The Course of Physiological Processes, Yielding, and Grain Quality of Hybrid and Population Wheat as Affected by Integrated and Conventional Cropping Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Experimental Set-Up
- -
- Factor I—two cropping systems (CS): integrated (INTEG) and conventional (CONV) (Table 1),
- -
- Factor II—two winter wheat cultivars (C): hybrid Hymalaya and population Formacja.
| Specification | Cropping System (CS) | |
|---|---|---|
| INTEG | CONV | |
| Previous crop | pea | spring wheat |
| Harvest previous crop | straw chopped and incorporated | straw baled |
| Tillage | disk harrow (12 cm), plowing (20 cm) | shallow plowing (12 cm) + harrowing, plowing (25 cm) |
| N (ammonium nitrate) | 90 kg·ha−1 (three applications: 40 + 30 + 20) | 180 kg·ha−1 (four applications: 60 + 60 + 40 + 20) |
| P (superphosphate) | 50 kg·ha−1 | 90 kg·ha−1 |
| K (potassium salt) | 70 kg·ha−1 | 140 kg·ha−1 |
| Weed control | pendimethalin + isoproturon (2.0 dm3·ha−1, direct after sowing), mechanical 2× (after starting the growth) | pendimethalin + isoproturon (4.0 dm3·ha−1), 2,4-dichlorophenoxyacetic acid (3.0 dm3·ha−1) |
| Fungicides | propiconazole + fenpropidin (1.0 dm3·ha−1) | propiconazole + fenpropidin (1.0 dm3·ha−1), propiconazole + cyproconazole (0.5 dm3·ha−1) |
| Insecticides | – | dimethoate (0.5 dm3·ha−1) |
| Growth regulator | trinexapac-ethyl (0.2 dm3·ha−1) | trinexapac-ethyl (0.4 dm3·ha−1) |
2.2. Weather and Soil Condition
2.3. Physiological Measurements
2.3.1. Leaf Area Index Measurement
2.3.2. Relative Chlorophyll Content
2.3.3. Chlorophyll Fluorescence
2.3.4. Gas Exchange
2.4. Grain Yield
2.5. Quality Parameters
2.5.1. Protein Extraction and Analysis
- Albumins + globulins—double extraction of 1 cm3 of the mixture (0.4 mol/L NaCl + 0.067 mol/L HKNaPO4 with a pH of 7.6),
- Gliadins—triple extraction of 1 cm3 of the mixture (60% ethanol),
- Glutenins—double extraction of 1 cm3 of the mixture (50% propanol 1 + 2 mol/L urea 0.05 mol/L Tris-HCl (pH 7.5) + 1% DTE under nitrogen).
2.5.2. Analysis of Nutrients
2.6. Statistical Analysis
3. Results and Discussion
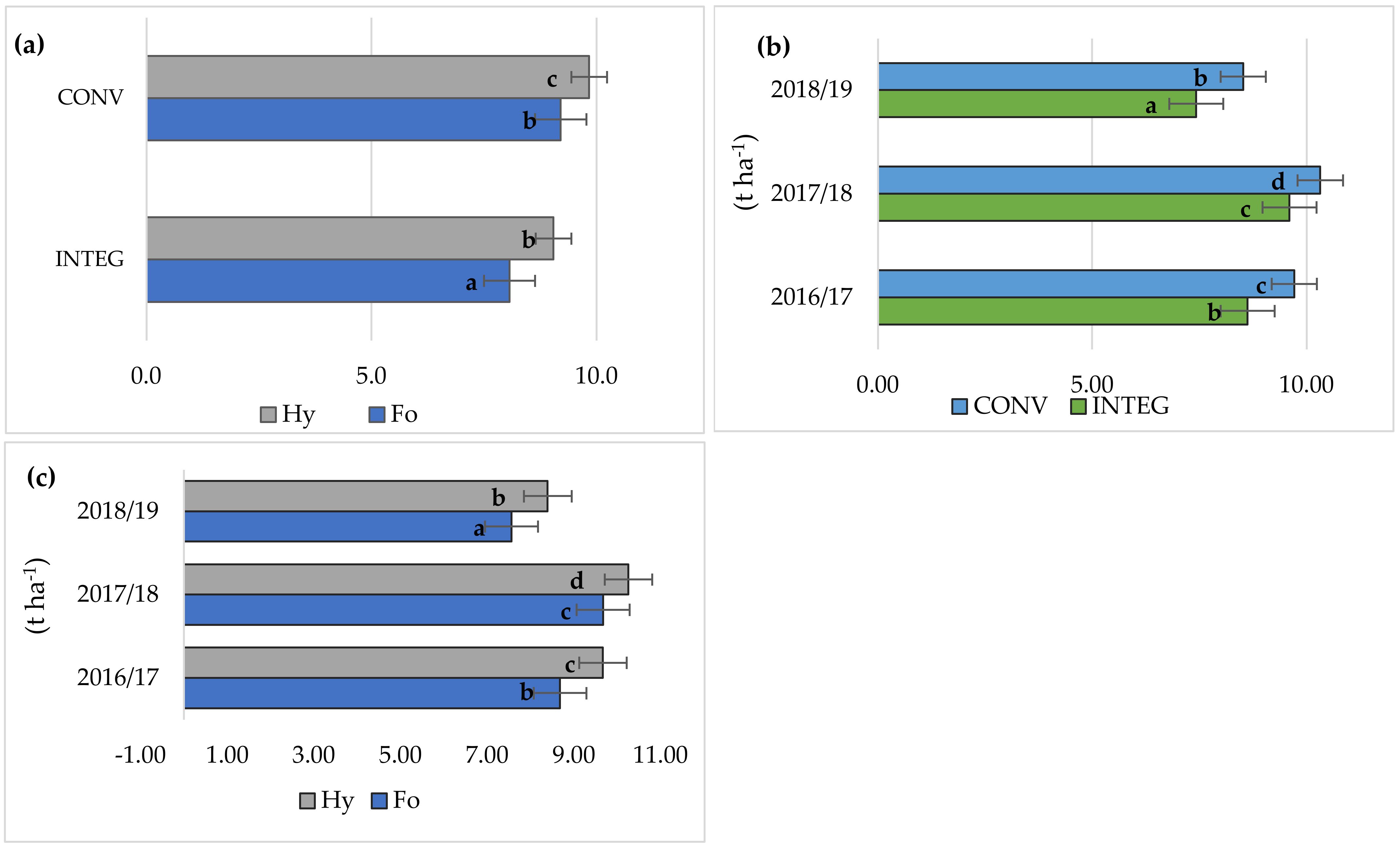
3.1. Grain Yield
3.2. Leaf Area Index (LAI)
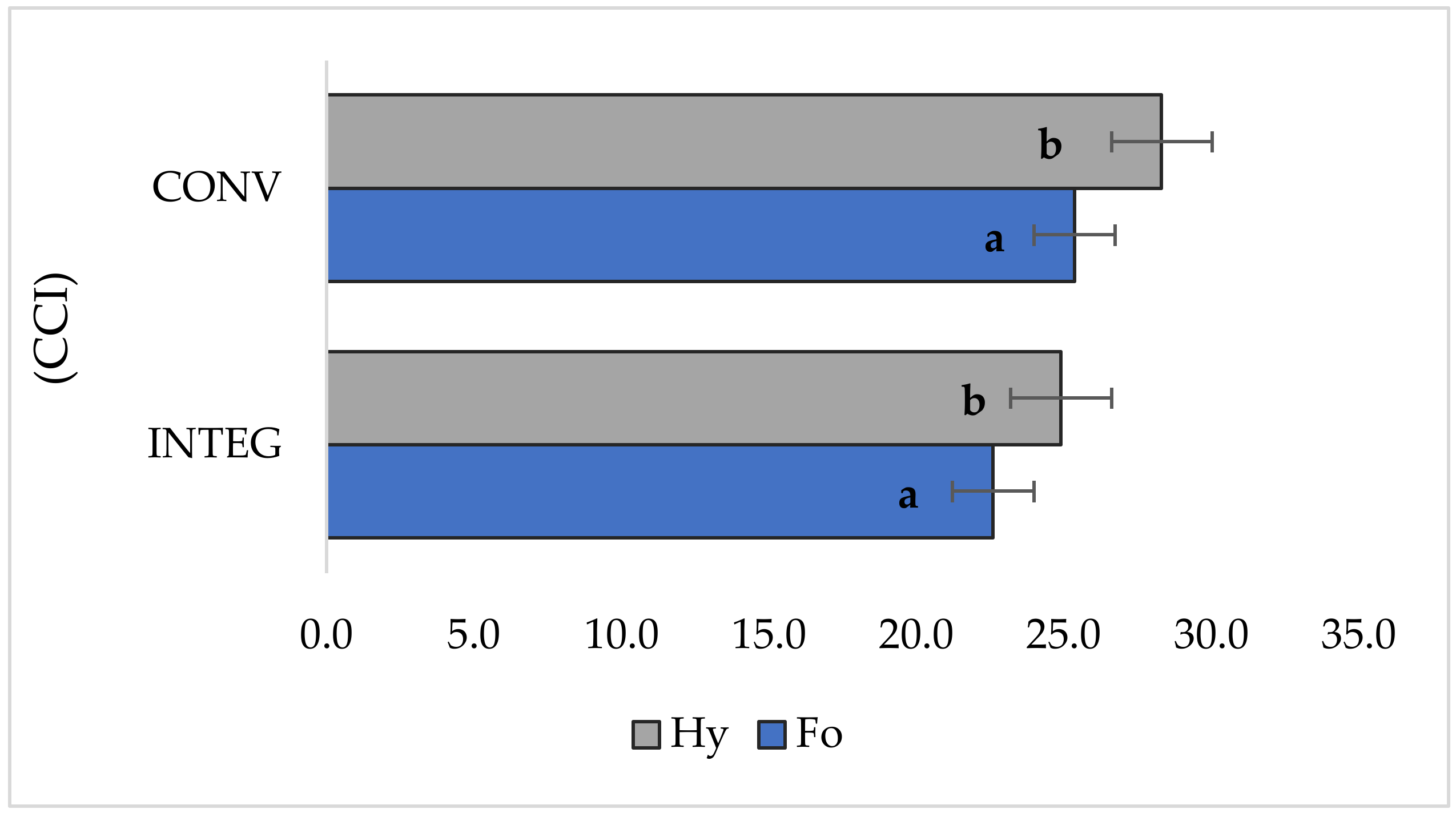
3.3. Relative Chlorophyll Content
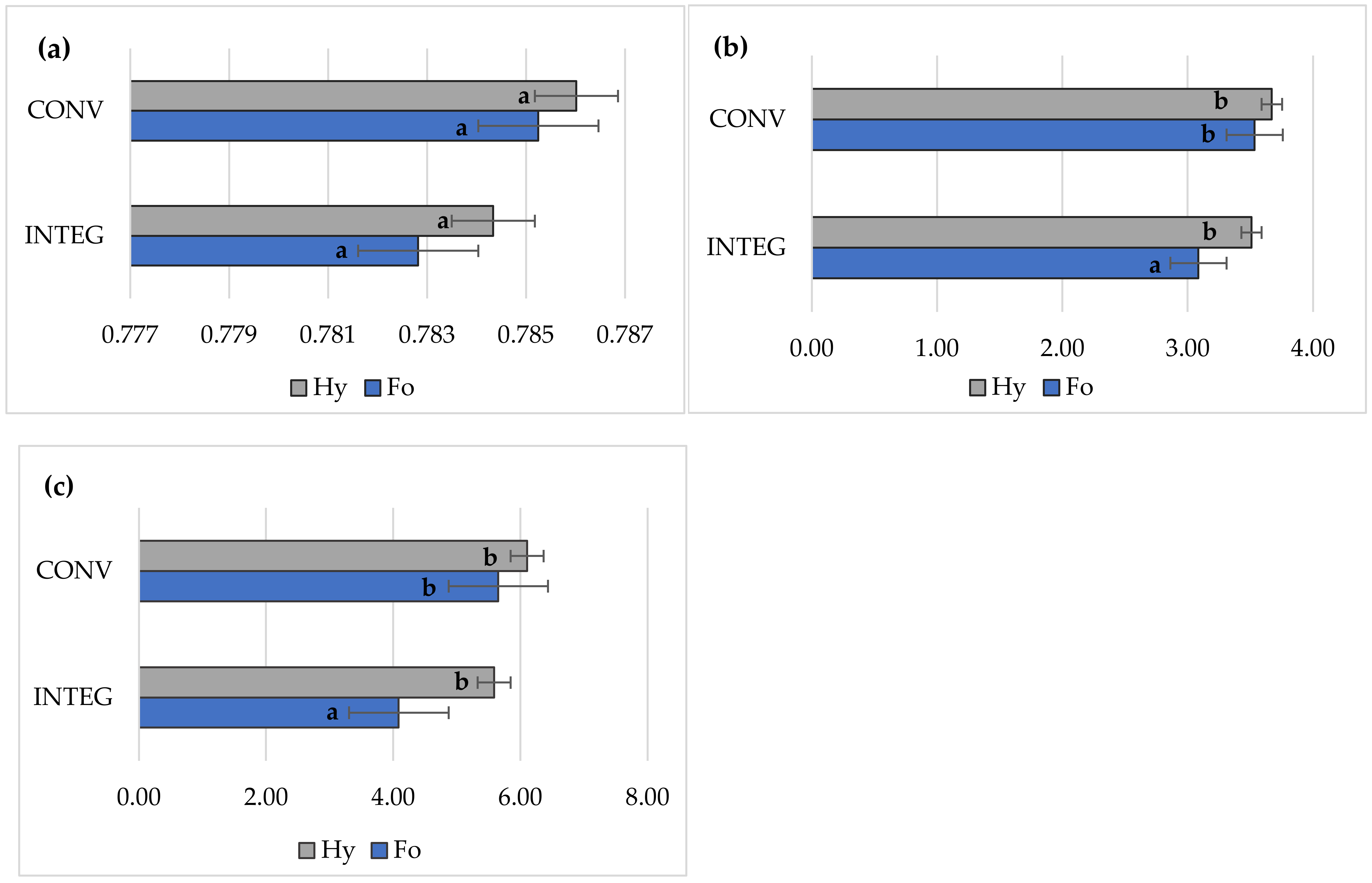
3.4. Chlorophyll Fluorescence
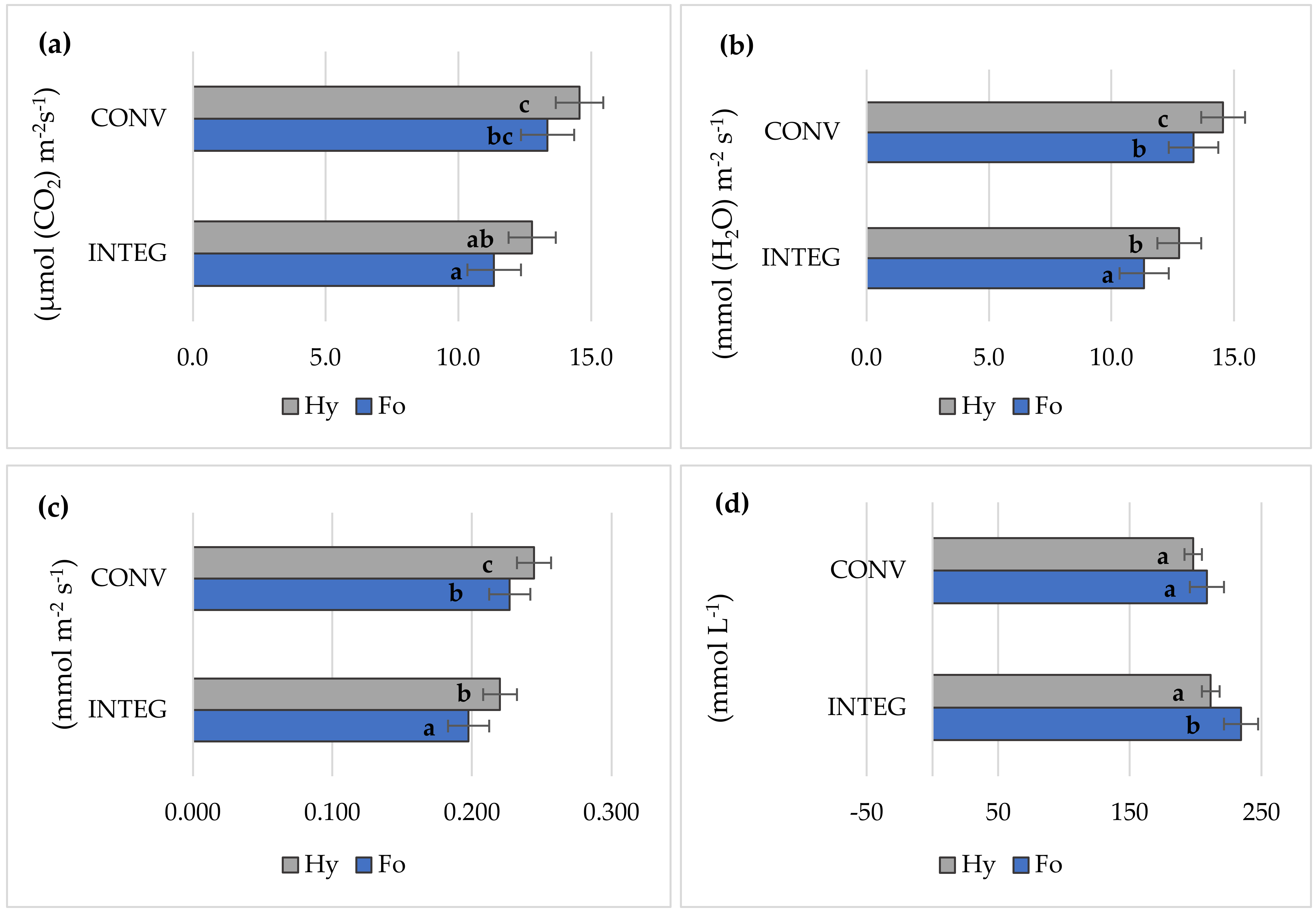
3.5. Gas Exchange
3.6. Quality Parameters
3.7. Protein Fraction Composition
3.8. Minerals Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Sułek, A. Economic evaluation of winter wheat production from different utility groups depending on the intensity of technology. Ann. Pol. Assoc. Agric. Aribus. Econ. 2017, 19, 226–231. [Google Scholar] [CrossRef]
- Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Gródek-Szostak, Z.; Kapusta-Duch, J.; Kuboń, M.; Komorowska, M.; Karcz, J. Impact of Integrated and Conventional Plant Production on Selected Soil Parameters in Carrot Production. Sustainability 2019, 11, 5612. [Google Scholar] [CrossRef]
- Dachraoui, M.; Sombrero, A. Effect of tillage systems and different rates of nitrogen fertilisation on the carbon footprint of irrigated maize in a semiarid area of Castile and Leon, Spain. Soil Tillage Res. 2020, 196, 104472. [Google Scholar] [CrossRef]
- Tuomisto, H.L.; Hodge, I.D.; Riordan, P.; Macdonald, D.W. Comparing global warming potential, energy use and land use of organic, conventional and integrated winter wheat production. Ann. Appl. Biol. 2012, 161, 116–126. [Google Scholar] [CrossRef]
- Grabiński, J. Productive and Economical Effects of Intensive and Integrated Technology Production of Winter Wheat and Spring Barley. Ann. Pol. Assoc. Agric. Aribus. Econ. 2015, 17, 94–99. Available online: http://ageconsearch.umn.edu/record/233486 (accessed on 4 March 2022). (In Polish).
- Bielski, S.; Romaneckas, K.; Novikova, A.; Šarauskis, E. Are Higher Input Levels to Triticale Growing Technologies Effective in Biofuel Production System? Sustainability 2019, 11, 5915. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating. Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 April 2022).
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Jiang, Y.; Schmidt, R.H.; Zhao, Y.; Reif, J.C. A quantitative genetic framework highlights the role of epistatic effects for grain-yield heterosis in bread wheat. Nat. Genet. 2017, 49, 1741–1746. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Gahlaut, V.; Saripalli, G.; Pal, B.; Basnet, B.R.; Joshi, A.K. Hybrid wheat: Past, present and future. Theor. Appl. Genet. 2019, 132, 2463–2483. [Google Scholar] [CrossRef]
- Selva, C.; Riboni, M.; Baumann, U.; Würschum, T.; Whitford, R.; Tucker, M.R. Hybrid breeding in wheat: How shaping floral biology can offer new perspectives. Funct. Plant Biol. 2020, 47, 675–694. [Google Scholar] [CrossRef]
- Živčák, M.; Olšovská, K.; Slamka, P.; Galambošová, J.; Rataj, V.; Shao, H.B.; Brestič, M. Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ. 2014, 60, 210–215. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, X.; Wang, Q.; Li, W.; Huang, M.; Zhou, Q.; Cai, J.; Wang, X.; Cao, W.; Dai, T.; et al. Increasing plant density improves grain yield, protein quality and nitrogen agronomic efficiency of soft wheat cultivars with reduced nitrogen rate. Field Crops Res. 2021, 267, 108145. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants; BBCH Monograph: Quedlinburg, Germany, 2018; p. 204. [Google Scholar]
- Skowera, B. Changes of hydrothermal conditions in the Polish area (1971–2010). Fragm. Agron. 2014, 31, 74–87. (In Polish) [Google Scholar]
- IUSS Working Group WRB. Reference Base for Soil Resources 2014; Update 2015; Word Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; pp. 172–173. [Google Scholar]
- Jankauskas, B.; Slepetiene, A.; Jankauskiene, G.; Fullen, A.M.; Booth, A.C. A comparative study of analytical methodologies to determine the soil organic matter content of Lithuanian Eutric Albeluvisols. Geoderma 2006, 136, 763–773. [Google Scholar] [CrossRef]
- Van Erp, P.J.; Houba, Y.J.G.; Van Beusichem, M.L. One hundredth molar calcium chloride extraction procedure. Part I: A review of soil chemical, analytical, and plant nutritional aspects. Commun. Soil Sci. Plant Anal. 1998, 29, 1603–1623. [Google Scholar] [CrossRef]
- Egner, H.; Riehm, H.; Domingo, R.W. Investigations on the chemical soil analysis as a basis for assessing the nutrient condition of the soil, II: Chemical extraction methods for phosphorus and potassium determination. K. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- Schachtschabel, P. The plant-available magnesium in the soil and its determination. Z. Pflanz. Düngung Bodenkd. 1954, 67, 9–23. [Google Scholar] [CrossRef]
- Rinkis, G.J. Micronutrients in Agriculture and Medicine; Naukova Dumka: Kijev, Ukraine, 1963. [Google Scholar]
- Hicks, S.; Lascano, R. Estimation of leaf area index for cotton canopies using the Li-Cor LAI 2000 plant canopy analyser. Agron. J. 1995, 87, 458–464. [Google Scholar] [CrossRef]
- Blackmer, T.M.; Schepers, J.S. Techniques for monitoring crop nitrogen status in corn. Commun. Soil Sci. Plant Anal. 1994, 25, 1791–1800. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosyn. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology; Elsevier: Oxford, UK, 2009. [Google Scholar] [CrossRef]
- AACC. American Association of Cereal Chemists (AACC) International Approved Methods of Analysis, 11th ed.; AACC: St. Paul, MN, USA, 2009. [Google Scholar]
- Wieser, H.; Antes, S.; Seilmeier, W. Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. Cereal Chem. 1998, 75, 544–650. [Google Scholar] [CrossRef]
- Konopka, I.; Tańska, M.; Pszczółkowska, A.; Fordoński, G.; Kozirok, W.; Olszewski, J. The effect of water stress on wheat kernel size, color and protein composition. Pol. J. Nat. Sci. 2007, 2, 157–171. [Google Scholar] [CrossRef]
- Sułek, A.; Cacak-Pietrzak, G. The influence of production technology on yield and selected quality parameters of spring wheat cultivars. Res. Rural Dev. 2018, 2, 42–48. [Google Scholar] [CrossRef]
- Kołodziejczyk, M.; Szmigiel, A.; Kulig, B. Yielding of selected spring wheat cultivars depending on technology level. Fragm. Agron. 2009, 26, 58–67. (In Polish) [Google Scholar]
- Plessis, A.; Ravel, C.; Bordes, J.; Balfourier, F.; Martre, P. Association study of wheat grain protein composition reveals that gliadin and glutenin composition are trans-regulated by different chromosome regions. J. Exp. Bot. 2013, 64, 3627–3644. [Google Scholar] [CrossRef]
- Whitford, R.; Fleury, D.; Reif, J.C.; Garcia, M.; Okada, T.; Korzun, V.; Langridge, P. Hybrid breeding in wheat: Technologies to improve hybrid wheat seed production. J. Exp. Bot. 2013, 64, 5411–5428. [Google Scholar] [CrossRef]
- Iwańska, M.; Paderewski, J.; Stępień, M.; Rodrigues, P.C. Adaptation of Winter Wheat Cultivars to Different Environments: A Case Study in Poland. Agronomy 2020, 10, 632. [Google Scholar] [CrossRef]
- Serrano, L.; Gamon, J.A.; Peńuelas, J. Estimation of canopy photosynthetic and nonphotosynthetic components from spectral transmittance. Ecology 2000, 81, 3149–3162. [Google Scholar] [CrossRef]
- Jameison, P.D.; Martin, R.J.; Francis, G.S.; Wilson, D.R. Drought effects on biomass production and radiation—Use efficiency in barley. Field Crops Res. 1995, 43, 77–86. [Google Scholar] [CrossRef]
- Bavec, M.; Vuković, K.; Grobelnik Mlakar, S.; Rozman, Č.; Bavec, F. Leaf area index in winter wheat: Response on seed rate and nitrogen application by different varieties. J. Cent. Eur. Agric. 2007, 8, 337–342. [Google Scholar]
- Olsen, J.; Weiner, J. The influence of Triticum aestivum density, sowing pattern and nitrogen fertilization on leaf area index and its spatial variation. Basic Appl. Ecol. 2007, 8, 252–257. [Google Scholar] [CrossRef]
- Skudra, I.; Ruza, A. Effect of nitrogen and sulphur fertilization on chlorophyll content in winter wheat. Rural Sustain. Res. 2017, 37, 29–37. [Google Scholar] [CrossRef]
- Kara, B.; Mujdeci, M. Influence of late-season nitrogen application on chlorophyll content and leaf area index in wheat. Sci. Res. Essays. 2010, 5, 2299–2303. [Google Scholar] [CrossRef]
- Sayed, O.H. Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 2003, 41, 321–330. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Whitney, S.M.; Houtz, R.L.; Alonso, H. Advancing our understanding and capacity to engineer nature’s CO2—Sequestering enzyme, Rubisco. Plant Physiol. 2011, 155, 27–35. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Shangguan, Z. Leaf gas exchange and fluorescence of two winter wheat varieties in response to drought stress and nitrogen supply. PLoS ONE 2016, 11, e0165733. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J.; Zhang, Q.; Li, L.; Kuang, T. Modification of photosystem II photochemistry in nitrogen deficient maize and wheat plants. J. Plant Physiol. 2001, 158, 1423–1430. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Ge, Q.; Li, B.; Tong, Y.; Li, Z.; Kuang, T.; Lu, C. Characterization of photosynthesis of flag leaves in a wheat hybrid and its parents grown under field conditions. J. Plant Physiol. 2007, 164, 318–326. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Saifullah Ahmad, A. Water stress and nitrogen managment effects on gas exchange, water relations, and water use efficiency in wheat. J. Plant Nutr. 2011, 34, 1867–1882. [Google Scholar] [CrossRef]
- Ciompi, S.; Gentili, E.; Guidi, L.; Soldatini, G.F. The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower. Plant Sci. 1996, 118, 177–184. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Y.I.; Liu, Y.; Shi, P. Simulation of the stomatal conductance of winter wheat in response to light, temperature and CO2 changes. Ann. Bot. 2004, 93, 435–441. [Google Scholar] [CrossRef]
- Rachoń, L.; Szumiło, G.; Brodowska, M.; Woźniak, A. Nutritional value and mineral composition of grain of selected wheat species depending on the intensity of a production technology. J. Elem. 2015, 3, 705–7015. [Google Scholar] [CrossRef][Green Version]
- Wojtkowiak, K.; Stępień, A. Nutritive value of spelt (Triticum aestivum spp. spelta L.) as influenced by the foliar application of copper, zinc and manganese. Zemdirbyste-Agriculture 2015, 24, 389–396. [Google Scholar] [CrossRef][Green Version]
- Mader, P.; Hahn, D.; Dubois, D.; Gunst, L.; Alfoldi, T.; Bergmann, H.; Oehme, M.; Amado, R.; Schneider, H.; Graf, U.; et al. Wheat quality in organic and conventional farming: Results of a 21 year field experiment. J. Sci. Food Agric. 2007, 87, 1826–1835. [Google Scholar] [CrossRef]
- García-Molina, D.M.; Barro, F. Characterization of changes in gluten proteins in low-gliadin transgenic wheat lines in response to application of different nitrogen regimes. Front. Plant Sci. 2017, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Rachoń, L. Effect of tillage systems on the yield and quality of winter wheat grain and soil properties. Agriculture 2020, 10, 405. [Google Scholar] [CrossRef]
- Shewry, P.R. Improving the protein content and composition of cereal grain. J. Cereal Sci. 2007, 46, 239–250. [Google Scholar] [CrossRef]
- Krejčířová, L.; Capouchová, I.; Petr, J.; Bicanová, E.; Faměra, O. The effect of organic and conventional growing systems on quality and storage protein composition of winter wheat. Plant Soil Environ. 2007, 53, 499–505. [Google Scholar] [CrossRef]
- Horvat, D.; Drezner, G.; Sudar, R.; Šimic, G.; Dvojkovic, K.; Španic, V.; Magdic, D. Distribution of wheat protein components under different genetic backgrounds and environments. Turk. J. Field Crops. 2015, 20, 150–154. [Google Scholar] [CrossRef][Green Version]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Stępień, A.; Wojtkowiak, K. Composition of gluten proteins in spring and winter wheat grain cultivated under conditions of varied fertilization. Acta Agric. Scand. B Soil Plant. Sci. 2013, 63, 588–594. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Dvojković, K.; Ivić, M.; Plavšin, I.; Novoselović, D. Gluten protein compositional changes in response to nitrogen application rate. Agronomy 2021, 11, 325. [Google Scholar] [CrossRef]
- Podolska, G.; Aleksandrowicz, E.; Szafrańska, A. Bread making potential of Triticum aestivum and Triticum spelta species. Open Life Sci. 2020, 15, 30–40. [Google Scholar] [CrossRef]
- Ciołek, A.; Makarska, E.; Wesołowski, M.; Cierpiała, R. Content of selected nutrients in wheat, barley and oat grain from organic and conventional farming. J. Elem. 2012, 2, 181–189. [Google Scholar] [CrossRef]
- Ryan, M.; Derrick, J.; Dann, P. Grain mineral concentrations and yield of wheat grown under organic and conventional management. J. Sci. Food Agri. 2004, 84, 207–216. [Google Scholar] [CrossRef]
- Suchowilska, E.; Wiwart, M.; Kandler, W.; Krska, R. A comparison of macro- and microelement concentrations in the whole grain of four Triticum species. Plant Soil Environ. 2012, 58, 141–147. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.Z.; Li, F.; Li, L.; Yang, N.; Yang, Y.; Huang, D.; Liang, D.; Zhao, H.; Mao, H.; et al. Grain iron and zinc concentrations of wheat and their relationships to yield in major wheat production areas in China. Field Crops Res. 2014, 156, 151–160. [Google Scholar] [CrossRef]
- Garnet, P.T.; Graham, D.G. Distribution and remobilization of iron and copper in wheat. Ann. Bot. 2005, 95, 817–826. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.P.; Huerta-Espino, J.; Peña, R.J. Breeding for enhanced zinc and iron concentration in CIMMYT spring wheat germplasm. Czech J. Genet. Plant Breed. 2011, 47, 174–177. [Google Scholar] [CrossRef]





| Traits | Years | ||
|---|---|---|---|
| 2016/2017 | 2017/2018 | 2018/2019 | |
| Value | |||
| pH KCl | 7.32 | 6.58 | 6.75 |
| organic carbon (g·kg−1) | 10.7 | 10.5 | 10.9 |
| Nmin (kg·ha−1) | 61.2 | 58.3 | 66.0 |
| Content of available nutrients (mg·kg−1 soil): | |||
| phosphorus (P) | 202.0 | 131.5 | 83.0 |
| potassium (K) | 272.5 | 190.1 | 245.2 |
| magnesium (Mg) | 128.6 | 142.4 | 231.7 |
| iron (Fe) | 2291.4 | 2529.1 | 2235.8 |
| zinc (Zn) | 15.3 | 14.1 | 13.9 |
| manganese (Mn) | 379.8 | 241.3 | 275.3 |
| copper (Cu) | 6.8 | 6.1 | 6.5 |
| Factor | Grain Yield | LAI | Relative Chlorophyll Content | Gas Exchange | Chlorophyll Fluorescence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cropping System (CS) | Cultivar (C) | Pn | E | gs | Ci | WUE | Fv/Fm | Fv/F0 | PI | |||
| INTEG | 8.55 a | 4.22 a | 23.8 a | 12.1 a | 3.17 a | 0.209 a | 223 b | 3.94 b | 0.757 a | 3.30 a | 4.84 a | |
| CONV | 9.52 b | 4.75 b | 26.8 b | 14.0 b | 3.91 b | 0.236 b | 203 a | 3.58 a | 0.812 b | 3.60 a | 5.88 b | |
| Formacja | 8.64 a | 4.23 a | 24.0 a | 12.3 a | 3.30 a | 0.212 a | 222 b | 3.87 a | 0.764 a | 3.31 a | 4.87 a | |
| Hymalaya | 9.44 b | 4.74 b | 26.6 b | 13.7 b | 3.78 b | 0.232 b | 205 a | 3.66 a | 0.805 b | 3.59 a | 5.85 b | |
| Year | ||||||||||||
| 2016/2017 | 9.17 b | 4.28 a | 24.6 b | 11.7 a | 3.30 b | 0.246 b | 220 b | 4.01 b | 0.780 a | 3.53 a | 5.38 b | |
| 2017/2018 | 9.96 c | 4.88 b | 28.6 c | 12.6 a | 4.36 c | 0.205 a | 202 a | 3.90 b | 0.772 a | 3.63 a | 6.09 c | |
| 2018/2019 | 7.98 a | 4.30 a | 22.7 a | 14.7 b | 2.96 a | 0.216 a | 218 b | 3.38 a | 0.801 a | 3.19 a | 4.60 a | |
| Mean | 9.04 | 4.48 | 25.3 | 13.0 | 3.54 | 0.222 | 213 | 3.76 | 0.785 | 3.45 | 5.36 | |
| CS | *** | *** | *** | *** | *** | *** | *** | ** | *** | ns | *** | |
| C | *** | *** | *** | *** | *** | *** | *** | ns | ** | ns | *** | |
| Y | *** | *** | *** | ** | *** | *** | ** | ** | ns | ns | *** | |
| CS × C | * | ns | ns | ns | ** | ns | ns | * | ns | ns | ** | |
| CS × Y | ns | ns | * | ns | ns | ns | ns | * | ns | ns | ns | |
| C × Y | ns | ns | ** | ns | ns | ns | ns | ns | ns | ns | ns | |
| CS × C × Y | ns | ns | * | ns | ns | ns | ns | ns | ns | ns | ns | |
| Factor | Protein [g·kg−1] | Gluten [%] | A + B | Gli | Glu | Gli/ Glu | |
|---|---|---|---|---|---|---|---|
| Cropping System (CS) | Cultivar (C) | mAU·s−1 | |||||
| INTEG | Formacja | 124.4 a | 25.5 a | 12.1 b | 28.4 b | 17.6 a | 1.47 b |
| Hymalaya | 131.9 b | 28.1 bc | 11.7 ab | 26.5 a | 23.2 b | 1.29 a | |
| CONV | Formacja | 127.5 a | 26.8 ab | 11.6 a | 28.9 b | 21.4 b | 1.33 a |
| Hymalaya | 137.3 c | 29.9 c | 11.7 ab | 28.8 b | 27.6 c | 1.27 a | |
| INTEG | 128.2 a | 26.8 a | 11.9 a | 27.5 a | 20.4 a | 1.38 a | |
| CONV | 132.4 b | 28.4 b | 11.7 a | 28.9 b | 24.5 b | 1.30 b | |
| Formacja | 126.0 a | 26.2 a | 11.9 a | 28.7 a | 19.5 a | 1.40 a | |
| Hymalaya | 134.6 b | 29.0 b | 11.7 a | 27.6 b | 25.4 b | 1.28 b | |
| Year (Y) | |||||||
| 2016/2017 | 120.5 a | 25.5 a | 11.1 a | 25.2 a | 19.8 a | 1.37 a | |
| 2017/2018 | 130.2 b | 27.3 b | 12.1 b | 29.4 b | 22.9 b | 1.35 a | |
| 2018/2019 | 140.2 c | 29.9 c | 12.1 b | 29.9 b | 24.6 c | 1.29 a | |
| Mean | 130.3 | 27.6 | 11.8 | 28.2 | 22.4 | 1.34 | |
| CS | *** | *** | ns | *** | *** | ** | |
| C | *** | *** | ns | *** | *** | *** | |
| Y | *** | *** | *** | *** | *** | * | |
| CS × C | * | ns | ns | *** | ns | * | |
| CS × Y | ** | ns | ns | *** | *** | ** | |
| C × Y | *** | * | ns | *** | ** | ns | |
| CS × C × Y | ns | ns | ns | *** | ns | ns | |
| Factor | α/β Gli | γ Gli | ω Gli | HMW Glu | LMW Glu | HMW/ LMW | |
|---|---|---|---|---|---|---|---|
| Cropping System (CS) | Cultivar (C) | mAU·s−1 | |||||
| INTEG | Formacja | 15.0 b | 8.4 b | 5.0 b | 4.1 a | 13.5 a | 0.30 b |
| Hymalaya | 14.4 a | 7.6 a | 4.5 a | 5.4 b | 17.8 b | 0.30 b | |
| CONV | Formacja | 14.8 ab | 9.1 c | 5.1 b | 4.4 a | 17.1 b | 0.26 a |
| Hymalaya | 14.8 ab | 9.2 c | 4.8 ab | 8.0 c | 19.6 c | 0.40 c | |
| INTEG | 14.7 a | 8.0 a | 4.8 a | 4.7 a | 15.7 a | 0.30 a | |
| CONV | 14.8 a | 9.1 b | 4.9 a | 6.2 b | 18.3 b | 0.33 b | |
| Formacja | 14.9 a | 8.7 a | 5.0 a | 4.2 a | 15.3 a | 0.28 a | |
| Hymalaya | 14.6 b | 8.4 b | 4.6 b | 6.7 b | 18.7 b | 0.35 b | |
| Year (Y) | |||||||
| 2016/2017 | 13.2 a | 7.8 a | 4.3 a | 4.7 a | 15.1 a | 0.31 a | |
| 2017/2018 | 15.5 b | 9.1 b | 4.9 b | 5.5 b | 17.4 b | 0.31 a | |
| 2018/2019 | 15.6 b | 8.9 b | 5.4 c | 6.2 c | 18.5 b | 0.33 a | |
| Mean | 14.7 | 8.6 | 4.8 | 5.5 | 17.0 | 0.32 | |
| CS | ns | *** | * | *** | *** | *** | |
| C | * | * | *** | *** | *** | *** | |
| Y | *** | *** | *** | *** | *** | ns | |
| CS × C | * | ** | ns | *** | * | *** | |
| CS × Y | ** | * | *** | *** | * | * | |
| C × Y | ** | *** | *** | *** | ns | * | |
| CS × C × Y | ns | ns | ns | ns | ns | ns | |
| Year (Y) | Cultivar (C) | Protein [g·kg−1] | Gluten [%] | A + B | Gli | Glu | Gli/ Glu |
|---|---|---|---|---|---|---|---|
| mAU·s−1 | |||||||
| 2016/2017 | Formacja | 117.7 a | 23.8 a | 11.2 a | 25.0 a | 17.3 a | 1.41 d |
| Hymalaya | 123.3 b | 27.1 b | 11.1 a | 25.3 a | 22.3 b | 1.34 c | |
| 20117/2018 | Formacja | 128.4 c | 26.5 b | 12.2 b | 29.6 c | 20.4 b | 1.42 d |
| Hymalaya | 132.0 c | 28.2 b | 12.0 b | 29.3 c | 25.5 c | 1.28 b | |
| 2018/2019 | Formacja | 131.8 c | 28.2 b | 12.2 b | 31.5 d | 20.8 b | 1.37 c |
| Hymalaya | 148.7 d | 31.7 c | 12.0 b | 28.3 b | 28.4 d | 1.21 a | |
| Year (Y) | Cultivar (C) | α/β Gli | γ Gli | ω Gli | HMW Glu | LMW Glu | HMW/ LMW |
|---|---|---|---|---|---|---|---|
| mAU·s−1 | |||||||
| 2016/2017 | Formacja | 13.2 a | 7.5 a | 4.3 a | 3.9 a | 13.5 a | 0.29 a |
| Hymalaya | 13.1 a | 8.0 ab | 4.2 a | 5.6 c | 16.7 bc | 0.33 b | |
| 20117/2018 | Formacja | 15.4 bc | 9.3 d | 4.9 b | 4.2 ab | 16.1 b | 0.27 a |
| Hymalaya | 15.1 b | 8.9 cd | 4.9 b | 6.7 d | 18.7 cd | 0.35 b | |
| 2018/2019 | Formacja | 16.1 c | 9.5 d | 5.9 c | 4.6 b | 16.3 b | 0.29 a |
| Hymalaya | 15.6 bc | 8.4 bc | 4.8 b | 7.8 e | 20.7 d | 0.37 b | |
| Factor | Iron (Fe) | Cooper (Cu) | Zinc (Zn) | Manganese (Mn) | Magnesium (Mg) | |
|---|---|---|---|---|---|---|
| Cropping System (CS) | Cultivar (C) | mg·kg−1 | g·kg−1 | |||
| INTEG | Formacja | 52.1 ab | 2.48 a | 38.37 a | 28.12 b | 0.92 a |
| Hymalaya | 54.9 a | 3.11 b | 39.93 a | 32.54 c | 1.02 b | |
| CONV | Formacja | 50.2 a | 2.59 a | 38.35 a | 25.84 a | 0.94 ab |
| Hymalaya | 52.9 ab | 3.05 b | 39.45 a | 28.34 b | 0.95 ab | |
| INTEG | 53.5 a | 2.79 a | 39.15 a | 30.33 a | 0.97 a | |
| CONV | 51.5 b | 2.82 a | 38.90 a | 27.09 b | 0.94 a | |
| Formacja | 51.1 a | 2.53 a | 38.36 a | 26.98 a | 0.93 a | |
| Hymalaya | 53.9 b | 3.08 b | 39.69 a | 30.44 b | 0.99 b | |
| 2016/2017 | 48.7 a | 2.50 a | 39.79 a | 27.85 a | 0.89 a | |
| 2017/2018 | 55.1 b | 3.06 b | 41.20 b | 30.34 b | 1.02 b | |
| 2018/2019 | 53.8 b | 2.86 b | 36.08 b | 27.93 a | 0.97 b | |
| Mean | 52.50 | 2.81 | 39.02 | 28.71 | 0.96 | |
| CS | ** | ns | ns | *** | * | |
| C | *** | *** | * | *** | * | |
| Y | *** | *** | *** | *** | *** | |
| CS × C | ns | ns | ns | *** | ns | |
| CS × Y | ns | ns | ns | ns | ns | |
| C × Y | ns | ns | ns | ns | ns | |
| CS × C × Y | ns | ns | ns | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jańczak-Pieniążek, M.; Buczek, J.; Kwiatkowski, C.A.; Harasim, E. The Course of Physiological Processes, Yielding, and Grain Quality of Hybrid and Population Wheat as Affected by Integrated and Conventional Cropping Systems. Agronomy 2022, 12, 1345. https://doi.org/10.3390/agronomy12061345
Jańczak-Pieniążek M, Buczek J, Kwiatkowski CA, Harasim E. The Course of Physiological Processes, Yielding, and Grain Quality of Hybrid and Population Wheat as Affected by Integrated and Conventional Cropping Systems. Agronomy. 2022; 12(6):1345. https://doi.org/10.3390/agronomy12061345
Chicago/Turabian StyleJańczak-Pieniążek, Marta, Jan Buczek, Cezary A. Kwiatkowski, and Elżbieta Harasim. 2022. "The Course of Physiological Processes, Yielding, and Grain Quality of Hybrid and Population Wheat as Affected by Integrated and Conventional Cropping Systems" Agronomy 12, no. 6: 1345. https://doi.org/10.3390/agronomy12061345
APA StyleJańczak-Pieniążek, M., Buczek, J., Kwiatkowski, C. A., & Harasim, E. (2022). The Course of Physiological Processes, Yielding, and Grain Quality of Hybrid and Population Wheat as Affected by Integrated and Conventional Cropping Systems. Agronomy, 12(6), 1345. https://doi.org/10.3390/agronomy12061345









