −1.5 | q-u | 1.5 | k-r | 1.8 | a-j | 2.3 | a-h | 4317 | e-j | 2001 | m-s | 3 | a | 28 | b-d |
| CC208 | 52 | a-f | 30 | j | 24 | h-t | 24 | a | 48 | a-k | 60 | a | 0.89 | a-d | 0.73 | a | −1.0 | h-u | 2.3 | c-q | 1.8 | b-j | 1.8 | d-h | 4455 | d-j | 2111 | k-s | 0 | a | 1 | h |
| CC202 | 51 | a-h | 47 | a-i | 35 | a-k | 29 | a | 56 | a-i | 60 | a | 0.88 | a-d | 0.68 | a | 0.5 | a-d | 2.6 | b-o | 2.2 | a-h | 2.8 | a-h | 5751 | a-j | 2303 | i-s | 2 | a | 13 | c-h |
| CC189 | 48 | a-j | 50 | a-g | 36 | a-j | 23 | a | 56 | a-h | 64 | a | 0.87 | a-d | 0.70 | a | 0.3 | a-f | 2.6 | b-n | 2.1 | a-i | 2.9 | a-h | 5345 | a-j | 2146 | j-s | 2 | a | 9 | e-h |
| CC187 | 41 | b-q | 44 | a-i | 27 | b-s | 30 | a | 40 | b-l | 61 | a | 0.87 | a-d | 0.72 | a | 0.2 | a-g | 1.2 | n-r | 1.3 | e-j | 2.4 | a-h | 4603 | c-j | 3056 | c-q | 2 | a | 27 | b-e |
| CC157 | 43 | a-p | 36 | c-i | 31 | a-r | 23 | a | 50 | a-k | 60 | a | 0.88 | a-d | 0.80 | a | −0.1 | a-k | 2.6 | b-n | 1.8 | a-j | 2.6 | a-h | 4884 | a-j | 2890 | c-r | 1 | a | 0 | h |
| CC155 | 47 | a-l | 32 | ij | 26 | e-t | 27 | a | 47 | a-k | 67 | a | 0.88 | a-d | 0.73 | a | −0.7 | e-u | 2.6 | b-n | 1.9 | a-j | 2.8 | a-h | 5482 | a-j | 2434 | f-s | 2 | a | 2 | gh |
| CC149 | 51 | a-g | 43 | a-i | 28 | b-s | 25 | a | 42 | a-l | 63 | a | 0.88 | a-d | 0.70 | a | −0.8 | e-u | 2.3 | c-q | 2.0 | a-j | 2.8 | a-h | 3835 | g-j | 2566 | d-s | 2 | a | 2 | gh |
| CC125 | 45 | a-n | 43 | a-i | 34 | a-l | 29 | a | 54 | a-j | 65 | a | 0.89 | a-d | 0.76 | a | −1.0 | h-u | 2.6 | b-n | 1.7 | b-j | 2.3 | a-h | 7035 | a-h | 3406 | b-l | 1 | a | 4 | gh |
| CC119 | 50 | a-h | 46 | a-i | 29 | b-s | 25 | a | 48 | a-k | 63 | a | 0.87 | a-d | 0.74 | a | −0.5 | b-r | 1.6 | j-r | 2.1 | a-i | 2.8 | a-h | 6081 | a-j | 3175 | c-m | 2 | a | 3 | gh |
| CC115A | 10 | uv | 46 | a-i | 20 | m-t | 27 | a | 32 | i-l | 70 | a | 0.80 | e | 0.71 | a | −0.9 | g-u | 3.2 | a-e | 1.0 | h-j | 1.6 | gh | 4873 | a-j | 3808 | b-f | 1 | a | 2 | gh |
| CC112 | 50 | a-h | 52 | a-d | 38 | a-g | 28 | a | 53 | a-j | 57 | a | 0.88 | a-d | 0.72 | a | −1.1 | i-u | 1.6 | j-r | 2.1 | a-i | 3.5 | ab | 5644 | a-j | 3552 | b-i | 2 | a | 18 | b-h |
| CC087 | 50 | a-i | 47 | a-i | 34 | a-l | 27 | a | 54 | a-j | 63 | a | 0.87 | a-d | 0.58 | a | −0.4 | b-q | 2.6 | b-o | 1.9 | a-j | 2.6 | a-h | 4640 | b-j | 3815 | b-f | 2 | a | 2 | gh |
| CC082 | 46 | a-n | 48 | a-h | 31 | a-r | 28 | a | 48 | a-k | 67 | a | 0.87 | a-d | 0.72 | a | −1.4 | m-u | 2.8 | a-k | 1.9 | a-j | 2.5 | a-h | 4157 | f-j | 2403 | g-s | 9 | a | 30 | bc |
| CC080 | 48 | a-j | 55 | a-c | 33 | a-m | 26 | a | 54 | a-j | 60 | a | 0.87 | a-d | 0.79 | a | −0.4 | b-q | 3.2 | a-e | 1.8 | a-j | 2.9 | a-h | 4165 | f-j | 2148 | j-s | 1 | a | 25 | b-f |
| CC075 | 46 | a-n | 44 | a-i | 31 | a-r | 29 | a | 51 | a-k | 64 | a | 0.87 | a-d | 0.75 | a | −0.5 | b-q | 2.4 | c-q | 2.1 | a-i | 2.9 | a-h | 3852 | f-j | 2588 | d-s | 2 | a | 3 | gh |
| CC068 | 52 | a-g | 52 | a-d | 40 | a-d | 32 | a | 57 | a-h | 74 | a | 0.87 | a-d | 0.70 | a | 0.1 | a-h | 1.8 | d-r | 2.1 | a-i | 2.6 | a-h | 4942 | a-j | 3935 | b-d | 1 | a | 2 | gh |
| CC053 | 51 | a-h | 46 | a-i | 41 | ab | 29 | a | 60 | a-e | 59 | a | 0.88 | a-d | 0.75 | a | −0.5 | b-q | 2.6 | b-n | 2.3 | a-g | 3.2 | a-f | 4748 | a-j | 2832 | c-r | 0 | a | 2 | gh |
| CC047 | 49 | a-j | 44 | a-i | 33 | a-m | 28 | a | 53 | a-j | 65 | a | 0.86 | a-d | 0.74 | a | −0.1 | a-k | 1.6 | j-r | 2.6 | a-d | 3.3 | a-d | 6132 | a-j | 3560 | b-i | 2 | a | 2 | gh |
| CC041 | 49 | a-j | 44 | a-i | 30 | a-r | 30 | a | 42 | a-l | 62 | a | 0.88 | a-d | 0.74 | a | −0.1 | a-k | 2.0 | d-r | 1.8 | a-j | 3.3 | a-d | 5101 | a-j | 1699 | q-s | 1 | a | 20 | b-g |
| CC038 | 45 | a-n | 35 | d-i | 36 | a-j | 29 | a | 52 | a-j | 64 | a | 0.88 | a-d | 0.72 | a | −0.4 | b-q | 4.1 | a | 2.4 | a-e | 3.4 | a-c | 5915 | a-j | 1290 | s | 0 | a | 52 | a |
| CC033 | 46 | a-n | 37 | c-i | 32 | a-p | 25 | a | 50 | a-k | 58 | a | 0.89 | a-d | 0.77 | a | −1.0 | g-u | 2.4 | c-q | 2.1 | a-i | 2.8 | a-h | 5543 | a-j | 2395 | g-s | 4 | a | 17 | b-h |
| CC016 | 48 | a-k | 51 | a-e | 40 | a-e | 28 | a | 57 | a-h | 61 | a | 0.88 | a-d | 0.78 | a | −0.3 | a-q | 2.2 | d-r | 2.2 | a-h | 3.4 | a-c | 4233 | f-j | 2870 | c-r | 5 | a | 17 | b-h |
| CC012 | 50 | a-h | 42 | a-i | 32 | a-n | 32 | a | 46 | a-l | 62 | a | 0.88 | a-d | 0.71 | a | −0.7 | e-u | 1.7 | h-r | 2.3 | a-g | 2.9 | a-h | 4966 | a-j | 2574 | d-s | 1 | a | 9 | e-h |
| C76-16 | 44 | a-o | 33 | g-j | 25 | f-t | 24 | a | 54 | a-j | 58 | a | 0.90 | a-c | 0.73 | a | 0.2 | a-g | 3.1 | a-i | 1.2 | f-j | 1.8 | d-h | 8407 | ab | 4753 | b | 1 | a | 0 | h |
| SWR | 44 | a-o | . | | 27 | c-s | . | | 59 | a-f | . | | 0.86 | a-d | . | | −0.3 | a-q | . | | 1.8 | a-j | . | | 5713 | a-j | . | | 1 | a | . | |
| Sullivan | 37 | g-s | . | | 22 | i-t | . | | 39 | c-l | . | | 0.89 | a-d | . | | −0.7 | d-t | . | | 1.0 | h-j | . | | 7651 | a-f | . | | 0 | a | . | |
| OLE | 52 | a-f | . | | 34 | a-l | . | | 52 | a-j | . | | 0.88 | a-d | . | | −0.8 | f-u | . | | 1.9 | a-j | . | | 6973 | a-h | . | | 0 | a | . | |
| GA09B | 40 | c-q | . | | 18 | q-t | . | | 47 | a-l | . | | 0.89 | a-d | . | | −1.0 | h-u | . | | 0.9 | ij | . | | 8105 | a-e | . | | 1 | a | . | |
| CC763 | 22 | s-u | . | | 21 | l-t | . | | 34 | h-l | . | | 0.87 | a-d | . | | −1.3 | k-u | . | | 1.3 | e-j | . | | 2606 | j | . | | 1 | a | . | |
| CC631 | 9 | uv | . | | 16 | st | . | | 28 | kl | . | | 0.85 | b-d | . | | −0.5 | b-r | . | | 1.3 | e-j | . | | 3502 | g-j | . | | 4 | a | . | |
| CC610 | 6 | v | . | | 18 | r-t | . | | 34 | g-l | . | | 0.71 | f | . | | −0.3 | a-q | . | | 1.7 | b-j | . | | 3971 | f-j | . | | 1 | a | . | |
| CC552B | 30 | o-t | . | | 26 | f-t | . | | 39 | c-l | . | | 0.88 | a-d | . | | −0.7 | e-u | . | | 2.0 | a-j | . | | 3337 | h-j | . | | 1 | a | . | |
| CC552A | 27 | q-t | . | | 28 | b-s | . | | 44 | a-l | . | | 0.87 | a-d | . | | −0.9 | f-u | . | | 2.3 | a-f | . | | 3535 | g-j | . | | 2 | a | . | |
| CC516B | 38 | d-q | . | | 25 | f-t | . | | 45 | a-l | . | | 0.88 | a-d | . | | −0.2 | a-m | . | | 1.7 | b-j | . | | 5233 | a-j | . | | 3 | a | . | |
| CC516A | 54 | a-c | . | | 36 | a-i | . | | 59 | a-f | . | | 0.86 | b-d | . | | 0.2 | a-g | . | | 2.2 | a-h | . | | 5644 | a-j | . | | 2 | a | . | |
| CC433A | 32 | m-s | . | | 24 | f-t | . | | 43 | a-l | . | | 0.88 | a-d | . | | 0.1 | a-i | . | | 2.0 | a-j | . | | 4433 | e-j | . | | 1 | a | . | |
| CC132 | 22 | s-u | . | | 27 | d-s | . | | 45 | a-l | . | | 0.86 | a-d | . | | 0.1 | a-h | . | | 1.8 | a-j | . | | 3335 | h-j | . | | 1 | a | . | |
| CC115B | 15 | t-v | . | | 12 | t | . | | 24 | l | . | | 0.78 | e | . | | −0.4 | b-q | . | | 1.1 | g-j | . | | 3535 | g-j | . | | 0 | a | . | |
| CC050 | 34 | j-s | . | | 22 | i-t | . | | 36 | f-l | . | | 0.88 | a-d | . | | −0.2 | a-l | . | | 2.1 | a-i | . | | 3167 | ij | . | | 3 | a | . | |
| Mean | 44 | | 44 | | 29 | | 27 | | 48 | | 63 | | 0.9 | | 0.73 | | −0.5 | | 2.3 | | 1.7 | | 2.5 | | 5334 | | 2886 | | 2 | | 10 | |
| p-value | <0.0001 | <0.0001 | <0.0001 | 0.998 | <0.0001 | 0.436 | <0.0001 | 0.421 | 0.0006 | 0.004 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.098 | <0.0001 |
Table 6.
Disease and insect damage parameters (spotted wilt (TSWV), southern stem rot (SSR), sclerotinia blight (SB), cylindrocladium black rot (CBR), and thrips damage) of 104 mini-core genotypes of peanut in 2017, and 88 in 2018. Values for TSWV, SSR, SB, and CBR, are averages over both measurement dates (10 and 12 WAP). The values followed by the same letters are not significantly different using Fisher’s protected LSD at α = 0.05.
Table 6.
Disease and insect damage parameters (spotted wilt (TSWV), southern stem rot (SSR), sclerotinia blight (SB), cylindrocladium black rot (CBR), and thrips damage) of 104 mini-core genotypes of peanut in 2017, and 88 in 2018. Values for TSWV, SSR, SB, and CBR, are averages over both measurement dates (10 and 12 WAP). The values followed by the same letters are not significantly different using Fisher’s protected LSD at α = 0.05.
| Genotypes | TSWV (%) † | SSR (%) | SB (%) | CBR (%) | Thrips Damage
(0–10) ‡ |
|---|
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 |
|---|
| Wynne | 4.4 | f-g | 17.3 | b | 0.0 | a | 3 | k-n | 0.5 | a | 3 | a | 0 | a | 4.9 | a | 0 | a | 3.8 | a |
| Walton | 5.4 | e-g | 17.1 | b | 0.5 | a | 2.9 | l-n | 1.5 | a | 2.9 | a | 0 | a | 2.9 | a | 0 | a | 2.8 | a |
| TVOL14 | 16.7 | b-g | 48.6 | ab | 0.0 | a | 8.4 | e-n | 2 | a | 14 | a | 0 | a | 4.6 | a | 0 | a | 3.8 | a |
| TS90 | 18.6 | b-g | 38.7 | b | 0.0 | a | 14.1 | a-j | 2.5 | a | 3.8 | a | 1.5 | a | 8.5 | a | 0 | a | 2.5 | a |
| TROL11 | 10.8 | b-g | 40.1 | b | 0.0 | a | 5.3 | h-n | 1.5 | a | 4.3 | a | 0 | a | 4.3 | a | 0 | a | 3.3 | a |
| NMVAL | 23.2 | a-g | 38.3 | b | 0.7 | a | 10.7 | c-n | 4 | a | 18.3 | a | 2.2 | a | 10.6 | a | 0 | a | 3.3 | a |
| CC812 | 16.7 | b-g | 34.3 | b | 1.0 | a | 10 | c-n | 1.5 | a | 4.4 | a | 0 | a | 3.5 | a | 0 | a | 3.1 | a |
| CC808 | 34.8 | a-d | 43.2 | b | 0.5 | a | 12 | b-n | 2.9 | a | 14.8 | a | 2 | a | 8.2 | a | 0 | a | 4.0 | a |
| CC805 | 24.0 | a-g | 40.8 | b | 1.5 | a | 8.4 | e-n | 2.5 | a | 7.4 | a | 0 | a | 4.6 | a | 0 | a | 2.7 | a |
| CC802 | 16.7 | b-g | 39.4 | b | 1.5 | a | 13.7 | a-l | 4.9 | a | 2.4 | a | 0.5 | a | 6.2 | a | 0 | a | 2.8 | a |
| CC798 | 21.1 | a-g | 38.3 | b | 0.0 | a | 12.8 | a-n | 2 | a | 5.2 | a | 0 | a | 7.2 | a | 0 | a | 2.2 | a |
| CC787 | 25.5 | a-g | 37.8 | b | 0.5 | a | 2.3 | n | 2.9 | a | 10.7 | a | 0 | a | 5.1 | a | 0 | a | 3.3 | a |
| CC781 | 49.5 | a | 48.8 | ab | 1.0 | a | 23.2 | a | 1.5 | a | 6.2 | a | 0 | a | 4.3 | a | 0 | a | 3.2 | a |
| CC775 | 32.4 | a-f | 38.3 | b | 0.0 | a | 19.8 | a-d | 4.4 | a | 5.4 | a | 0.5 | a | 8.3 | a | 0 | a | 2.3 | a |
| CC760 | 33.8 | a-e | 37.2 | b | 0.5 | a | 5.8 | g-n | 2.9 | a | 4.9 | a | 0 | a | 3.9 | a | 0 | a | 4.2 | a |
| CC755 | 25.5 | a-g | 32.6 | b | 0.0 | a | 15.8 | a-h | 4.9 | a | 1.8 | a | 0 | a | 8.3 | a | 0 | a | 2.4 | a |
| CC740 | 24.0 | a-g | 38.2 | b | 0.0 | a | 9.1 | d-n | 0.5 | a | 9.2 | a | 1 | a | 5.2 | a | 0 | a | 3.2 | a |
| CC725 | 24.0 | a-g | 40.6 | b | 0.0 | a | 15.2 | a-i | 2 | a | 4.8 | a | 0.5 | a | 6.7 | a | 0 | a | 2.8 | a |
| CC711 | 27.9 | a-g | 53.0 | ab | 0.0 | a | 7.2 | f-n | 3.4 | a | 5.3 | a | 0 | a | 4.3 | a | 0 | a | 3.2 | a |
| CC703B | 7.4 | c-g | 42.8 | b | 0.0 | a | 6.5 | g-n | 0 | a | 2.7 | a | 0 | a | 2.7 | a | 0 | a | 3.3 | a |
| CC703A | 16.7 | b-g | 42.0 | b | 1.5 | a | 17.6 | a-f | 4.4 | a | 10 | a | 0 | a | 3.4 | a | 0 | a | 2.8 | a |
| CC698 | 9.3 | b-g | 38.7 | b | 0.0 | a | 5.9 | g-n | 3.4 | a | 3.1 | a | 0 | a | 5 | a | 0 | a | 2.8 | a |
| CC678 | 8.8 | b-g | 39.1 | b | 0.0 | a | 3.5 | j-n | 0 | a | 5.4 | a | 0 | a | 4.4 | a | 0 | a | 2.6 | a |
| CC673 | 22.6 | a-g | 29.9 | b | 2.5 | a | 7.4 | f-n | 2 | a | 6.5 | a | 0 | a | 11.1 | a | 0 | a | 2.8 | a |
| CC650 | 12.3 | b-g | 30.5 | b | 0.5 | a | 12.6 | a-n | 4.9 | a | 6.1 | a | 0 | a | 6.1 | a | 0 | a | 3.0 | a |
| CC643 | 24.0 | a-g | 36.5 | b | 0.0 | a | 13.9 | a-l | 3.9 | a | 4.5 | a | 0 | a | 8.3 | a | 0 | a | 3.5 | a |
| CC605 | 32.8 | a-f | 42.0 | b | 0.0 | a | 6.2 | g-n | 4.9 | a | 13.8 | a | 0 | a | 3.2 | a | 0 | a | 4.1 | a |
| CC588 | 18.1 | b-g | 47.9 | ab | 0.0 | a | 7.2 | f-n | 8.3 | a | 8.1 | a | 0.5 | a | 10.1 | a | 0 | a | 2.6 | a |
| CC580 | 35.8 | a-c | 41.0 | b | 2.0 | a | 9.9 | c-n | 2.5 | a | 9 | a | 1 | a | 3.4 | a | 0 | a | 2.4 | a |
| CC579 | 20.1 | b-g | 36.8 | b | 1.0 | a | 13.2 | a-n | 3.4 | a | 4.9 | a | 0 | a | 4.9 | a | 0 | a | 3.1 | a |
| CC559 | 22.1 | a-g | 52.7 | ab | 0.5 | a | 4 | j-n | 3.9 | a | 6.8 | a | 0 | a | 8.8 | a | 0 | a | 3.2 | a |
| CC553 | 22.1 | a-g | 36.5 | b | 2.0 | a | 11 | b-n | 1.5 | a | 9.1 | a | 1 | a | 8.2 | a | 0 | a | 2.8 | a |
| CC548 | 11.3 | b-g | 42.2 | b | 0.0 | a | 3.4 | j-n | 1 | a | 2.5 | a | 0 | a | 5.3 | a | 0 | a | 2.9 | a |
| CC546 | 26.0 | a-g | 48.1 | ab | 1.5 | a | 8.5 | e-n | 1.5 | a | 7.6 | a | 1 | a | 6.6 | a | 0 | a | 3.2 | a |
| CC535 | 25.0 | a-g | 36.7 | b | 2.0 | a | 4.7 | h-n | 2.9 | a | 2.9 | a | 0 | a | 7.6 | a | 0 | a | 2.5 | a |
| CC529 | 8.8 | b-g | 37.0 | b | 0.0 | a | 10.8 | b-n | 2.5 | a | 5 | a | 0 | a | 5 | a | 0 | a | 2.9 | a |
| CC526 | 14.7 | b-g | 40.0 | b | 0.0 | a | 15.2 | a-i | 0 | a | 9.4 | a | 0 | a | 5.5 | a | 0 | a | 2.9 | a |
| CC508 | 31.4 | a-f | 52.3 | ab | 2.0 | a | 10.5 | c-n | 3.9 | a | 2.8 | a | 0 | a | 14.1 | a | 0 | a | 3.6 | a |
| CC488 | 18.1 | b-g | 38.1 | b | 0.0 | a | 6.6 | f-n | 0 | a | 9.4 | a | 0 | a | 12.4 | a | 0 | a | 3.8 | a |
| CC485 | 22.1 | a-g | 44.1 | ab | 2.0 | a | 2.5 | mn | 6.4 | a | 10.1 | a | 0 | a | 6.3 | a | 0 | a | 3.4 | a |
| CC481 | 29.9 | a-g | 39.6 | b | 2.5 | a | 4.7 | h-n | 2 | a | 6.7 | a | 0 | a | 2.9 | a | 0 | a | 3.0 | a |
| CC477 | 18.6 | b-g | 33.5 | b | 1.5 | a | 9.1 | d-n | 1 | a | 10.9 | a | 0 | a | 10.9 | a | 0 | a | 3.9 | a |
| CC458 | 13.2 | b-g | 44.4 | ab | 0.5 | a | 3.4 | j-n | 0.5 | a | 7.2 | a | 0 | a | 3.4 | a | 0 | a | 3.5 | a |
| CC446 | 23.0 | a-g | 44.5 | ab | 0.0 | a | 2.4 | mn | 5.9 | a | 8 | a | 0 | a | 5.3 | a | 0 | a | 3.1 | a |
| CC431 | 22.6 | a-g | 45.3 | ab | 0.0 | a | 16.7 | a-g | 1.5 | a | 5.2 | a | 0 | a | 6.1 | a | 0 | a | 3.3 | a |
| CC408 | 22.1 | a-g | 43.0 | b | 0.5 | a | 12.7 | a-n | 0 | a | 11.7 | a | 0 | a | 6.9 | a | 0 | a | 3.7 | a |
| CC406 | 21.6 | a-g | 35.5 | b | 0.0 | a | 9.3 | d-n | 2.5 | a | 2.7 | a | 0 | a | 6.5 | a | 0 | a | 2.6 | a |
| CC388 | 19.1 | b-g | 40.9 | b | 0.0 | a | 15.2 | a-i | 0.5 | a | 6.4 | a | 1 | a | 13.1 | a | 0 | a | 3.1 | a |
| CC384 | 33.3 | a-f | 31.8 | b | 0.0 | a | 8.1 | e-n | 4.9 | a | 2.5 | a | 1.5 | a | 5.4 | a | 0 | a | 3.3 | a |
| CC381 | 15.7 | b-g | 33.2 | b | 0.5 | a | 5.9 | g-n | 1 | a | 6.9 | a | 0 | a | 2.2 | a | 0 | a | 2.5 | a |
| CC342 | 19.6 | b-g | 43.3 | ab | 0.5 | a | 5.6 | h-n | 1.5 | a | 4.6 | a | 0 | a | 5.6 | a | 0 | a | 3.1 | a |
| CC338 | 22.1 | a-g | 37.8 | b | 1.0 | a | 9.7 | c-n | 2 | a | 6.9 | a | 3.4 | a | 12.4 | a | 0 | a | 2.8 | a |
| CC310 | 29.9 | a-g | 46.0 | ab | 0.0 | a | 18.5 | a-e | 1.5 | a | 3.2 | a | 1.5 | a | 7.9 | a | 0 | a | 3.2 | a |
| CC296 | 24.5 | a-g | 46.2 | ab | 0.5 | a | 5 | h-n | 2 | a | 9.8 | a | 1.5 | a | 11.8 | a | 0 | a | 2.8 | a |
| CC287 | 11.3 | b-g | 44.7 | ab | 0.0 | a | 7.4 | f-n | 0 | a | 2.6 | a | 1.5 | a | 2.6 | a | 0 | a | 3.6 | a |
| CC277 | 14.7 | b-g | 29.0 | b | 0.0 | a | 4.4 | i-n | 2.5 | a | 2.5 | a | 0 | a | 3.5 | a | 0 | a | 2.9 | a |
| CC266 | 29.9 | a-g | 42.8 | b | 1.0 | a | 13.4 | a-m | 1.5 | a | 8.6 | a | 0 | a | 4.8 | a | 0 | a | 3.5 | a |
| CC249 | 22.1 | a-g | 48.9 | ab | 0.0 | a | 12.8 | a-n | 3.9 | a | 7.2 | a | 2.5 | a | 5.2 | a | 0 | a | 3.5 | a |
| CC246 | 6.4 | d-g | 29.8 | b | 0.0 | a | 4.4 | i-n | 0 | a | 6.2 | a | 0 | a | 8 | a | 0 | a | 3.4 | a |
| CC233 | 8.3 | b-g | 40.6 | b | 0.0 | a | 8.9 | d-n | 0 | a | 3.2 | a | 0 | a | 11.8 | a | 0 | a | 2.9 | a |
| CC230 | 6.9 | c-g | 39.0 | b | 0.0 | a | 12.8 | a-n | 0 | a | 4.4 | a | 0 | a | 5.4 | a | 0 | a | 2.8 | a |
| CC227 | 10.3 | b-g | 45.5 | ab | 0.0 | a | 3.5 | j-n | 0.5 | a | 11.1 | a | 0 | a | 11.1 | a | 0 | a | 2.5 | a |
| CC223 | 7.8 | c-g | 53.6 | ab | 5.4 | a | 14.2 | a-j | 0 | a | 13.3 | a | 0.5 | a | 5.8 | a | 0 | a | 2.4 | a |
| CC221 | 20.1 | b-g | 39.6 | b | 1.0 | a | 20.7 | a-c | 2.5 | a | 2.9 | a | 0 | a | 6.6 | a | 0 | a | 3.6 | a |
| CC208 | 23.5 | a-g | 45.1 | ab | 1.5 | a | 8.1 | e-n | 0.5 | a | 2.5 | a | 0 | a | 6.3 | a | 0 | a | 3.7 | a |
| CC202 | 21.6 | a-g | 37.9 | b | 2.0 | a | 9.4 | d-n | 2.9 | a | 12.4 | a | 3.4 | a | 8.5 | a | 0 | a | 3.3 | a |
| CC189 | 26.5 | a-g | 38.9 | b | 1.0 | a | 21.9 | ab | 0 | a | 14.4 | a | 0 | a | 3.9 | a | 0 | a | 2.5 | a |
| CC187 | 21.1 | a-g | 39.2 | b | 0.5 | a | 10.9 | b-n | 0 | a | 6.2 | a | 0.5 | a | 10.9 | a | 0 | a | 3.3 | a |
| CC157 | 31.4 | a-f | 45.3 | ab | 2.5 | a | 10.9 | b-n | 2 | a | 5 | a | 0 | a | 3.1 | a | 0 | a | 3.3 | a |
| CC155 | 15.2 | b-g | 41.3 | b | 0.0 | a | 7 | f-n | 1.5 | a | 6.1 | a | 0 | a | 8 | a | 0 | a | 3.3 | a |
| CC149 | 27.0 | a-g | 45.6 | ab | 2.0 | a | 14 | a-k | 2.5 | a | 8.3 | a | 0 | a | 7.2 | a | 0 | a | 3.3 | a |
| CC125 | 29.4 | a-g | 40.3 | b | 0.5 | a | 11.1 | b-n | 0.5 | a | 7.2 | a | 0 | a | 6.3 | a | 0 | a | 3.5 | a |
| CC119 | 24.0 | a-g | 39.2 | b | 0.0 | a | 7.3 | f-n | 2 | a | 4.5 | a | 1.5 | a | 8.2 | a | 0 | a | 2.3 | a |
| CC115A | 9.3 | b-g | 38.0 | b | 0.0 | a | 15.2 | a-i | 0.5 | a | 2.9 | a | 0 | a | 6.7 | a | 0 | a | 3.3 | a |
| CC112 | 16.2 | b-g | 39.6 | b | 0.0 | a | 11.6 | b-n | 0 | a | 10.8 | a | 0 | a | 3.1 | a | 0 | a | 4.5 | a |
| CC087 | 25.0 | a-g | 42.1 | b | 0.0 | a | 8.9 | d-n | 2 | a | 14.7 | a | 0 | a | 7.1 | a | 0 | a | 3.7 | a |
| CC082 | 17.6 | b-g | 36.3 | b | 0.0 | a | 13.8 | a-l | 2.9 | a | 4.1 | a | 0 | a | 6 | a | 0 | a | 3.8 | a |
| CC080 | 27.5 | a-g | 33.9 | b | 0.0 | a | 6.6 | f-n | 1 | a | 9.5 | a | 0 | a | 5.7 | a | 0 | a | 3.1 | a |
| CC075 | 23.5 | a-g | 35.3 | b | 0.5 | a | 5.5 | h-n | 1.5 | a | 6.5 | a | 0 | a | 11.3 | a | 0 | a | 2.8 | a |
| CC068 | 19.6 | b-g | 33.9 | b | 4.4 | a | 7.6 | e-n | 3.4 | a | 10.4 | a | 0 | a | 3.8 | a | 0 | a | 3.8 | a |
| CC053 | 28.4 | a-g | 95.7 | a | 0.0 | a | 5 | h-n | 0 | a | 10.7 | a | 1 | a | 3.1 | a | 0 | a | 3.3 | a |
| CC047 | 19.1 | b-g | 36.4 | b | 0.0 | a | 3.6 | j-n | 2.5 | a | 3.6 | a | 1 | a | 5.5 | a | 0 | a | 2.7 | a |
| CC041 | 27.0 | a-g | 44.6 | ab | 0.0 | a | 5.6 | h-n | 0.5 | a | 16.1 | a | 0 | a | 5.7 | a | 0 | a | 3.6 | a |
| CC038 | 29.4 | a-g | 46.9 | ab | 0.0 | a | 10.1 | c-n | 1 | a | 13.7 | a | 1 | a | 4.4 | a | 0 | a | 3.3 | a |
| CC033 | 21.6 | a-g | 35.6 | b | 1.0 | a | 2.6 | mn | 3.9 | a | 4.5 | a | 1.5 | a | 9.2 | a | 0 | a | 2.3 | a |
| CC016 | 18.1 | b-g | 44.0 | ab | 0.5 | a | 12.7 | a-n | 2.9 | a | 12.8 | a | 1 | a | 5.2 | a | 0 | a | 4.0 | a |
| CC012 | 18.1 | b-g | 32.0 | b | 0.0 | a | 5.1 | h-n | 1.5 | a | 1.4 | a | 0 | a | 6.1 | a | 0 | a | 3.0 | a |
| C76-16 | 6.9 | c-g | 30.2 | b | 1.0 | a | 4.7 | i-n | 1.5 | a | 2.8 | a | 0 | a | 5.6 | a | 0 | a | 2.2 | a |
| SWR | 13.7 | b-g | . | | 0.0 | a | . | | 3.4 | a | . | | 0 | a | . | | 0 | a | . | |
| Sullivan | 4.4 | fg | . | | 0.0 | a | . | | 0.5 | a | . | | 0 | a | . | | 0 | a | . | |
| OLE | 16.7 | b-g | . | | 0.5 | a | . | | 1 | a | . | | 0 | a | . | | 0 | a | . | |
| GA09B | 2.0 | g | . | | 0.0 | a | . | | 0 | a | . | | 0 | a | . | | 0 | a | . | |
| CC763 | 25.5 | a-g | . | | 1.0 | a | . | | 1.5 | a | . | | 1 | a | . | | 0 | a | . | |
| CC631 | 17.2 | b-g | . | | 0.0 | a | . | | 1 | a | . | | 0.5 | a | . | | 0 | a | . | |
| CC610 | 11.8 | b-g | . | | 0.0 | a | . | | 1.5 | a | . | | 0 | a | . | | 0 | a | . | |
| CC552B | 26.5 | a-g | . | | 0.0 | a | . | | 2.5 | a | . | | 0.5 | a | . | | 0 | a | . | |
| CC552A | 26.5 | a-g | . | | 0.5 | a | . | | 0 | a | . | | 0 | a | . | | 0 | a | . | |
| CC516B | 25.0 | a-g | . | | 0.5 | a | . | | 0 | a | . | | 0 | a | . | | 0 | a | . | |
| CC516A | 21.6 | a-g | . | | 0.0 | a | . | | 3.4 | a | . | | 0 | a | . | | 0 | a | . | |
| CC433A | 16.2 | b-g | . | | 0.0 | a | . | | 3.4 | a | . | | 0 | a | . | | 0 | a | . | |
| CC132 | 25.5 | a-g | . | | 0.0 | a | . | | 2.9 | a | . | | 0 | a | . | | 0 | a | . | |
| CC115B | 15.7 | b-g | . | | 0.0 | a | . | | 0 | a | . | | 0 | a | . | | 0 | a | . | |
| CC050 | 37.3 | ab | . | | 0.0 | a | . | | 1 | a | . | | 1 | a | . | | 0 | a | . | |
| Mean | 20.5 | | 40.4 | | 0.6 | | 9.42 | | 2 | | 6.98 | | 0.4 | | 6.48 | | 0 | | 3.1 | |
| p-value | <0.0001 | 0.041 | 0.785 | <0.001 | 0.092 | 0.109 | 0.391 | 0.479 | - | 0.495 |
Table 7.
The 2017 and 2018 stand count, plant height, lateral growth, Normalized Difference Vegetation Index (NDVI), canopy temperature depression (CTD), leaf wilting, tomato spotted wilt (TSW), southern stem rot (SSR), sclerotinia blight (SB), cylindrocladium black rot (CBR), thrips damage, pod yield, and post-harvest sprouting by varieties of the U.S. mini-core peanut collection. The plant height, lateral growth, and NDVI, are measurements taken at maximum vegetative growth (6 weeks after planting (WAP)). Leaf wilting and canopy temperature depression (CTD) are the average of two dates (10 and 12 WAP in 2017 and 5 to 7 WAP in 2018), with the highest values corresponding to sudden droughts. Values for TSW, SSR, SB, and CBR, are averages over both measurement dates (10 and 12 WAP). The values followed by the same letters are not significantly different using Fisher’s protected LSD at α = 0.1.
Table 7.
The 2017 and 2018 stand count, plant height, lateral growth, Normalized Difference Vegetation Index (NDVI), canopy temperature depression (CTD), leaf wilting, tomato spotted wilt (TSW), southern stem rot (SSR), sclerotinia blight (SB), cylindrocladium black rot (CBR), thrips damage, pod yield, and post-harvest sprouting by varieties of the U.S. mini-core peanut collection. The plant height, lateral growth, and NDVI, are measurements taken at maximum vegetative growth (6 weeks after planting (WAP)). Leaf wilting and canopy temperature depression (CTD) are the average of two dates (10 and 12 WAP in 2017 and 5 to 7 WAP in 2018), with the highest values corresponding to sudden droughts. Values for TSW, SSR, SB, and CBR, are averages over both measurement dates (10 and 12 WAP). The values followed by the same letters are not significantly different using Fisher’s protected LSD at α = 0.1.
| Varieties | Stand Count (Plants/Plot) | Plant Height (cm) | Lateral Growth (cm) | NDVI | CTD (°C) | Leaf Wilting
(0–5) | TSW (%) | SSR (%) | SB (%) | CBR (%) | Thrips Damage (0–10) | Pod Yield (kg ha−1) | Sprouting (#/Plot) |
|---|
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 |
|---|
| Fastigiata | 42 | a | 45 | a | 29 | ab | 28 | a | 48 | a | 63 | a | 0.88 | a | 0.73 | a | −0.5 | a | 2.1 | a | 2 | bc | 3 | ab | 22 | a | 41 | a | 0.4 | a | 10.5 | a | 1.9 | a | 5.8 | a | 0.3 | a | 6.8 | a | 0 | a | 3.3 | a | 5312 | a | 2836 | a | 2.4 | a | 13.0 | a |
| Hypogaea | 44 | a | 43 | a | 28 | b | 27 | a | 48 | a | 63 | a | 0.88 | a | 0.73 | a | −0.5 | a | 2.4 | a | 2 | c | 2 | b | 18 | a | 40 | a | 0.7 | a | 9.4 | a | 2.0 | a | 6.7 | a | 0.5 | a | 6.4 | a | 0 | a | 3.1 | a | 5569 | a | 2921 | a | 1.9 | a | 6.9 | b |
| Peruvian | 49 | a | 38 | a | 27 | b | 26 | a | 45 | a | 65 | a | 0.88 | a | 0.71 | a | −0.7 | a | 2.5 | a | 2 | a | 3 | a | 21 | a | 44 | a | 1.0 | a | 10.5 | a | 2.0 | a | 7.2 | a | 0.0 | a | 7.6 | a | 0 | a | 3.3 | a | 4658 | a | 2500 | a | 1.7 | a | 1.8 | b |
| Vulgaris | 46 | a | 46 | a | 32 | a | 28 | a | 50 | a | 62 | a | 0.87 | a | 0.71 | a | −0.5 | a | 2.3 | a | 2 | ab | 3 | ab | 23 | a | 40 | a | 0.5 | a | 8.5 | a | 2.0 | a | 8.5 | a | 0.3 | a | 6.2 | a | 0 | a | 3.1 | a | 5037 | a | 2903 | a | 2.2 | a | 12.6 | a |
| Mean | 45 | | 43 | | 29 | | 27 | | 48 | | 63 | | 0.88 | | 0.72 | | −0.5 | | 2.3 | | 2 | | 3 | | 21 | | 41 | | 1 | | 9.7 | | 2.0 | | 7.1 | | 0.3 | | 6.8 | | 0 | | 3.2 | | 5144 | | 2790 | | 2.0 | | 8.6 | |
| p-value | 0.665 | 0.785 | <0.0001 | 0.679 | 0.998 | 0.569 | 0.556 | 0.384 | 0.893 | 0.183 | 0.0003 | 0.009 | 0.266 | 0.938 | 0.652 | 0.486 | 0.997 | 0.082 | 0.266 | 0.823 | - | 0.572 | 0.420 | 0.802 | 0.647 | 0.005 |
Table 8.
Heatmap correlation matrix of aerial reflectance, color space indices, and their derived vegetation indices, with physiological, morphological, and agronomic traits of peanuts in 2017.
Table 8.
Heatmap correlation matrix of aerial reflectance, color space indices, and their derived vegetation indices, with physiological, morphological, and agronomic traits of peanuts in 2017.
| | Hypogaea | Fastigiata | Vulgaris |
|---|
| Indices | Stand Count | Plant Height | Lateral Growth | Ground NDVI | CTD | Wilting | TSW | SSR | SB | CBR | Yield | Sprouting | Stand Count | Plant Height | Lateral Growth | Ground NDVI | CTD | Wilting | TSW | SSR | SB | CBR | Yield | Sprouting | Stand Count | Plant Height | Lateral Growth | Ground NDVI | CTD | Wilting | TSW | SSR | SB | CBR | Yield | Sprouting |
|---|
| Red | −0.72 | −0.29 | −0.76 | −0.74 | 0.41 | 0.59 | 0.48 | −0.12 | 0.27 | 0.06 | −0.57 | 0.35 | −0.69 | −0.62 | −0.60 | −0.71 | 0.45 | 0.45 | 0.45 | 0.25 | 0.34 | 0.23 | −0.40 | 0.14 | −0.75 | −0.64 | −0.74 | −0.81 | 0.36 | 0.30 | 0.24 | 0.17 | −0.04 | 0.04 | −0.58 | 0.18 |
| Green | −0.72 | −0.42 | −0.74 | −0.74 | 0.38 | 0.49 | 0.44 | −0.12 | 0.22 | 0.01 | −0.59 | 0.33 | −0.64 | −0.60 | −0.44 | −0.65 | 0.49 | 0.65 | 0.50 | 0.34 | 0.31 | 0.23 | −0.40 | 0.12 | −0.62 | −0.66 | −0.62 | −0.69 | 0.33 | 0.19 | 0.18 | 0.13 | −0.11 | 0.05 | −0.54 | 0.18 |
| Blue | −0.80 | −0.40 | −0.78 | −0.77 | 0.40 | 0.57 | 0.32 | −0.17 | 0.15 | 0.11 | −0.46 | 0.20 | −0.68 | −0.71 | −0.60 | −0.76 | 0.41 | 0.44 | 0.25 | 0.02 | 0.44 | 0.18 | −0.30 | −0.33 | −0.75 | −0.70 | −0.77 | −0.83 | 0.33 | 0.24 | 0.10 | 0.07 | −0.24 | 0.07 | −0.43 | −0.03 |
| NIR | −0.05 | −0.39 | 0.37 | 0.02 | 0.14 | 0.20 | 0.15 | 0.32 | 0.09 | 0.10 | 0.27 | 0.03 | −0.17 | −0.25 | 0.16 | −0.09 | 0.25 | 0.40 | 0.14 | 0.09 | 0.30 | 0.16 | 0.14 | 0.43 | −0.27 | −0.58 | −0.16 | −0.24 | 0.18 | −0.01 | 0.26 | −0.01 | 0.35 | 0.11 | 0.41 | −0.08 |
| NDVI | 0.35 | −0.07 | 0.69 | 0.46 | −0.16 | −0.15 | −0.15 | 0.28 | −0.07 | 0.03 | 0.54 | −0.23 | 0.36 | 0.26 | 0.52 | 0.44 | −0.14 | −0.19 | −0.32 | −0.18 | −0.03 | −0.08 | 0.57 | 0.19 | 0.35 | 0.04 | 0.45 | 0.43 | −0.13 | −0.08 | 0.11 | −0.07 | 0.26 | 0.04 | 0.55 | −0.14 |
| BGI | −0.63 | −0.05 | −0.27 | −0.26 | 0.12 | −0.13 | −0.45 | −0.01 | −0.25 | 0.16 | 0.03 | −0.05 | −0.07 | −0.21 | −0.31 | −0.21 | −0.18 | −0.47 | −0.38 | −0.45 | 0.13 | −0.08 | 0.03 | −0.39 | −0.19 | −0.05 | −0.28 | −0.23 | −0.04 | 0.08 | −0.17 | −0.12 | −0.29 | 0.04 | −0.20 | −0.12 |
| RGR | −0.36 | 0.33 | 0.11 | 0.14 | −0.01 | −0.01 | 0.29 | −0.10 | 0.29 | 0.22 | −0.36 | 0.34 | 0.25 | 0.26 | −0.03 | 0.22 | −0.35 | −0.39 | −0.01 | −0.24 | 0.17 | 0.09 | −0.26 | 0.17 | 0.24 | 0.43 | 0.24 | 0.29 | −0.19 | 0.24 | 0.27 | 0.20 | 0.29 | −0.02 | −0.56 | 0.20 |
| NPPR | 0.56 | −0.15 | 0.06 | 0.04 | −0.06 | 0.05 | 0.13 | 0.03 | −0.01 | −0.23 | 0.07 | −0.19 | −0.08 | −0.01 | 0.19 | 0.01 | 0.27 | 0.40 | 0.29 | 0.44 | −0.16 | 0.02 | 0.03 | 0.24 | −0.05 | −0.24 | −0.02 | −0.06 | 0.12 | −0.15 | −0.04 | −0.02 | 0.04 | −0.02 | 0.31 | −0.04 |
| NGRDI | 0.35 | −0.33 | −0.12 | −0.14 | 0.02 | 0.01 | −0.29 | 0.09 | −0.29 | −0.22 | 0.33 | −0.35 | −0.25 | −0.26 | 0.04 | −0.21 | 0.34 | 0.38 | 0.01 | 0.23 | −0.17 | −0.10 | 0.25 | −0.16 | −0.24 | −0.43 | −0.24 | −0.28 | 0.18 | −0.23 | −0.28 | −0.19 | −0.29 | 0.02 | 0.55 | −0.21 |
| PPR | 0.61 | 0.05 | 0.26 | 0.25 | −0.12 | 0.13 | 0.44 | 0.01 | 0.24 | −0.16 | −0.08 | 0.04 | 0.08 | 0.22 | 0.31 | 0.22 | 0.17 | 0.46 | 0.38 | 0.45 | −0.13 | 0.08 | −0.06 | 0.41 | 0.20 | 0.05 | 0.28 | 0.23 | 0.03 | −0.08 | 0.17 | 0.12 | 0.29 | −0.04 | 0.16 | 0.09 |
| NCPI | 0.54 | 0.43 | 0.47 | 0.48 | −0.18 | 0.59 | 0.57 | −0.04 | 0.38 | −0.04 | −0.27 | 0.22 | 0.37 | 0.55 | 0.34 | 0.50 | −0.16 | 0.36 | 0.41 | 0.40 | −0.07 | 0.12 | −0.18 | 0.44 | 0.53 | 0.60 | 0.62 | 0.62 | −0.19 | 0.68 | 0.28 | 0.20 | 0.39 | −0.05 | −0.04 | 0.17 |
| SRI | 0.33 | −0.09 | 0.68 | 0.44 | −0.14 | −0.19 | −0.21 | 0.32 | −0.14 | 0.04 | 0.53 | −0.24 | 0.36 | 0.27 | 0.53 | 0.43 | −0.14 | −0.23 | −0.35 | −0.18 | −0.04 | −0.11 | 0.58 | 0.25 | 0.35 | 0.04 | 0.45 | 0.43 | −0.12 | −0.06 | 0.02 | −0.11 | 0.26 | 0.06 | 0.60 | −0.20 |
| GRVI | 0.38 | 0.02 | 0.68 | 0.46 | −0.14 | −0.12 | −0.17 | 0.30 | −0.10 | 0.07 | 0.50 | −0.15 | 0.44 | 0.36 | 0.48 | 0.49 | −0.27 | −0.17 | −0.35 | −0.24 | −0.01 | −0.08 | 0.50 | 0.36 | 0.40 | 0.26 | 0.49 | 0.49 | −0.19 | −0.02 | 0.06 | −0.08 | 0.30 | 0.06 | 0.54 | −0.15 |
| IO | 0.53 | 0.43 | 0.47 | 0.48 | −0.17 | 0.59 | 0.57 | −0.03 | 0.38 | −0.05 | −0.31 | 0.18 | 0.37 | 0.55 | 0.34 | 0.50 | −0.15 | 0.36 | 0.41 | 0.41 | −0.07 | 0.12 | −0.20 | 0.45 | 0.52 | 0.60 | 0.62 | 0.62 | −0.19 | 0.68 | 0.27 | 0.20 | 0.38 | −0.05 | −0.09 | 0.10 |
| GNDVI | 0.40 | 0.04 | 0.69 | 0.48 | −0.15 | −0.09 | −0.11 | 0.27 | −0.04 | 0.06 | 0.51 | −0.17 | 0.43 | 0.34 | 0.46 | 0.48 | −0.26 | −0.14 | −0.33 | −0.23 | 0.01 | −0.06 | 0.50 | 0.30 | 0.41 | 0.26 | 0.48 | 0.49 | −0.19 | −0.05 | 0.13 | −0.05 | 0.29 | 0.04 | 0.51 | −0.12 |
| BNDVI | 0.42 | 0.06 | 0.73 | 0.53 | −0.19 | −0.07 | −0.01 | 0.30 | 0.03 | 0.02 | 0.42 | −0.11 | 0.46 | 0.42 | 0.59 | 0.57 | −0.18 | 0.14 | −0.07 | 0.06 | −0.06 | 0.00 | 0.27 | 0.54 | 0.49 | 0.28 | 0.60 | 0.59 | −0.18 | −0.05 | 0.17 | −0.02 | 0.32 | 0.02 | 0.42 | −0.01 |
| CIG | 0.38 | 0.02 | 0.68 | 0.46 | −0.14 | −0.12 | −0.17 | 0.30 | −0.10 | 0.07 | 0.50 | −0.15 | 0.44 | 0.36 | 0.48 | 0.49 | −0.27 | 0.36 | −0.35 | −0.24 | −0.01 | −0.08 | 0.50 | 0.36 | 0.40 | 0.26 | 0.49 | 0.49 | −0.19 | −0.02 | 0.06 | −0.08 | 0.30 | 0.06 | 0.54 | −0.15 |
| CVI | 0.55 | 0.43 | 0.46 | 0.48 | −0.18 | 0.59 | 0.58 | −0.04 | 0.38 | −0.04 | −0.20 | 0.24 | 0.37 | 0.55 | 0.33 | 0.50 | −0.16 | 0.42 | 0.41 | 0.40 | −0.08 | 0.13 | −0.17 | 0.44 | 0.53 | 0.61 | 0.62 | 0.62 | −0.19 | 0.68 | 0.29 | 0.20 | 0.39 | −0.05 | 0.00 | 0.20 |
| GLI | 0.57 | −0.16 | 0.07 | 0.05 | −0.06 | 0.07 | 0.15 | 0.05 | 0.01 | −0.24 | 0.13 | −0.18 | −0.10 | −0.03 | 0.19 | 0.00 | 0.29 | 0.24 | 0.29 | 0.44 | −0.17 | 0.02 | 0.07 | 0.21 | −0.06 | −0.25 | −0.02 | −0.07 | 0.13 | −0.16 | −0.04 | −0.03 | 0.04 | −0.02 | 0.36 | −0.02 |
| GBNDVI | 0.40 | 0.04 | 0.71 | 0.50 | −0.16 | −0.09 | −0.09 | 0.29 | −0.03 | 0.05 | 0.48 | −0.15 | 0.45 | 0.38 | 0.52 | 0.53 | −0.24 | −0.04 | −0.25 | −0.14 | −0.02 | −0.04 | 0.44 | 0.44 | 0.45 | 0.27 | 0.54 | 0.54 | −0.19 | −0.05 | 0.13 | −0.05 | 0.31 | 0.03 | 0.49 | −0.09 |
| GRNDVI | 0.38 | −0.01 | 0.70 | 0.47 | −0.15 | −0.12 | −0.14 | 0.28 | −0.07 | 0.05 | 0.52 | −0.20 | 0.41 | 0.31 | 0.50 | 0.47 | −0.22 | −0.17 | −0.33 | −0.21 | −0.01 | −0.07 | 0.55 | 0.27 | 0.40 | 0.19 | 0.49 | 0.48 | −0.17 | −0.06 | 0.10 | −0.07 | 0.28 | 0.04 | 0.54 | −0.15 |
| RBNDVI | 0.25 | −0.17 | 0.53 | 0.28 | −0.07 | −0.13 | −0.15 | 0.22 | −0.02 | 0.02 | 0.58 | −0.25 | 0.12 | −0.09 | 0.21 | 0.11 | −0.08 | −0.40 | −0.44 | −0.37 | 0.05 | −0.08 | 0.51 | −0.06 | 0.00 | −0.28 | 0.02 | 0.01 | −0.05 | −0.10 | 0.19 | −0.03 | 0.18 | 0.05 | 0.41 | −0.06 |
| mSR | 0.37 | −0.01 | 0.70 | 0.49 | −0.16 | −0.15 | −0.14 | 0.33 | −0.09 | 0.03 | 0.46 | −0.17 | 0.42 | 0.39 | 0.58 | 0.53 | −0.17 | −0.05 | −0.22 | −0.06 | −0.06 | −0.07 | 0.41 | 0.52 | 0.45 | 0.21 | 0.57 | 0.54 | −0.15 | −0.04 | 0.06 | −0.08 | 0.32 | 0.05 | 0.51 | −0.13 |
| GARI | 0.38 | −0.01 | 0.71 | 0.49 | −0.17 | −0.13 | −0.10 | 0.30 | −0.05 | 0.03 | 0.49 | −0.19 | 0.41 | 0.34 | 0.56 | 0.51 | −0.17 | −0.06 | −0.23 | −0.09 | −0.05 | −0.05 | 0.47 | 0.40 | 0.43 | 0.16 | 0.54 | 0.52 | −0.15 | −0.06 | 0.12 | −0.05 | 0.29 | 0.03 | 0.51 | −0.10 |
| Intensity | −0.85 | −0.65 | −0.82 | −0.93 | 0.56 | 0.27 | 0.16 | −0.20 | −0.06 | 0.02 | −0.56 | 0.32 | −0.83 | −0.86 | −0.78 | −0.92 | 0.45 | 0.48 | 0.32 | 0.19 | 0.09 | 0.11 | −0.45 | 0.05 | −0.82 | −0.87 | −0.86 | −0.93 | 0.37 | 0.25 | −0.16 | −0.01 | −0.16 | 0.01 | −0.48 | 0.12 |
| Hue | 0.79 | 0.64 | 0.64 | 0.83 | −0.65 | −0.40 | −0.22 | 0.23 | 0.00 | −0.05 | 0.54 | −0.36 | 0.70 | 0.69 | 0.70 | 0.82 | −0.28 | −0.62 | −0.22 | −0.15 | 0.20 | −0.06 | 0.55 | −0.33 | 0.69 | 0.46 | 0.57 | 0.74 | −0.40 | −0.27 | 0.10 | −0.04 | −0.07 | 0.02 | 0.63 | −0.27 |
| Saturation | 0.83 | 0.52 | 0.76 | 0.69 | −0.26 | 0.12 | 0.35 | 0.22 | 0.42 | −0.05 | 0.09 | 0.12 | 0.55 | 0.72 | 0.56 | 0.59 | −0.38 | 0.44 | 0.37 | 0.35 | 0.13 | 0.14 | −0.09 | 0.43 | 0.55 | 0.72 | 0.72 | 0.67 | −0.19 | −0.35 | 0.33 | 0.14 | 0.29 | −0.04 | 0.18 | 0.14 |
| Lightness | −0.82 | −0.63 | −0.80 | −0.92 | 0.56 | 0.31 | 0.24 | −0.18 | 0.01 | 0.01 | −0.59 | 0.34 | −0.84 | −0.84 | −0.77 | −0.92 | 0.45 | 0.57 | 0.43 | 0.30 | 0.15 | 0.15 | −0.51 | 0.13 | −0.82 | −0.87 | −0.85 | −0.92 | 0.38 | 0.24 | −0.10 | 0.02 | −0.15 | 0.01 | −0.50 | 0.17 |
| a* | −0.72 | −0.69 | −0.77 | −0.88 | 0.56 | −0.04 | −0.36 | −0.23 | −0.39 | 0.09 | −0.22 | 0.05 | −0.78 | −0.91 | −0.83 | −0.89 | 0.37 | −0.36 | −0.46 | −0.40 | −0.31 | −0.19 | −0.09 | −0.06 | −0.80 | −0.73 | −0.85 | −0.92 | 0.35 | 0.36 | −0.41 | −0.17 | −0.06 | −0.02 | −0.43 | −0.02 |
| b* | 0.60 | 0.46 | 0.69 | 0.57 | −0.17 | 0.46 | 0.61 | 0.06 | 0.46 | −0.06 | −0.31 | 0.29 | 0.41 | 0.61 | 0.41 | 0.43 | −0.29 | 0.64 | 0.53 | 0.44 | 0.21 | 0.20 | −0.23 | 0.30 | 0.48 | 0.60 | 0.64 | 0.58 | −0.15 | −0.17 | 0.48 | 0.28 | 0.18 | 0.01 | −0.12 | 0.27 |
| u* | −0.69 | −0.66 | −0.67 | −0.84 | 0.60 | 0.05 | −0.24 | −0.24 | −0.32 | 0.09 | −0.31 | 0.16 | −0.75 | −0.82 | −0.80 | −0.88 | 0.33 | −0.20 | −0.41 | −0.37 | −0.34 | −0.18 | −0.21 | 0.06 | −0.74 | −0.61 | −0.73 | −0.83 | 0.35 | 0.33 | −0.37 | −0.13 | −0.02 | −0.03 | −0.49 | 0.08 |
| v* | 0.41 | 0.42 | 0.62 | 0.50 | −0.13 | 0.47 | 0.56 | −0.03 | 0.34 | −0.05 | −0.46 | 0.32 | 0.33 | 0.55 | 0.32 | 0.34 | −0.23 | 0.66 | 0.54 | 0.43 | 0.22 | 0.20 | −0.28 | 0.24 | 0.44 | 0.51 | 0.58 | 0.53 | −0.13 | 0.03 | 0.36 | 0.24 | 0.02 | 0.03 | −0.33 | 0.28 |
| GA | 0.89 | 0.70 | 0.81 | 0.91 | −0.60 | 0.04 | 0.08 | 0.22 | 0.25 | −0.01 | 0.43 | −0.09 | 0.82 | 0.91 | 0.83 | 0.92 | −0.46 | 0.29 | 0.28 | 0.07 | 0.47 | 0.05 | 0.32 | −0.17 | 0.86 | 0.79 | 0.87 | 0.97 | −0.42 | −0.20 | 0.33 | 0.10 | 0.18 | 0.01 | 0.46 | 0.06 |
| GGA | 0.85 | 0.62 | 0.80 | 0.90 | −0.61 | −0.25 | −0.11 | 0.24 | 0.08 | −0.07 | 0.55 | −0.34 | 0.78 | 0.86 | 0.82 | 0.89 | −0.36 | −0.49 | −0.12 | 0.00 | 0.15 | 0.02 | 0.52 | −0.28 | 0.75 | 0.63 | 0.78 | 0.86 | −0.33 | −0.36 | 0.14 | 0.00 | 0.00 | −0.02 | 0.58 | −0.25 |
| CSI | −0.67 | −0.42 | −0.66 | −0.81 | 0.63 | 0.44 | 0.25 | −0.20 | 0.07 | 0.13 | −0.55 | 0.38 | −0.55 | −0.43 | −0.50 | −0.64 | 0.14 | 0.64 | 0.29 | 0.04 | 0.05 | 0.00 | −0.30 | 0.27 | −0.18 | 0.00 | −0.15 | −0.22 | 0.07 | 0.37 | 0.04 | 0.08 | 0.17 | 0.02 | −0.56 | 0.28 |
| ab | −0.71 | −0.67 | −0.80 | −0.84 | 0.46 | −0.28 | −0.54 | −0.17 | −0.46 | 0.09 | 0.06 | −0.16 | −0.72 | −0.91 | −0.79 | −0.82 | 0.38 | −0.53 | −0.51 | −0.46 | −0.25 | −0.19 | 0.10 | −0.26 | −0.76 | −0.79 | −0.90 | −0.90 | 0.30 | 0.30 | −0.48 | −0.26 | −0.11 | −0.01 | −0.16 | −0.20 |
| uv | −0.63 | −0.66 | −0.69 | −0.84 | 0.57 | −0.22 | −0.47 | −0.18 | −0.41 | 0.10 | −0.07 | −0.07 | −0.77 | −0.87 | −0.82 | −0.89 | 0.36 | −0.47 | −0.50 | −0.46 | −0.30 | −0.20 | −0.01 | −0.16 | −0.75 | −0.66 | −0.79 | −0.86 | 0.34 | 0.27 | −0.48 | −0.24 | −0.03 | −0.04 | −0.29 | −0.13 |
| abI | −0.72 | −0.61 | −0.58 | −0.78 | 0.61 | 0.43 | 0.25 | −0.23 | 0.03 | 0.05 | −0.56 | 0.36 | −0.67 | −0.68 | −0.70 | −0.79 | 0.25 | 0.62 | 0.21 | 0.14 | −0.20 | 0.06 | −0.56 | 0.33 | −0.64 | −0.44 | −0.56 | −0.70 | 0.34 | 0.29 | −0.04 | 0.06 | 0.10 | −0.02 | −0.66 | 0.30 |
| uvI | −0.73 | −0.64 | −0.64 | −0.83 | 0.62 | 0.36 | 0.14 | −0.25 | −0.07 | 0.05 | −0.52 | 0.35 | −0.72 | −0.75 | −0.75 | −0.85 | 0.29 | 0.49 | 0.05 | 0.00 | −0.25 | −0.01 | −0.50 | 0.28 | −0.71 | −0.54 | −0.66 | −0.79 | 0.36 | 0.33 | −0.14 | 0.01 | 0.01 | −0.01 | −0.62 | 0.26 |
| auI | −0.27 | 0.40 | 0.56 | 0.55 | −0.34 | 0.18 | 0.04 | −0.01 | 0.02 | 0.10 | 0.11 | 0.04 | 0.19 | 0.26 | 0.20 | 0.17 | −0.30 | 0.13 | −0.23 | −0.19 | −0.38 | −0.14 | −0.30 | 0.28 | 0.18 | 0.16 | 0.07 | 0.23 | −0.08 | 0.18 | −0.35 | −0.08 | −0.07 | −0.05 | 0.03 | 0.08 |
| bvI | 0.77 | 0.48 | 0.73 | 0.69 | −0.26 | −0.28 | −0.13 | 0.23 | 0.07 | −0.01 | 0.49 | −0.14 | 0.58 | 0.70 | 0.56 | 0.61 | −0.40 | −0.28 | −0.19 | −0.06 | −0.08 | −0.07 | 0.27 | 0.42 | 0.52 | 0.72 | 0.68 | 0.63 | −0.18 | −0.32 | 0.10 | 0.00 | 0.23 | −0.05 | 0.39 | 0.04 |
| NDabI | 0.69 | 0.48 | 0.55 | 0.65 | −0.44 | −0.53 | −0.36 | 0.25 | −0.12 | −0.06 | 0.68 | −0.38 | 0.54 | 0.66 | 0.66 | 0.66 | −0.13 | −0.63 | −0.20 | −0.15 | 0.20 | −0.09 | 0.54 | −0.34 | 0.49 | 0.29 | 0.48 | 0.55 | −0.17 | −0.31 | −0.12 | −0.09 | −0.16 | −0.01 | 0.70 | −0.31 |
| NDuvI | 0.71 | 0.59 | 0.63 | 0.76 | −0.54 | −0.45 | −0.20 | 0.26 | 0.01 | −0.06 | 0.61 | −0.38 | 0.65 | 0.76 | 0.75 | 0.77 | −0.23 | −0.51 | −0.05 | 0.00 | 0.25 | −0.02 | 0.53 | −0.28 | 0.62 | 0.47 | 0.63 | 0.70 | −0.25 | −0.36 | 0.04 | −0.04 | −0.06 | −0.01 | 0.68 | −0.28 |
| NDLab | 0.71 | 0.60 | 0.55 | 0.77 | −0.62 | −0.41 | −0.25 | 0.22 | −0.03 | −0.04 | 0.53 | −0.36 | 0.68 | 0.65 | 0.68 | 0.80 | −0.26 | −0.65 | −0.27 | −0.19 | 0.16 | −0.08 | 0.55 | −0.33 | 0.64 | 0.44 | 0.54 | 0.69 | −0.37 | −0.27 | 0.05 | −0.06 | −0.09 | 0.02 | 0.65 | −0.29 |
| NDLuv | 0.75 | 0.65 | 0.63 | 0.85 | −0.64 | −0.32 | −0.13 | 0.24 | 0.08 | −0.04 | 0.45 | −0.33 | 0.76 | 0.72 | 0.73 | 0.88 | −0.33 | −0.53 | −0.11 | −0.04 | 0.22 | −0.01 | 0.44 | −0.27 | 0.74 | 0.56 | 0.64 | 0.81 | −0.43 | −0.30 | 0.16 | 0.00 | 0.01 | 0.02 | 0.60 | −0.25 |
| GI | 0.68 | 0.42 | 0.66 | 0.80 | −0.62 | −0.44 | −0.26 | 0.20 | −0.08 | −0.12 | 0.55 | −0.38 | 0.55 | 0.43 | 0.50 | 0.63 | −0.14 | −0.64 | −0.29 | −0.04 | −0.06 | 0.00 | 0.30 | −0.27 | 0.17 | 0.00 | 0.14 | 0.20 | −0.06 | −0.38 | −0.09 | −0.10 | −0.20 | −0.03 | 0.57 | −0.30 |
| GPI | 0.78 | 0.61 | 0.78 | 0.84 | −0.53 | −0.18 | −0.06 | 0.25 | 0.13 | −0.05 | 0.54 | −0.31 | 0.73 | 0.90 | 0.85 | 0.83 | −0.35 | −0.36 | 0.00 | 0.02 | 0.27 | 0.03 | 0.52 | −0.29 | 0.71 | 0.69 | 0.85 | 0.85 | −0.25 | −0.34 | 0.19 | 0.02 | 0.06 | −0.02 | 0.56 | −0.21 |
| NDGI | −0.66 | −0.43 | −0.66 | −0.79 | 0.62 | 0.42 | 0.24 | −0.20 | 0.06 | 0.12 | −0.52 | 0.36 | −0.57 | −0.43 | −0.50 | −0.66 | 0.17 | 0.64 | 0.29 | 0.03 | 0.06 | −0.01 | −0.22 | 0.25 | −0.17 | −0.01 | −0.15 | −0.21 | 0.07 | 0.37 | 0.05 | 0.10 | 0.19 | 0.02 | −0.53 | 0.28 |
Table 9.
Heatmap correlation matrix of aerial reflectance, color space indices, and their derived vegetation indices, with physiological, morphological, and agronomic traits of peanuts in 2018.
Table 9.
Heatmap correlation matrix of aerial reflectance, color space indices, and their derived vegetation indices, with physiological, morphological, and agronomic traits of peanuts in 2018.
| | Hypogaea | Fastigiata | Vulgaris |
|---|
| Indices | Stand Count | Plant Height | Lateral Growth | Ground NDVI | CTD | Wilting | TSW | SSR | SB | CBR | Yield | Sprouting | Stand Count | Plant Height | Lateral Growth | Ground NDVI | CTD | Wilting | TSW | SSR | SB | CBR | Yield | Sprouting | Stand Count | Plant Height | Lateral Growth | Ground NDVI | CTD | Wilting | TSW | SSR | SB | CBR | Yield | Sprouting |
|---|
| Red | −0.40 | −0.50 | −0.26 | −0.25 | 0.34 | −0.31 | 0.12 | 0.27 | 0.34 | 0.18 | −0.31 | 0.36 | 0.04 | 0.07 | 0.06 | −0.32 | −0.23 | −0.27 | −0.15 | 0.19 | 0.13 | 0.16 | −0.56 | −0.38 | 0.13 | −0.10 | −0.33 | −0.27 | 0.18 | −0.31 | 0.05 | −0.05 | 0.10 | −0.36 | −0.46 | 0.36 |
| Green | −0.57 | −0.60 | −0.27 | −0.40 | 0.37 | −0.46 | −0.01 | 0.25 | 0.17 | 0.13 | −0.23 | 0.35 | −0.07 | −0.05 | 0.03 | −0.50 | −0.11 | −0.40 | −0.09 | 0.29 | 0.18 | 0.05 | −0.52 | −0.42 | 0.26 | −0.26 | −0.33 | −0.51 | 0.18 | −0.33 | 0.06 | −0.16 | 0.01 | −0.34 | −0.47 | 0.34 |
| Blue | −0.55 | −0.62 | −0.24 | −0.37 | 0.28 | −0.34 | 0.03 | 0.00 | −0.09 | 0.15 | −0.26 | −0.17 | −0.36 | −0.08 | 0.11 | −0.43 | −0.10 | −0.27 | 0.02 | −0.02 | −0.09 | 0.36 | −0.28 | −0.49 | 0.19 | −0.39 | −0.48 | −0.52 | 0.39 | −0.45 | −0.02 | 0.13 | 0.28 | −0.13 | −0.39 | 0.44 |
| NIR | 0.35 | 0.31 | 0.38 | 0.57 | −0.39 | −0.02 | 0.14 | 0.04 | 0.05 | 0.03 | 0.02 | −0.01 | 0.01 | −0.07 | −0.17 | 0.18 | −0.02 | 0.12 | 0.18 | 0.25 | 0.33 | 0.08 | −0.01 | 0.00 | 0.28 | 0.25 | 0.28 | 0.19 | −0.08 | 0.04 | 0.28 | 0.18 | −0.17 | 0.14 | −0.03 | 0.11 |
| NDVI | 0.48 | 0.51 | 0.43 | 0.59 | −0.49 | 0.14 | −0.04 | −0.19 | −0.26 | −0.15 | 0.23 | −0.29 | −0.05 | −0.08 | −0.17 | 0.27 | 0.09 | 0.20 | 0.18 | −0.08 | −0.01 | −0.13 | 0.34 | 0.09 | 0.14 | 0.23 | 0.40 | 0.27 | −0.13 | 0.18 | 0.04 | 0.13 | −0.15 | 0.37 | 0.33 | −0.24 |
| BGI | −0.47 | −0.56 | −0.19 | −0.29 | 0.15 | −0.20 | 0.06 | −0.11 | −0.13 | 0.10 | −0.22 | −0.30 | −0.46 | −0.13 | 0.16 | −0.27 | −0.03 | −0.08 | 0.09 | −0.12 | −0.10 | 0.40 | −0.02 | −0.45 | 0.05 | −0.32 | −0.39 | −0.41 | 0.46 | −0.49 | −0.05 | 0.16 | 0.26 | 0.02 | −0.24 | 0.33 |
| RGR | 0.44 | 0.33 | 0.11 | 0.38 | −0.13 | 0.38 | 0.22 | 0.25 | 0.45 | 0.20 | −0.45 | 0.31 | 0.14 | 0.23 | 0.05 | 0.41 | −0.21 | 0.32 | −0.14 | 0.12 | 0.15 | 0.31 | −0.53 | −0.19 | −0.20 | 0.24 | 0.03 | 0.39 | 0.08 | 0.00 | 0.06 | 0.05 | 0.16 | −0.27 | −0.36 | 0.32 |
| NPPR | 0.18 | 0.34 | 0.11 | 0.05 | −0.05 | −0.05 | −0.22 | −0.12 | −0.24 | −0.22 | 0.32 | −0.02 | 0.41 | −0.02 | −0.16 | 0.00 | 0.14 | −0.09 | 0.03 | −0.05 | −0.11 | −0.49 | 0.34 | 0.41 | 0.10 | 0.20 | 0.43 | 0.09 | −0.37 | 0.42 | 0.01 | −0.07 | −0.28 | 0.16 | 0.36 | −0.31 |
| NGRDI | −0.44 | −0.32 | −0.11 | −0.38 | 0.13 | −0.38 | −0.22 | −0.24 | −0.44 | −0.20 | 0.43 | −0.31 | −0.13 | −0.23 | −0.05 | −0.41 | 0.21 | −0.32 | 0.13 | −0.14 | −0.19 | −0.32 | 0.52 | 0.21 | 0.20 | −0.23 | −0.03 | −0.39 | −0.08 | −0.01 | −0.07 | −0.01 | −0.16 | 0.27 | 0.36 | −0.30 |
| PPR | 0.46 | 0.56 | 0.19 | 0.29 | −0.14 | 0.20 | −0.06 | 0.11 | 0.14 | −0.11 | 0.20 | 0.32 | 0.46 | 0.14 | −0.16 | 0.27 | 0.02 | 0.08 | −0.08 | 0.12 | 0.10 | −0.40 | 0.06 | 0.45 | −0.04 | 0.32 | 0.40 | 0.41 | −0.46 | 0.49 | 0.06 | −0.17 | −0.25 | −0.03 | 0.23 | −0.31 |
| NCPI | 0.55 | 0.59 | 0.20 | 0.39 | −0.17 | 0.32 | 0.05 | 0.20 | 0.32 | −0.01 | −0.03 | 0.41 | 0.41 | 0.24 | −0.12 | 0.46 | −0.08 | 0.23 | −0.14 | 0.18 | 0.19 | −0.22 | −0.23 | 0.44 | −0.11 | 0.33 | 0.30 | 0.51 | −0.50 | 0.39 | 0.10 | −0.17 | −0.18 | −0.14 | 0.07 | −0.24 |
| SRI | 0.46 | 0.48 | 0.44 | 0.57 | −0.47 | 0.14 | −0.06 | −0.25 | −0.26 | −0.14 | 0.23 | −0.31 | 0.03 | −0.05 | −0.15 | 0.28 | 0.07 | 0.23 | 0.12 | −0.19 | −0.19 | −0.23 | 0.40 | 0.02 | 0.13 | 0.24 | 0.40 | 0.29 | −0.16 | 0.19 | 0.00 | 0.16 | −0.15 | 0.32 | 0.32 | −0.23 |
| GRVI | 0.53 | 0.52 | 0.43 | 0.62 | −0.46 | 0.22 | 0.09 | −0.18 | −0.09 | −0.08 | 0.08 | −0.26 | 0.06 | 0.00 | −0.12 | 0.35 | 0.01 | 0.28 | 0.14 | −0.11 | −0.01 | −0.03 | 0.25 | −0.07 | 0.03 | 0.33 | 0.39 | 0.42 | −0.15 | 0.21 | 0.11 | 0.22 | −0.10 | 0.37 | 0.25 | −0.21 |
| IO | 0.55 | 0.59 | 0.20 | 0.38 | −0.17 | 0.33 | 0.07 | 0.19 | 0.35 | −0.07 | −0.03 | 0.45 | 0.42 | 0.25 | −0.11 | 0.45 | −0.11 | 0.22 | −0.12 | 0.17 | 0.17 | −0.23 | −0.13 | 0.40 | −0.11 | 0.32 | 0.31 | 0.51 | −0.50 | 0.39 | 0.07 | −0.20 | −0.15 | −0.15 | 0.11 | −0.23 |
| GNDVI | 0.55 | 0.55 | 0.42 | 0.64 | −0.48 | 0.23 | 0.10 | −0.13 | −0.07 | −0.08 | 0.07 | −0.24 | −0.01 | −0.02 | −0.15 | 0.34 | 0.04 | 0.26 | 0.18 | −0.06 | 0.09 | 0.00 | 0.22 | −0.05 | 0.04 | 0.33 | 0.39 | 0.40 | −0.12 | 0.19 | 0.12 | 0.25 | −0.11 | 0.39 | 0.24 | −0.20 |
| BNDVI | 0.57 | 0.61 | 0.36 | 0.57 | −0.41 | 0.23 | 0.03 | 0.04 | 0.11 | −0.13 | 0.18 | 0.17 | 0.18 | 0.02 | −0.18 | 0.36 | 0.05 | 0.22 | 0.06 | 0.09 | 0.17 | −0.31 | 0.06 | 0.07 | 0.00 | 0.43 | 0.52 | 0.46 | −0.30 | 0.33 | 0.10 | −0.08 | −0.32 | 0.15 | 0.36 | −0.29 |
| CIG | 0.53 | 0.52 | 0.43 | 0.62 | −0.46 | 0.22 | 0.09 | −0.18 | −0.09 | −0.08 | 0.08 | −0.26 | 0.06 | 0.00 | −0.12 | 0.35 | 0.01 | 0.28 | 0.14 | −0.11 | −0.01 | −0.03 | 0.25 | −0.07 | 0.03 | 0.33 | 0.39 | 0.42 | −0.15 | 0.21 | 0.11 | 0.22 | −0.10 | 0.37 | 0.25 | −0.21 |
| CVI | 0.56 | 0.59 | 0.19 | 0.39 | −0.17 | 0.31 | 0.04 | 0.20 | 0.31 | 0.01 | −0.01 | 0.39 | 0.40 | 0.23 | −0.13 | 0.46 | −0.06 | 0.24 | −0.15 | 0.18 | 0.20 | −0.20 | −0.30 | 0.46 | −0.12 | 0.33 | 0.29 | 0.52 | −0.51 | 0.38 | 0.11 | −0.15 | −0.19 | −0.13 | 0.05 | −0.24 |
| GLI | 0.18 | 0.35 | 0.11 | 0.05 | −0.06 | −0.05 | −0.21 | −0.11 | −0.24 | −0.22 | 0.42 | −0.02 | 0.40 | −0.02 | −0.15 | 0.00 | 0.14 | −0.10 | 0.03 | −0.02 | −0.06 | −0.47 | 0.34 | 0.39 | 0.09 | 0.20 | 0.43 | 0.09 | −0.37 | 0.43 | 0.01 | −0.12 | −0.28 | 0.15 | 0.37 | −0.34 |
| GBNDVI | 0.56 | 0.57 | 0.41 | 0.61 | −0.45 | 0.23 | 0.08 | −0.09 | −0.02 | −0.12 | 0.14 | −0.11 | 0.12 | 0.01 | −0.16 | 0.35 | 0.03 | 0.25 | 0.13 | −0.01 | 0.11 | −0.16 | 0.19 | −0.01 | 0.02 | 0.40 | 0.48 | 0.44 | −0.20 | 0.26 | 0.15 | 0.15 | −0.26 | 0.36 | 0.38 | −0.31 |
| GRNDVI | 0.52 | 0.53 | 0.43 | 0.62 | −0.49 | 0.19 | 0.02 | −0.18 | −0.18 | −0.12 | 0.16 | −0.28 | −0.01 | −0.04 | −0.15 | 0.32 | 0.05 | 0.24 | 0.17 | −0.09 | 0.00 | −0.09 | 0.29 | 0.01 | 0.08 | 0.29 | 0.40 | 0.35 | −0.13 | 0.19 | 0.07 | 0.18 | −0.13 | 0.38 | 0.30 | −0.23 |
| RBNDVI | −0.19 | −0.20 | 0.24 | 0.13 | −0.30 | −0.17 | 0.02 | −0.14 | −0.23 | −0.11 | 0.11 | −0.33 | −0.18 | −0.26 | −0.03 | −0.18 | 0.03 | −0.01 | 0.24 | −0.03 | 0.15 | 0.05 | 0.44 | 0.12 | 0.16 | −0.28 | −0.20 | −0.19 | 0.20 | −0.18 | 0.04 | 0.23 | −0.01 | 0.35 | 0.18 | −0.11 |
| mSR | 0.53 | 0.56 | 0.41 | 0.57 | −0.42 | 0.21 | −0.06 | −0.17 | −0.15 | −0.18 | 0.28 | −0.14 | 0.23 | 0.02 | −0.15 | 0.35 | 0.03 | 0.25 | 0.05 | −0.11 | −0.09 | −0.34 | 0.32 | 0.06 | 0.03 | 0.41 | 0.52 | 0.44 | −0.27 | 0.31 | 0.09 | 0.09 | −0.27 | 0.29 | 0.39 | −0.29 |
| GARI | 0.53 | 0.57 | 0.41 | 0.59 | −0.45 | 0.20 | −0.03 | −0.14 | −0.17 | −0.17 | 0.26 | −0.17 | 0.10 | −0.02 | −0.17 | 0.32 | 0.06 | 0.22 | 0.14 | −0.04 | 0.03 | −0.24 | 0.27 | 0.09 | 0.06 | 0.39 | 0.52 | 0.38 | −0.22 | 0.27 | 0.08 | 0.08 | −0.25 | 0.35 | 0.40 | −0.30 |
| Intensity | −0.39 | −0.37 | −0.38 | −0.38 | 0.30 | −0.34 | 0.07 | 0.24 | 0.23 | 0.19 | −0.26 | 0.28 | 0.20 | 0.14 | −0.14 | −0.03 | 0.09 | −0.11 | −0.09 | 0.18 | 0.10 | 0.21 | −0.46 | −0.44 | −0.25 | 0.11 | −0.13 | −0.39 | 0.24 | −0.35 | 0.05 | −0.04 | 0.15 | −0.35 | −0.47 | 0.41 |
| Hue | 0.50 | 0.48 | 0.40 | 0.54 | −0.32 | 0.32 | −0.18 | −0.27 | −0.47 | −0.18 | 0.51 | −0.39 | 0.52 | 0.27 | −0.04 | 0.22 | −0.13 | 0.13 | 0.19 | −0.14 | −0.16 | −0.15 | 0.29 | 0.08 | 0.23 | 0.33 | 0.34 | 0.53 | −0.24 | 0.17 | −0.09 | −0.06 | −0.03 | 0.23 | 0.41 | −0.35 |
| Saturation | 0.45 | 0.38 | 0.32 | 0.45 | −0.40 | 0.33 | −0.01 | 0.16 | 0.22 | −0.07 | 0.09 | 0.36 | −0.32 | 0.14 | 0.26 | −0.30 | −0.45 | −0.36 | −0.11 | 0.14 | 0.14 | −0.34 | 0.10 | 0.49 | −0.17 | 0.19 | 0.19 | 0.34 | −0.23 | 0.37 | 0.08 | −0.15 | −0.23 | −0.07 | 0.10 | −0.32 |
| Lightness | −0.35 | −0.34 | −0.37 | −0.34 | 0.28 | −0.33 | 0.02 | 0.26 | 0.21 | 0.14 | −0.25 | 0.35 | 0.19 | 0.20 | −0.14 | −0.02 | 0.05 | −0.14 | −0.09 | 0.27 | 0.18 | 0.09 | −0.51 | −0.41 | −0.27 | 0.15 | −0.08 | −0.35 | 0.23 | −0.34 | 0.07 | −0.13 | 0.03 | −0.35 | −0.48 | 0.35 |
| a* | −0.48 | −0.46 | −0.36 | −0.52 | 0.31 | −0.27 | 0.27 | 0.15 | 0.43 | 0.18 | −0.48 | 0.14 | −0.49 | −0.28 | 0.03 | −0.18 | 0.17 | −0.07 | −0.15 | −0.11 | −0.06 | 0.34 | −0.14 | −0.07 | −0.06 | −0.31 | −0.35 | −0.51 | 0.25 | −0.15 | −0.01 | 0.21 | 0.24 | −0.14 | −0.15 | 0.27 |
| b* | 0.43 | 0.32 | 0.13 | 0.49 | −0.40 | 0.19 | 0.00 | 0.24 | 0.25 | 0.04 | −0.15 | 0.42 | −0.19 | 0.32 | 0.11 | −0.21 | −0.38 | −0.42 | −0.13 | 0.28 | 0.23 | −0.22 | −0.41 | 0.09 | −0.30 | 0.29 | 0.18 | 0.26 | −0.16 | 0.21 | 0.07 | −0.18 | −0.17 | −0.21 | −0.13 | −0.12 |
| u* | −0.46 | −0.46 | −0.37 | −0.50 | 0.28 | −0.27 | 0.25 | 0.21 | 0.47 | 0.17 | −0.50 | 0.26 | −0.53 | −0.20 | 0.04 | −0.22 | 0.09 | −0.15 | −0.20 | −0.06 | −0.04 | 0.22 | −0.24 | −0.05 | −0.24 | −0.14 | −0.25 | −0.49 | 0.26 | −0.12 | 0.00 | 0.19 | 0.19 | −0.24 | −0.28 | 0.28 |
| v* | 0.29 | 0.21 | 0.00 | 0.36 | −0.25 | 0.04 | −0.02 | 0.25 | 0.20 | 0.07 | −0.17 | 0.41 | −0.09 | 0.38 | 0.03 | −0.13 | −0.32 | −0.36 | −0.11 | 0.32 | 0.25 | −0.15 | −0.57 | −0.13 | −0.32 | 0.30 | 0.17 | 0.22 | −0.10 | 0.09 | 0.07 | −0.19 | −0.14 | −0.25 | −0.23 | −0.02 |
| GA | 0.54 | 0.50 | 0.43 | 0.56 | −0.36 | 0.37 | −0.11 | −0.31 | −0.28 | −0.26 | 0.50 | 0.02 | 0.59 | 0.14 | −0.02 | 0.34 | −0.01 | 0.23 | 0.18 | 0.15 | 0.10 | −0.09 | 0.31 | 0.03 | 0.27 | 0.42 | 0.61 | 0.54 | −0.24 | 0.24 | 0.02 | −0.40 | −0.13 | 0.14 | 0.42 | −0.44 |
| GGA | 0.50 | 0.47 | 0.45 | 0.57 | −0.41 | 0.26 | −0.19 | −0.27 | −0.49 | −0.15 | 0.41 | −0.43 | 0.57 | 0.15 | −0.12 | 0.32 | 0.08 | 0.18 | 0.13 | −0.15 | −0.16 | −0.19 | 0.32 | 0.23 | 0.38 | 0.17 | 0.38 | 0.42 | −0.31 | 0.18 | −0.01 | 0.02 | −0.09 | 0.37 | 0.31 | −0.16 |
| CSI | −0.34 | −0.34 | −0.26 | −0.41 | 0.37 | 0.05 | 0.17 | 0.26 | 0.50 | 0.12 | −0.42 | 0.45 | −0.38 | −0.01 | 0.23 | −0.18 | −0.24 | −0.01 | −0.12 | 0.18 | 0.18 | 0.17 | −0.33 | −0.20 | −0.32 | 0.08 | −0.18 | 0.03 | 0.27 | 0.06 | 0.02 | −0.03 | 0.09 | −0.35 | −0.25 | 0.13 |
| ab | −0.48 | −0.46 | −0.33 | −0.52 | 0.32 | −0.26 | 0.19 | −0.07 | 0.09 | 0.09 | −0.25 | −0.24 | −0.36 | −0.31 | 0.01 | −0.13 | 0.22 | 0.01 | −0.05 | −0.28 | −0.26 | 0.35 | 0.08 | −0.09 | 0.06 | −0.34 | −0.36 | −0.49 | 0.24 | −0.16 | −0.07 | 0.26 | 0.25 | 0.04 | −0.01 | 0.21 |
| uv | −0.45 | −0.46 | −0.37 | −0.48 | 0.27 | −0.27 | 0.26 | 0.12 | 0.38 | 0.13 | −0.47 | 0.09 | −0.49 | −0.16 | 0.04 | −0.23 | 0.07 | −0.16 | −0.20 | −0.20 | −0.21 | 0.21 | −0.16 | −0.02 | −0.23 | −0.05 | −0.24 | −0.47 | 0.25 | −0.11 | −0.06 | 0.27 | 0.24 | −0.15 | −0.19 | 0.22 |
| abI | −0.47 | −0.46 | −0.38 | −0.51 | 0.29 | −0.28 | 0.18 | 0.27 | 0.46 | 0.18 | −0.49 | 0.39 | −0.54 | −0.23 | 0.05 | −0.23 | 0.10 | −0.15 | −0.19 | 0.15 | 0.16 | 0.14 | −0.31 | −0.03 | −0.26 | −0.21 | −0.23 | −0.50 | 0.25 | −0.13 | 0.08 | 0.04 | 0.02 | −0.25 | −0.41 | 0.30 |
| uvI | −0.47 | −0.46 | −0.37 | −0.51 | 0.29 | −0.28 | 0.20 | 0.27 | 0.47 | 0.20 | −0.50 | 0.36 | −0.55 | −0.23 | 0.04 | −0.21 | 0.11 | −0.14 | −0.17 | 0.11 | 0.12 | 0.21 | −0.31 | −0.09 | −0.24 | −0.23 | −0.26 | −0.50 | 0.26 | −0.14 | 0.06 | 0.10 | 0.09 | −0.25 | −0.39 | 0.34 |
| auI | 0.07 | 0.05 | 0.17 | 0.16 | −0.04 | 0.19 | 0.06 | 0.15 | 0.31 | 0.03 | −0.07 | 0.12 | 0.03 | 0.02 | −0.09 | 0.08 | 0.08 | −0.11 | −0.23 | −0.11 | −0.21 | 0.01 | 0.18 | 0.09 | 0.09 | 0.13 | −0.25 | 0.12 | −0.02 | 0.14 | 0.01 | −0.57 | −0.35 | 0.09 | 0.06 | −0.17 |
| bvI | 0.25 | 0.20 | 0.26 | 0.21 | −0.26 | 0.29 | 0.06 | 0.07 | 0.24 | −0.08 | 0.15 | 0.21 | −0.40 | −0.23 | 0.30 | −0.32 | −0.22 | −0.16 | −0.12 | −0.09 | −0.04 | −0.34 | 0.38 | 0.56 | 0.00 | −0.04 | 0.08 | 0.16 | −0.25 | 0.39 | 0.04 | −0.02 | −0.24 | 0.06 | 0.46 | −0.46 |
| NDabI | 0.49 | 0.48 | 0.40 | 0.53 | −0.31 | 0.31 | −0.15 | −0.19 | −0.37 | −0.16 | 0.43 | −0.28 | 0.52 | 0.26 | −0.06 | 0.25 | −0.09 | 0.17 | −0.09 | −0.02 | −0.25 | −0.15 | 0.21 | 0.14 | 0.28 | 0.21 | 0.20 | 0.52 | −0.26 | 0.13 | −0.13 | 0.06 | −0.11 | 0.16 | 0.41 | −0.28 |
| NDuvI | 0.49 | 0.47 | 0.40 | 0.53 | −0.31 | 0.31 | −0.20 | −0.23 | −0.44 | −0.19 | 0.44 | −0.32 | 0.54 | 0.26 | −0.05 | 0.23 | −0.11 | 0.15 | 0.15 | −0.18 | −0.26 | −0.25 | 0.44 | 0.18 | 0.26 | 0.23 | 0.25 | 0.51 | −0.27 | 0.14 | −0.11 | 0.05 | −0.10 | 0.23 | 0.38 | −0.30 |
| NDLab | 0.46 | 0.46 | 0.36 | 0.50 | −0.27 | 0.26 | −0.18 | −0.28 | −0.47 | −0.17 | 0.50 | −0.40 | 0.54 | 0.20 | −0.05 | 0.22 | −0.09 | 0.16 | 0.19 | −0.12 | −0.12 | −0.12 | 0.29 | −0.01 | 0.26 | 0.18 | 0.22 | 0.49 | −0.25 | 0.12 | −0.07 | −0.08 | −0.02 | 0.25 | 0.42 | −0.30 |
| NDLuv | 0.45 | 0.45 | 0.35 | 0.49 | −0.27 | 0.25 | −0.19 | −0.29 | −0.47 | −0.19 | 0.52 | −0.37 | 0.56 | 0.18 | −0.04 | 0.19 | −0.10 | 0.13 | 0.18 | −0.07 | −0.06 | −0.18 | 0.25 | 0.03 | 0.23 | 0.21 | 0.27 | 0.49 | −0.26 | 0.13 | −0.05 | −0.17 | −0.10 | 0.23 | 0.42 | −0.36 |
| GI | 0.34 | 0.34 | 0.28 | 0.41 | −0.37 | −0.04 | −0.17 | −0.26 | −0.49 | −0.12 | 0.42 | −0.45 | 0.38 | 0.03 | −0.23 | 0.18 | 0.23 | 0.02 | 0.13 | −0.18 | −0.18 | −0.18 | 0.34 | 0.19 | 0.32 | −0.08 | 0.18 | −0.04 | −0.27 | −0.06 | −0.02 | 0.04 | −0.08 | 0.35 | 0.26 | −0.13 |
| GPI | 0.51 | 0.48 | 0.48 | 0.57 | −0.40 | 0.34 | −0.19 | −0.27 | −0.49 | −0.17 | 0.38 | −0.40 | 0.60 | 0.22 | −0.08 | 0.33 | 0.00 | 0.22 | 0.14 | −0.13 | −0.16 | −0.20 | 0.33 | 0.26 | 0.35 | 0.27 | 0.42 | 0.47 | −0.30 | 0.21 | 0.01 | 0.01 | −0.09 | 0.39 | 0.35 | −0.17 |
| NDGI | −0.36 | −0.36 | −0.27 | −0.41 | 0.38 | 0.03 | 0.17 | 0.25 | 0.48 | 0.09 | −0.45 | 0.44 | −0.36 | 0.00 | 0.23 | −0.17 | −0.23 | −0.01 | −0.18 | 0.15 | 0.11 | 0.15 | −0.41 | −0.11 | −0.26 | 0.07 | −0.22 | 0.05 | 0.27 | 0.07 | 0.00 | −0.03 | 0.12 | −0.32 | −0.23 | 0.13 |
Table 10.
Heritability of physiological, morphological, disease, sprouting, and yield measurements, of peanuts over different growth stages. Heritability values range from 0 to 1; the closer the values to 1 the higher the heritability.
Table 10.
Heritability of physiological, morphological, disease, sprouting, and yield measurements, of peanuts over different growth stages. Heritability values range from 0 to 1; the closer the values to 1 the higher the heritability.
| | Weeks after Planting |
|---|
| Traits | 4 | 5 | 6 | 7 | 9 | 10 | 11 | 12 | 16 | Average † |
|---|
| | Vegetative Phase | Beginning Bloom | Beginning Peg | Beginning Pod | Full Pod | Beginning Seed | Seed Development | Full Seed | Harvest | |
|---|
| Stand count | 0.87 | . | . | . | . | . | . | . | . | 0.87 |
| Thrips | 0.01 | . | . | . | . | . | . | . | . | 0.01 |
| Plant height | 0.18 | 0.22 | 0.45 | . | 0.94 | . | . | . | . | 0.37 |
| Lateral growth | 0.32 | 0.03 | 0.07 | . | . | . | . | . | . | 0.07 |
| NDVI | 0.95 | 0.91 | 0.25 | 0.80 | 0.04 | 0.06 | . | 0.08 | . | 0.02 |
| CTD | . | 0.97 | 0.03 | 0.79 | 0.11 | 0.33 | . | 0.26 | . | 0.07 |
| Wilting | . | . | . | 0.65 | . | 0.05 | . | 0.16 | . | 0.32 |
| TSW | . | . | . | . | . | 0.21 | 0.52 | 0.26 | . | 0.15 |
| SSR | . | . | . | . | . | 0.58 | 0.33 | 0.15 | . | 0.10 |
| SB | . | . | . | . | . | 0.24 | 0.66 | 0.52 | . | 0.20 |
| CBR | . | . | . | . | . | 0.38 | 0.56 | 0.27 | . | 0.09 |
| Sprouting | . | . | . | . | . | . | . | . | 0.26 | 0.26 |
| Yield | . | . | . | . | . | . | . | . | 0.14 | 0.14 |
Table 11.
Heritability of aerially derived vegetation indices over different growth stages. Heritability values range from 0 to 1; the closer the values to 1 the higher the heritability.
Table 11.
Heritability of aerially derived vegetation indices over different growth stages. Heritability values range from 0 to 1; the closer the values to 1 the higher the heritability.
| | Weeks after Planting | | Weeks after Planting |
|---|
| Indices | 4 | 6 | 8 | 10 | 12 | 14 | Avg. † | Indices | 4 | 6 | 8 | 10 | 12 | 14 | Avg. |
|---|
| | Vegetative Phase | Beginning Peg | Pod Development | Beginning Seed | Full Seed | Pod Maturity | | | Vegetative Phase | Beginning Peg | Pod Development | Beginning Seed | Full Seed | Pod Maturity | |
|---|
| Red | 0.09 | 0.01 | 0.07 | 0.33 | 0.17 | 0.1 | 0.52 | Intensity | 0.31 | 0.33 | 0.25 | 0.49 | 0.23 | 0.13 | 0.42 |
| Green | 0.36 | 0.01 | 0.1 | 0.25 | 0.11 | 0.41 | 0.61 | Hue | 0.39 | 0.48 | 0.56 | 0.02 | 0.13 | 0.03 | 0.29 |
| Blue | 0.67 | 0.03 | 0.08 | 0.11 | 0.17 | 0.1 | 0.53 | Saturation | 0.3 | 0.38 | 0.24 | 0.05 | 0.05 | 0.21 | 0.49 |
| NIR | 0.22 | 0.7 | 0.07 | 0.47 | 0.21 | 0.48 | 0.45 | Lightness | 0.27 | 0.29 | 0.24 | 0.41 | 0.12 | 0.26 | 0.37 |
| NDVI | 0.25 | 0.52 | 0.05 | 0.23 | 0.13 | 0.22 | 0.42 | a* | 0.38 | 0.51 | 0.59 | 0.05 | 0.16 | 0.02 | 0.5 |
| BGI | 0.33 | 0.21 | 0.22 | 0.24 | 0.05 | 0.08 | 0.56 | b* | 0.17 | 0.24 | 0.3 | 0.29 | 0.06 | 0.58 | 0.34 |
| RGR | 0.06 | 0.72 | 0.87 | 0.02 | 0.45 | 0.02 | 0.54 | u* | 0.25 | 0.4 | 0.63 | 0.02 | 0.49 | 0.02 | 0.33 |
| NPPR | 0.63 | 0.26 | 0.53 | 0.08 | 0.1 | 0.03 | 0.39 | v* | 0.15 | 0.21 | 0.37 | 0.56 | 0.07 | 0.69 | 0.32 |
| NGRDI | 0.06 | 0.72 | 0.86 | 0.02 | 0.49 | 0.02 | 0.6 | GA | 0.4 | 0.36 | 0.51 | 0.9 | 0.19 | 0.04 | 0.24 |
| PPR | 0.33 | 0.21 | 0.22 | 0.24 | 0.06 | 0.09 | 0.5 | GGA | 0.12 | 0.44 | 0.39 | 0.53 | 0.07 | 0.02 | 0.05 |
| NCPI | 0.09 | 0.29 | 0.13 | 0.03 | 0.06 | 0.62 | 0.62 | CSI | 0.06 | 0.16 | 0.39 | 0.42 | 0.07 | 0.02 | 0.04 |
| SRI | 0.19 | 0.52 | 0.04 | 0.21 | 0.13 | 0.23 | 0.4 | ab | 0.44 | 0.54 | 0.46 | 0.27 | 0.06 | 0.05 | 0.52 |
| GRVI | 0.35 | 0.61 | 0.04 | 0.61 | 0.09 | 0.26 | 0.39 | uv | 0.2 | 0.36 | 0.63 | 0.07 | 0.14 | 0.02 | 0.52 |
| IO | 0.09 | 0.29 | 0.12 | 0.03 | 0.08 | 0.54 | 0.39 | abI | 0.3 | 0.43 | 0.52 | 0.02 | 0.13 | 0.03 | 0.28 |
| GNDVI | 0.43 | 0.61 | 0.05 | 0.67 | 0.09 | 0.25 | 0.35 | uvI | 0.31 | 0.45 | 0.6 | 0.02 | 0.19 | 0.03 | 0.25 |
| BNDVI | 0.64 | 0.63 | 0.04 | 0.37 | 0.25 | 0.16 | 0.38 | auI | 0.78 | 0.82 | 0.92 | 0.22 | 0.66 | 0.93 | 0.8 |
| CIG | 0.35 | 0.61 | 0.04 | 0.61 | 0.09 | 0.26 | 0.39 | bvI | 0.48 | 0.3 | 0.2 | 0.05 | 0.14 | 0.32 | 0.49 |
| CVI | 0.09 | 0.3 | 0.13 | 0.03 | 0.06 | 0.66 | 0.74 | NDabI | 0.33 | 0.42 | 0.43 | 0.87 | 0.44 | 0.06 | 0.87 |
| GLI | 0.63 | 0.26 | 0.52 | 0.08 | 0.1 | 0.03 | 0.46 | NDuvI | 0.32 | 0.44 | 0.54 | 0.03 | 0.3 | 0.03 | 0.5 |
| GBNDVI | 0.49 | 0.61 | 0.04 | 0.57 | 0.18 | 0.2 | 0.39 | NDLab | 0.27 | 0.41 | 0.54 | 0.02 | 0.11 | 0.04 | 0.18 |
| GRNDVI | 0.3 | 0.57 | 0.04 | 0.59 | 0.1 | 0.24 | 0.38 | NDLuv | 0.28 | 0.45 | 0.65 | 0.03 | 0.17 | 0.03 | 0.2 |
| RBNDVI | 0.53 | 0.55 | 0.15 | 0.09 | 0.1 | 0.41 | 0.34 | GI | 0.06 | 0.15 | 0.39 | 0.42 | 0.08 | 0.02 | 0.04 |
| mSR | 0.41 | 0.59 | 0.04 | 0.63 | 0.28 | 0.16 | 0.35 | GPI | 0.18 | 0.46 | 0.43 | 0.61 | 0.06 | 0.02 | 0.05 |
| GARI | 0.35 | 0.57 | 0.04 | 0.54 | 0.23 | 0.17 | 0.38 | NDGI | 0.06 | 0.17 | 0.39 | 0.44 | 0.1 | 0.02 | 0.03 |