The Impact of Salinity Stress on Antioxidant Response and Bioactive Compounds of Nepeta cataria L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Climate Conditions
2.3. Soil Characterization
2.4. Morphological Analysis
2.5. Physiological Analysis
2.6. Biochemical Analyses
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yield of Nepeta Cultures
3.2. Morphological and Physiological Analyses
3.3. Biochemical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, T.; Adams, R.M. Salt and Silt in Ancient Mesopotamian Agriculture. Science 1958, 128, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Dagar, J.C.; Yadav, R.K.; Singh, A.; Singh, N.T. Historical Perspectives and Dynamics of Nature, Extent, Classification and Management of Salt-affected Soils and Waters. In Research Developments in Saline Agriculture; Springer: Singapore, 2019; pp. 3–52. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Saeidnejad, A.H.; Kafi, M.; Pessarakli, M. Interactive Effects of Salinity Stress and Zn Availability on Physiological Properties, Antioxidant Activity, and Micronutrients Content of Wheat (Triticum aestivum) Plants. Commun. Soil Sci. Plant Anal. 2016, 47, 1048–1057. [Google Scholar] [CrossRef]
- Alnusairi, G.S.H.; Mazrou, Y.S.A.; Qari, S.H.; Elkelish, A.A.; Soliman, M.H.; Eweis, M.; Abdelaal, K.; El-Samad, G.A.; Ibrahim, M.F.M.; ElNahhas, N. Exogenous Nitric Oxide Reinforces Photosynthetic Efficiency, Osmolyte, Mineral Uptake, Antioxidant, Expression of Stress-Responsive Genes and Ameliorates the Effects of Salinity Stress in Wheat. Plants 2021, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.N.; Sadras, V.O.; Tester, M. A water-centred framework to assess the effects of salinity on the growth and yield of wheat and barley. Plant Soil 2010, 336, 377–389. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription Factors Interact with ABA through Gene Expression and Signaling Pathways to Mitigate Drought and Salinity Stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, J.; Zhang, Y.; Fan, F.; Li, W.; Wang, F.; Zhong, W.; Wang, C.; Yang, J. Comparative transcriptome analysis reveals molecular response to salinity stress of salt-tolerant and sensitive genotypes of indica rice at seedling stage. Sci. Rep. 2018, 8, 2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formisano, C.; Rigano, D.; Senatore, F. Chemical Constituents and Biological Activities of Nepeta Species. Chem. Biodivers. 2011, 8, 1783–1818. [Google Scholar] [CrossRef] [PubMed]
- Reichert, W.; Villani, T.; Pan, M.H.; Ho, C.T.; Simon, J.E.; Wu, Q.L. Phytochemical analysis and anti-inflammatory activity of Nepeta cataria accessions. J. Med. Active Plants 2018, 7, 19–27. [Google Scholar] [CrossRef]
- Bandh, S.A.; Kamili, A.N.; Ganai, B.A.; Lone, B.A.; Saleem, S. Evaluation of antimicrobial activity of aqueous extracts of Nepeta cataria. J. Pharm. Res. 2011, 4, 3141–3142. [Google Scholar]
- Zomorodian, K.; Saharkhiz, M.J.; Rahimi, M.J.; Shariatifard, S.; Pakshir, K.; Khashei, R. Chemical composition and antimi-crobial activities of essential oils from Nepeta cataria L. against commons causes of oral infections. J. Dent. 2013, 10, 329–337. [Google Scholar]
- Duda, S.C.; Marghitas, L.A.; Dezmirean, D.S.; Bobis, O.; Duda, M.M. Nepeta cataria: Medicinal plant of interest in phytotherapy and bee keeping. Hop. Med. Plants 2015, 23, 34–38. [Google Scholar]
- Patience, G.S.; Karirekinyana, G.; Galli, F.; Patience, N.A.; Kubwabo, C.; Collin, G.; Bizimana, J.C.; Boffito, D.C. Sustainable manufacture of insect repellents derived from Nepeta cataria. Sci. Rep. 2018, 8, 2235. [Google Scholar] [CrossRef] [Green Version]
- Handjieva, N.V.; Popov, S.S.; Evstatieva, L.N. Constituents of Essential Oils from Nepeta cataria L., N. grandiflora M.B. and N. nuda L. J. Essent. Oil Res. 1996, 8, 639–643. [Google Scholar] [CrossRef]
- Gomes, E.N.; Allen, K.; Jaworski, K.; Zorde, M.; Lockhart, A.; Besancon, T.; Brown, T.; Reichert, W.; Wu, Q.; Simon, J.E. Catnip (Nepeta cataria L.): Recent Advances in Horticulture and Production. In Medicinal and Aromatic Plants of North America; Springer: Berlin/Heidelberg, Germany, 2020; pp. 247–284. [Google Scholar]
- Emami, S.A.; Asili, J.; HosseinNia, S.; Yazdian-Robati, R.; Sahranavard, M.; Tayarani-Najaran, Z. Growth Inhibition and Apoptosis Induction of Essential Oils and Extracts of Nepeta cataria L. on Human Prostatic and Breast Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2016, 17, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Wesołowska, A.; Jadczak, D.; Grzeszczuk, M. GC-MS analysis of lemon catnip (Nepeta cataria L. var. citriodora Balbis) essential oil. Acta Chromatogr. 2011, 23, 169–180. [Google Scholar] [CrossRef]
- Giarratana, F.; Muscolino, D.; Ziino, G.; Lo Presti, V.; Rao, R.; Chiofalo, V.; Giuffrida, A.; Panebianco, A. Activity of Catmint (Nepeta cataria) essential oil against Anisakis larvae. Trop. Biomed. 2017, 34, 22–31. [Google Scholar]
- Regnier, F.; Waller, G.; Eisenbraun, E. Studies on the composition of the essential oils of three Nepeta species. Phytochemistry 1967, 6, 1281–1289. [Google Scholar] [CrossRef]
- Gilani, A.H.; Shah, A.J.; Zubair, A.; Khalid, S.; Kiani, J.; Ahmed, A.; Rasheed, M.; Ahmad, V.U. Chemical composition and mechanisms underlying the spasmolytic and bronchodilatory properties of the essential oil of Nepeta cataria L. J. Ethnopharmacol. 2009, 121, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Frolova, N.; Ukrainets, A.; Korablova, O.; Voitsekhivskyi, V. Plants of Nepeta cataria var. citriodora Beck. and essential oils from them for food industry. Potravin. Slovak J. Food Sci. 2019, 13, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Li, J.; Ma, J.; Qiao, F. Hepatoprotective effect of essential oils of Nepeta cataria L. on acetaminophen-induced liver dysfunction. Biosci. Rep. 2019, 39, BSR20190697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.A.; Abourashed, E.A. Leung’s Encyclopedia of Common Natural Ingredients, 3rd ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 159–161. [Google Scholar]
- Acimovic, M.; Zeremski, T.; Kiprovski, B.; Brdar-Jokanovic, M.; Popovic, V.; Koren, A.; Sikora, V. Nepeta cataria—Cultivation, chemical composition and biological activity. J. Agron. Technol. Eng. Manag. 2021, 4, 620–634. [Google Scholar]
- Mohammadi, S.; Saharkhiz, M.J.; Javanmardi, J. Evaluation of interaction effects of spermidine and salinity on physiological and morphological trait of catnip (Nepeta cataria L.). Z. Arznei-Gewürzpflanzen 2017, 22, 104–109. [Google Scholar]
- Mohammadizad, H.A.; Khazaei, I.; Ghafari, M.; Fazel, M.; Sinehsar, F.; Barzegar, R. Effect of salt and drought stress on seed germination and early seedling growth of Nepeta persica. Int. J. Farm. Allied Sci. 2013, 2, 895–899. [Google Scholar]
- Mojarab, S.; Moghaddam, M.; Narimani, R. The effect of pretreatment of salicylic acid on seed germination, total phenoland antioxidant activity of Nepeta nuda L. under salt stress. Eco-Phytochem. J. Med. Plants 2018, 6, 21–31. [Google Scholar]
- Onofrei, V.; Teliban, G.-C.; Burducea, M.; Lobiuc, A.; Sandu, C.B.; Tocai, M.; Robu, T. Organic foliar fertilization increases polyphenol content of Calendula officinalis L. Ind. Crop. Prod. 2017, 109, 509–513. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Teliban, G.-C.; Burducea, M.; Zheljazkov, V.; Dincheva, I.; Badjakov, I.; Munteanu, N.; Mihalache, G.; Cojocaru, A.; Popa, L.-D.; Stoleru, V. The Effect of Myco-Biocontrol Based Formulates on Yield, Physiology and Secondary Products of Organically Grown Basil. Agriculture 2021, 11, 180. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.-M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Contents in Acyanic and Cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [Green Version]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Kulak, M.; Gul, F.; Sekeroglu, N. Changes in growth parameter and essential oil composition of sage (Salvia officinalis L.) leaves in response to various salt stresses. Ind. Crop. Prod. 2020, 145, 112078. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crop. Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Herron, S. Catnip, Nepeta cataria, a morphological comparison of mutant and wild type speciments to gain an ethnobotanical perspective. Econ. Bot. 2003, 57, 135–142. [Google Scholar] [CrossRef]
- Karray-Bouraoui, N.; Rabhi, M.; Neffati, M.; Baldan, B.; Ranieri, A.; Marzouk, B.; Lachaâl, M.; Smaoui, A. Salt effect on yield and composition of shoot essential oil and trichome morphology and density on leaves of Mentha pulegium. Ind. Crop. Prod. 2009, 30, 338–343. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanam, D.; Mohammad, F. Plant growth regulators ameliorate the ill effect of salt stress through improved growth, photosynthesis, antioxidant system, yield and quality attributes in Mentha piperita L. Acta Physiol. Plant. 2018, 40, 188. [Google Scholar] [CrossRef]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Ehauser, M.-T. Molecular basis of natural variation and environmental control of trichome patterning. Front. Plant Sci. 2014, 5, 320. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-E.; Harada, E.; Kim, G.-H.; Yoon, E.-S.; Sano, H. Distribution of elements on tobacco trichomes and leaves under cadmium and sodium stresses. J. Plant Biol. 2004, 47, 75–82. [Google Scholar] [CrossRef]
- Gonzáles, W.L.; Negritto, M.A.; Suárez, L.H.; Gianoli, E. Induction of glandular and non-glandular trichomes by damage in leaves of Madia sativa under contrasting water regimes. Acta Oecol. 2008, 33, 128–132. [Google Scholar] [CrossRef]
- Oksanen, E. Trichomes form an important first line of defence against adverse environment-New evidence for ozone stress mitigation. Plant Cell Environ. 2018, 41, 1497–1499. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; El-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy 2019, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Melesse, T.; Caesar, K. Stomatal and Non-stomatal Effects of Salinity on Photosynthesis in Faba Beans (Vicia faba L.). J. Agron. Crop Sci. 1992, 168, 345–353. [Google Scholar] [CrossRef]
- Mihaylova, D.; Georgieva, L.; Pavlov, A. In Vitro Antioxidant Activity and Phenolic Composition of Nepeta cataria L. Extracts. Int. J. Agric. Sci. Technol. 2013, 1, 74–79. [Google Scholar]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Tomás-Barberán, F.A.; García-Villalba, R. Structural Diversity of Polyphenols and Distribution in Foods. Diet. Polyphen. Metab. Health Eff. 2020, 1–29. [Google Scholar] [CrossRef]
- Aelenei, P.; Rimbu, C.M.; Horhogea, C.E.; Lobiuc, A.; Neagu, A.-N.; Dunca, S.I.; Motrescu, I.; Dimitriu, G.; Aprotosoaie, A.C.; Miron, A. Prenylated phenolics as promising candidates for combination antibacterial therapy: Morusin and kuwanon G. Saudi Pharm. J. 2020, 28, 1172–1181. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]



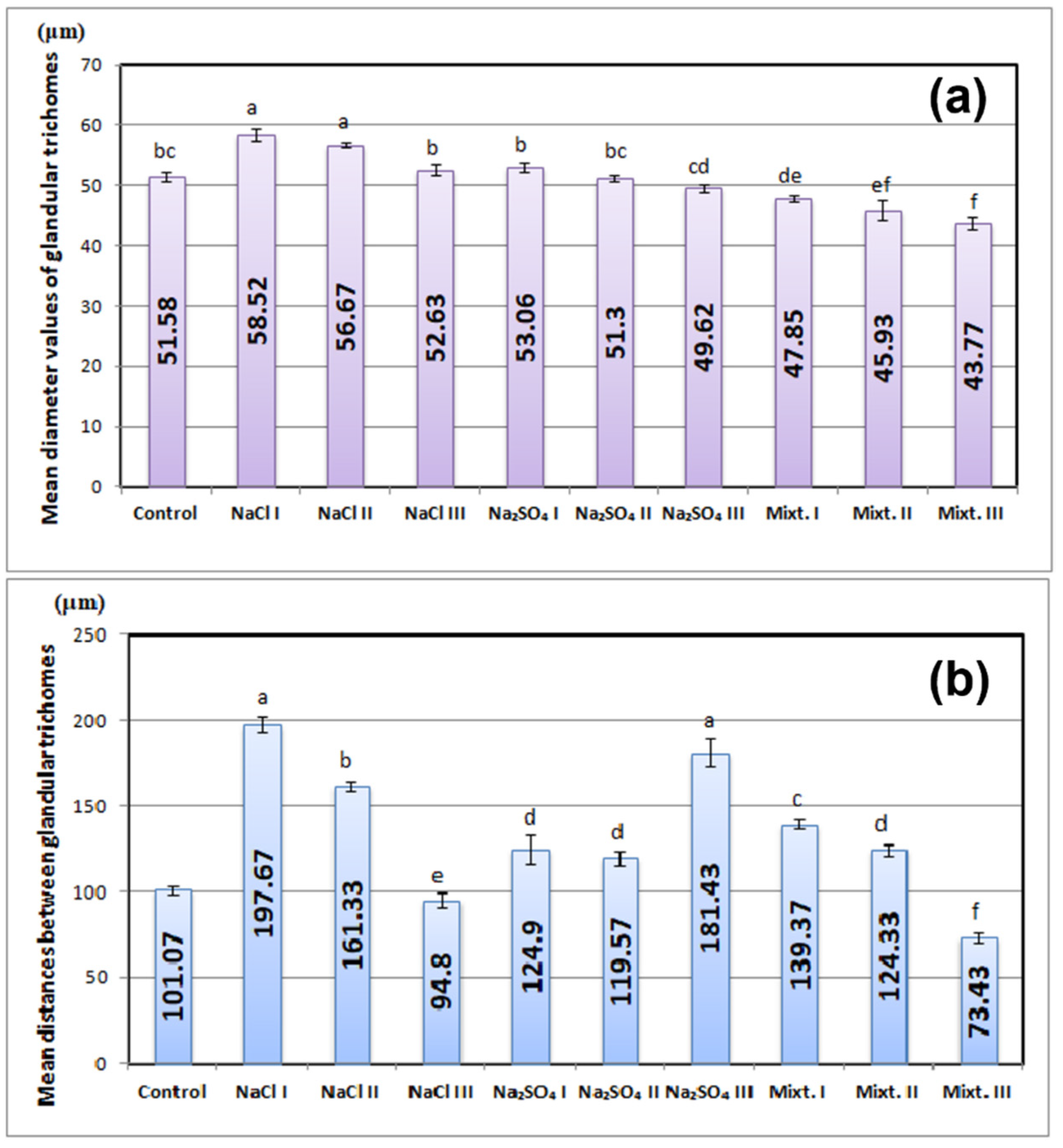


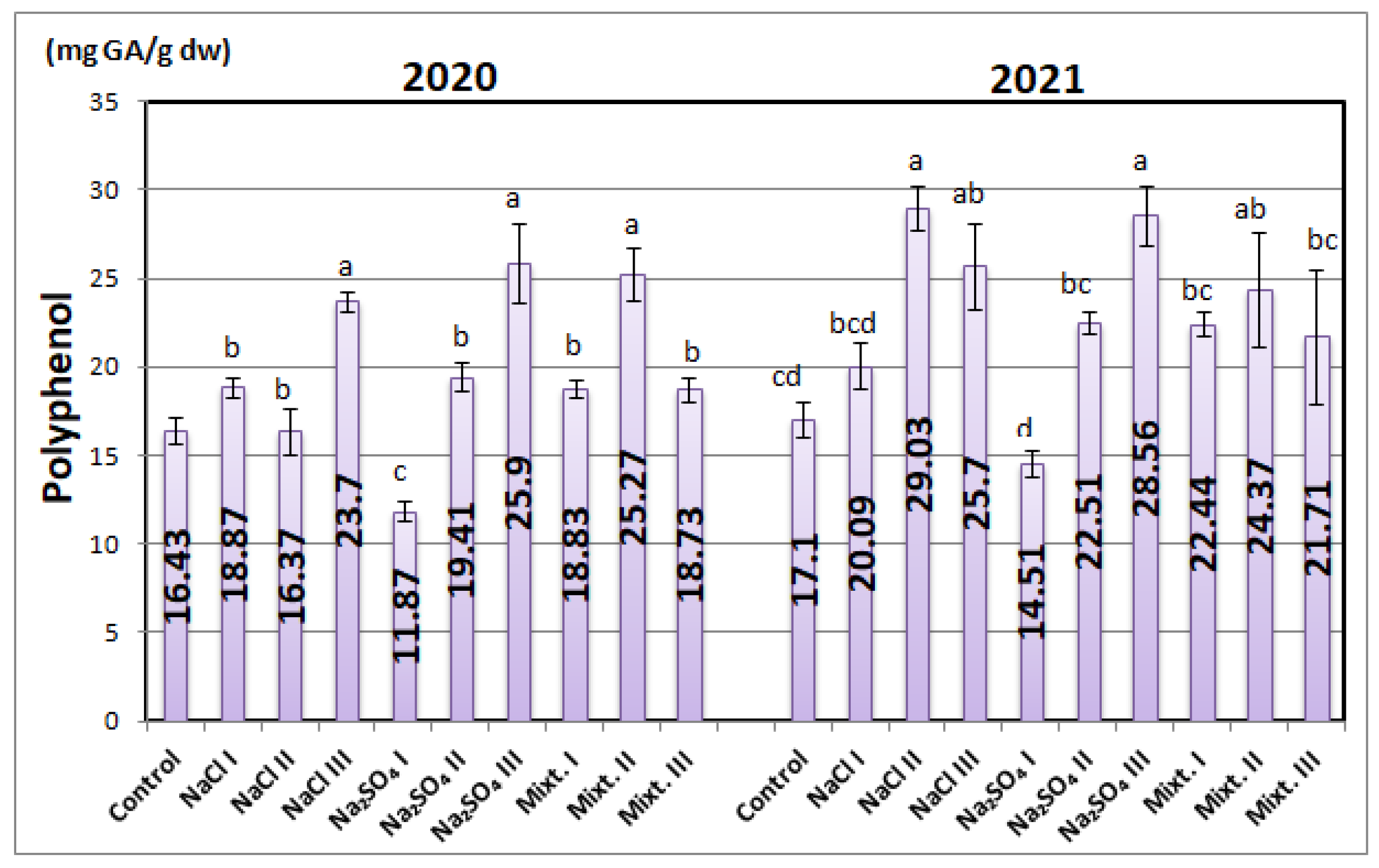


| Variant | v1 | v2 | v3 | v4 | v5 | v6 | v7 | v8 | v9 | v10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | NaCl I | NaCl II | NaCl III | Na2SO4 I | Na2SO4 II | Na2SO4 III | Mixt. I | Mixt. II | Mixt. III | ||
| Salt content (mg/100 g of soil) | Cl− | 0 | 18 | 39 | 60 | 0 | 0 | 0 | 9 | 19 | 30 |
| SO4−2 | 0 | 0 | 0 | 0 | 50 | 85 | 120 | 25 | 43 | 60 | |
| Average Air Temperature (°C) | Average Precipitations (mm) | |||
|---|---|---|---|---|
| Month/Years | 2020 | 2021 | 2020 | 2021 |
| March | 7.34 | 3.7 | 65.4 | 29.2 |
| April | 11.01 | 8.3 | 56.4 | 44.8 |
| May | 14.29 | 15.2 | 87 | 55.8 |
| June | 20.97 | 19.8 | 115 | 83.7 |
| July | 22.42 | 23.2 | 71.6 | 71.3 |
| August | 23.27 | 20.9 | 155.4 | 57.4 |
| September | 19.20 | 14.6 | 12.4 | 47.0 |
| Soil Horizon | pH | ppm | Humus % | CaCO3 % | Ntotal % | Corganic | C/N | CTSS mg/100 g Soil | ||
|---|---|---|---|---|---|---|---|---|---|---|
| P | P Corrected | K | ||||||||
| Am | 7.9 | 31 | 17 | 229 | 3.49 | 4.77 | 0.176 | 2.02 | 9.84 | 53 |
| 2020 | Variant | Polyphenol | Antioxidant Activity | Stomatal Conductivity | Chlorophyll a | Chlorophyll b | Carotenoids | Yield |
|---|---|---|---|---|---|---|---|---|
| Variant | 1 | |||||||
| Polyphenol | 0.49 ns | 1 | ||||||
| Antioxidant activity | 0.41 ns | 0.41 ns | 1 | |||||
| Stomatal conductivity | 0.60 ns | 0.27 ns | 0.04 ns | 1 | ||||
| Chlorophyll a | −0.43 ns | −0.62 ns | −0.30 ns | −0.02 ns | 1 | |||
| Chlorophyll b | −0.65 * | −0.56 ns | −0.20 ns | −0.19 ns | 0.90 ** | 1 | ||
| Carotenoids | −0.41 ns | −0.57 ns | 0.01 ns | 0.06 ns | 0.82 ns | 0.83 ** | 1 | |
| Yield | −0.63 ** | −0.32 ns | −0.22 ns | −0.68 * | 0.04 ns | 0.04 ns | −0.04 ns | 1 |
| 2021 | Variant | Polyphenol | Antioxidant activity | Stomatal conductivity | Chlorophyll a | Chlorophyll b | Carotenoids | Yield |
| Variant | 1 | |||||||
| Polyphenol | 0.44 ns | 1 | ||||||
| Antioxidant activity | 0.41 ns | 0.92 ** | 1 | |||||
| Stomatal conductivity | 0.62 ns | 0.34 ns | 0.18 ns | 1 | ||||
| Chlorophyll a | −0.45 ns | −0.39 ns | −0.28 ns | −0.09 ns | 1 | |||
| Chlorophyll b | −0.62 * | −0.47 ns | −0.35 ns | −0.26 ns | 0.94 ** | 1 | ||
| Carotenoids | −0.33 ns | −0.32 ns | −0.23 ns | 0.02 ns | 0.98 ** | 0.86 ** | 1 | |
| Yield | −0.68 ** | −0.41 ns | −0.26 ns | −0.85 ** | −0.01 ns | 0.19 ns | −0.16 ns | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungoci, C.; Motrescu, I.; Filipov, F.; Jitareanu, C.D.; Teliban, G.-C.; Ghitau, C.S.; Puiu, I.; Robu, T. The Impact of Salinity Stress on Antioxidant Response and Bioactive Compounds of Nepeta cataria L. Agronomy 2022, 12, 562. https://doi.org/10.3390/agronomy12030562
Lungoci C, Motrescu I, Filipov F, Jitareanu CD, Teliban G-C, Ghitau CS, Puiu I, Robu T. The Impact of Salinity Stress on Antioxidant Response and Bioactive Compounds of Nepeta cataria L. Agronomy. 2022; 12(3):562. https://doi.org/10.3390/agronomy12030562
Chicago/Turabian StyleLungoci, Constantin, Iuliana Motrescu, Feodor Filipov, Carmenica Doina Jitareanu, Gabriel-Ciprian Teliban, Carmen Simona Ghitau, Ioan Puiu, and Teodor Robu. 2022. "The Impact of Salinity Stress on Antioxidant Response and Bioactive Compounds of Nepeta cataria L." Agronomy 12, no. 3: 562. https://doi.org/10.3390/agronomy12030562
APA StyleLungoci, C., Motrescu, I., Filipov, F., Jitareanu, C. D., Teliban, G.-C., Ghitau, C. S., Puiu, I., & Robu, T. (2022). The Impact of Salinity Stress on Antioxidant Response and Bioactive Compounds of Nepeta cataria L. Agronomy, 12(3), 562. https://doi.org/10.3390/agronomy12030562








