Abstract
Water stress affects the growth, development, and yield of crops. The objective of this study is to evaluate the positive effects of nutrients (calcium, potassium, and boron) on durum wheat facing drought stress. Two treatments of calcium, potassium, and boron were used under drought and well-watered conditions on two varieties of durum wheat (V1—Preco; V2—Kronos). The data depict that the exogenous application of these nutrients gave significantly different (p < 0.05) results. The percentage increase in shoot length and root length was 29% and 35% compared to the untreated, drought-facing plants. There is also an increase in the synthesis of photosynthetic pigments and osmolytes. The foliar spray of nutrients enhances the synthesis of antioxidants, including superoxide dismutase, catalase, and peroxidase, which reduce the production of free radicals. It also helps to maintain the stability of membranes and other cell organelles. The spray application enhances the yield of durum wheat, i.e., the percentage increase in the number of grains per spike and 1000-grain weight was 23% and 25% compared to the untreated, drought-facing plants. The use of these nutrients considerably improves the functioning of antioxidant machinery, helping combat the adverse effects of drought. Additionally, they improve the growth- and yield-related parameters. Hence, these sprays can be used as a plant growth regulator.
1. Introduction
Drought is the most critical threat to world food security as there is a limited supply of fresh water and the demand for food is increasing with time. The major proportion of global land area belongs to the arid and semi-arid zone. About 30% of the area in developing countries consists of arid and semi-arid environments that suffer from large fluctuations in the amount and frequency of rainfall. Wheat is commonly grown in these drought-prone areas. Although drought affects the performance of plants at all growth stages, it is more critically reducing the yields by affecting the flowering and grain-filling stage. Water deficiency alters the physiological and biochemical processes of plants. Water stress reduces relative water content, water potential, osmotic potential, turgor pressure, and yield. Drought decreases the productivity of crops by up to 50% [1,2,3]. The effect of stress also depends upon plant type and its genotype, while the extent of damage due to drought depends upon stress intensity and length of the stress period. Drought tolerance is important to increase yield and productivity. Durum wheat is important for its high drought tolerance ability as it contains drought-related genes. It is the hardest wheat, as it contains a high protein content and is better suited under low annual precipitation compared to bread wheat. The drought tolerance of durum wheat has been analyzed through the study of physiological characters under different environmental conditions [4]. There is a high demand for durum wheat and its production should be enhanced to meet the needs of the growing population. Durum is mostly cultivated in rain-fed areas and is exposed to drought stress during the hot season(s). The scarcity of water significantly decreases the uptake of nutrients and the growth of plants [5]. In response to drought, durum wheat shows changes in the water relation of tissues, maintains stomatal conductance, and carries out ion accumulation. Different physiological mechanisms help the plant to tolerate water shortage and maintain growth and productivity. When the ability of the plant to use water becomes increased, it gives maximum yield and the plant becomes more tolerant to drought. Durum wheat breeders are trying to increase the yield under drought stress [6].
Plants have adopted some mechanisms to cope with drought stress that include an increase in water uptake by the deep root system, closure of stomata, as well as the production and accumulation of osmolytes, compatible solutes, and antioxidants. There are some essential nutrients that plants can use to reduce the damaging effects of water scarcity. The transport of nutrients to the plant roots occurs by diffusion, but low soil moisture reduces the nutrient availability [7,8]. Water deficiency decreases the uptake and translocation of nutrients, but when they are applied in the form of foliar spray, plants effectively uptake them. It is one of the important methods because the consumption of nutrients for plants becomes easy and quick by penetrating the stoma and enters in the cells so the drought potential of the plant can be increased by foliar application of nutrients. When nutrients are applied as a foliar spray on crops, their rate of absorption is dependent on the plant’s chemical properties, and if the valiancy is high, its mobility reduces. Different studies have been carried out to illustrate the efficiency of different nutrients under drought stress [9,10]. Potassium is an important nutrient that maintains turgor pressure in plants; however, its uptake decreases with decreasing soil water content. Calcium is another nutrient that acts as a signaling molecule during stress and activates different enzymes. Calcium is responsible for the upregulation of stress-responsive genes in the plants. The deficiency of boron is also a common phenomenon under drought. This is related to boron’s low mineralization because much of the boron is in the form of organic matter. The foliar spray of nutrients helps the plant to mitigate the negative effects of drought and enhance its growth and yield [11,12].
The excess use of chemical fertilizer as nutrient supplements degrades the ecosystem and causes soil and water pollution as well. Chemical fertilizers cause soil acidification, and soil pH changes [13,14]. Kizilgeci et al. [15] experimented to find the appropriate dose of nitrogen for durum wheat. The results showed that the application of nitrogen at the rate of 100 kg/ha gives good results. The investigation of Pačuta et al. [16] showed that foliar application of bioactive compounds such as humic acid and seaweeds improve the grain yield and quality. The foliar application of zinc enhances the bioavailability of micronutrients in the grains of wheat compared to the control. So it decreases the chances of nutrient deficiency in seeds which is a serious concern in various countries [17].
Foliar spray was selected in this research as a suitable strategy to supply nutrients to plants. The present study was designed to evaluate the role of nutrients and their positive effects on durum wheat facing drought stress.
Durum wheat is drought tolerant so we selected two (Preco and Kronos) varieties which have a good yield. The novelty of this research includes the comparison of two drought-tolerant varieties of durum wheat to select the best one to be used in agricultural fields facing water shortages.
2. Materials and Methods
2.1. Experimental Details
Two varieties of durum wheat, i.e., Preco (V1) and Kronos (V2), were selected and their seeds were sterilized with 0.01% of mercuric chloride and washed with distilled water. Solutions of calcium sulfate (4 mM and 8 mM), potassium sulfate (2% and 4%), and borax (10 mg and 20 mg) were prepared. The soil was collected from PMAS, Arid Agriculture University Rawalpindi, Pakistan, and its analysis was carried out by following the method of Li et al. [18] (Table 1). To determine soil texture, 40 g of soil was mixed with 1% solution of sodium hexa meta-phosphate (40 mL). Approximately, 40 mL of distilled water was added and kept for 12 h. A Bouyoucos hydrometer was used to measure particle size and the ISSS triangle was used to determine the soil texture. To measure the potassium in the soil, 5 g of soil was mixed with 33 mL of NH4CH3CO2 and after being shaken for 5 min, it was centrifuged at 4000 rpm to obtain a transparent supernatant. The extract was taken and mixed with 100 mL of NH4CH3CO2 and the mixture was subjected to a flame photometer to analyze the potassium content [19]. To measure the calcium, 1 g of soil was mixed with (0.2 N) ammonium oxalate solution and placed on a shaker at 250 rpm for 2 h. After filtration, 10 mL of suspension was mixed with 5 mL of H2SO4 and 75 mL of distilled water and the mixture was heated at 70 °C. Then, (0.2 N) potassium permanganate was used to titrate the hot solution. It was observed for the appearance of pink color. A blank was also prepared. The following formula was used for the calculation:
where N is the normality of potassium permanganate, V1 is the total volume used (mL) and V2 is the volume of the extract (mL) [20].
Active calcium carbonate = (Vblank − V sample) × N × V1/V2 × 100/Wt. of soil × 5/1000

Table 1.
Analysis of soil used in the experiment.
To measure the boron content in the soil, 10 g of soil was mixed with (0.2 g) activated charcoal and (20 mL) deionized water and boiled for 5 min on a hot plate. The mixture was filtered and 1 mL of the filtrate was added to a tube which contained 2 mL of azomethine and (2 mL) buffer solution. The absorbance was recorded after 30 min at 420 nm. The calculation was carried out using the following formula [20]:
Boron content = B from a standard curve (ppm) × Vol. of soil extract/Wt. of soil
To measure the soil pH, 50 g of soil was mixed with 50 mL of distilled water and after 1 h its pH was recorded using a pH meter. In total, 2 g of soil was mixed with 10 mL (1 N) of K2Cr2O7. Approximately, 20 mL of sulfuric acid was added to the mixture and kept for 30 min. After 30 min, 10 mL of phosphoric acid, 200 mL of distilled water, and 1 mL of diphenylamine were added to the mixture. Titration was carried out using (0.5 N) FeSO4 until the color turned green. The calculation was carried out using the formula [21].
Organic Matter = mL for blank − mL for soil/ wt. of soil (g) × N FeSO4 × 0.69
Plastic pots (6 cm diameter and length) were filled with 7 kg of soil and 8 seeds were sown in each pot in November. The weather was dry, with 36% humidity and 21 °C ± 5 temperature and the length of daylight was 10 h. The experimental design was factorial. After germination, thinning was carried out to maintain 5 plants/pot. Pots were irrigated daily to maintain relative soil humidity. Drought stress was applied by maintaining the field capacity at 50% at the anthesis stage. The solution of calcium, potassium, and boron was applied in the form of foliar spray with a gap of 4 days (3-time spray) and 3 replicates per treatment were maintained. After 10 days of foliar application, leaf samples were collected and used to measure different morphological, physiological, and biochemical attributes of the plants. The field capacity was maintained at 50% until the collection of yield.
2.2. Growth Attributes
A meter rod was used to measure the length of the shoots and roots of plants. One plant from each replicate was uprooted and after removing dust, its fresh weight was measured using weight balance. The samples were dried in an oven at 70 °C at 72 h to measure the constant dry weight of the roots and shoots [22].
2.3. Chlorophyll Content
Leaves (100 mg) were placed in a 12 mL vial containing 7 mL of dimethyl sulfoxide and it was incubated at 65 °C for 30 min. The sample was filtered and transferred to a 25 mL tube and dimethyl sulfoxide was added to make the final volume 10 mL. Then, the absorbance was recorded at 645 and 663 nm [23]. The calculation was carried out using the following formula:
Chlorophyll a content = 12.7 (OD 663) − 2.69 (OD 645)
Chlorophyll b content = 22.9 (OD 645) − 4.68 (OD 663)
2.4. Proline Content and Sugar Content
The leaf sample of 0.5 g was homogenized in 10 mL of sulfosalicylic acid. The sample was filtered and an equal volume of glacial acetic acid and ninhydrin reagent was added to the filtrate. The contents were transferred to a separating funnel and 4 mL of toluene was added and mixed vigorously. The colored toluene fraction was separated and optical density was measured at 520 nm by spectrophotometer [23]. To measure the sugar content, 0.1 g of fresh leaves was taken in a test tube which contained 5 mL of 80% ethanol and placed in a water bath at 95 °C for 10 min, then it was centrifuged at 2500 rpm for 5 min. The extract was collected (2 mL) and mixed with 4% phenol (2 mL) and 96% sulfuric acid (2 mL) and kept in the dark for 30 min. Absorbance was measured by spectrophotometer and a standard curve was used for the calculation [24].
2.5. Membrane Stability Index and Relative Water Content
Leaves samples (T1) were washed and placed in deionized water in an incubator for 24 h at 10 °C. After incubation, they were kept at room temperature for 1 h, and their electrical conductivity was measured. All samples were covered with plastic sheets and placed in an autoclave for 15 min. Then, the conductivity of the samples was measured again and the membrane stability index was calculated [25]. To measure relative water content, the fresh weight (FW) of the leaves was recorded and then they were hydrated to full turgidity for 3 h at room temperature. After hydration, their turgid weight was measured (TW). Then, samples were oven-dried at 80 °C for 24 h and their dry weight (DW) was measured. The following equation was used [26]:
Relative Water Content (RWC) = [(FW − DW)/(TW − DW)] × 100
2.6. Antioxidants Activity
To measure the activity of superoxide dismutase, the leaf (0.5 g) was ground in 10 mL of sodium phosphate buffer and allowed to settle. The extract (1 mL) was transferred in a separate test tube and 0.1 mL of riboflavin and 3 mL of phosphate buffer were added to it. The mixture was placed under a fluorescent lamp for 8 min to start the reaction. Then, the absorbance was recorded at 560 nm using a spectrophotometer [27]. To measure peroxidase and catalase activity, the sample was homogenized in extraction buffer, and after centrifugation, the supernatant was collected and used for the analysis of antioxidants. The enzyme extract (250 µL) was mixed with 50 mM K-phosphate buffer (200 µL), 60 ηM H2O2 (100 µL), and 450 µL of DH2O. The addition of H2O2 was carried out at the end and a decrease in the absorbance was recorded for 3 min. To measure peroxidase activity, a reaction mixture was prepared by mixing K-phosphate buffer (200 µL), 100 mM guaiacol (100 µL), 100 µL of enzyme extract, DH2O (500 µL), and 100 µL of H2O2 (27.5 mM). The blank has the same composition except for the enzyme extract, which had an extra buffer added in the sample. The absorbance was measured at 470 nm using a spectrophotometer [28].
2.7. Yield Attributes
To determine the yield attributes, we carried out an analysis of the number of grains per spike and the 1000-grain weight of durum wheat [29].
2.8. Analysis of Ca, K, and B in Plant Samples
To measure Ca and K in the samples, 1 g of the sample was boiled in perchloric acid (10 mL) for 30 min. Deionized water was added to the sample to make the final volume 1 L. The digested solution was collected and the analysis of K and Ca was carried out in a flame photometer. The standard curves were prepared by using KOH and CaOH [30]. To determine B content, a 0.5 g of the dried ground sample was placed in a muffle furnace at 550 °C. The ash was collected and dissolved in 0.1 N HCl (5 mL) and distilled water was added to make the total volume 20 mL. The 1 mL solution was mixed with 4 mL of curcumin oxalic acid solution in a beaker. The beaker was kept in the water bath at 55 °C to evaporate the liquid. When the sample was dried completely, 95% ethyl alcohol (25 mL) was added to it, and after filtration, readings were taken with a colorimeter [31].
2.9. Statistical Analysis
Statistics was applied to the data to analyze the results. The software used for the analysis of results was Statistix 8.1. The experimental design was factorial and LSD (least significant difference) was used at the level of α = 0.05. The standard error in the mean values was calculated using replicates.
3. Results
3.1. Effect of Nutrients on Plant Height and Biomass
In this study, two durum wheat varieties, i.e., Preco (V1) and Kronos (V2), have been used. The data were analyzed statistically and it was observed that the application of nutrients increased the shoot length of the plants (Figure 1a–f). The percentage increase in shoot length with the low dose of Ca, K, and B was 29%, 47%, and 9% in V1 and 33%, 50%, and 13% in V2, respectively. The percentage increase in shoot length with a high dose of these nutrients was recorded as 38%, 52%, and 19% in V1 and 43%, 53%, and 21% in V2, respectively. Drought stress affects the vegetative growth of both varieties of durum wheat (Figure 1a–f). The percent decrease in plant height was 31% and 38% in V1 and V2, respectively. The exogenous application of Ca, K, and B showed ameliorative potential and enhanced plant growth significantly (p < 0.05). The percentage increase in shoot length with the application of a low dose of Ca, K, and B was 13%, 26%, and 6% in V1 and 19%, 27%, and 7% in V2 compared to untreated (drought exposed) plants. The high dose of these nutrients further increased the shoot length by 18%, 29%, and 14% in V1 and 22%, 30%, and 18% in V2, respectively, compared to their control plants. Drought stress also increased the root length of durum wheat so that the plants absorb water from deeper layers of soil. The maximum increase in root length was reported in plants receiving K as a supplement, i.e., the percentage increase was 35% and 43% with a low and high dose of K, respectively.
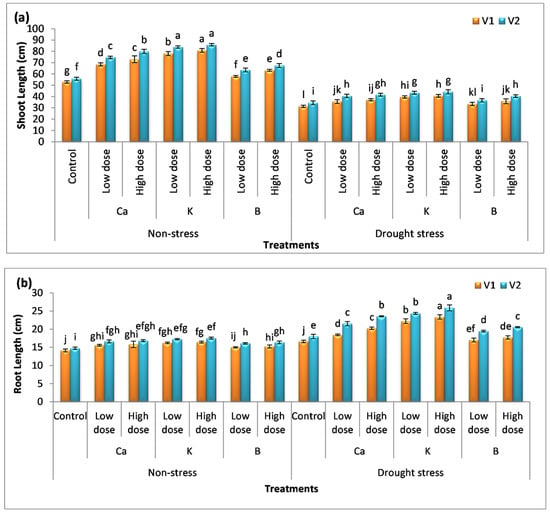
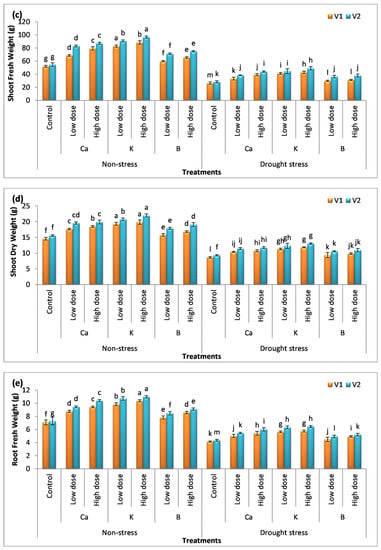
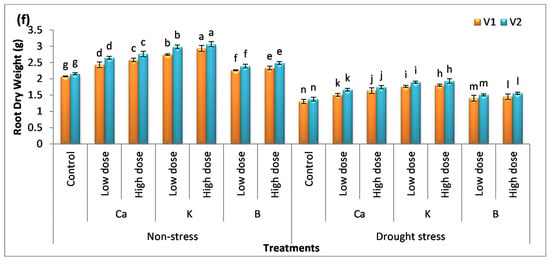
Figure 1.
Effect of Ca, K, and B on growth and biomass of durum wheat. The data include (a) shoot length, (b) root length, (c) shoot fresh weight, (d) shoot dry weight, (e) root fresh weight, and (f) root dry weight of durum wheat, where V1 = Preco, and V2 = Kronos. The different letter shows significant difference among the mean values of treatments.
The foliar application of nutrients augmented biomass production in the plants (Figure 1c–f). An increase in the fresh weight and dry weight of the plants was observed. The analysis of data showed that the improvement in plants biomass receiving the exogenous application of nutrients was in the order of B < Ca < K. The percentage increase in the fresh weight of the shoots with the treatment of K was 70% and 76%, while root fresh weight was enhanced by 46% and 50% in V1 and V2, respectively. The increase in dry weight has also been found to be significant (p < 0.05). The use of a low dose of Ca, K, and B increases the dry weight of the shoots by 21%, 32%, and 8% in V1 and 25%, 33%, and 14% in V2, respectively. However, the application of a high dose of these nutrients increased the dry weight of the shoots by 26%, 36%, and 15% in V1 and 27%, 41%, and 22% in V2, respectively. The maximum percentage increase in the dry weight of the root was recorded with the application of K, i.e., a 41% increase in V1 and a 42% increase in V2. The analysis of data showed a significant decrease in plant biomass under drought stress compared to the control. It was observed that the application of Ca, K, and B increased the fresh weight and dry weight of plants significantly (p < 0.05). The low dose of these nutrients enhanced the fresh weight of the shoots by 26%, 56%, and 13% in V1 and 36%, 57%, and 28% in V2, whereas the percentage increase in the fresh weight of root was 20%, 34%, and 8% in V1 and 25%, 44%, and 12% in V2 compared to the (untreated) drought-exposed plants. There was a considerable difference among the means values of treatments which showed that these nutrients have different roles in plants. The percentage increase in the fresh weight of the shoots with the high dose of Ca, K, and B was 49%, 63%, and 18% in V1 and 55%, 73%, and 34% in V2. The dry weight was also analyzed and the data revealed that the use of K gave more pronounced results compared to other treatments. The increase in the dry weight of the shoots with the application of K was 30% and 36% in V1 and 32% and 40% in V2, respectively. The percentage increase in the dry weight of the roots in plants receiving the treatment of K was 30% and 38% in V1 and 37% and 40% in V2, respectively. It was also observed that the difference among the mean values of both varieties was significant. Variety V2 gave more pronounced results compared to V1.
3.2. Effect of Nutrients on Chlorophyll Content
The amount of chlorophyll content indicates the photosynthetic capacity of plants. The percentage increase in chlorophyll a with the application of a low dose of Ca, K, and B was 17%, 28%, and 8% in V1 and 18%, 30%, and 11% in V2 (Figure 2a–c). The high dose increased chlorophyll a content by 22%, 31%, and 14% in V1 and 24%, 34%, and 15% in V2, respectively. It was observed that the use of K gave better results. The same trend B < Ca < K was observed in the case of chlorophyll b and chlorophyll a + b content. Drought stress decreased the amount of chlorophyll a content by 34% and 33%, while the decrease in the content of chlorophyll b was 48% and 46% compared to the control. The application of selective nutrients (Ca, K, and B) assuaged the deteriorating effects of drought stress and enhanced the amount of chlorophyll content in both varieties. The maximum increase was reported in plants treated with a high dose of K compared to other treatments, i.e., the amount of chlorophyll a, chlorophyll b, and chlorophyll a + b was augmented to 35%, 49%, and 44% in V1 and 37%, 52%, and 49 in V2 compared to the untreated (drought) plants.
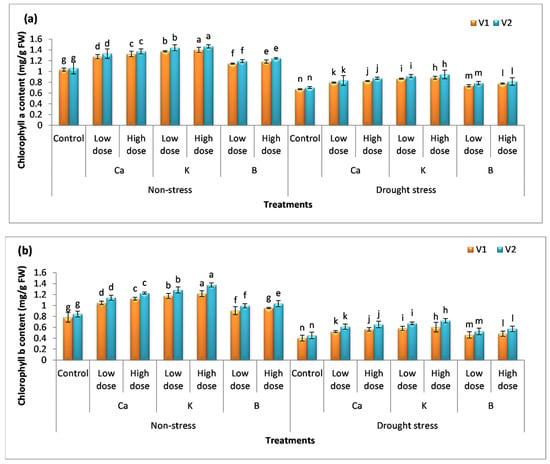
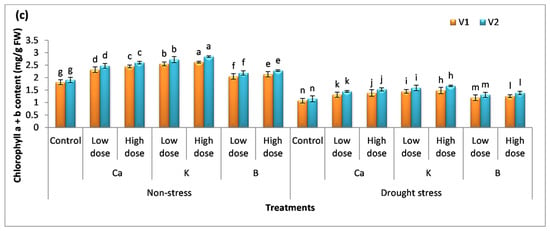
Figure 2.
Effect of Ca, K, and B on the physiological attributes of durum wheat. The figure includes (a) chlorophyll a content, (b) chlorophyll b content, and (c) chlorophyll a + b content of durum wheat, where V1 = Preco and V2 = Kronos. The different letter shows significant difference among the mean values of treatments.
3.3. Effect of Nutrients on Osmolyte Production
The content of proline and sugar was analyzed in plants, and it was observed that there was no significant difference among the mean values for proline content, while a significant difference was observed for the sugar content in non-stress plants compared to the control (Figure 3a,b). The percentage increase in sugar content was more pronounced in plants receiving K spray, i.e., the percentage increase was 29% (V1) and 40% (V2) with a low dose of K and 32% (V1) and 44% (V2) with a high dose. The foliar application of Ca, K, and B significantly (p < 0.05) enhanced the rate of osmolyte production in both varieties. The low dose of these nutrients increased proline content by 20%, 41%, and 8% in V1 and 22%, 45%, and 11% in V2 compared to the untreated (drought-exposed) plants, whereas the high dose of these supplements enhanced the proline content by 36%, 54%, and 36% in V1 and 39%, 62%, and 20% in V2. The data show that B < Ca < K and the same trend was observed in the case of sugar content in both varieties.
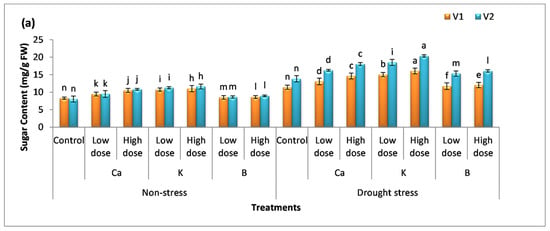
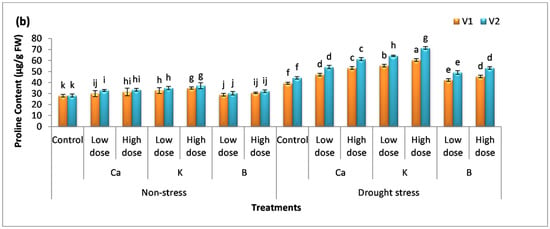
Figure 3.
Effect of Ca, K, and B on the physiological attributes of durum wheat. The figures include (a) sugar content and (b) proline content of durum wheat, where V1 = Preco and V2 = Kronos. The different letter shows significant difference among the mean values of treatments.
3.4. Effect of Nutrients on Membrane Stability Index and Relative Water Content
Drought stress drastically affects the membrane stability of cells and organelles as well. The integrity of the membrane is vital for the normal functioning of plants cells. In non-stressed plants, the membrane of cells was stable and the plants had enough water availability; therefore, there was no significant difference among the mean values compared to the control (Figure 4a,b). On the other hand, a decrease of 49% and 45% was observed for the membrane stability index in plants facing water stress in comparison to the control. The supplementation of Ca, K, and B reduced cell damage which indicates a high rate of membrane stability in plants. The percentage increase in membrane stability index with the low dose of these nutrients was 39%, 50%, and 13% in V1 and 41%, 54%, and 17% in V2 compared to the untreated (stress-facing) plants. The high dose of these supplements further augmented membrane stability by 46%, 60%, 18%, 51%, 67%, and 24% in V1 and V2, respectively, compared to the untreated (drought) plants.
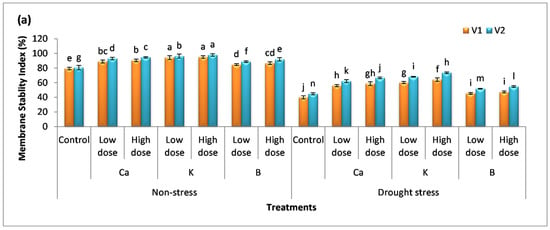
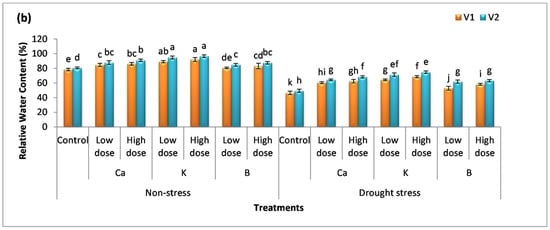
Figure 4.
Effect of Ca, K, and B on the physiological attributes of durum wheat. (a) Membrane stability index and (b) relative water content of durum wheat, where V1 = Preco and V2 = Kronos. The different letter shows significant difference among the mean values of treatments.
Cell turgidity is one of the crucial factors required for cell division and a balanced amount of water content is also important for maintaining homeostasis in plants. The percentage decrease in the amount of relative water content by drought stress was 41% and 39% compared to the control. The maximum percentage increase in relative water content was observed in the case of K application, i.e., 39% and 48% in V1 and 45% and 52% in V2 compared to the untreated (drought) plants. V2 gave better results compared to V1.
3.5. Effect of Nutrients on Antioxidant Production
When durum wheat was subjected to water stress, the activity of antioxidants was enhanced due to the upregulation of genes responsible for the defense system in plants (Figure 5a–c). The data show that V2 has a high rate of antioxidant production compared to V1. The percentage increase in superoxide dismutase with the application of a low dose of Ca, K, and B was 41%, 76%, and 28%, while a high dose of these nutrients further augmented the superoxide dismutase activity by 53%, 95%, and 36% compared to the untreated (drought) plants. The activity of peroxidase and catalase was also increased in plants receiving the dose of these nutrients. The increase in peroxidase activity with the low dose of these nutrients was 38%, 76%, and 24% in V1 and 60%, 77%, and 36% in V2, respectively. However, the high dose enhanced peroxidase activity by 56%, 81%, and 35% in V1 and 60%, 87%, and 50% in V2, respectively, compared to the untreated (drought) plants. A similar trend B < Ca < K was observed in plants in the case of catalase activity (Figure 5c). The application of K gave a maximum activity of catalase, i.e., a 49%, 53%, 55%, and 59% increase was recorded in V1 and V2, respectively.
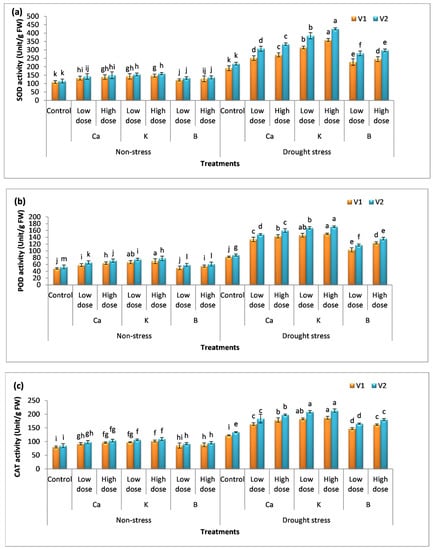
Figure 5.
Effect of Ca, K, and B on antioxidant activity of durum wheat. The figures include (a) superoxide dismutase activity, (b) peroxidase, and (c) catalase activity in durum wheat, where V1 = Preco and V2 = Kronos. The different letter shows significant difference among the mean values of treatments.
3.6. Effect of Nutrients on Yield Attributes
Drought stress aborts ovaries and decreases the yield by reducing grain number and size (Figure 6a,b). The percentage decrease in the number of grains per spike and 1000-grain weight was 49%, 38%, 54%, and 55% in V1 and V2, respectively, compared to the control. The application of Ca, K, and B enhanced the number of grains per spike by 8%, 18%, and 3% in V1 and 13%, 21%, and 7% in V2 compared to the untreated (drought) plants. The high dose of these supplements further enhanced the number of grains per spike by 15%, 19%, and 6% in V1 and 17%, 23%, and 10% in V2, respectively. A pronounced increase in 1000-grain weight was also observed in plants receiving the application of Ca, K, and B in the direction of B < Ca < K. The maximum increase in 1000-grain weight was observed in plants receiving the high dose of K, i.e., a 24% and a 25% increase was noted in V1 and V2.
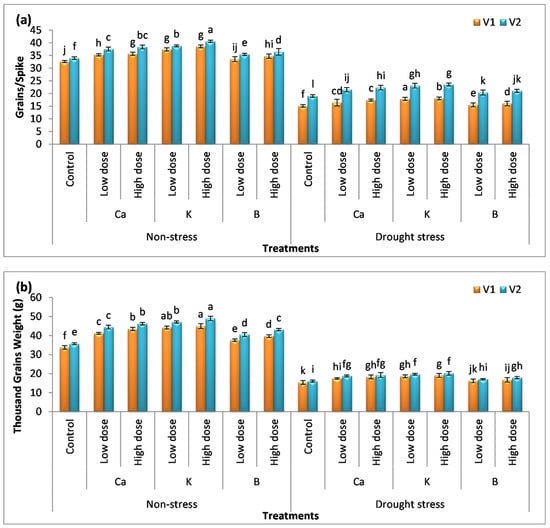
Figure 6.
Effect of Ca, K, and B on yield attributes of durum wheat. The figures include (a) number of grains per spike and (b) 1000-grain weight of durum wheat, where V1 = Preco and V2 = Kronos. The different letter shows significant difference among the mean values of treatments.
3.7. Amount of Ca, K, and B in the Root, Shoot, and Seeds of Durum Wheat
The amount of Ca, K, and B was calculated using the analysis of different parts of the plants to support our findings. The maximum amount of Ca was observed in plants receiving the foliar spray of Ca. The percentage increase in Ca content was 52% (grains), 41% (shoot), and 30% (root) in plants treated with a low dose of Ca, while the plants receiving a high dose of Ca showed an increase of 60% (grains), 48% (shoot), and 33% (root) compared to the control. The amount of K and B was also high in plants receiving the exogenous application of these nutrients compared to the control. Drought stress declines the uptake of Ca, K, and B in both varieties of durum wheat, while exogenous application of these nutrients enhanced their amount in the root, shoot, and seeds which further plays an important role in mitigating the deleterious effects of drought stress and also enhanced the nutritional value of the grains (Table 2, Table 3 and Table 4). The variety V2 performed better than V1. The maximum amount of Ca was observed in the plants receiving the exogenous application of Ca, i.e., the percentage increase was 26% (root), 28% (shoot), and 30% (grain) with a low dose of Ca, whereas the percent increase with a high dose of Ca was 29% (root), 33% (shoot), and 35% (grain). The K content was also significant in plants treated with K salt, i.e., the amount of K in plants receiving a low dose of K was 32% (root), 33% (shoot), and 66% (grain), while a 42% (root), 62% (shoot), and 80% (grain) increase was observed in plants treated with a high dose of K, respectively. The B content was also increased by 28% (root), 52% (shoot), and 63% (grain) in plants receiving a low dose of B, while a high dose of B salt increased B content to 33% (root), 67% (shoot), and 85% (grain), compared to the untreated (drought) plants.

Table 2.
Analysis of Ca content in the root, shoot, and grains of durum wheat. Different letters shows significant difference among the treatments. Standard error was calculated by using the replicates.

Table 3.
Analysis of K content in the root, shoot, and grains of durum wheat. Different letters shows significant difference among the treatments. Standard error was calculated by using the replicates.

Table 4.
Analysis of B content in the root, shoot, and grains of durum wheat. Different letters shows significant difference among the treatments. Standard error was calculated by using the replicates.
4. Discussion
During this research, the foliar spray of calcium, potassium, and boron showed conspicuous effects on the different parameters of two selected cultivars of durum wheat. The spray significantly enhanced the length and biomass of the plants. This is likely due to the active absorption of these nutrients that maintain the turgor in the cells, which forms the basic requirement of cell division. It is reported in the literature [32,33,34] that calcium increases cell wall cohesion and improves the level of cell hydration that makes the cells drought tolerant. Potassium is categorized as a stress alleviator as it enhances cell turgor pressure in unfavorable conditions and improves plant development. It plays a key role in different metabolic activities and creates a balance in osmotic and turgor pressure in plants [35]. It also assists the process of photosynthesis by maintaining the process of electron transport in photochemical reactions. The study of Santos et al. [36] showed that potassium supplementation enhances shoot biomass, water use efficiency, gas exchange, and also improved chlorophyll content in Eucalyptus urophyll facing water stress. The growth of the plants was affected under water stress due to the decrease in cell turgidity that in turn decreased cell expansion and cell division. Boron is another nutrient that improves the rate of photosynthesis, stomatal conductance, and water absorption in plants. It maintains turgor pressure and decreases the evaporation of water [37]. The extended root system was very important for the better absorption of water and nutrients from the soil. Underwater scarcity, root length increases so that roots penetrate deep in the soil and absorb the moisture. During this research, the root length of the plants facing drought stress was also increased compared to the control, while the application of nutrients also gave significant results. The increase in root length was possibly due to the fact that these nutrients maintain turgor pressure, improve cell division, elongation, sugar translocation, and growth of meristematic tissues [38,39]. Our results are in agreement with Shehzad et al. [40] who reported that application of B increases root length, the number of roots, and improves root hair development. The application of boron enhances the expression of certain stress-related genes which plays a significant role in plant protection. It also has a prominent role in flowering, pollen germination, and the movement of water and other nutrients from the roots to aerial parts [41].
The production of reactive oxygen species in the chloroplast is a common phenomenon during water deficiency. These reactive oxygen species are very harmful even at low concentrations because they inhibit the Calvin cycle enzymes and reduce photosynthetic carbon dioxide assimilation. Reactive oxygen species reduce the amount of chlorophyll in plants. Drought stress decreases the amount of chlorophyll b more than chlorophyll a. The application of nutrients enhanced the synthesis of chlorophyll. This is due to nutrients increasing the concentration of antioxidants by signaling certain pathways and these antioxidants scavenge the reactive oxygen species and protect the structure and functions of chlorophyll [42]. The application of calcium enhances the root length, plant height, number of leaves, nodule formation, and the yield of Arachis hypogea. Calcium maintains the pH of the cell which is one of the requirements of electron transports in the process of photosynthesis, so it is indirectly involved in the process of photosynthesis. It also enhances the rate of fertilization and helps in the development of the ovule [43]. Yao et al. [44] reported that the application of calcium enhances the photochemical efficiency, photochemical quenching index, and maintains a high rate of photosynthesis in plants. It was documented by Siddiqui et al. [45] that the application of potassium maintains the rate of carbon assimilation, chlorophyll metabolism, improves chlorophyll content, and maintains the optimum rate of photosynthesis in plants. The application of potassium and salicylic acid enhanced root length (44.5% increase) for the absorption of water and decreased electrolyte leakage, which improved chlorophyll a, chlorophyll b, and total chlorophyll content of Spinacia oleracea by 72%, 115%, and 88% in water-stressed conditions [46].
Production of osmolytes is one of the most frequent drought responses. Proline is an important compatible solute and its high concentration in the cytoplasm creates osmotic adjustment of the cells and it does not interfere with the cellular structures or metabolism [47]. A high concentration of proline maintains water balance in plants, protecting the plants from injury by scavenging reactive oxygen species. Sugar is another stress marker that plays an important role in plant protection against different stresses. It acts as a regulator of gene expression and signaling molecules. It produces energy which is required for the synthesis of different compounds that were involved in stress tolerance/resistance [48,49]. Potassium acts as an inorganic osmoticum by mediating potassium transporters in the cell membrane, thus maintaining the osmotic potential under drought. There is a positive correlation between potassium application and the production of proline. It triggers the synthesis of proline and protects the cells from the damaging effects of water stress [50]. The application of potassium silicate enhanced proline, soluble sugar, phenol, ascorbic acid, catalase, and ascorbate peroxidase in Zea mays under water-stressed conditions [51]. The application of potassium and chitosan considerably enhanced chlorophyll content and the yield attributes of Helianthus annuus facing water stress [52].
The cell membrane protects the cell organelles and performs several functions; thus, its integrity is important for the plant. During drought stress, it is also involved in signal transduction and ion homeostasis. Drought stress produces reactive oxygen species that disrupt the structure of the membrane and reduce its stability [53]. The amount of water absorption is also reduced under stress conditions. The plants with high relative water content are more drought tolerant. Water deficiency is a major physiological mechanism for growth reduction. When the moisture in the soil is reduced, it affects nutrient uptake and their transport from root to shoot due to impaired membrane permeability and restricted transpiration rates. The foliar spray of nutrients was effective in improving membrane stability and also increased the relative water content and alleviated the negative effects of drought [54,55]. Application of potassium in the form of foliar spray helps the plant to cope with soil moisture stress as potassium is important for maintaining osmotic potential and causes stomatal closure which results in water conservation. It is also involved in the activation of a wide range of enzymes that regulate water use efficiency under water shortage. It increases the translocation and maintains water balance within the plants, thus mitigating the adverse effects of water stress. It was reported by Wasaya et al. [56] that foliar application of potassium on Z. mays diminished the negative effects of drought and increased the growth attributes, relative water content, and proline by 9%, 10%, and 12%, respectively, compared to the control. The foliar application of calcium also plays an important role in the alleviation of drought stress in plants. It prevents the damage caused by cellular dehydration by balancing the osmotic potential, postpones the oxidative damage, and increases the concentration of antioxidants and osmolytes. The foliar application of calcium increases the biomass and chlorophyll content of plants, even the low applied concentration of calcium effectively contributes to the alleviation of drought stress [57]. It acts as a secondary messenger and ameliorates water stress. It is involved in the signaling of oxidative stress by participating in hydrogen peroxide perception and induction of antioxidant genes in plants. It regulates specific protein kinase activity and downstream gene expression. There are several calcium-binding proteins such as calmodulin, calreticulin, calcium-sensing receptors, and calcium-dependent protein kinase. The amount of these proteins is changed in response to drought. During water stress, due to an abundance of calmodulin and calreticulin, the rate of survival of the plant was increased. Plants under drought stress try to regulate their osmotic potential through several important osmotic homeostasis-related proteins such as dehydrins, betaine aldehyde dehydrogenase, and late embryogenesis abundant protein, which help the plant to regulate osmotic potential. The level of these proteins increased in leaves during water deficiency and calcium plays a very important role in regulating late embryogenesis abundant protein in plants [58,59].
Plants have specific antioxidant mechanisms to cope with drought stress. Superoxide dismutase (SOD) is a low molecular weight antioxidant and its concentration in the plants is correlated with water deficiency. The exogenous supply of nutrients enhances antioxidant production and reduces the production of reactive oxygen species. The results of this study were in line with the finding of Tadayon [11] who reported that the application of calcium, boron, and potassium increases the rate of antioxidant activity and also enhances the quality of fruits of the Punica granatum plant. The application of potassium and SiO2 increased catalase, superoxide dismutase, soluble sugar, carbohydrate, ascorbate peroxidase, and chlorophyll content in Oryza sativa under drought-stressed conditions [60]. Boron also has the potential to trigger antioxidant activity in plants but its very low concentration is effective [61]. These antioxidants induce the removal of reactive oxygen species and reduce the chances of lipid peroxidation. Hussain et al. [62] documented that the exogenous application of boron enhanced the production of catalase, ascorbate peroxidase, peroxidase, and superoxide dismutase by 20%, 8%, 13%, and 17% compared to the control.
Water deficiency significantly decreases the yield and yield components in durum wheat. The decrease in yield could be due to the reduction in photosynthesis, less availability of water, and the increased production of reactive oxygen species that damage the membranes and other organelles of cells. Additionally, it also disrupts the photosynthetic machinery. The nutrients that were supplied through leaves by foliar spray mitigates the negative effects of water deficiency. Boron plays an important role in cell division, the elongation of meristematic tissues, maintains membrane integrity, and improves ion absorption and water relation [9]. Boron deficiency during reproductive development causes yield loss and sterility. The basic mechanism through which boron deficiency leads to the sterility of wheat includes the poor development of anthers and pollen as well as the failure of pollen germination, so the exogenous supply of boron during reproductive development can fix this problem [63,64]. Balanced nutrition is one of the key factors for improving the yield of crops. They maintain cell structures and improve metabolic activities. Nutrients are integral structural and functional components of plants and they perform various roles in a plant’s life cycle [65]. In the present research, durum wheat was treated with two doses of calcium, potassium, and boron, and a significant increase was recorded in the number of grains per spike and 1000-grain weight. The supplementation of these nutrients plays a significant role in improving the yield attributes of plants. The maximum increase in the yield and yield components was reported with a high dose of potassium application. The application of a high dose of boron had a significant effect on the number of grains per spike. This may be due to the fact that boron reduces sterility and helps in the grain setting of durum wheat. These results were in harmony with those reported by Sofi et al. [66] who reported that the application of boron enhanced the panicle initiation and significantly increased the yield of O. sativa. The increase in 1000-grain weight could be due to the high rate of photosynthesis and the number of grains per spike was increased as the nutrients enhanced seed setting in plants. The main purpose of foliar spray is to increase the absorption of nutrients through leaves and to maximize their utilization. Grain weight determines the yield and it has a direct relationship with productivity; therefore, the higher the grain weight, the higher the yield of crops. During this study, a statistically significant difference was observed in the 1000-grain weight of plants treated with boron, both under well-watered and drought conditions. When compared with non-treated drought-exposed plants, the maximum increase in the 1000-grain weight was reported when a high dose of potassium was applied. These results were as documented by Ali et al. [67], who reported that the application of nutrients increased spike length, the number of grains per spike, and 1000-grain weight in T. aestivum. These results may be due to the sufficient availability of potassium, calcium, and boron in plants at the reproductive stage because these three nutrients also play a role in maintaining the fertility of the reproductive parts in durum wheat. The analysis of these nutrients in the roots, shoots, and seeds also supports our findings. The application of nutrients improves the synthesis and accumulation of photoassimilates, flowering, seed filling, and the production of secondary metabolites. It was observed that the application of nutrient fertilizer improved the physiochemical attributes of the plants and the yield of Brassica napus was augmented by 30% [68]. Salman et al. [69] reported that the foliar application of calcium, boron, and zinc improves the fruit quality and antioxidant activity in Fragaria vesca. The availability of these nutrients is vital for the improvement of fruit quality and quantity. The use of boron increases the number of leaves, chlorophyll content, and number of pods in Sesamum indicum. It enhances the yield by 0.30 t/ha and seed oil content by 53%, compared to the control.
5. Conclusions
This research shows that drought stress has drastic effects on the growth of plants. Foliar spray is one of the best strategies as it enables the plants to directly absorb nutrients from their leaves and through other openings as well. They fulfill the nutrient deficiency and, to some extent, the plants tried to carry out their normal metabolic processes. The data collected in this study show that the foliar spray of nutrients enhances plant height, plant biomass, and the amount of chlorophyll synthesis as well. The increase in yield was also significant. It can be concluded that the foliar application of calcium, potassium, and boron is a good strategy to reduce the negative effects of drought. Thus, farmers should use this technique for better output. We also recommend that farmers should grow the Kronos variety of durum wheat as it is drought tolerant and will give a better yield.
Author Contributions
Conceptualization, N.A. and N.I.; methodology, M.A.; software, T.A.M.; validation, D.I.H.; formal analysis, B.L.J.; investigation, N.A.; resources, N.I.; data curation, M.A.; writing—original draft preparation, N.I.; writing—review and editing, P.A.; visualization, P.A.; supervision, P.A.; project administration, N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the conclusions of this article are included within the article. Any queries regarding these data may be directed to the corresponding author.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/168), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hosseyni Moghaddam, M.S.; Safaie, N.; Soltani, J.; Hagh-Doust, N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021, 160, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Wasaya, A.; Abbas, T.; Yasir, T.A.; Sarwar, N.; Aziz, A.; Javaid, M.M.; Akram, S. Mitigating Drought Stress in Sunflower (Helianthus annuus L.) Through Exogenous Application of β-Aminobutyric Acid. J. Soil Sci. Plant Nutr. 2021, 21, 936–948. [Google Scholar] [CrossRef]
- Pamungkas, S.S.T.; Farid, N. Drought Stress: Responses and Mechanism in Plants. Rev. Agric. Sci. 2022, 10, 168–185. [Google Scholar] [CrossRef]
- Phakela, K.; van Biljon, A.; Wentzel, B.; Guzman, C.; Labuschagne, M.T. Gluten protein response to heat and drought stress in durum wheat as measured by reverse phase—High performance liquid chromatography. J. Cereal Sci. 2021, 100, 103267. [Google Scholar] [CrossRef]
- Laddomada, B.; Blanco, A.; Mita, G.; D’Amico, L.; Singh, R.P.; Ammar, K.; Crossa, J.; Guzmán, C. Drought and Heat Stress Impacts on Phenolic Acids Accumulation in Durum Wheat Cultivars. Foods 2021, 10, 2142. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R. The Effect of Drought Stress on the Superoxide Dismutase and Chlorophyll Content in Durum Wheat Genotypes. Adv. Life Sci. 2021, 8, 119–123. [Google Scholar]
- Rea, R.S.; Islam, M.R.; Rahman, M.M.; Nath, B.; Mix, K. Growth, Nutrient Accumulation, and Drought Tolerance in Crop Plants with Silicon Application: A Review. Sustainability 2022, 14, 4525. [Google Scholar] [CrossRef]
- Xi, N.; Chen, D.; Bahn, M.; Wu, H.; Chu, C.; Cadotte, M.W.; Bloor, J.M. Drought soil legacy alters drivers of plant diversity-productivity relationships in oldfield systems. Sci. Adv. 2022, 8, 3368. [Google Scholar] [CrossRef]
- Akbarimehr, S.; Sayfzadeh, S.; Shahsavari, N.; Valad Abadi, S.A.; Masouleh, E.H. Cycocel, iron and zinc effects on yield and physiological characteristics of wheat under drought stress conditions. J. Plant Nutr. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Anwar, S.; Khalilzadeh, R.; Khan, S.; Bashir, R.; Pirzad, A.; Malik, A. Mitigation of Drought Stress and Yield Improvement in Wheat by Zinc Foliar Spray Relates to Enhanced Water Use Efficiency and Zinc Contents. Int. J. Plant Prod. 2021, 15, 377–389. [Google Scholar] [CrossRef]
- Tadayon, M.S. Effect of foliar nutrition with calcium, boron, and potassium on amelioration of aril browning in pomegranate (Punica granatum cv. ‘Rabab’). J. Hortic. Sci. Biotechnol. 2021, 96, 372–382. [Google Scholar] [CrossRef]
- Kohli, S.K.; Kaur, H.; Khanna, K.; Handa, N.; Bhardwaj, R.; Rinklebe, J.; Ahmad, P. Boron in plants: Uptake, deficiency and biological potential. Plant Growth Regul. 2022, 1–16. [Google Scholar] [CrossRef]
- Kandpal, D.G. Review on Impact of Chemical Fertilizers on Environment. Int. J. Mod. Agric. 2021, 10, 758–763. [Google Scholar]
- Sotayo, F.O.; Donli, P.O. A Comparative Study of the Effects of Chemical fertilizer (NPK) and Organic Manure (Cow Dung) on the Growth of Bambara Groundnut in Borno State, Nigeria. DUJOPAS 2021, 7, 182–189. [Google Scholar]
- Kizilgeci, F.; Yildirim, M.; Islam, M.S.; Ratnasekera, D.; Iqbal, M.A.; Sabagh, A.E. Normalized difference vegetation index and chlorophyll content for precision nitrogen management in durum wheat cultivars under semi-arid conditions. Sustainability 2021, 13, 3725. [Google Scholar] [CrossRef]
- Pačuta, V.; Rašovský, M.; Michalska-Klimczak, B.; Wyszyňski, Z. Grain yield and quality traits of durum wheat (Triticum durum Desf.) treated with seaweed-and humic acid-based biostimulants. Agronomy 2021, 11, 1270. [Google Scholar] [CrossRef]
- Hao, B.; Ma, J.; Jiang, L.; Wang, X.; Bai, Y.; Zhou, C.; Ren, S.; Li, C.; Wang, Z. Effects of foliar application of micronutrients on concentration and bioavailability of zinc and iron in wheat landraces and cultivars. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Li, R.; Tao, R.; Ling, N. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field. Soil Tillage Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Knudsen, D.; Peterson, G.; Pratt, P. Lithium, sodium, and potassium. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 225–246. [Google Scholar]
- Estefan, G. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013. [Google Scholar]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils—effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Dawar, K.; Khan, H.; Zaman, M.; Muller, C.; Alam, S.S.; Fahad, S.; Alwahibi, M.S.; Alkahtani, J.; Saeed, B.; Saud, S.; et al. The Effect of Biochar and Nitrogen Inhibitor on Ammonia and Nitrous Oxide Emissions and Wheat Productivity. J. Plant Growth Regul. 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Weatherley, P.E. Studies in the Water Relations Of The Cotton Plant: I. The Field Measurement of Water Deficits in Leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Ullah, N.; Haq, I.U.; Safdar, N.; Mirza, B. Physiological and biochemical mechanisms of allelopathy mediated by the allelochemical extracts of Phytolacca latbenia (Moq.) H. Walter. Toxicol. Ind. Health 2015, 31, 931–937. [Google Scholar] [CrossRef]
- Roy, A.; Kumar, A.; Singh, A.; Mandi, A.; Barman, M. Analysis of genetic diversity and correlation studies on grain yield and its component characters in bread wheat (Triticum aestivum L. em Thell) genotypes. Pharm. Innov. J. 2021, 10, 341–345. [Google Scholar]
- Abdul Majid, S.; Asghar, R.; Murtaza, G. Potassium-calcium Interrelationship Linked to Drought Tolerance in Wheat (Triticum aestivum L.). Pak. J. Bot. 2007, 39, 1609–1621. [Google Scholar]
- Wu, Z.; Wang, X.; Song, B.; Zhao, X.; Du, J.; Huang, W. Responses of Photosynthetic Performance of Sugar Beet Varieties to Foliar Boron Spraying. Sugar Technol. 2021, 23, 1332–1339. [Google Scholar] [CrossRef]
- Abbas, M.; Abdel-Lattif, H.; Shahba, M. Ameliorative Effects of Calcium Sprays on Yield and Grain Nutritional Composition of Maize (Zea mays L.) Cultivars under Drought Stress. Agriculture 2021, 11, 285. [Google Scholar] [CrossRef]
- Mohammed, I.S.; Al-Maliki, S. Interaction Between Calcium Silicate and Pseudomonas Fluorescens and Irrigation Intervals Affecting Soil Microbial Biomass and Yield of Wheat. Nveo-Nat. Volatiles Essent. Oils J. NVEO 2022, 9, 628–645. [Google Scholar]
- Mustafa, H.; Ilyas, N.; Akhtar, N.; Raja, N.I.; Zainab, T.; Shah, T.; Ahmad, A.; Ahmad, P. Biosynthesis and characterization of titanium dioxide nanoparticles and its effects along with calcium phosphate on physicochemical attributes of wheat under drought stress. Ecotoxicol. Environ. Saf. 2021, 223, 112519. [Google Scholar] [CrossRef]
- Ul-Allah, S.; Ijaz, M.; Nawaz, A.; Sattar, A.; Sher, A.; Naeem, M.; Shahzad, U.; Farooq, U.; Nawaz, F.; Mahmood, K. Potassium Application Improves Grain Yield and Alleviates Drought Susceptibility in Diverse Maize Hybrids. Plants 2020, 9, 75. [Google Scholar] [CrossRef]
- Santos, E.F.; Mateus, N.S.; Rosário, M.O.; Garcez, T.B.; Mazzafera, P.; Lavres, J. Enhancing potassium content in leaves and stems improves drought tolerance of eucalyptus clones. Physiol. Plant 2021, 172, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Páez, S.E.; Cueto-Niño, Y.A.; Sánchez-Reinoso, A.D.; Garces-Varon, G.; Chávez-Arias, C.C.; Restrepo-Díaz, H. Foliar boron compounds applications mitigate heat stress caused by high daytime temperatures in rice (Oryza sativa L.). Boron mitigates heat stress in rice. J. Plant Nutr. 2021, 44, 2514–2527. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Ge, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Mur, L.A.J.; Jiang, D. The different root apex zones contribute to drought priming induced tolerance to a reoccurring drought stress in wheat. Crop J. 2021, 9, 1088–1097. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Naz, M.; Hussain, S.; Javed, T.; Aslam, S.; Raza, A.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z.; et al. Exogenous salicylic acid-induced drought stress tolerance in wheat (Triticum aestivum L.) grown under hydroponic culture. PLoS ONE 2021, 16, e0260556. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Maqsood, M.; Nawaz, F.; Abbas, T.; Yasin, S. Boron-induced improvement in physiological, biochemical and growth attributes in sunflower (Helianthus annuus L.) exposed to terminal drought stress. J. Plant Nutr. 2018, 41, 943–955. [Google Scholar] [CrossRef]
- Aydin, M.; Tombuloglu, G.; Sakcali, M.S.; Hakeem, K.R.; Tombuloglu, H. Boron alleviates drought stress by enhancing gene expression and antioxidant enzyme activity. J. Soil Sci. Plant Nutr. 2019, 19, 545–555. [Google Scholar] [CrossRef]
- Zrig, A.; AbdElgawad, H.; Touneckti, T.; Mohamed, H.B.; Hamouda, F.; Khemira, H. Potassium and calcium improve salt tolerance of Thymus vulgaris by activating the antioxidant systems. Sci. Hortic. 2021, 277, 109812. [Google Scholar] [CrossRef]
- Hemon, A.F. The Rhizobium and calcium fertilizer application to peanut plant in dry land. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Virtual, 16–18 August 2021; p. 012011. [Google Scholar]
- Yao, P.; Tun, H.; Fenge, Z.; Hua, L.S. Effects of calcium on physiology and photosynthesis of paris polyphylla sm. under high temperature and strong light stress. Pak. J. Bot. 2022, 54, 809–816. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Khan, M.N.; Mukherjee, S.; Alamri, S.; Basahi, R.A.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedhi, B.M.A.; Ali, H.M.; Almohisen, I.A.A. Hydrogen sulfide (H2S) and potassium (K+) synergistically induce drought stress tolerance through regulation of H+-ATPase activity, sugar metabolism, and antioxidative defense in tomato seedlings. Plant Cell Rep. 2021, 1, 1–22. [Google Scholar] [CrossRef]
- Gilani, M.; Danish, S.; Ahmed, N.; Rahi, A.A.; Akrem, A.; Younis, U.; Irshad, I.; Khalid Iqbal, R. Mitigation of Drought Stress In Spinach Using Individual And Combined Applications Of Salicylic Acid And Potassium. Pak. J. Bot. 2020, 52, 1505–1513. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N. Role of nanosilicab to boost the activities of metabolites in Triticum aestivum facing drought stress. Plant Soil 2022, 1–17. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N.; Yasmin, H.; Sayyed, R.Z.; Hasnain, Z.A.; Elsayed, E.; El Enshasy, H.A. Role of Bacillus cereus in improving the growth and phytoextractability of Brassica nigra (L.) K. Koch in chromium contaminated soil. Molecules 2021, 26, 1569. [Google Scholar] [CrossRef]
- Miranda, M.T.; Silva, D.S.F.; Silveira, N.M.; Pereira, L.; Machado, E.C.; Ribeiro, V.R. Root Osmotic Adjustment and Stomatal Control of Leaf Gas Exchange are Dependent on Citrus Rootstocks Under Water Deficit. J. Plant Growth Regul. 2020, 40, 11–19. [Google Scholar] [CrossRef]
- Aksu, G.; Altay, H. The Effects of Potassium Applications on Drought Stress in Sugar Beet. Sugar Technol. 2020, 22, 1092–1102. [Google Scholar] [CrossRef]
- Ibrahim, M.F.M.; El-Samad, G.A.; Ashour, H.; El-Sawy, A.M.; Hikal, M.; Elkelish, A.; El-Gawad, H.A.; El-Yazied, A.A.; Hozzein, W.N.; Farag, R. Regulation of Agronomic Traits, Nutrient Uptake, Osmolytes and Antioxidants of Maize as Influenced by Exogenous Potassium Silicate under Deficit Irrigation and Semiarid Conditions. Agronomy 2020, 10, 1212. [Google Scholar] [CrossRef]
- Sohail, M.A.; Nawaz, F.; Aziz, M.; Ahmad, W. Effect of supplemental potassium and chitosan on growth and yield of sunflower (Helianthus annuus L.) under drought stress. Agric. Sci. J. 2021, 3, 56–71. [Google Scholar]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; El-Mageed, T.A.A.; El-Mageed, S.A.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Anokye, E.; Lowor, S.T.; Dogbatse, J.A.; Padi, F.K. Potassium Application Positively Modulates Physiological Responses of Cocoa Seedlings to Drought Stress. Agronomy 2021, 11, 563. [Google Scholar] [CrossRef]
- Azad, N.; Rezayian, M.; Hassanpour, H.; Niknam, V.; Ebrahimzadeh, H. Physiological Mechanism of Salicylic Acid in Mentha pulegium L. under salinity and drought stress. Braz. J. Bot. 2021, 44, 359–369. [Google Scholar] [CrossRef]
- Wasaya, A.; Affan, M.; Yasir, T.A.; Atiqueur, R.; Mubeen, K.; Rehman, H.U.; Ali, M.; Nawaz, F.; Galal, A.; Iqbal, M.A.; et al. Foliar Potassium Sulfate Application Improved Photosynthetic Characteristics, Water Relations and Seedling Growth of Drought-Stressed Maize. Atmosphere 2021, 12, 663. [Google Scholar] [CrossRef]
- Raina, M.; Kumar, A.; Yadav, N.; Kumari, S.; Yusuf, M.A.; Mustafiz, A.; Kumar, D. StCaM2, a calcium binding protein, alleviates negative effects of salinity and drought stress in tobacco. Plant Mol. Biol. 2021, 106, 85–108. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, Y.; Yan, J.; Di, P.; Li, J.; Sun, X.; Han, G.; Ni, L.; Jiang, M.; Yuan, J.; et al. Brassinosteroid-signaling Kinase 1 Phosphorylating Calcium/calmodulin-dependent Protein Kinase Functions in Drought Tolerance in Maize. New Phytol. 2021, 231, 695–712. [Google Scholar] [CrossRef]
- Nurwahyuni, E.; Putra, E.T.S. The role of calcium in drought stress response induced through antioxidant activity in oil palm (Elaeis guineensis Jacq.) seedlings. E-J. Menara Perkeb. 2021, 89, 1. [Google Scholar] [CrossRef]
- Ali, L.G.; Nulit, R.; Ibrahim, M.H.; Yien, C.Y.S. Efficacy of KNO3, SiO2 and SA priming for improving emergence, seedling growth and antioxidant enzymes of rice (Oryza sativa), under drought. Sci. Rep. 2021, 11, 3864. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Yang, G.; Rizwan, M.; Ali, S.; Zhou, Y.; Wang, Q.; Deng, L.; Wang, Y.; et al. Boron supply alleviates cadmium toxicity in rice (Oryza sativa L.) by enhancing cadmium adsorption on cell wall and triggering antioxidant defense system in roots. Chemosphere 2021, 266, 128938. [Google Scholar] [CrossRef]
- Hussain, S.; Irfan, M.; Sattar, A.; Hussain, S.; Ullah, S.; Abbas, T.; Ur-Rehman, H.; Nawaz, F.; Al-Hashimi, A.; Elshikh, M.S. Alleviation of cadmium stress in wheat through the combined application of boron and biochar via regulating morpho-physiological and antioxidant defense mechanisms. Agronomy 2022, 12, 434. [Google Scholar] [CrossRef]
- Domingos, C.d.S.; Besen, M.R.; Neto, M.E.; Costa, E.J.O. Can calcium and boron leaf application increase soybean yield and seed quality? Acta Agric. Scand. Sect. B Soil Plant Sci. 2021, 71, 171–181. [Google Scholar] [CrossRef]
- Sharafi, Y.; Letters, M.R.N.A.S. Effect of Boron on Pollen Attributes in Different Cultivars of Malus domestica L. Natl. Acad. Sci. Lett. 2021, 44, 189–194. [Google Scholar] [CrossRef]
- Nawaz, F.; Shehzad, M.A.; Majeed, S.; Ahmad, K.S.; Aqib, M.; Usmani, M.M.; Shabbir, R.N. Role of mineral nutrition in improving drought and salinity tolerance in field crops. In Agronomic Crops; Springer: Berlin/Heidelberg, Germany, 2020; pp. 129–147. [Google Scholar]
- Sofi, K.A.; Gulzar, A.; Islam, T.; Gulzar, R. Response of Boron Nutrition on Growth and Yield of Rice Grown under Temperate Conditions of Kashmir Valley. Asian J. Adv. Res. 2021, 6, 7–11. [Google Scholar]
- Ali, S.; Shah, S.; Arif, M. Agronomic Biofortification with Zinc and Iron for the Improvement of Wheat Phenology and Yield. Sarhad J. Agric. 2021, 37, 714–1097. [Google Scholar] [CrossRef]
- Aslam, M.M.; Farhat, F.; Siddiqui, M.A.; Yasmeen, S.; Khan, M.T.; Sial, M.A.; Khan, I.A. Exploration of physiological and biochemical processes of canola with exogenously applied fertilizers and plant growth regulators under drought stress. PLoS ONE 2021, 16, e0260960. [Google Scholar] [CrossRef]
- Salman, M.; Ullah, S.; Razzaq, K.; Rajwana, I.A.; Akhtar, G.; Faried, H.N.; Hussain, A.; Amin, M.; Khalid, S. Combined foliar application of calcium, zinc, boron and time influence leaf nutrient status, vegetative growth, fruit yield, fruit biochemical and anti-oxidative attributes of “Chandler” strawberry. J. Plant Nutr. 2022, 45, 1–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).