Industrial Hemp Clone Selection Method under LED Smart Farm Condition Based on CBD Production per Cubic Meter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generating the F1 Population
2.2. Cultivation Conditions
2.3. Sample for F1 Hybrid Selection
2.4. Statistical Analysis
3. Results and Discussion
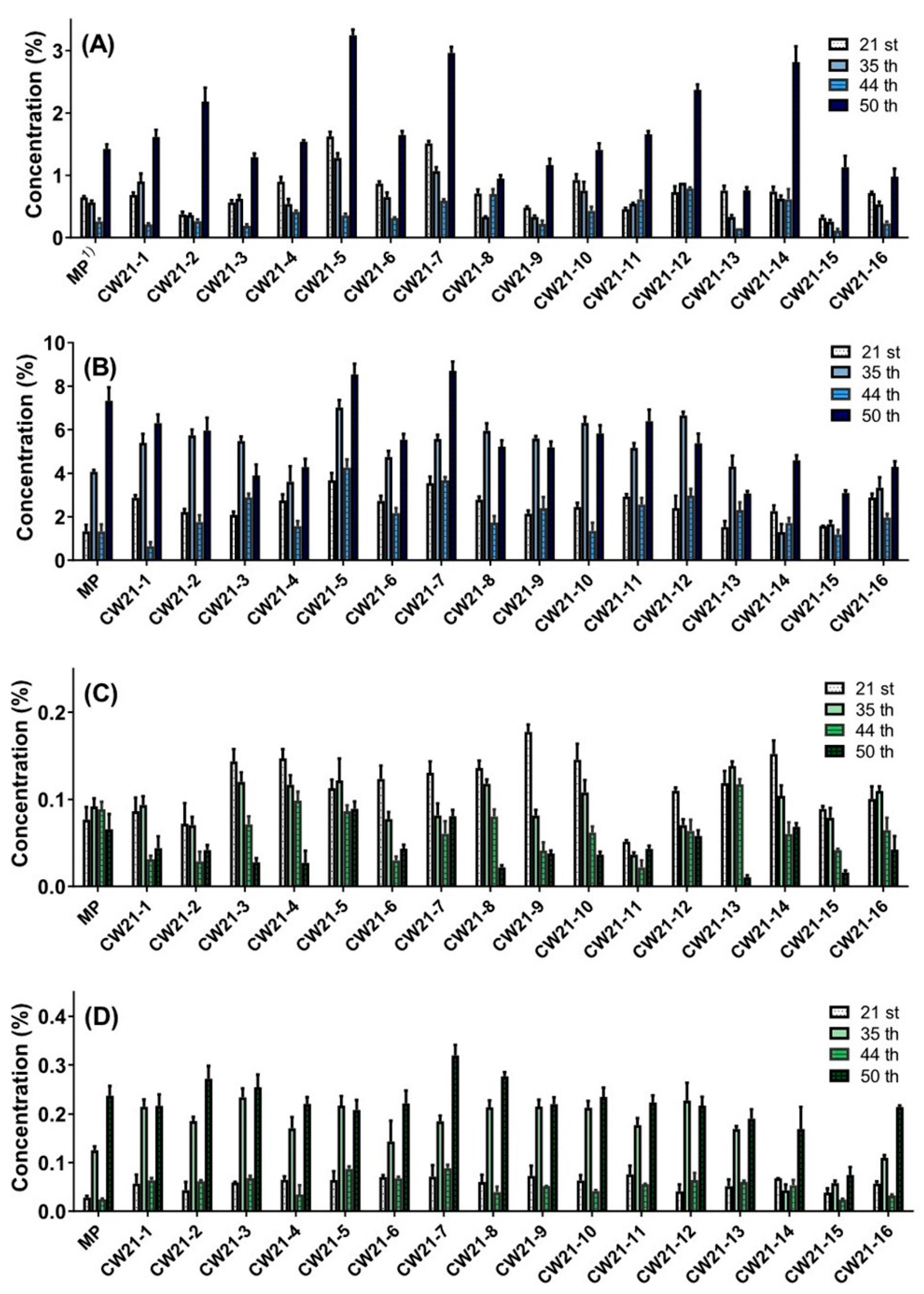
3.1. Selection according to Cannabidiol Content
3.2. Component and Morphological Correlation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Clarke, R.C. Marijuana Botany: An Advanced Study: The Propagation and Breeding of Distinctive Cannabis; Ronin Publishing: Berkeley, CA, USA, 1981; ISBN 1579511090. [Google Scholar]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and Medicinal Chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Page, J.E.; Nagel, J. Biosynthesis of Terpenophenolic Metabolites in Hop and Cannabis. Recent Adv. Phytochem. 2006, 40, 179–210. [Google Scholar]
- Śledziński, P.; Nowak-Terpiłowska, A.; Zeyland, J. Cannabinoids in Medicine: Cancer, Immunity, and Microbial Diseases. Int. J. Mol. Sci. 2021, 22, 263. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.R.; Islam, M.J.; Azad, M.O.K.; Go, E.-J.; Rahman, M.H.; Rana, M.S.; Lim, Y.-S.; Lim, J.-D. Conversion Characteristics of Some Major Cannabinoids from Hemp (Cannabis sativa L.) Raw Materials by New Rapid Simultaneous Analysis Method. Molecules 2021, 26, 4113. [Google Scholar] [CrossRef] [PubMed]
- Hindley, G.; Beck, K.; Borgan, F.; Ginestet, C.E.; McCutcheon, R.; Kleinloog, D.; Ganesh, S.; Radhakrishnan, R.; D’Souza, D.C.; Howes, O.D. Psychiatric Symptoms Caused by Cannabis Constituents: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2020, 7, 344–353. [Google Scholar] [CrossRef]
- Stuyt, E. The Problem with the Current High Potency THC Marijuana from the Perspective of an Addiction Psychiatrist. Mo. Med. 2018, 115, 482–486. [Google Scholar] [PubMed]
- Sarris, J.; Sinclair, J.; Karamacoska, D.; Davidson, M.; Firth, J. Medicinal Cannabis for Psychiatric Disorders: A Clinically-Focused Systematic Review. BMC Psychiatry 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yep, B.; Gale, N.V.; Zheng, Y. Comparing Hydroponic and Aquaponic Rootzones on the Growth of Two Drug-Type Cannabis sativa L. Cultivars during the Flowering Stage. Ind. Crops Prod. 2020, 157, 112881. [Google Scholar] [CrossRef]
- Elsaid, S.; Kloiber, S.; Le Foll, B. Chapter Two—Effects of Cannabidiol (CBD) in Neuropsychiatric Disorders: A Review of Pre-Clinical and Clinical Findings. In Molecular Basis of Neuropsychiatric Disorders: From Bench to Bedside; Rahman, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 167, pp. 25–75. ISSN 1877-1173. [Google Scholar]
- Crippa, J.A.; Guimarães, F.S.; Campos, A.C.; Zuardi, A.W. Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front. Immunol. 2018, 9, 2009. [Google Scholar] [CrossRef] [Green Version]
- Fellermeier, M.; Eisenreich, W.; Bacher, A.; Zenk, M.H. Biosynthesis of Cannabinoids: Incorporation Experiments with 13C-labeled Glucoses. Eur. J. Biochem. 2001, 268, 1596–1604. [Google Scholar] [CrossRef]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Shoyama, Y.; Morimoto, S. Phytocannabinoids in Cannabis sativa: Recent Studies on Biosynthetic Enzymes. Chem. Biodivers. 2007, 4, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The Biosynthesis of the Cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Muntendam, R.; Happyana, N.; Erkelens, T.; Bruining, F.; Kayser, O. Time Dependent Metabolomics and Transcriptional Analysis of Cannabinoid Biosynthesis in Cannabis sativa Var. Bedrobinol and Bediol Grown under Standardized Condition and with Genetic Homogeneity. Online Int. J. Med. Plant Res 2012, 1, 31–40. [Google Scholar]
- Mandolino, G.; Bagatta, M.; Carboni, A.; Ranalli, P.; de Meijer, E. Qualitative and Quantitative Aspects of the Inheritance of Chemical Phenotype in Cannabis. J. Ind. Hemp 2003, 8, 51–72. [Google Scholar] [CrossRef]
- Hillig, K.W.; Mahlberg, P.G. A Chemotaxonomic Analysis of Cannabinoid Variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahlberg, P.G.; Hemphill, J.K. Effect of Light Quality on Cannabinoid Content of Cannabis sativa L. (Cannabaceae). Bot. Gaz. 1983, 144, 43–48. [Google Scholar] [CrossRef]
- Zhang, W.J.; Björn, L.O. The Effect of Ultraviolet Radiation on the Accumulation of Medicinal Compounds in Plants. Fitoterapia 2009, 80, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, W.; van Damme, P.; Meert, N. Factors Determining Yield and Quality of Illicit Indoor Cannabis (Cannabis Spp.) Production. Forensic Sci. Int. 2011, 212, 158–163. [Google Scholar] [CrossRef]
- Wenger, J.P.; Dabney III, C.J.; ElSohly, M.A.; Chandra, S.; Radwan, M.M.; Majumdar, C.G.; Weiblen, G.D. Validating a Predictive Model of Cannabinoid Inheritance with Feral, Clinical, and Industrial Cannabis sativa. Am. J. Bot. 2020, 107, 1423–1432. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahlberg, P.G.; Kim, E.S. Accumulation of Cannabinoids in Glandular Trichomes of Cannabis (Cannabaceae). J. Ind. Hemp 2004, 9, 15–36. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichomes Alter Morphology and Metabolite Content during Flower Maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Schachtsiek, J.; Warzecha, H.; Kayser, O.; Stehle, F. Current Perspectives on Biotechnological Cannabinoid Production in Plants. Planta Med. 2018, 84, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef]
- Berthold, E.C.; Yang, R.; Sharma, A.; Kamble, S.H.; Kanumuri, S.R.; King, T.I.; Popa, R.; Freeman, J.H.; Brym, Z.T.; Avery, B.A. Regulatory Sampling of Industrial Hemp Plant Samples (Cannabis sativa L.) Using UPLC-MS/MS Method for Detection and Quantification of Twelve Cannabinoids. J. Cannabis Res. 2020, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.C.; Merlin, M.D. Cannabis Domestication, Breeding History, Present-Day Genetic Diversity, and Future Prospects. Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Moliterni, V.M.C.; Cattivelli, L.; Ranalli, P.; Mandolino, G. The Sexual Differentiation of Cannabis sativa L.: A Morphological and Molecular Study. Euphytica 2004, 140, 95–106. [Google Scholar] [CrossRef]
- Schilling, S.; Dowling, C.A.; Shi, J.; Ryan, L.; Hunt, D.; OReilly, E.; Perry, A.S.; Kinnane, O.; McCabe, P.F.; Melzer, R. The Cream of the Crop: Biology, Breeding and Applications of Cannabis sativa. Authorea Prepr. 2020, 1–46. [Google Scholar] [CrossRef]
- Mead, A. The Legal Status of Cannabis (Marijuana) and Cannabidiol (CBD) under US Law. Epilepsy Behav. 2017, 70, 288–291. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, P.; Faro, A.F.L.; Pirani, F.; Berretta, P.; Pacifici, R.; Pichini, S.; Busardò, F.P. Pharmacology and Legal Status of Cannabidiol. Ann. Dell’istituto Super. Sanità 2020, 56, 285–291. [Google Scholar]
- Bifulco, M.; Pisanti, S. Medicinal Use of Cannabis in E Urope: The Fact That More Countries Legalize the Medicinal Use of Cannabis Should Not Become an Argument for Unfettered and Uncontrolled Use. EMBO Rep. 2015, 16, 130–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Areesantichai, C.; Perngparn, U.; Pilley, C. Current Cannabis-Related Situation in the Asia-Pacific Region. Curr. Opin. Psychiatry 2020, 33, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.; Wadsworth, E.; Leos-Toro, C.; Hammond, D. Prevalence and Forms of Cannabis Use in Legal vs. Illegal Recreational Cannabis Markets. Int. J. Drug Policy 2020, 76, 102658. [Google Scholar] [CrossRef] [PubMed]
- Chae, C.-J.; Cho, H.-J. Enhanced Secure Device Authentication Algorithm in P2P-Based Smart Farm System. Peer-to-Peer Netw. Appl. 2018, 11, 1230–1239. [Google Scholar] [CrossRef]
- Lee, S.; Hong, Y.; Lee, E.; Han, Y.; Kim, E.; Park, J.; Lee, S.; Jung, Y.; You, Y. Effects of Sources and Quality of LED Light on Response of Lycium Chinense of Photosynthetic Rate, Transpiration Rate, and Water Use Efficiency in the Smart Farm. Korean J. Ecol. Environ. 2019, 52, 171–177. [Google Scholar] [CrossRef]
- Lohchab, V.; Kumar, M.; Suryan, G.; Gautam, V.; Das, R.K. A Review of IoT Based Smart Farm Monitoring. In Proceedings of the 2018 Second International Conference on Inventive Communication and Computational Technologies (ICICCT), Coimbatore, India, 20–21 April 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1620–1625. [Google Scholar]
- Kanjilal, D.; Singh, D.; Reddy, R.; Mathew, J. Smart Farm: Extending Automation to the Farm Level. Int. J. Sci. Technol. Res. 2014, 3, 109–113. [Google Scholar]
- Yoon, C.; Huh, M.; Kang, S.-G.; Park, J.; Lee, C. Implement Smart Farm with IoT Technology. In Proceedings of the 2018 20th International Conference on Advanced Communication Technology (ICACT), Chuncheon, Korea, 11–14 February 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 749–752. [Google Scholar]
- Islam, M.J.; Ryu, B.R.; Azad, M.O.K.; Rahman, M.H.; Cheong, E.J.; Lim, J.-D.; Lim, Y.-S. Cannabinoids Accumulation in Hemp (Cannabis sativa L.) Plants under LED Light Spectra and Their Discrete Role as a Stress Marker. Biology 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Ryu, B.R.; Azad, M.O.K.; Rahman, M.H.; Rana, M.S.; Kang, C.-W.; Lim, J.-D.; Lim, Y.-S. Comparative Growth, Photosynthetic Pigments, and Osmolytes Analysis of Hemp (Cannabis sativa L.) Seedlings under an Aeroponics System with Different LED Light Sources. Horticulturae 2021, 7, 239. [Google Scholar] [CrossRef]
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; da Silva Benevenute, S.; Brym, Z.T.; Freeman, J.H. Development of Cannabinoids in Flowers of Industrial Hemp (Cannabis sativa L.): A Pilot Study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between Chemistry and Morphology in Medical Cannabis (Cannabis sativa L.). Ind. Crops Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Burgel, L.; Hartung, J.; Schibano, D.; Graeff-Hönninger, S. Impact of Different Phytohormones on Morphology, Yield and Cannabinoid Content of Cannabis sativa L. Plants 2020, 9, 725. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Feathers, C.; Huscher, E.L.; Holmes, B.; Haas, J.A.; Kane, N.C. Widely Assumed Phenotypic Associations in Cannabis sativa Lack a Shared Genetic Basis. PeerJ 2021, 9, e10672. [Google Scholar] [CrossRef] [PubMed]
- Abot, A.; Bonnafous, C.; Touchard, F.; Thibault, F.; Chocinski-Arnault, L.; Lemoine, R.; Dedaldechamp, F. Effects of Cultural Conditions on the Hemp (Cannabis sativa) Phloem Fibres: Biological Development and Mechanical Properties. J. Compos. Mater. 2013, 47, 1067–1077. [Google Scholar] [CrossRef]
- Barcaccia, G.; Palumbo, F.; Scariolo, F.; Vannozzi, A.; Borin, M.; Bona, S. Potentials and Challenges of Genomics for Breeding Cannabis Cultivars. Front. Plant Sci. 2020, 11, 1472. [Google Scholar] [CrossRef]
- Moon, Y.H.; Koo, B.C.; Choi, Y.H.; Bark, S.T.; Ahn, S.H.; Cha, Y.L.; An, G.H.; Kim, J.K.; Suh, S.J. Seed production by induction of male flowers on female plants of hemp (Cannabis sativa L.). Korean J. Crop Sci. 2010, 55, 327–332. [Google Scholar]
- Silver, R.; Wakshalg, J.; Wynn, S.; Kramer, K. Nutritional Analysis of Cannabis. In Cannabis Therapy in Veterinary Medicine; Springer: Berlin/Heidelberg, Germany, 2021; pp. 271–293. [Google Scholar]
- Mölleken, H.; Theimer, R.R. Survey of Minor Fatty Acids in Cannabis sativa L. Fruits of Various Origins. J. Int. Hemp Assoc. 1997, 4, 13–17. [Google Scholar]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic Fingerprinting of Cannabis sativa L., Cannabinoids and Terpenoids for Chemotaxonomic and Drug Standardization Purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. Temperature Response of Photosynthesis in Different Drug and Fiber Varieties of Cannabis sativa L. Physiol. Mol. Biol. Plants 2011, 17, 297–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, R.C.; Vergara, D.; Tittes, S.; White, K.; Schwartz, C.J.; Gibbs, M.J.; Ruthenburg, T.C.; deCesare, K.; Land, D.P.; Kane, N.C. Genomic and Chemical Diversity in Cannabis. Crit. Rev. Plant Sci. 2016, 35, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]

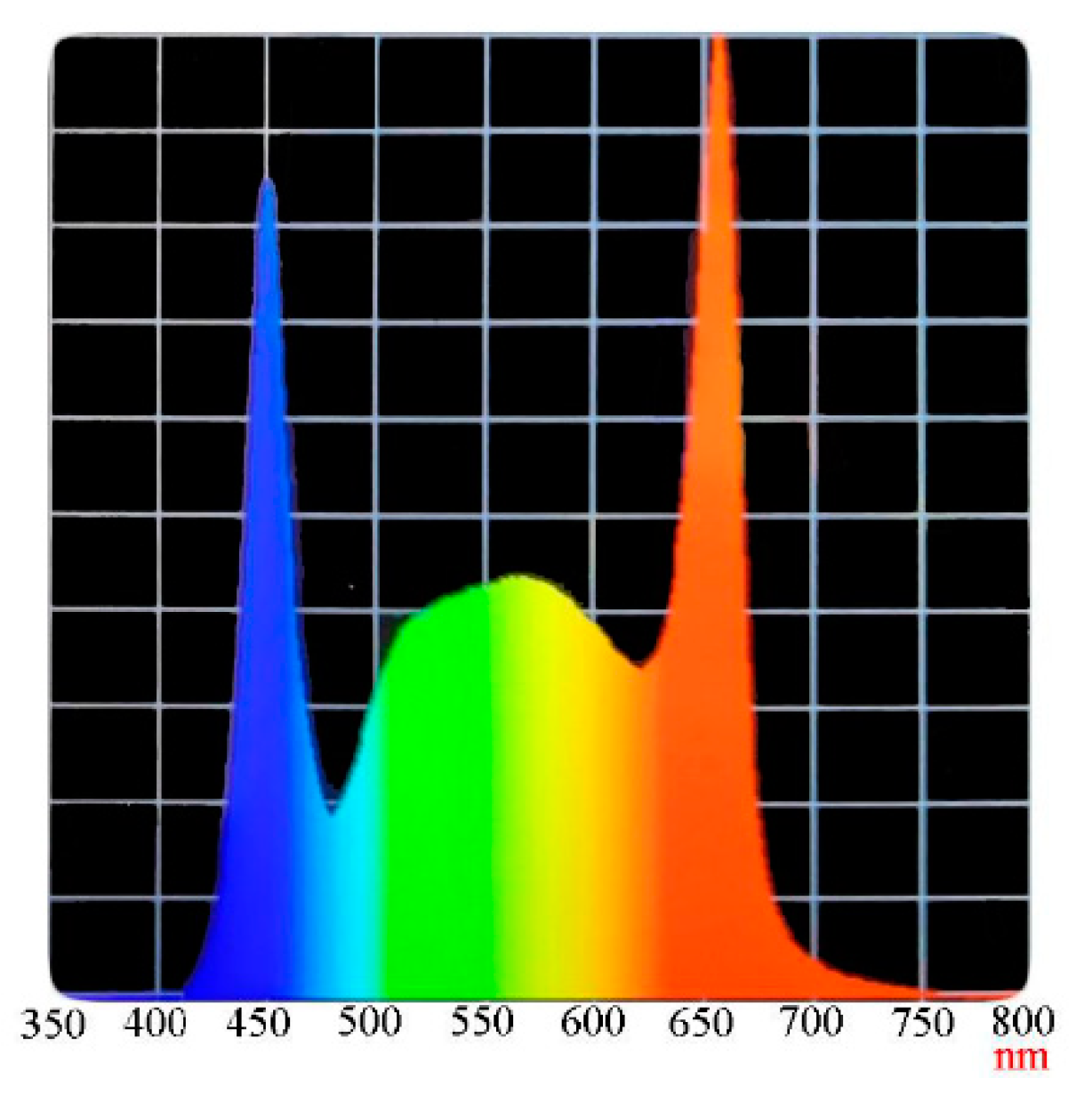



| Nutrient Name | A Solution (50 L) | B Solution (50 L) |
|---|---|---|
| Ca(NO3)·4H2O | 1.50 kg | |
| KNO3 | 3.79 kg | 3.79 kg |
| (NH4)H2PO4 | 1.60 kg | |
| MgSO4 | 4.30 kg | |
| Fe-EDTA | 460.0 g | |
| MnSO4 | 30.8 g | |
| H3BO3 | 57.2 g | |
| ZnSO4 | 3.6 g | |
| CuSO4 | 1.3 g | |
| (NH4)6Mo7O24·4H2O | 0.4 g |
| Equipment | Condition |
|---|---|
| Instrument | Shimadzu LC-20AT HPLC system |
| Column | Reverse phase Zorbax SB-C18 (4.6 mm × 100 mm, 3.5 µm) |
| Detector | UV-Vis detector (220 nm) |
| Elution type | Gradient |
| Mobile phase | Solvent A: Water containing 0.05% TFA Solvent B: Acetonitrile containing 0.05% TFA |
| Flow rate | 1.5 mL/min |
| Oven | 27 °C |
| Injection volume | 10 µL |
| Plant Line | Plant Height (cm) | Leaf Length (cm) | Leaf Width (cm) | Branch Number | Stem Diameter (mm) | Inf. Number | Inf. Length (1) | Petiole Color (Red%) | Stem Color (Red%) | Whole Plant Diameter (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mother plant | 78.1 ± 5.1 | 12 ± 2 | 1.1 ± 0.3 | 17 ± 2 | 10.17 ± 3.70 | 410 ± 112 | ++++ | 40 | 0 | 60 ± 11 |
| CW21-1 | 44.1 ± 4.3 | 18 ± 2 | 3.2 ± 0.6 | 12 ± 3 | 15.40 ± 3.12 | 198 ± 25 | ++ | 60 | 5 | 66 ± 5 |
| CW21-2 | 52.5 ± 8.1 | 12 ± 3 | 3.0 ± 0.6 | 10 ± 2 | 8.62 ± 2.75 | 320 ± 49 | +++ | 95 | 75 | 68 ± 13 |
| CW21-3 | 50.2 ± 3.2 | 23 ± 4 | 5.1 ± 0.6 | 26 ± 4 | 14.86 ± 3.34 | 76 ± 14 | + | 15 | 5 | 37 ± 18 |
| CW21-4 | 84.0 ± 8.4 | 14 ± 4 | 2.5 ± 0.4 | 23 ± 6 | 10.44 ± 3.11 | 146 ± 43 | ++ | 50 | 5 | 50 ± 16 |
| CW21-5 | 68.2 ± 4.5 | 15 ± 5 | 3.1 ± 0.5 | 19 ± 3 | 13.06 ± 2.02 | 205 ± 51 | ++++ | 30 | 5 | 43 ± 11 |
| CW21-6 | 69.3 ± 4.4 | 12 ± 2 | 1.4 ± 0.2 | 20 ± 3 | 7.04 ± 3.55 | 330 ± 44 | ++ | 40 | 50 | 58 ± 21 |
| CW21-7 | 78.9 ± 5.7 | 14 ± 4 | 1.8 ± 0.2 | 20 ± 5 | 10.50 ± 2.13 | 460 ± 37 | +++ | 40 | 20 | 78 ± 17 |
| CW21-8 | 38.5 ± 6.1 | 12 ± 2 | 1.5 ± 0.4 | 12 ± 2 | 10.09 ± 4.00 | 248 ± 44 | ++ | 5 | 0 | 87 ± 21 |
| CW21-9 | 62.1 ± 6.6 | 11 ± 3 | 1.3 ± 0.2 | 17 ± 6 | 13.27 ± 2.76 | 512 ± 85 | ++++ | 5 | 0 | 90 ± 18 |
| CW21-10 | 47.0 ± 4.3 | 19 ± 8 | 2.1 ± 0.4 | 14 ± 5 | 8.15 ± 1.11 | 245 ± 64 | ++++ | 85 | 80 | 70 ± 20 |
| CW21-11 | 72.0 ± 11.1 | 13 ± 1 | 2.0 ± 0.3 | 14 ± 5 | 6.84 ± 1.04 | 179 ± 51 | ++ | 80 | 80 | 70 ± 11 |
| CW21-12 | 54.2 ± 5.3 | 15 ± 4 | 1.8 ± 0.6 | 16 ± 4 | 8.51 ± 1.31 | 295 ± 52 | ++++ | 75 | 60 | 73 ± 17 |
| CW21-13 | 59.0 ± 4.0 | 15 ± 4 | 2.8 ± 0.5 | 14 ± 6 | 11.06 ± 2.34 | 349 ± 105 | ++ | 15 | 0 | 50 ± 12 |
| CW21-14 | 61.2 ± 7.7 | 10 ± 2 | 1.2 ± 0.4 | 11 ± 4 | 8.39 ± 1.08 | 300 ± 13 | ++++ | 30 | 30 | 89 ± 11 |
| CW21-15 | 88.9 ± 6.0 | 19 ± 5 | 4.0 ± 0.8 | 17 ± 3 | 11.23 ± 3.11 | 201 ± 91 | ++ | 30 | 0 | 58 ± 21 |
| CW21-16 | 69.3 ± 3.1 | 18 ± 6 | 3.1 ± 0.5 | 15 ± 7 | 7.51 ± 2.53 | 85 ± 37 | ++ | 40 | 0 | 68 ± 16 |
| Plant Name | Total CBD Content (g, CBD + CBDA) | ||
|---|---|---|---|
| Leaf | Inflorescence | Total | |
| Mother plant | 2.663 ± 0.211 f | 23.612 ± 0.861 c | 26.275 ± 0.724 e |
| CW21-1 | 7.791 ± 0.354 d | 30.310 ± 0.434 b | 38.102 ± 0.457 b |
| CW21-2 | 1.751 ± 0.133 g | 11.913 ± 0.060 f | 13.663 ± 0.119 i |
| CW21-3 | 8.680 ± 0.297 c | 20.916 ± 0.451 d | 29.596 ± 0.441 d |
| CW21-4 | 9.865 ± 0.188 b | 23.440 ± 0.510 c | 33.304 ± 0.444 c |
| CW21-5 | 14.936 ± 0.266 a | 38.066 ± 0.088 a | 53.002 ± 0.228 a |
| CW21-6 | 0.505 ± 0.037 ij | 5.767 ± 0.174 h | 6.272 ± 0.145 k |
| CW21-7 | 1.823 ± 0.157 g | 14.637 ± 0.354 e | 16.460 ± 0.316 h |
| CW21-8 | 1.040 ± 0.104 hi | 8.313 ± 0.396 g | 9.353 ± 0.334 j |
| CW21-9 | 1.238 ± 0.104 h | 14.759 ± 0.069 e | 15.998 ± 0.102 h |
| CW21-10 | 2.831 ± 0.097 f | 15.219 ± 0.160 e | 18.050 ± 0.153 g |
| CW21-11 | 1.016 ± 0.149 hi | 3.619 ± 0.186 i | 4.635 ± 0.194 m |
| CW21-12 | 4.096 ± 0.128 e | 14.991 ± 0.189 e | 19.087 ± 0.187 f |
| CW21-13 | 4.248 ± 0.096 e | 12.104 ± 0.306 f | 16.352 ± 0.262 h |
| CW21-14 | 0.661 ± 0.011 ij | 5.745 ± 0.095 h | 6.406 ± 0.078 k |
| CW21-15 | 1.840 ± 0.075 g | 3.314 ± 0.030 i | 5.154 ± 0.066 l |
| CW21-16 | 1.184 ± 0.095 h | 8.099 ± 0.303 g | 9.283 ± 0.259 j |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, B.-R.; Kim, C.-H.; Kwon, T.-H.; Han, J.-H.; Gim, G.-J.; Jahirul Islam, M.; Azad, M.O.K.; Hafizur Rahman, M.; Soyel Rana, M.; Lim, J.-D.; et al. Industrial Hemp Clone Selection Method under LED Smart Farm Condition Based on CBD Production per Cubic Meter. Agronomy 2022, 12, 1809. https://doi.org/10.3390/agronomy12081809
Ryu B-R, Kim C-H, Kwon T-H, Han J-H, Gim G-J, Jahirul Islam M, Azad MOK, Hafizur Rahman M, Soyel Rana M, Lim J-D, et al. Industrial Hemp Clone Selection Method under LED Smart Farm Condition Based on CBD Production per Cubic Meter. Agronomy. 2022; 12(8):1809. https://doi.org/10.3390/agronomy12081809
Chicago/Turabian StyleRyu, Byeong-Ryeol, Chang-Hyeug Kim, Tae-Hyung Kwon, Joon-Hee Han, Gyeong-Ju Gim, Md Jahirul Islam, Md Obyedul Kalam Azad, Md Hafizur Rahman, Md Soyel Rana, Jung-Dae Lim, and et al. 2022. "Industrial Hemp Clone Selection Method under LED Smart Farm Condition Based on CBD Production per Cubic Meter" Agronomy 12, no. 8: 1809. https://doi.org/10.3390/agronomy12081809
APA StyleRyu, B.-R., Kim, C.-H., Kwon, T.-H., Han, J.-H., Gim, G.-J., Jahirul Islam, M., Azad, M. O. K., Hafizur Rahman, M., Soyel Rana, M., Lim, J.-D., & Lim, Y.-S. (2022). Industrial Hemp Clone Selection Method under LED Smart Farm Condition Based on CBD Production per Cubic Meter. Agronomy, 12(8), 1809. https://doi.org/10.3390/agronomy12081809








