TMT-Based Quantitative Proteomic Analysis Reveals the Response of Tomato (Solanum lycopersicum L.) Seedlings to Ebb-and-Flow Subirrigation
Abstract
:1. Introduction
2. Materials and Methods
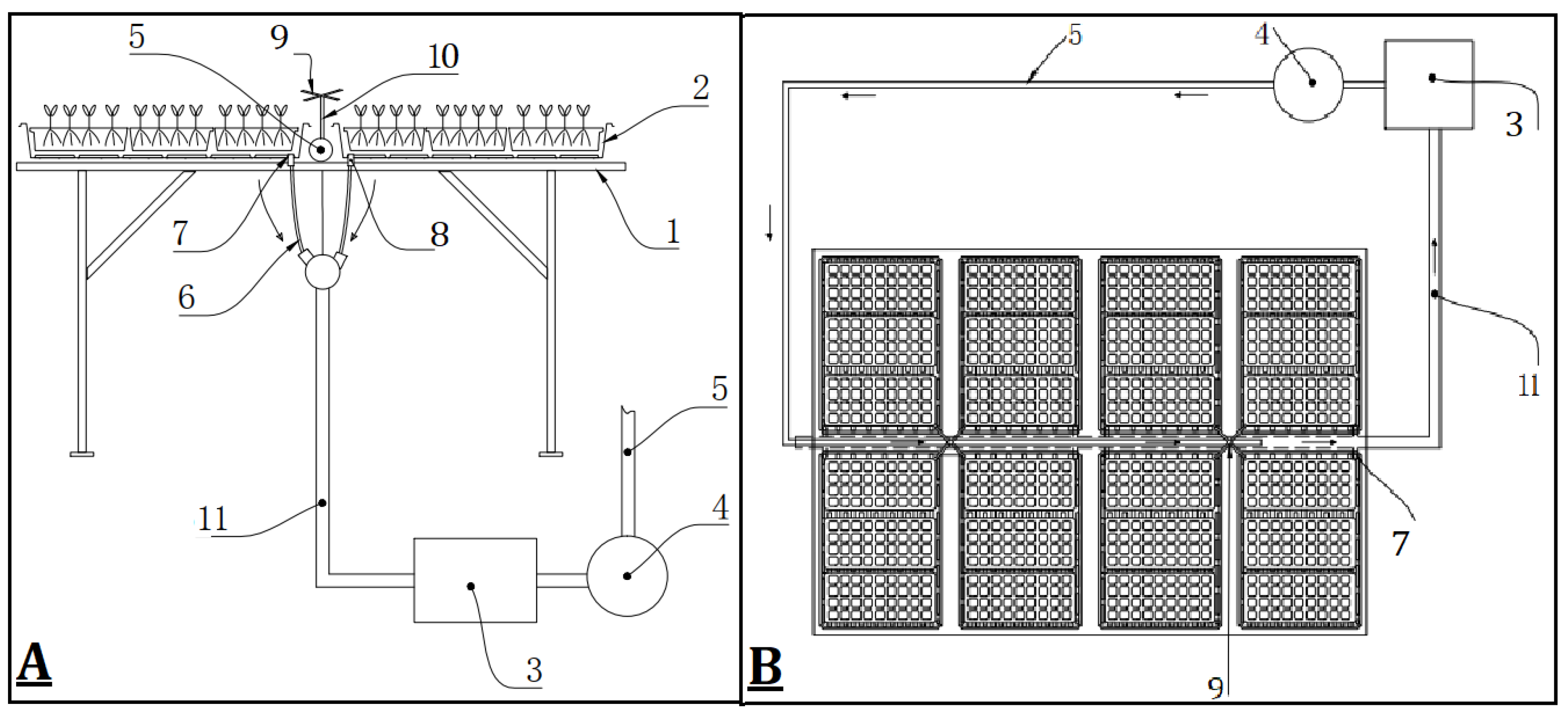
2.1. Plant Source, Experimental Design, and Irrigation Treatments
2.2. Enzymes’ Activity Assay
2.3. Protein Extraction, TMT Labeling, and Data Analysis
2.4. Data Analysis
3. Results
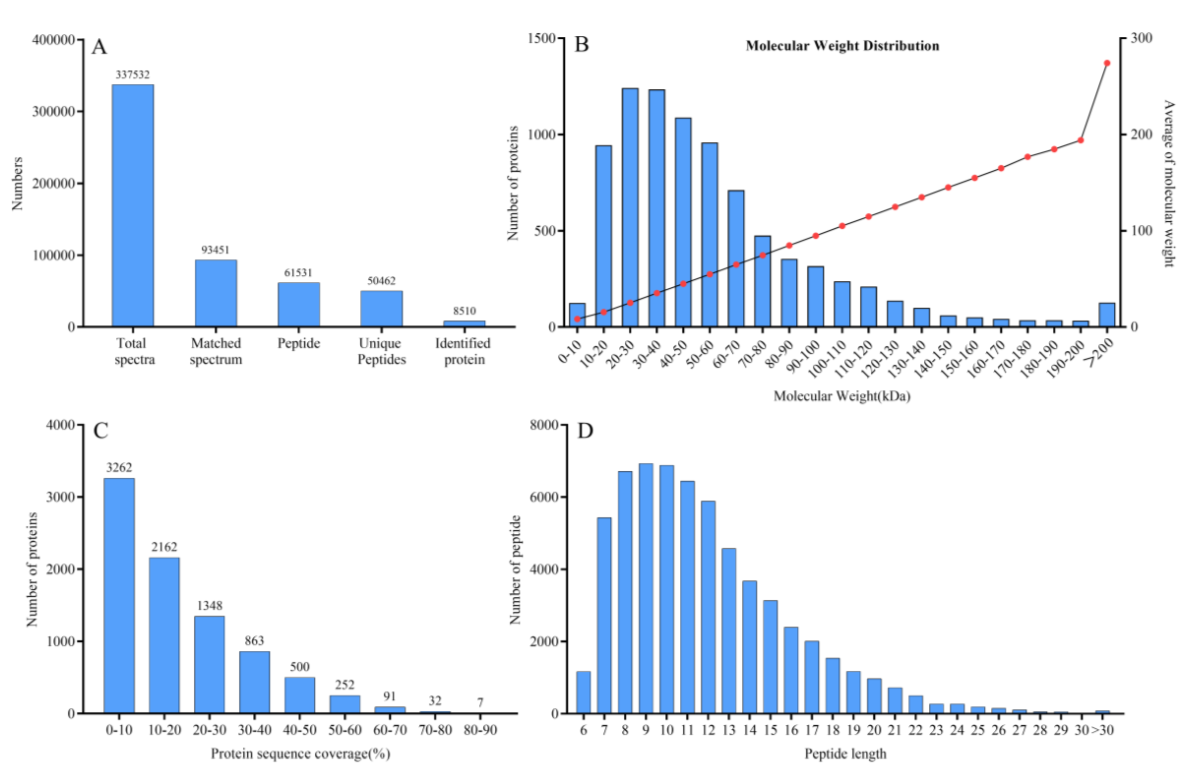
3.1. Primary Quantitative Proteome Analysis
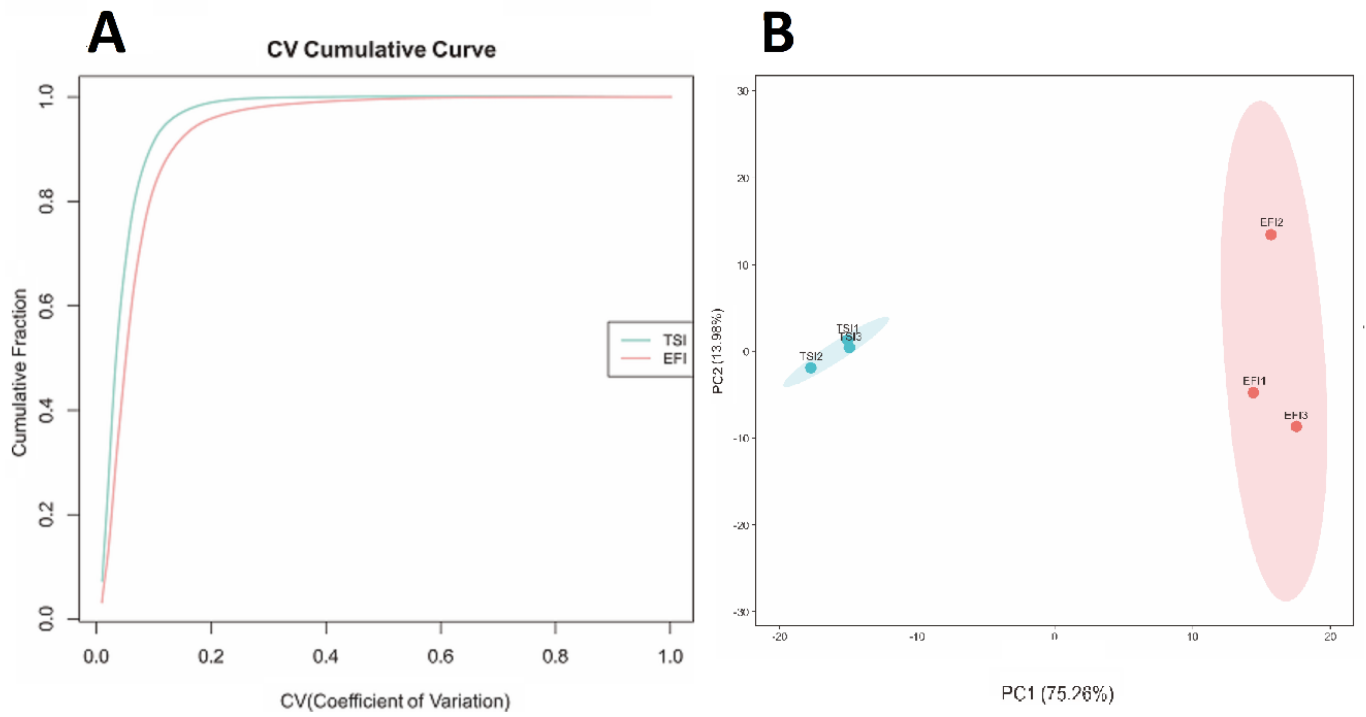
3.2. Effect of EFI Treatment on the Global Proteome of Tomato Seedlings
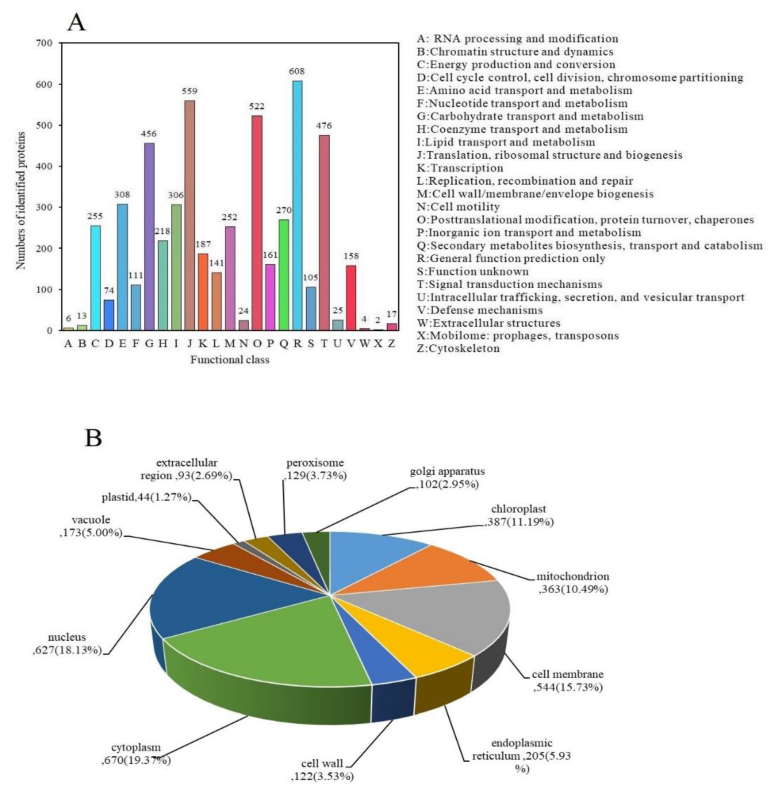
3.3. Enrichment Analysis of DAPs
3.4. Activities of Antioxidant Enzymes in Tomato Roots under Ebb-and-Flow Subirrigation
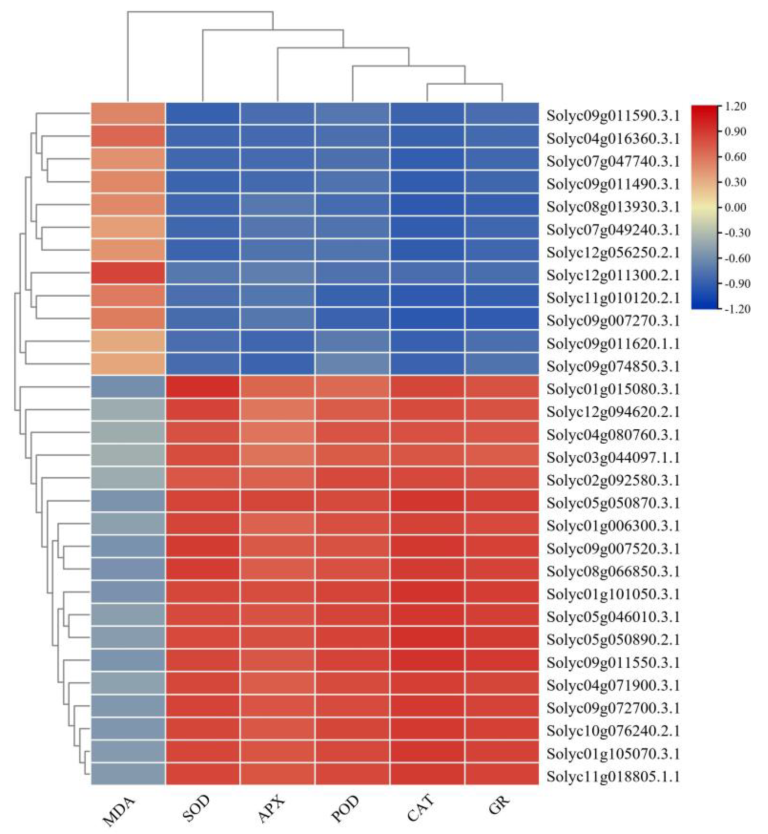
3.5. Correlation between Antioxidant Enzymes’ Activities and Their Metabolism-Related Proteins
4. Discussion
4.1. DAPs Participated in Carbohydrates and Energy Metabolism
4.2. DAPs Participated in Stress Resistance and Defense Response
4.3. DAPs Participated in Amino Acid Metabolism
4.4. DAPs Participated in Plant Hormones and Secondary Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, Y.; Lindhout, P. Domestication and Breeding of Tomatoes: What have We Gained and What Can We Gain in the Future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Gad, A.G.; Liang, D.; Binqi, L.; Kalaji, H.M.; Wróbel, J.; Xu, Y.; Chen, F. Light quality and quantity affect graft union formation of tomato plants. Sci. Rep. 2021, 11, 9870. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Gull, S.; Ali, M.M.; Yousef, A.F.; Ercisli, S.; Kalaji, H.M.; Telesiński, A.; Auriga, A.; Wróbel, J.; Radwan, N.S.; et al. Heat stress mitigation in tomato (Solanum lycopersicum L.) through foliar application of gibberellic acid. Sci. Rep. 2022, 12, 11324. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhtaseb, A.H.; Al-Harahsheh, M.; Hararah, M.; Magee, T.R.A. Drying characteristics and quality change of unutilized-protein rich-tomato pomace with and without osmotic pre-treatment. Ind. Crops Prod. 2010, 31, 171–177. [Google Scholar] [CrossRef]
- Ali, M.M.; Waleed Shafique, M.; Gull, S.; Afzal Naveed, W.; Javed, T.; Yousef, A.F.; Mauro, R.P. Alleviation of Heat Stress in Tomato by Exogenous Application of Sulfur. Horticulturae 2021, 7, 21. [Google Scholar] [CrossRef]
- Yousef, A.F.; Xu, Y.; Chen, F.; Lin, K.; Zhang, X.; Guiamba, H.; Ibrahim, M.M.; Rizwan, H.M.; Ali, M.M. The influence of LEDs light quality on the growth pigments biochemical and chlorophyll fluorescence characteristics of tomato seedlings (Solanum lycopersicum L.). Fresenius Environ. Bull. 2021, 30, 3575–3588. [Google Scholar]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Tadda, S.A.; Xu, Y.; Kalaji, H.M.; Yang, H.; Ahmed, M.A.A.; Wro, J.; Chen, F. Photosynthetic apparatus performance of tomato seedlings grown under various combinations of LED illumination. PLoS ONE 2021, 16, e0249373. [Google Scholar] [CrossRef]
- Ali, M.M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato. Agriculture 2020, 10, 498. [Google Scholar] [CrossRef]
- Richards, D.L.; Reed, D.W. New Guinea Impatiens Growth Response and Nutrient Release from Controlled-release Fertilizer in a Recirculating Subirrigation and Top-watering System. HortScience 2004, 39, 280–286. [Google Scholar] [CrossRef]
- Zheng, Y.; Graham, T.; Richard, S.; Dixon, M. Potted Gerbera Production in a Subirrigation System Using Low-concentration Nutrient Solutions. HortScience 2004, 39, 1283–1286. [Google Scholar] [CrossRef]
- Zheng, Y.; Graham, T.; Richard, S.; Dixon, M. Can Low Nutrient Strategies be used for Pot Gerbera Production in Closed-Loop Subirrigation? Acta Hortic. 2005, 691, 365–372. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Weaver, G.M.; van Iersel, M.W.; Testezlaf, R. Subirrigation: Historical Overview, Challenges, and Future Prospects. Horttechnology 2015, 25, 262–276. [Google Scholar] [CrossRef]
- Araus, J.L.; Rezzouk, F.Z.; Thushar, S.; Shahid, M.; Elouafi, I.A.; Bort, J.; Serret, M.D. Effect of irrigation salinity and ecotype on the growth, physiological indicators and seed yield and quality of Salicornia europaea. Plant Sci. 2021, 304, 110819. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2006, 58, 147–159. [Google Scholar] [CrossRef]
- Morison, J.I.; Baker, N.; Mullineaux, P.; Davies, W. Improving water use in crop production. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 639–658. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S.; Deb, S.K.; Ritchie, G.L.; Wallace, R.W. Effect of deficit irrigation on physiology, plant growth, and fruit yield of cucumber cultivars. Plant Stress 2021, 1, 100004. [Google Scholar] [CrossRef]
- Leskovar, D.I. Root and Shoot Modification by Irrigation. Horttechnology 1998, 8, 510–514. [Google Scholar] [CrossRef]
- Elmer, W.H.; Gent, M.P.N.; McAvoy, R.J. Partial saturation under ebb and flow irrigation suppresses Pythium root rot of ornamentals. Crop Prot. 2012, 33, 29–33. [Google Scholar] [CrossRef]
- James, E.; van Iersel, M. Ebb and Flow Production of Petunias and Begonias as Affected by Fertilizers with Different Phosphorus Content. HortScience 2001, 36, 282–285. [Google Scholar] [CrossRef]
- Buwalda, F.; Baas, R.; van Weel, P.A. A soilless ebb-and-flow system for all-year-round chrysanthemums. Acta Hortic. 1994, 361, 123–132. [Google Scholar] [CrossRef]
- Naghedifar, S.M.; Ziaei, A.N.; Ansari, H. Numerical analysis of sensor-based flood-floor ebb-and-flow subirrigation system with saline water. Arch. Agron. Soil Sci. 2021, 67, 1285–1299. [Google Scholar] [CrossRef]
- Poole, R.T.; Conover, C.A. Fertilizer Levels and Medium Affect Foliage Plant Growth in an Ebb and Flow Irrigation System. J. Environ. Hortic. 1992, 10, 81–86. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Rea, E.; Colla, G. The influence of irrigation system and nutrient solution concentration on potted geranium production under various conditions of radiation and temperature. Sci. Hortic. 2008, 118, 328–337. [Google Scholar] [CrossRef]
- Leskovar, D.I.; Boales, A.K. Plant Establishment Systems Affect Yield of Jalapeno Pepper. Acta Hortic. 1995, 412, 275–280. [Google Scholar] [CrossRef]
- Leskovar, D.I.; Cantliffe, D.J. Comparison of Plant Establishment Method, Transplant, or Direct Seeding on Growth and Yield of Bell Pepper. J. Am. Soc. Hortic. Sci. 1993, 118, 17–22. [Google Scholar] [CrossRef]
- Leskovar, D.I.; Cantliffe, D.J.; Stoffella, P.J. Transplant Production Systems Influence Growth and Yield of Fresh-market Tomatoes. J. Am. Soc. Hortic. Sci. 1994, 119, 662–668. [Google Scholar] [CrossRef]
- Mahgoub, N.A.; Ibrahim, A.M.; Ali, O.M. Effect of different irrigation systems on root growth of maize and cowpea plants in sandy soil. Eur. J. Soil Sci. 2017, 6, 374–379. [Google Scholar] [CrossRef]
- Xuewen, X.; Huihui, W.; Xiaohua, Q.; Qiang, X.; Xuehao, C. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci. Hortic. 2014, 179, 388–395. [Google Scholar] [CrossRef]
- Zhang, P.; Lyu, D.; Jia, L.; He, J.; Qin, S. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genom. 2017, 18, 649. [Google Scholar] [CrossRef]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.-J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and Waterlogging Stress in Plants: A Review Highlighting Research Opportunities and Understudied Aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Hasanuzzaman Oxidative Damage and Antioxidant Defense in Sesamum indicum after Different Waterlogging Durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef]
- Habibullah, M.; Sarkar, S.; Islam, M.M.; Ahmed, K.U.; Rahman, M.Z.; Awad, M.F.; ElSayed, A.I.; Mansour, E.; Hossain, M.S. Assessing the Response of Diverse Sesame Genotypes to Waterlogging Durations at Different Plant Growth Stages. Plants 2021, 10, 2294. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, D.; Ma, L.; Jin, X.; Yang, P.; Ye, F.; Liu, P.; Gong, Z.; Wei, C. TMT-based quantitative proteomics analysis reveals the response of tea plant (Camellia sinensis) to fluoride. J. Proteom. 2018, 176, 71–81. [Google Scholar] [CrossRef]
- Hao, J.; Guo, H.; Shi, X.; Wang, Y.; Wan, Q.; Song, Y.-B.; Zhang, L.; Dong, M.; Shen, C. Comparative proteomic analyses of two Taxus species (Taxus× media and Taxus mairei) reveals variations in the metabolisms associated with paclitaxel and other metabolites. Plant Cell Physiol. 2017, 58, 1878–1890. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, H.; Tong, Y.; Zhao, L.; Qiu, L.; Guo, W.; Shen, C.; Liu, H.; Yan, D.; Zheng, B. Comparative Proteomic Analysis of the Graft Unions in Hickory (Carya cathayensis) Provides Insights into Response Mechanisms to Grafting Process. Front. Plant Sci. 2017, 8, 676. [Google Scholar] [CrossRef]
- Kelei, W.; Youhe, Z.; Jianlei, S.; Zong’an, H.; Longjing, Z.; Jian, X. Application of dynamic water level management of ebb and flow irrigation for cucumber seedlings. Acta Agric. Zhejiangensis 2017, 29, 408–413. [Google Scholar]
- Chao, Y.-Y.; Chen, C.-Y.; Huang, W.-D.; Kao, C.H. Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 2010, 329, 327–337. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, D.; Cui, J.; Chen, X.; Wen, Z.; Zhang, J.; Liu, H. Exogenous GSH protects tomatoes against salt stress by modulating photosystem II efficiency, absorbed light allocation and H2O2-scavenging system in chloroplasts. J. Integr. Agric. 2018, 17, 2257–2272. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of Hydrogen Peroxide-Scavenging Enzymes in Germinating Wheat Seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Hao, P.; Zhu, J.; Gu, A.; Lv, D.; Ge, P.; Chen, G.; Li, X.; Yan, Y. An integrative proteome analysis of different seedling organs in tolerant and sensitive wheat cultivars under drought stress and recovery. Proteomics 2015, 15, 1544–1563. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Hecht, M.; Hamp, T.; Karl, T.; Yachdav, G.; Ahmed, N.; Altermann, U.; Angerer, P.; Ansorge, S.; Balasz, K.; et al. LocTree3 prediction of localization. Nucleic Acids Res. 2014, 42, W350–W355. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting Subcellular Localization of Proteins Based on their N-terminal Amino Acid Sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Xu, J. Abiotic stress responses in plant roots: A proteomics perspective. Front. Plant Sci. 2014, 5, 6. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, L.; Shang, H.; Liu, S.; Peng, J.; Gong, W.; Shi, Y.; Zhang, S.; Li, J.; Gong, J.; et al. iTRAQ-Based Quantitative Proteomic Analysis of Cotton Roots and Leaves Reveals Pathways Associated with Salt Stress. PLoS ONE 2016, 11, e0148487. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Wu, Y.Y. Phosphofructokinase and glucose-6-phosphate dehydrogenase in response to drought and bicarbonate stress at transcriptional and functional levels in mulberry. Russ. J. Plant Physiol. 2016, 63, 235–242. [Google Scholar] [CrossRef]
- Yu, X.; Ali, M.M.; Li, B.; Fang, T.; Chen, F. Transcriptome data-based identification of candidate genes involved in metabolism and accumulation of soluble sugars during fruit development in ‘Huangguan’ plum. J. Food Biochem. 2021, 45, e13878. [Google Scholar] [CrossRef]
- Pan, T.; Ali, M.M.; Gong, J.; She, W.; Pan, D.; Guo, Z.; Yu, Y.; Chen, F. Fruit Physiology and Sugar-Acid Profile of 24 Pomelo (Citrus grandis (L.) Osbeck) Cultivars Grown in Subtropical Region of China. Agronomy 2021, 11, 2393. [Google Scholar] [CrossRef]
- Park, J.-Y.; Canam, T.; Kang, K.-Y.; Ellis, D.D.; Mansfield, S.D. Over-expression of an arabidopsis family A sucrose phosphate synthase (SPS) gene alters plant growth and fibre development. Transgenic Res. 2008, 17, 181–192. [Google Scholar] [CrossRef]
- Tian, H.; Ma, L.; Zhao, C.; Hao, H.; Gong, B.; Yu, X.; Wang, X. Antisense repression of sucrose phosphate synthase in transgenic muskmelon alters plant growth and fruit development. Biochem. Biophys. Res. Commun. 2010, 393, 365–370. [Google Scholar] [CrossRef]
- Wang, K.; Ali, M.M.; Pan, K.; Su, S.; Xu, J.; Chen, F. Ebb-and-Flow Subirrigation Improves Seedling Growth and Root Morphology of Tomato by Influencing Root-Softening Enzymes and Transcript Profiling of Related Genes. Agronomy 2022, 12, 494. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S. Physiological and Proteomic Responses of Contrasting Alfalfa (Medicago sativa L.) Varieties to PEG-Induced Osmotic Stress. Front. Plant Sci. 2018, 9, 242. [Google Scholar] [CrossRef]
- Du, C.; Chai, L.; Wang, Z.; Fan, H. Response of proteome and morphological structure to short-term drought and subsequent recovery in Cucumis sativus leaves. Physiol. Plant. 2019, 167, 676–689. [Google Scholar] [CrossRef]
- Mathews, M.C.; Summers, C.B.; Felton, G.W. Ascorbate peroxidase: A novel antioxidant enzyme in insects. Arch. Insect Biochem. Physiol. 1997, 34, 57–68. [Google Scholar] [CrossRef]
- Choi, S.; Jeong, S.; Jeong, W.; Kwon, S.; Chow, W.; Park, Y.-I. Chloroplast Cu/Zn-superoxide dismutase is a highly sensitive site in cucumber leaves chilled in the light. Planta 2002, 216, 315–324. [Google Scholar] [CrossRef]
- Tu, Y.; Rochfort, S.; Liu, Z.; Ran, Y.; Griffith, M.; Badenhorst, P.; Louie, G.V.; Bowman, M.E.; Smith, K.F.; Noel, J.P.; et al. Functional Analyses of Caffeic Acid O-Methyltransferase and Cinnamoyl-CoA-Reductase Genes from Perennial Ryegrass (Lolium perenne). Plant Cell 2010, 22, 3357–3373. [Google Scholar] [CrossRef]
- Hu, W.; Wang, B.; Ali, M.M.; Chen, X.; Zhang, J.; Zheng, S.; Chen, F. Free Amino Acids Profile and Expression Analysis of Core Genes Involved in Branched-Chain Amino Acids Metabolism during Fruit Development of Longan (Dimocarpus longan Lour.) Cultivars with Different Aroma Types. Biology 2021, 10, 807. [Google Scholar] [CrossRef]
- Zhi, C.; Ali, M.M.; Zhang, J.; Shi, M.; Ma, S.; Chen, F. Effect of Paper and Aluminum Bagging on Fruit Quality of Loquat (Eriobotrya japonica Lindl.). Plants 2021, 10, 2704. [Google Scholar] [CrossRef]
- Cao, L.; Lu, X.; Zhang, P.; Wang, G.; Wei, L.; Wang, T. Systematic Analysis of Differentially Expressed Maize ZmbZIP Genes between Drought and Rewatering Transcriptome Reveals bZIP Family Members Involved in Abiotic Stress Responses. Int. J. Mol. Sci. 2019, 20, 4103. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Malik, A.U.; Khan, A.S.; Ahmad, S.; Hussain, Z.; Hasan, M.U.; Nasir, M.; Chen, F. Plant Growth and Fruit Quality Response of Strawberry is Improved After Exogenous Application of 24-Epibrassinolide. J. Plant Growth Regul. 2022, 41, 1786–1799. [Google Scholar] [CrossRef]
- Ali, M.M.; Yousef, A.F.; Li, B.; Chen, F. Effect of Environmental Factors on Growth and Development of Fruits. Trop. Plant Biol. 2021, 14, 226–238. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis Enzyme Family That Conjugates Amino Acids to Indole-3-Acetic Acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, H.; Wang, M.; Li, Q.; He, Z. Salicyloyl-aspartate synthesized by the acetyl-amido synthetase GH3.5 is a potential activator of plant immunity in Arabidopsis. Acta Biochim. Biophys. Sin. 2013, 45, 827–836. [Google Scholar] [CrossRef]
- Yang, Y.; Yue, R.; Sun, T.; Zhang, L.; Chen, W.; Zeng, H.; Wang, H.; Shen, C. Genome-wide identification, expression analysis of GH3 family genes in Medicago truncatula under stress-related hormones and Sinorhizobium meliloti infection. Appl. Microbiol. Biotechnol. 2015, 99, 841–854. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis Abscisic Acid Response Gene ABI5 Encodes a Basic Leucine Zipper Transcription Factor. Plant Cell 2000, 12, 599–609. [Google Scholar] [CrossRef]
- Zhao, W.; Guan, C.; Feng, J.; Liang, Y.; Zhan, N.; Zuo, J.; Ren, B. The Arabidopsis CROWDED NUCLEI genes regulate seed germination by modulating degradation of ABI5 protein. J. Integr. Plant Biol. 2016, 58, 669–678. [Google Scholar] [CrossRef]
- Mittal, A.; Gampala, S.S.L.; Ritchie, G.L.; Payton, P.; Burke, J.J.; Rock, C.D. Related to ABA-Insensitive3(ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotechnol. J. 2014, 12, 578–589. [Google Scholar] [CrossRef]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Ali, M.M.; Guo, T.; Su, S.; Chen, X.; Xu, J.; Chen, F. TMT-Based Quantitative Proteomic Analysis Reveals the Response of Tomato (Solanum lycopersicum L.) Seedlings to Ebb-and-Flow Subirrigation. Agronomy 2022, 12, 1880. https://doi.org/10.3390/agronomy12081880
Wang K, Ali MM, Guo T, Su S, Chen X, Xu J, Chen F. TMT-Based Quantitative Proteomic Analysis Reveals the Response of Tomato (Solanum lycopersicum L.) Seedlings to Ebb-and-Flow Subirrigation. Agronomy. 2022; 12(8):1880. https://doi.org/10.3390/agronomy12081880
Chicago/Turabian StyleWang, Kelei, Muhammad Moaaz Ali, Tianxin Guo, Shiwen Su, Xianzhi Chen, Jian Xu, and Faxing Chen. 2022. "TMT-Based Quantitative Proteomic Analysis Reveals the Response of Tomato (Solanum lycopersicum L.) Seedlings to Ebb-and-Flow Subirrigation" Agronomy 12, no. 8: 1880. https://doi.org/10.3390/agronomy12081880
APA StyleWang, K., Ali, M. M., Guo, T., Su, S., Chen, X., Xu, J., & Chen, F. (2022). TMT-Based Quantitative Proteomic Analysis Reveals the Response of Tomato (Solanum lycopersicum L.) Seedlings to Ebb-and-Flow Subirrigation. Agronomy, 12(8), 1880. https://doi.org/10.3390/agronomy12081880






