Response of Potted Hebe andersonii to Salinity under an Efficient Irrigation Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Conditions
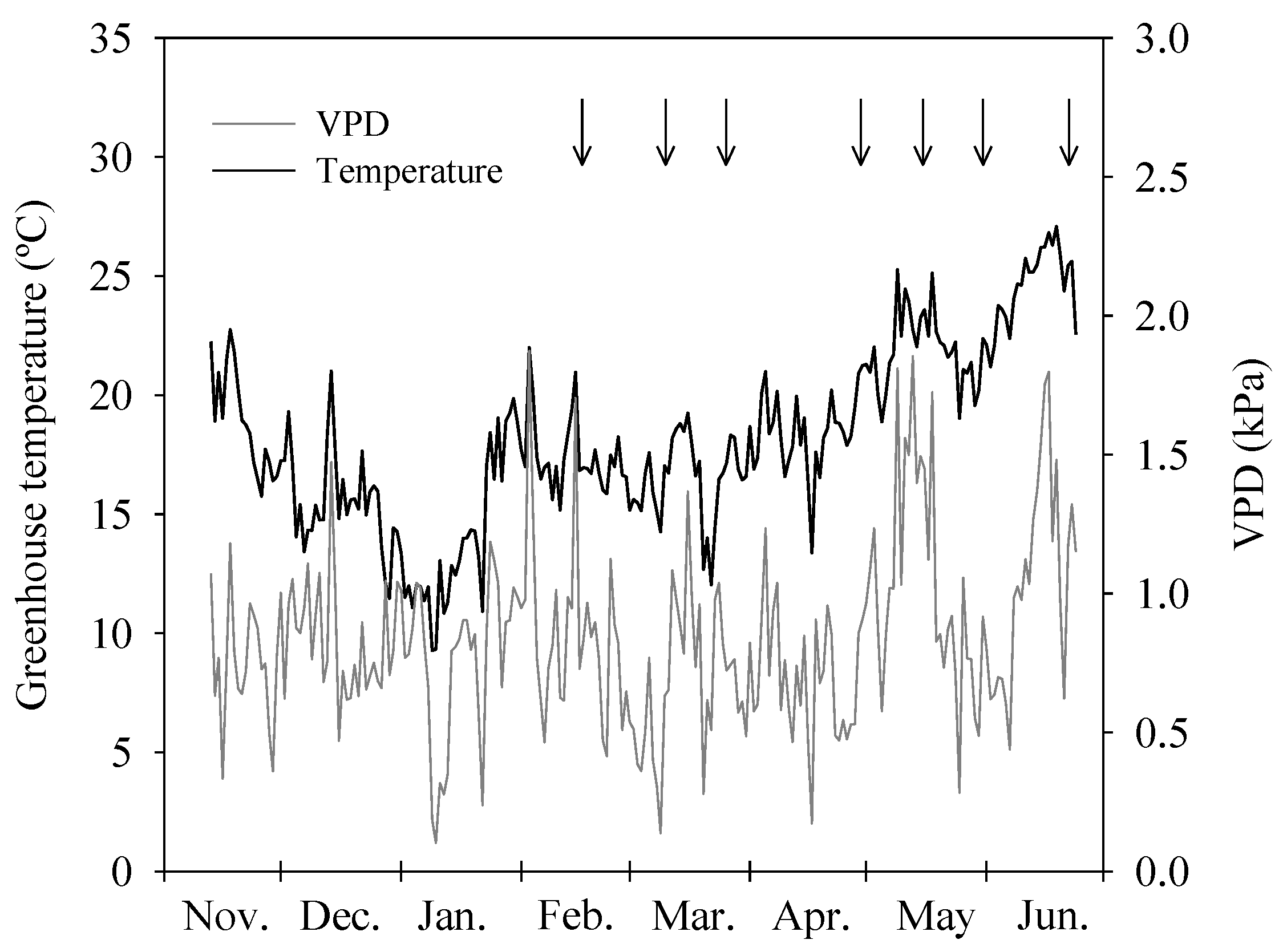
2.2. Treatments and Irrigation Parameters
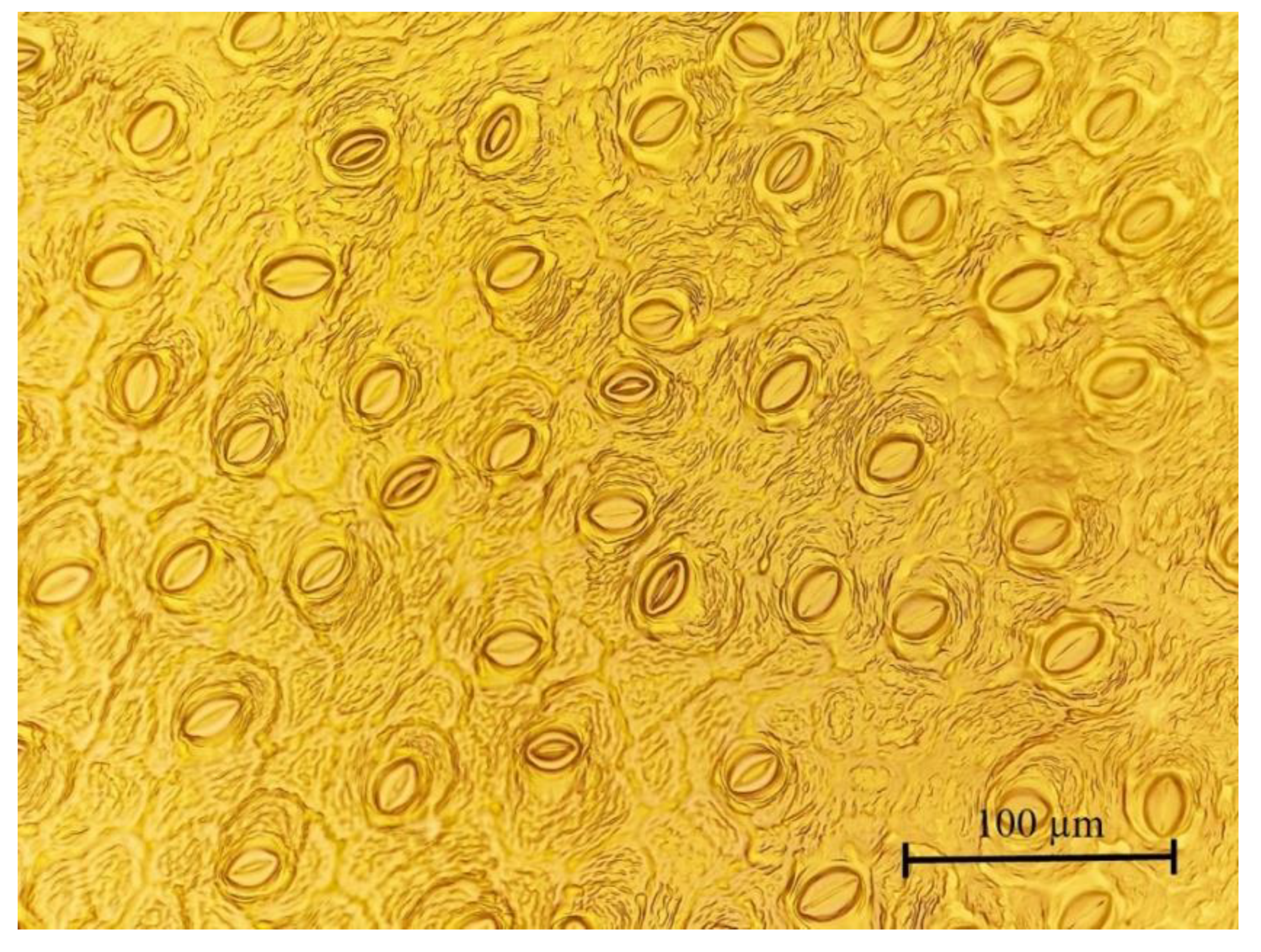
2.3. Plant Growth, Foliar Chlorophyll Content, and Stomatal Features
2.4. Leaf Water Potential
2.5. Gas Exchange and Photochemical Efficiency of the Photosystem II (PSII)
2.6. Leaf Temperature
2.7. Plant Mineral Content
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion
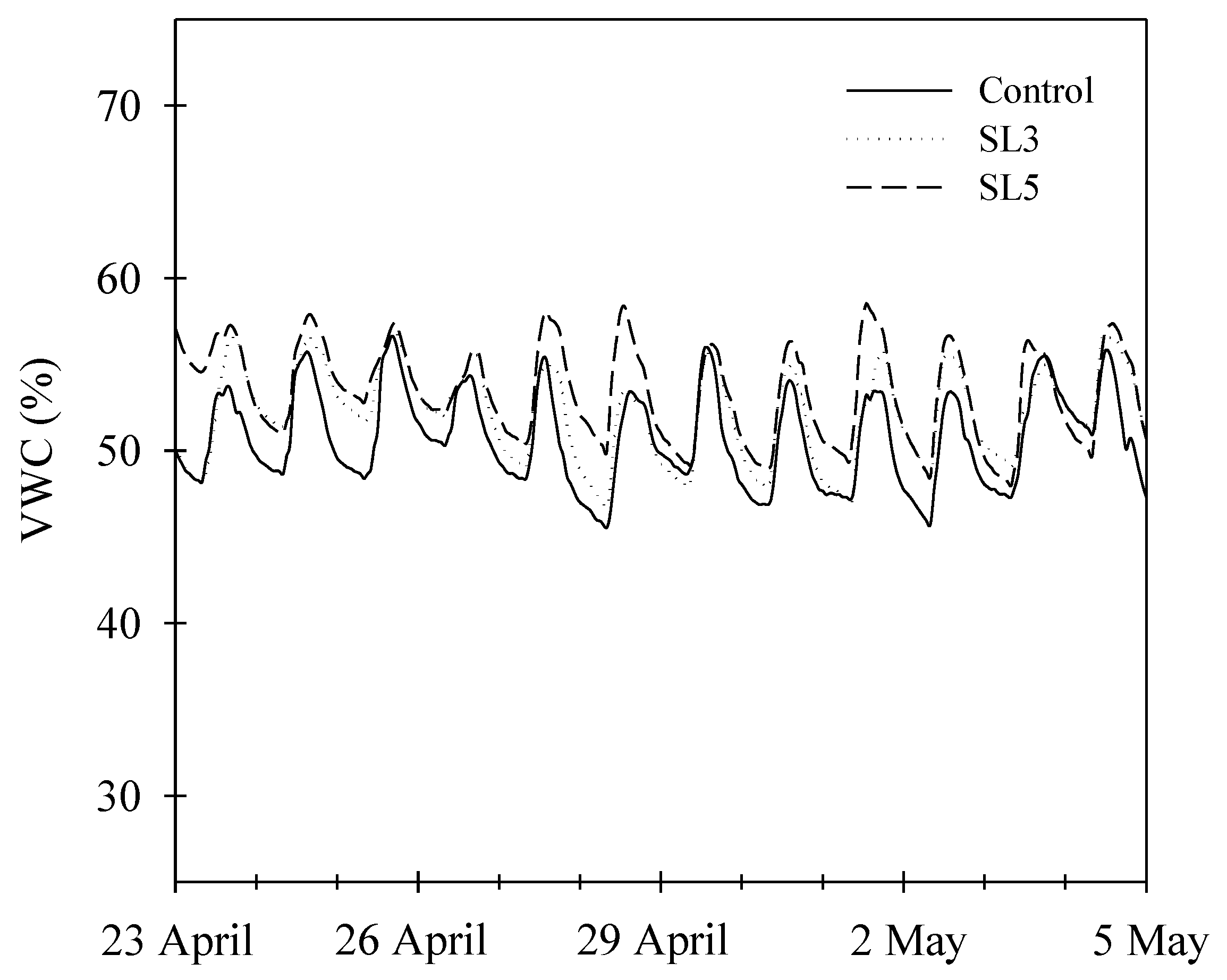
3.1. Water Consumption and Water-Use Efficiency
3.2. Plant Growth and Visual Quality
3.3. Mineral Ions and Substrate Solution EC
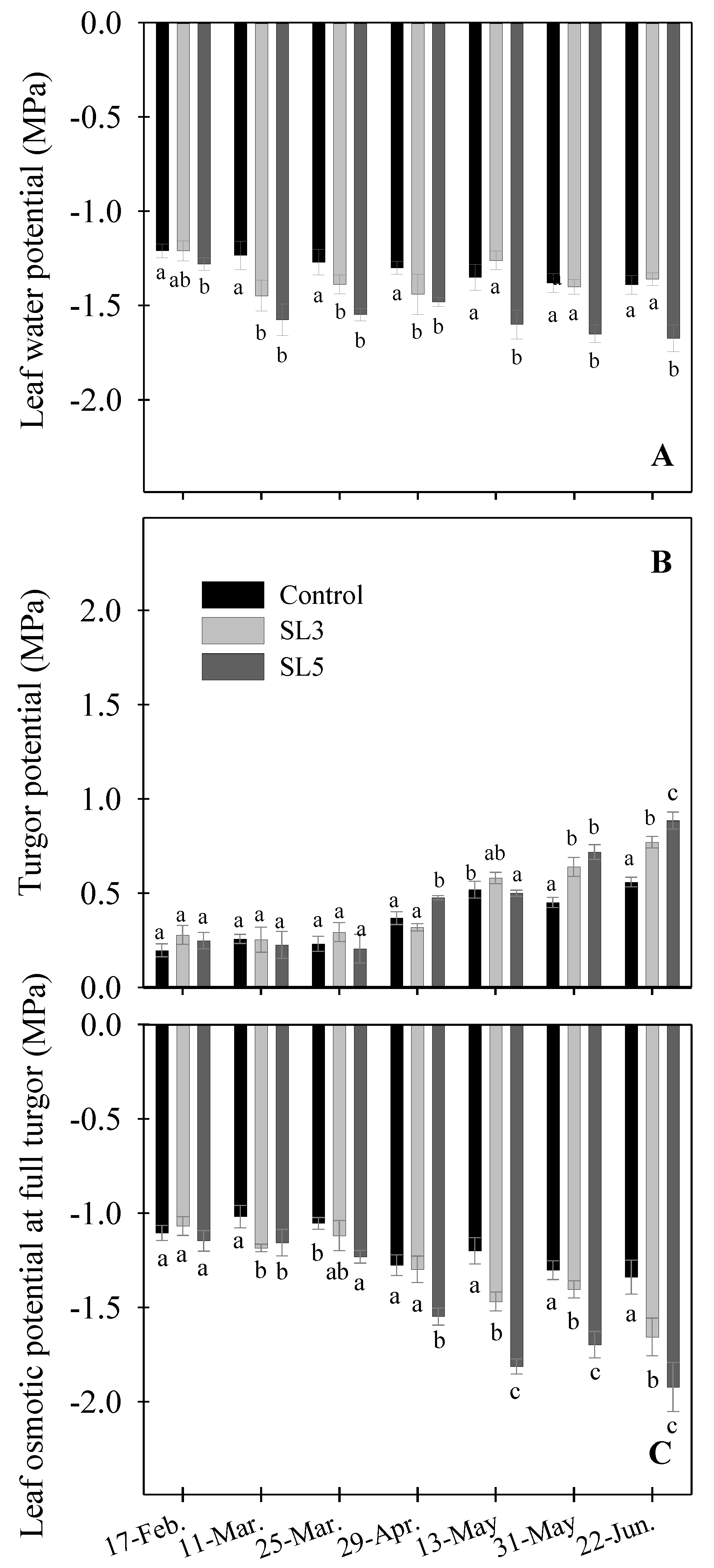
3.4. Plant Water Relations
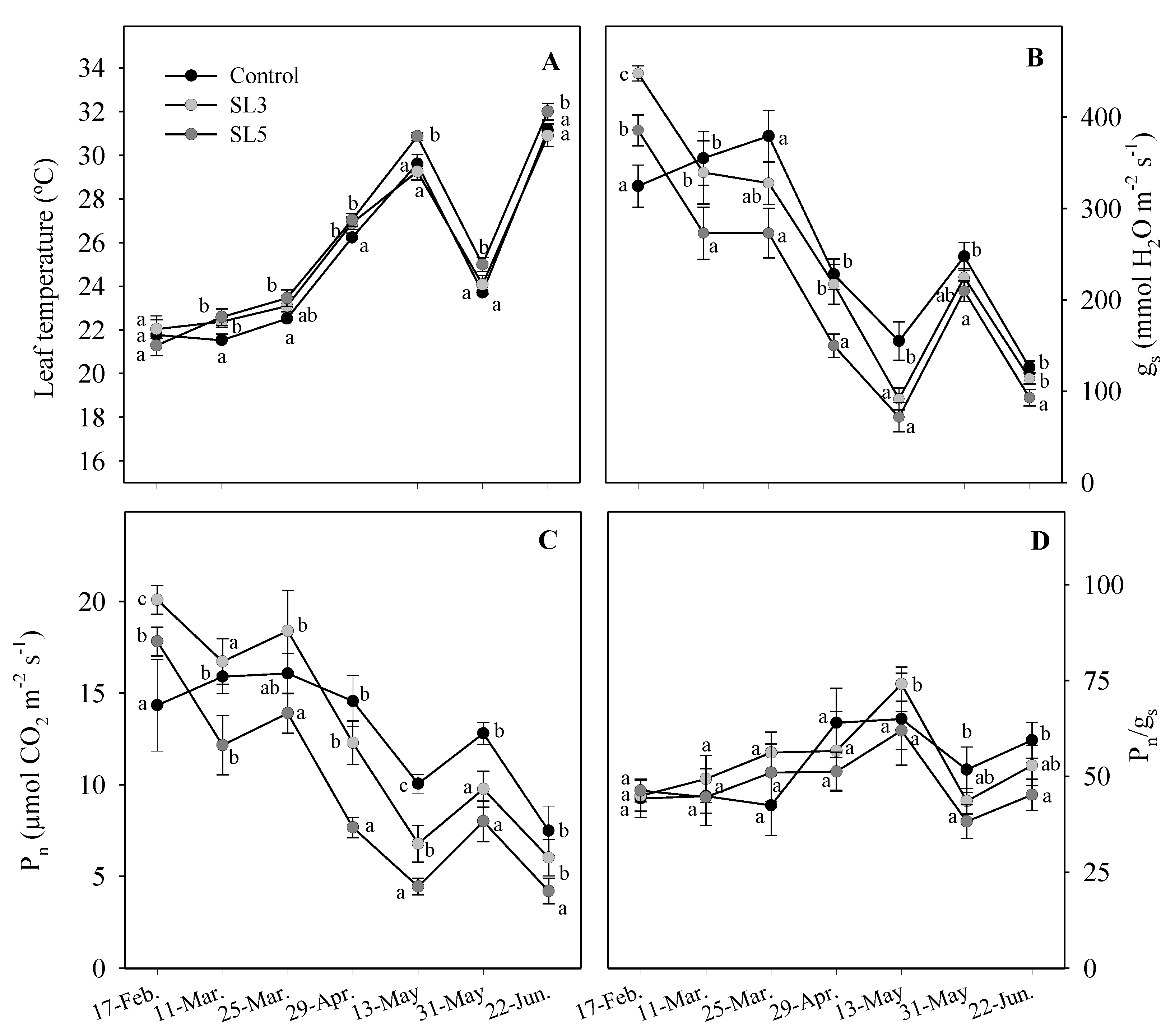
3.5. Leaf Temperature and Gas Exchange
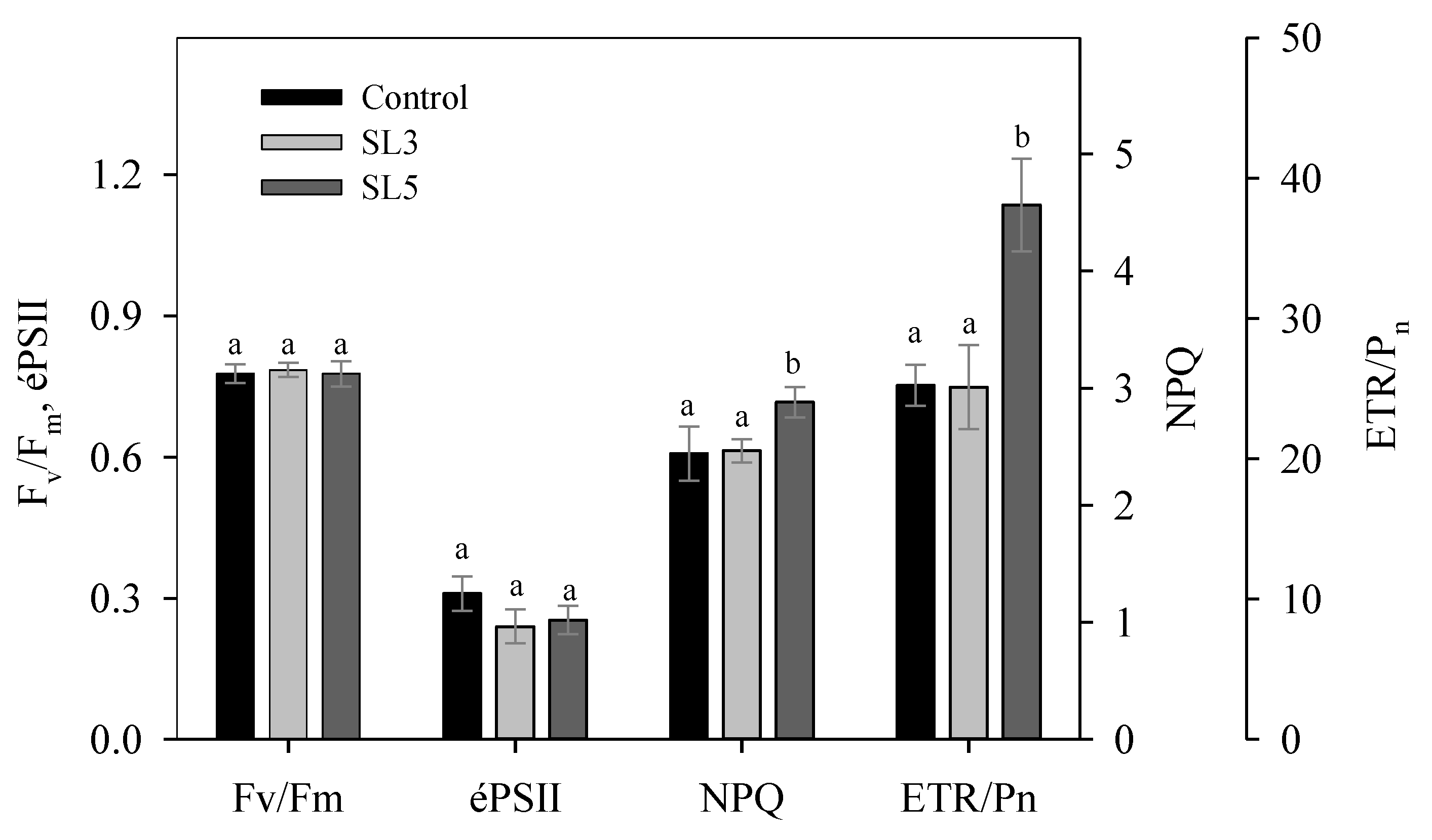
3.6. Chlorophyll Fluorescence
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernández García, I.; Lecina, S.; Ruiz-Sánchez, M.C.; Vera, J.; Conejero, W.; Conesa, M.R.; Domínguez, A.; Pardo, J.J.; Léllis, B.C.; Montesinos, P. Trends and Challenges in Irrigation Scheduling in the Semi-Arid Area of Spain. Water 2020, 12, 785. [Google Scholar] [CrossRef]
- Murtaza, G.; Zia-ur-Rehman, M.; Rashid, I.; Qadir, M. Use of Poor-Quality Water for Agricultural Production. In Research Developments in Saline Agriculture; Dagar, J.C., Yadav, R.K., Sharma, P.C., Eds.; Springer: Singapore, 2019; pp. 769–783. ISBN 9789811358326. [Google Scholar]
- Liu, L.; Wang, T.; Wang, L.; Wu, X.; Zuo, Q.; Shi, J.; Sheng, J.; Jiang, P.; Chen, Q.; Ben-Gal, A. Plant Water Deficit Index-Based Irrigation under Conditions of Salinity. Agric. Water Manag. 2022, 269, 107669. [Google Scholar] [CrossRef]
- Shahzad, B.; Fahad, S.; Tanveer, M.; Saud, S.; Khan, B. Plant Responses and Tolerance to Salt Stress. In Approaches for Enhancing Abiotic Stress Tolerance in Plants; CRC Press: Boca Raton, FL, USA, 2019; pp. 61–78. ISBN 978-1-351-10472-2. [Google Scholar]
- Hossain, M.A.; Kumar, V.; Burritt, D.J.; Fujita, M.; Mäkelä, P.S.A. Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Springer Nature: Cham, Switzerland, 2019; ISBN 978-3-030-27423-8. [Google Scholar]
- Cassaniti, C.; Romano, D.; Flowers, J.T. The Response of Ornamental Plants to Saline Irrigation Water. In Irrigation-Water Management, Pollution and Alternative Strategies; Garcia-Garizabal, I., Abrahao, R., Eds.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0421-6. [Google Scholar]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Álvarez, S.; Gómez-Bellot, M.J.; Castillo, M.; Bañón, S.; Sánchez-Blanco, M.J. Osmotic and Saline Effect on Growth, Water Relations, and Ion Uptake and Translocation in Phlomis purpurea Plants. Environ. Exp. Bot. 2012, 78, 138–145. [Google Scholar] [CrossRef]
- Villarino, G.H.; Mattson, N.S. Assessing Tolerance to Sodium Chloride Salinity in Fourteen Floriculture Species. HortTechnology 2011, 21, 539–545. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Valdés, R.; Miralles, J.; Ochoa, J.; Sánchez-Blanco, M.J.; Bañón Arias, S. Saline Reclaimed Wastewater Can Be Used to Produce Potted Weeping Fig (Ficus benjamina L.) with Minimal Effects on Plant Quality. Span. J. Agric. Res. 2012, 10, 1167. [Google Scholar] [CrossRef]
- Warrington, I.J.; Southward, R.C. Seasonal Frost Tolerance of Hebe Species and Cultivars. N. Z. J. Crop Hortic. Sci. 2010, 23, 437–445. [Google Scholar] [CrossRef][Green Version]
- Morrys, J. Creating a Drought Tolerant Garden. Available online: https://www.mercurynews.com/2014/06/25/creating-a-drought-tolerant-garden/ (accessed on 30 April 2022).
- Bañón, D.; Lorente, B.; Bañón, S.; Ortuño, M.F.; Sánchez-Blanco, M.J.; Alarcón, J.J. Control of Substrate Water Availability Using Soil Sensors and Effects of Water Deficit on the Morphology and Physiology of Potted Hebe Andersonii. Agronomy 2022, 12, 206. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ferrández, T.; Navarro, A.; Bañon, S.; Alarcón, J.J. Effects of Irrigation and Air Humidity Preconditioning on Water Relations, Growth and Survival of Rosmarinus Officinalis Plants during and after Transplanting. J. Plant Physiol. 2004, 161, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Hagen, E.; Nambuthiri, S.; Fulcher, A.; Geneve, R. Comparing Substrate Moisture-Based Daily Water Use and on-Demand Irrigation Regimes for Oakleaf Hydrangea Grown in Two Container Sizes. Sci. Hortic. 2014, 179, 132–139. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated Digital Image Analysis for Rapid and Accurate Measurement of Leaf Area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef] [PubMed]
- Romero-Trigueros, C.; Nortes, P.A.; Pedrero, F.; Mounzer, O.; Alarcón, J.J.; Bayona, J.M.; Nicolás, E. Assessment of the Viability of Using Saline Reclaimed Water in Grapefruit in Medium to Long Term. Span. J. Agric. Res. 2014, 12, 1137–1148. [Google Scholar] [CrossRef]
- Lorente, B.; Zugasti, I.; Ortuño, M.F.; Nortes, P.; Bañón, S.; Hernández, J.A.; Sánchez-Blanco, M.J. Substrate Composition Affects the Development of Water Stress and Subsequent Recovery by Inducing Physiological Changes in Cistus albidus Plants. Plant Physiol. Biochem. 2021, 158, 125–135. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of Arbuscular Mycorrhizae on Photosynthesis and Water Status of Maize Plants under Salt Stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M. PSII Fluorescence Techniques for Measurement of Drought and High Temperature Stress Signal in Crop Plants: Protocols and Applications. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 87–131. ISBN 978-81-322-0807-5. [Google Scholar]
- Akat Saraçoğlu, Ö.; Meriç, M.K.; Tüzel, İ.H.; Kukul Kurttaş, Y.S. Responses of Gerbera Jamesonii Plants to Different Salinity Levels and Leaching Ratios When Grown in Soilless Culture. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 357–379. ISBN 978-3-030-06117-3. [Google Scholar]
- Escalona, A.; Valverde, J.; Dos Santos Coutinho, C.; Salas, M.C. Preliminary Study of the Response of Ornamental Plants to the NaCl Case I. Tagetes erecta L. Acta Hortic. 2012, 937, 955–962. [Google Scholar] [CrossRef]
- Paradiso, R.; De Pascale, S. Morpho-Physiological Response of Garden Roses to Saline Aerosol Stress. Acta Hortic. 2021, 1, 131–138. [Google Scholar] [CrossRef]
- Patil, S.T.; Kadam, U.S.; Mane, M.S.; Mahale, D.M.; Dhekale, J.S. Hydroponic Growth Media (Substrate): A Review. Int. Res. J. Pure Appl. Chem. 2020, 21, 106–113. [Google Scholar] [CrossRef]
- Al-Busaidi, A.; Al-Rawahy, S.; Ahmed, M. Response of Different Tomato Cultivars to Diluted Seawater Salinity. Asian J. Crop Sci. 2009, 1, 77–86. [Google Scholar] [CrossRef]
- Álvarez, S.; Rodríguez, P.; Broetto, F.; Sánchez-Blanco, M.J. Long Term Responses and Adaptive Strategies of Pistacia lentiscus under Moderate and Severe Deficit Irrigation and Salinity: Osmotic and Elastic Adjustment, Growth, Ion Uptake and Photosynthetic Activity. Agric. Water Manag. 2018, 202, 253–262. [Google Scholar] [CrossRef]
- Valdés, R.; Miralles, J.; Ochoa, J.; Bañón, S.; Sánchez-Blanco, M.J. The Number of Emitters Alters Salt Distribution and Root Growth in Potted Gerbera. HortScience 2014, 49, 160–165. [Google Scholar] [CrossRef]
- Bolla, A.; Voyiatzis, D.; Koukourikou-Petridou, M.; Chimonidou, D. Photosynthetic Parameters and Cut-Flower Yield of Rose ‘Eurored’ (H.T.) Are Adversely Affected by Mild Water Stress Irrespective of Substrate Composition. Sci. Hortic. 2010, 126, 390–394. [Google Scholar] [CrossRef]
- Espadafor, M.; Orgaz Rosua, F.; Testi, L.; Lorite, I.J.; González-Dugo, M.P.; Fereres Castiel, E. Responses of Transpiration and Transpiration Efficiency of Almond Trees to Moderate Water Deficits. Sci. Hortic. 2017, 225, 6–14. [Google Scholar] [CrossRef]
- Vivaldi, G.A.; Camposeo, S.; Romero-Trigueros, C.; Pedrero, F.; Caponio, G.; Lopriore, G.; Álvarez, S. Physiological Responses of Almond Trees under Regulated Deficit Irrigation Using Saline and Desalinated Reclaimed Water. Agric. Water Manag. 2021, 258, 107172. [Google Scholar] [CrossRef]
- Omamt, E.N.; Hammes, P.S.; Robbertse, P.J. Differences in Salinity Tolerance for Growth and Water-use Efficiency in Some Amaranth (Amaranthus spp.) Genotypes. N. Z. J. Crop Hortic. Sci. 2006, 34, 11–22. [Google Scholar] [CrossRef]
- Lee, M.K.; van Iersel, M.W. Sodium Chloride Effects on Growth, Morphology, and Physiology of Chrysanthemum (Chrysanthemum × morifolium). HortScience 2008, 43, 1888–1891. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Lao, M.T. Effects of Salinity on Growth, Water-Use Efficiency, and Nutrient Leaching of Three Containerized Ornamental Plants. Commun. Soil Sci. Plant Anal. 2017, 48, 1221–1230. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Lao, M.T. The Effects of Salt Stress on Ornamental Plants and Integrative Cultivation Practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Álvarez, S.; Navarro, A.; Bañón, S.; Sánchez-Blanco, M.J. Regulated Deficit Irrigation in Potted Dianthus Plants: Effects of Severe and Moderate Water Stress on Growth and Physiological Responses. Sci. Hortic. 2009, 122, 579–585. [Google Scholar] [CrossRef]
- Miralles, J.; Valdés, R.; Franco, J.A.; Bañón, S.; Sánchez-Blanco, M.J. Irrigation of Hydrangea with Saline Reclaimed Wastewater: Effects of Fresh Water Flushing. Acta Hortic. 2013, 1000, 229–236. [Google Scholar] [CrossRef]
- De Pascale, S.; Ruggiero, C.; Barbieri, G.; Maggio, A. Physiological Responses of Pepper to Salinity and Drought. J. Am. Soc. Hortic. Sci. 2003, 128, 48–54. [Google Scholar] [CrossRef]
- Nandy, P.; Dasgupta, N.; Das, S. Differential Expression of Physiological and Biochemical Characters of Some Indian Mangroves towards Salt Tolerance. Physiol. Mol. Biol. Plants 2009, 15, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Stützel, H. Biomass Partitioning, Specific Leaf Area, and Water Use Efficiency of Vegetable Amaranth (Amaranthus spp.) in Response to Drought Stress. Sci. Hortic. 2004, 102, 15–27. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Ač, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in Pigment Composition, Photosynthetic Rates and Chlorophyll Fluorescence Images of Sun and Shade Leaves of Four Tree Species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef]
- Orsini, F.; Alnayef, M.; Bona, S.; Maggio, A.; Gianquinto, G. Low Stomatal Density and Reduced Transpiration Facilitate Strawberry Adaptation to Salinity. Environ. Exp. Bot. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Shabala, L.; Mackay, A.; Tian, Y.; Jacobsen, S.-E.; Zhou, D.; Shabala, S. Oxidative Stress Protection and Stomatal Patterning as Components of Salinity Tolerance Mechanism in Quinoa (Chenopodium quinoa). Physiol. Plant. 2012, 146, 26–38. [Google Scholar] [CrossRef]
- Loescher, W.; Chan, Z.; Grumet, R. Options for Developing Salt-Tolerant Crops. HortScience 2011, 46, 1085–1092. [Google Scholar] [CrossRef]
- Tanaka, T.; Kurokawa, C.; Oikawa, S. Leaf Shedding Increases the Photosynthetic Rate of the Canopy in N2-Fixing and Non-N2-Fixing Woody Species. Tree Physiol. 2018, 38, 1903–1911. [Google Scholar] [CrossRef]
- Moura, R.d.S.; Soares, T.L.; Lima, L.K.S.; Gheyi, H.R.; Jesus, O.N.; Coelho Filho, M.A. Salinity-Induced Changes in Biometric, Physiological and Anatomical Parameters of Passiflora edulis Sims Plants Propagated by Different Methods. Arch. Agron. Soil Sci. 2020, 66, 1692–1706. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S.; Aguiniga, L.; Mackay, W. Salinity Tolerance of Lupinus havardii and Lupinus texensis. HortScience 2007, 42, 526–528. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Improvement in Growth, Chlorophyll Pigments and Photosynthetic Performance in Salt-Stressed Plants of Sunflower (Helianthus annuus L.) by Foliar Application of 5-Aminolevulinic Acid. Agrochimica 2011, 55, 94–104. [Google Scholar]
- Khan, M.A.; Shirazi, M.U.; Khan, M.A.; Mujtaba, S.M.; Islam, E.; Mumtaz, S.; Shereen, A.; Ashraf, M.Y. Role of Proline, K/NA Ratio and Chlorophyll Content in Salt Tolerance of Wheat (Triticum aestivum L.). Pak. J. Bot. 2009, 41, 633–638. [Google Scholar]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity Effects on Nitrogen Metabolism in Plants—Focusing on the Activities of Nitrogen Metabolizing Enzymes: A Review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- De Garcia, G.O.; Ferreira, P.A.; dos Santos, D.B.; de Oliveira, F.G.; Miranda, G.V. Saline Stress in Maize Plants: I-Anionic Macronutrients and Their Relationships with Chloride. Rev. Bras. Eng. Agríc. E Ambient. 2005, 9, 26–30. [Google Scholar] [CrossRef]
- Shiyab, S. Effects of NaCl Application to Hydroponic Nutrient Solution on Macro and Micro Elements and Protein Content of Hot Pepper (Capsicum annuum L.). J. Food Agric. Amp Environ. 2011, 9, 350–356. [Google Scholar]
- Sacała, E.; Demczuk, A.; Grzyś, E. The Response of Maize Seedlings to Salt Stress under Increasing Levels of Phosphorus. J. Elem. 2016, 21, 185–194. [Google Scholar] [CrossRef]
- Rahi, T.S.; Singh, B. Salinity Tolerance in Chrysanthemum morifolium. J. Appl. Hortic. 2011, 13, 30–36. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Growth, Sodium, and Potassium Uptake and Translocation in Salt Stressed Tomato. J. Plant Nutr. 2000, 23, 369–379. [Google Scholar] [CrossRef]
- Navarro, A.; Bañón, S.; Conejero, W.; Sánchez-Blanco, M.J. Ornamental Characters, Ion Accumulation and Water Status in Arbutus Unedo Seedlings Irrigated with Saline Water and Subsequent Relief and Transplanting. Environ. Exp. Bot. 2008, 62, 364–370. [Google Scholar] [CrossRef]
- Grieve, C.M.; Poss, J.A. Response of Ornamental Sunflower Cultivars ‘Sunbeam’ and ‘Moonbright’ to Irrigation with Saline Wastewaters. J. Plant Nutr. 2010, 33, 1579–1592. [Google Scholar] [CrossRef]
- Salachna, P.; Zawadzi, A. Response of Ornithogalum saundersiae Bak. to Salinity Stress. Acta Sci. Pol. Hortorum Cultus 2016, 15, 123–134. [Google Scholar]
- Acosta-Motos, J.-R.; Diaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sanchez-Blanco, M.J.; Hernández, J.A. Physiological and Biochemical Mechanisms of the Ornamental Eugenia myrtifolia L. Plants for Coping with NaCl Stress and Recovery. Planta 2015, 242, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Fornes, F.; Belda, R.M.; Carrión, C.; Noguera, V.; García-Agustín, P.; Abad, M. Pre-Conditioning Ornamental Plants to Drought by Means of Saline Water Irrigation as Related to Salinity Tolerance. Sci. Hortic. 2007, 113, 52–59. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Wang, S.; Fritz, E.; Hüttermann, A.; Altman, A. Effects of NaCl on Shoot Growth, Transpiration, Ion Compartmentation, and Transport in Regenerated Plants of Populus euphratica and Populus tomentosa. Can. J. For. Res. 2003, 33, 967–975. [Google Scholar] [CrossRef]
- Cuin, T.A.; Tian, Y.; Betts, S.A.; Chalmandrier, R.; Shabala, S.; Cuin, T.A.; Tian, Y.; Betts, S.A.; Chalmandrier, R.; Shabala, S. Ionic Relations and Osmotic Adjustment in Durum and Bread Wheat under Saline Conditions. Funct. Plant Biol. 2009, 36, 1110–1119. [Google Scholar] [CrossRef]
- Paz, R.C.; Rocco, R.A.; Reinoso, H.; Menéndez, A.B.; Pieckenstain, F.L.; Ruiz, O.A. Comparative Study of Alkaline, Saline, and Mixed Saline–Alkaline Stresses with Regard to Their Effects on Growth, Nutrient Accumulation, and Root Morphology of Lotus tenuis. J. Plant Growth Regul. 2012, 31, 448–459. [Google Scholar] [CrossRef]
- Cassaniti, C.; Leonardi, C.; Flowers, T.J. The Effects of Sodium Chloride on Ornamental Shrubs. Sci. Hortic. 2009, 122, 586–593. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Khan, A.H.; Azmi, A.R. Cell Membrane Stability and Its Relation with Some Physiological Processes in Wheat. Acta Agron. Hung. 1992, 41, 183. [Google Scholar]
- Camberato, D.M.; Lopez, R.; Mickelbart, M. PH and Electrical Conductivity Measurements in Soilless Substrates. Purdue Univ. Ext. 2009, HO-237-W, 1–7. [Google Scholar]
- García-Caparrós, P.; Llanderal, A.; Pestana, M.; Correia, P.J.; Lao, M.T. Tolerance Mechanisms of Three Potted Ornamental Plants Grown under Moderate Salinity. Sci. Hortic. 2016, 201, 84–91. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Nortes, P.A.; Sánchez-Blanco, M.J.; Ortuño, M.F. Sensitivity of Thermal Imaging and Infrared Thermometry to Detect Water Status Changes in Euonymus Japonica Plants Irrigated with Saline Reclaimed Water. Biosyst. Eng. 2015, 133, 21–32. [Google Scholar] [CrossRef]
- Sirault, X.R.R.; James, R.A.; Furbank, R.T. A New Screening Method for Osmotic Component of Salinity Tolerance in Cereals Using Infrared Thermography. Funct. Plant Biol. FPB 2009, 36, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Urrestarazu, M. Infrared Thermography Used to Diagnose the Effects of Salinity in a Soilless Culture. Quant. InfraRed Thermogr. J. 2013, 10, 1–8. [Google Scholar] [CrossRef][Green Version]
- Azevedo Neto, A.D.d.; Prisco, J.T.; Enéas-Filho, J.; Lacerda, C.F.d.; Silva, J.V.; da Costa, P.H.A.; Gomes-Filho, E. Effects of Salt Stress on Plant Growth, Stomatal Response and Solute Accumulation of Different Maize Genotypes. Braz. J. Plant Physiol. 2004, 16, 31–38. [Google Scholar] [CrossRef]
- Long, R.W.; D’Antonio, C.M.; Dudley, T.L.; Hultine, K.R. Variation in Salinity Tolerance and Water Use Strategies in an Introduced Woody Halophyte (Tamarix spp.). J. Ecol. 2021, 109, 3807–3817. [Google Scholar] [CrossRef]
- Sussmilch, F.C.; Roelfsema, M.R.G.; Hedrich, R. On the Origins of Osmotically Driven Stomatal Movements. New Phytol. 2019, 222, 84–90. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S.; Wang, Y.-T. Impact of Drought and Temperature on Growth and Leaf Gas Exchange of Six Bedding Plant Species Under Greenhouse Conditions. HortScience 2006, 41, 1408–1411. [Google Scholar] [CrossRef]
- Passioura, J.B. Plant–Water Relations. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd: Chichester, UK, 2010; Volume 2, ISBN 978-0-470-01590-2. [Google Scholar]
- Centritto, M.; Brilli, F.; Fodale, R.; Loreto, F. Different Sensitivity of Isoprene Emission, Respiration and Photosynthesis to High Growth Temperature Coupled with Drought Stress in Black Poplar (Populus nigra) Saplings. Tree Physiol. 2011, 31, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Dong, W.Y.; He, K.N.; Yu, Y.; Tan, G.D.; Han, L.; Dong, M.; Zhang, Y.Y.; Zhang, D.; Li, A.Z.; et al. NaCl Salinity-Induced Changes in Water Status, Ion Contents and Photosynthetic Properties of Shepherdia argentea (Pursh) Nutt. Seedlings. Plant Soil Environ. 2010, 56, 325–332. [Google Scholar] [CrossRef]
- Ammar, A.; Aissa, I.B.; Gouiaa, M.; Mars, M. Fig (Ficus carica L.) Vulnerability to Climate Change: Combined Effects of Water Stress and High Temperature on Ecophysiological Behaviour of Different Cultivars. S. Afr. J. Bot. 2022, 147, 482–492. [Google Scholar] [CrossRef]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Cifre, J.; Mariano Escalona, J.; Galmés, J.; Gulías, J.; Lefi, E.-K.; Florinda Martínez-Cañellas, S.; Teresa Moreno, M.; Ribas-Carbó, M.; et al. Understanding Down-Regulation of Photosynthesis under Water Stress: Future Prospects and Searching for Physiological Tools for Irrigation Management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Saibo, N.J.M.; Lourenço, T.; Oliveira, M.M. Transcription Factors and Regulation of Photosynthetic and Related Metabolism under Environmental Stresses. Ann. Bot. 2009, 103, 609–623. [Google Scholar] [CrossRef]
- Mittal, S.; Kumari, N.; Sharma, V. Differential Response of Salt Stress on Brassica Juncea: Photosynthetic Performance, Pigment, Proline, D1 and Antioxidant Enzymes. Plant Physiol. Biochem. 2012, 54, 17–26. [Google Scholar] [CrossRef]
- Veiga, T.A.M.; Silva, S.C.; Francisco, A.-C.; Filho, E.R.; Vieira, P.C.; Fernandes, J.B.; Silva, M.F.G.F.; Müller, M.W.; Lotina-Hennsen, B. Inhibition of Photophosphorylation and Electron Transport Chain in Thylakoids by Lasiodiplodin, a Natural Product from Botryosphaeria Rhodina. J. Agric. Food Chem. 2007, 55, 4217–4221. [Google Scholar] [CrossRef]
- Omoto, E.; Taniguchi, M.; Miyake, H. Effects of Salinity Stress on the Structure of Bundle Sheath and Mesophyll Chloroplasts in NAD-Malic Enzyme and PCK Type C4 Plants. Plant Prod. Sci. 2010, 13, 169–176. [Google Scholar] [CrossRef]
- Veatch-Blohm, M.E.; Malinowski, M.; Keefer, D. Leaf Water Status, Osmotic Adjustment and Carbon Assimilation in Colored Calla Lilies in Response to Saline Irrigation. Sci. Hortic. 2012, 144, 65–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, J.; Wu, X.; Dong, L. Na+/K+ Balance and Transport Regulatory Mechanisms in Weedy and Cultivated Rice (Oryza sativa L.) Under Salt Stress. BMC Plant Biol. 2018, 18, 375. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Sánchez-Blanco, M.J. Long-Term Effect of Salinity on Plant Quality, Water Relations, Photosynthetic Parameters and Ion Distribution in Callistemon citrinus. Plant Biol. 2013, 16, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.W.F.; Harrison-Murray, R.S.; Atkinson, C.J.; Judd, H.L. Regulated Deficit Irrigation—A Means to Control Growth in Woody Ornamentals. J. Hortic. Sci. Biotechnol. 2006, 81, 435–443. [Google Scholar] [CrossRef]
- Chaves, M.M.; Miguel Costa, J.; Madeira Saibo, N.J. Chapter 3—Recent Advances in Photosynthesis Under Drought and Salinity. In Advances in Botanical Research; Turkan, I., Ed.; Plant Responses to Drought and Salinity Stress; Academic Press: Cambridge, USA, 2011; Volume 57, pp. 49–104. [Google Scholar]
- Najar, R.; Aydi, S.; Sassi-Aydi, S.; Zarai, A.; Abdelly, C. Effect of Salt Stress on Photosynthesis and Chlorophyll Fluorescence in Medicago truncatula. Plant Biosyst. 2019, 153, 88–97. [Google Scholar] [CrossRef]
- Da Silva, E.; Ribeiro, R.; Ferreira-Silva, S.; Viégas, R.; Silveira, J. Salt Stress Induced Damages on the Photosynthesis of Physic Nut Young Plants. Sci. Agric. 2011, 68, 62–68. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zou, Y.-N.; He, X.-H. Contributions of Arbuscular Mycorrhizal Fungi to Growth, Photosynthesis, Root Morphology and Ionic Balance of Citrus Seedlings under Salt Stress. Acta Physiol. Plant. 2010, 32, 297–304. [Google Scholar] [CrossRef]
- Akram, M.S.; Ashraf, M. Exogenous Application of Potassium Dihydrogen Phosphate Can Alleviate the Adverse Effects of Salt Stress on Sunflower. J. Plant Nutr. 2011, 34, 1041–1057. [Google Scholar] [CrossRef]
- Saleem, A.; Ashraf, M.; Akram, N.A. Salt (NaCl)-Induced Modulation in Some Key Physio-Biochemical Attributes in Okra (Abelmoschus esculentus L.). J. Agron. Crop Sci. 2011, 197, 202–213. [Google Scholar] [CrossRef]
- Abdeshahian, M.; Nabipour, M.; Meskarbashee, M. Chlorophyll Fluorescence as Criterion for the Diagnosis Salt Stress in Wheat (Triticum aestivum) Plants. Int. J. Nucl. Quantum Eng. 2010, 4, 839–841. [Google Scholar] [CrossRef]
- Al-Taweel, K.; Iwaki, T.; Yabuta, Y.; Shigeoka, S.; Murata, N.; Wadano, A. A Bacterial Transgene for Catalase Protects Translation of D1 Protein during Exposure of Salt-Stressed Tobacco Leaves to Strong Light. Plant Physiol. 2007, 145, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Peñuelas, J.; Llusià, J.; Asensio, D.; Estiarte, M. Effects of Long-Term Experimental Night-Time Warming and Drought on Photosynthesis, Fv/Fm and Stomatal Conductance in the Dominant Species of a Mediterranean Shrubland. Acta Physiol. Plant. 2009, 31, 729–739. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Yamakawa, H.; Itoh, S. Dissipation of Excess Excitation Energy by Drought-Induced Nonphotochemical Quenching in Two Species of Drought-Tolerant Moss: Desiccation-Induced Acceleration of Photosystem II Fluorescence Decay. Biochemistry 2013, 52, 4451–4459. [Google Scholar] [CrossRef]
- Chen, C.-I.; Lin, K.-H.; Huang, M.-Y.; Yang, C.-K.; Lin, Y.-H.; Hsueh, M.-L.; Lee, L.-H.; Wang, C.-W. Photosynthetic Physiology Comparisons between No Tillage and Sod Culture of Citrus Farming in Different Seasons under Various Light Intensities. Agronomy 2021, 11, 1805. [Google Scholar] [CrossRef]
- D’Ambrosio, N.; Arena, C.; De Santo, A.V. Temperature Response of Photosynthesis, Excitation Energy Dissipation and Alternative Electron Sinks to Carbon Assimilation in Beta vulgaris L. Environ. Exp. Bot. 2006, 55, 248–257. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, L.; Virzo de Santo, A. Photosynthesis and Photoprotective Strategies in Laurus nobilis L. and Quercus ilex L. under Summer Drought and Winter Cold. Plant Biosyst. 2008, 142, 472–479. [Google Scholar] [CrossRef]
| Measurements | Treatments | ||
|---|---|---|---|
| Control | SL3 | SL5 | |
| pH | 7.38 | 7.59 | 7.60 |
| EC (dS m−1) | 1.81 | 3.32 | 4.88 |
| NO3− (mg L−1) | 280.23 | 272.45 | 240.93 |
| NH4+ (mg L−1) | 1.89 | 1.72 | 1.81 |
| H2PO4− (mg L−1) | 17.09 | 15.60 | 14.23 |
| K+ (mg L−1) | 56.06 | 55.13 | 59.26 |
| Ca2+ (mg L−1) | 125.72 | 117.93 | 116.50 |
| Mg2+ (mg L−1) | 57.18 | 53.90 | 53.37 |
| SO42− (mg L−1) | 258.99 | 258.72 | 258.17 |
| Cl− (mg L−1) | 245.28 | 650.55 | 1220.95 |
| Na+ (mg L−1) | 161.37 | 444.00 | 781.57 |
| HCO3− (mg L−1) | 105.11 | 111.23 | 108.99 |
| B3+ (mg L−1) | 0.61 | 0.55 | 0.52 |
| Mn2+ (mg L−1) | 0.45 | 0.43 | 0.41 |
| Fe3+ (mg L−1) | 0.07 | 0.05 | 0.06 |
| Zn2+ (mg L−1) | 0.07 | 0.05 | 0.05 |
| Cu2+ (mg L−1) | 0.09 | 0.08 | 0.08 |
| Control | SL3 | SL5 | |
|---|---|---|---|
| Substrate VWC (%) | 49.04 a | 50.02 a | 49.03 a |
| Total applied water (L pot−1) | 22.95 | 17.20 | 12.25 |
| ET (mL pot−1 day−1) | 117.86 | 88.32 | 62.89 |
| WUE (g L−1) | 3.27 b | 3.50 c | 4.31 a |
| Measurements | Control | SL3 | SL5 |
|---|---|---|---|
| Growth index (cm) | 42.65 ± 1.83 a | 38.9 ± 1.04 b | 36.83 ± 1.11 c |
| Plant height (cm) | 42.25 ± 2.12 a | 40.27 ± 2.25 a | 34.92 ± 2.09 b |
| Plant width (cm) | 39.50 ± 1.64 a | 36.17 ± 1.49 b | 33.33 ± 1.34 c |
| No. of shoots per plant | 10.00 ± 0.87 a | 9.67 ± 0.46 a | 8.83 ± 0.33 b |
| No. of inflorescences per plant | 24.07 ± 1.33 a | 26.33 ± 1.94 a | 18.83 ± 1.94 b |
| Total plant dry weight (g) | 74.97 ± 6.46 a | 60.22 ± 4.11 b | 52.77 ± 3.14 c |
| Leaf dry weight (g) | 29.38 ± 0.48 a | 27.19 ± 0.91 b | 23.2 ± 1.63 c |
| Stem dry weight (g) | 20.09 ± 1.65 a | 17.99 ± 1.22 b | 15.76 ± 1.03 c |
| Root dry weight (g) | 25.5 ± 2.63 a | 15.05 ± 1.69 b | 13.8 ± 1.14 b |
| Root to shoot ratio | 0.52 ± 0.06 a | 0.33 ± 0.04 b | 0.35 ± 0.04 b |
| No. of leaves per plant | 336.0 ± 4.33 a | 287.4 ± 2.97 b | 269.1 ± 3.11 b |
| Average blade area (cm2) | 10.38 ± 0.74 a | 9.15 ± 0.86 a | 7.23 ± 0.77 b |
| Leaf area (dm2) | 34.86 ± 2.73 a | 26.3 ± 2.43 b | 19.45 ± 2.82 c |
| Specific leaf area (cm2 g−1) | 118.7 ± 7.23 a | 96.8 ± 4.17 b | 83.8 ± 6.32 c |
| Stomatal density (no. mm−2) | 318 ± 6.86 a | 326 ± 4.54 a | 331 ± 6.36 a |
| Stomatal pore area (µm2) | 214 ± 3.99 a | 206 ± 6.39 a | 210 ± 5.08 a |
| Leaf chlorophyll content (mg g−1) | 0.75 ± 0.04 a | 0.71 ± 0.06 ab | 0.68 ± 0.03 b |
| Element (mg g−1) | Plant Organ | Control | SL3 | SL5 |
|---|---|---|---|---|
| N | Leaf | 20.49 ± 1.98 a | 21.38 ± 2.15 a | 19.00 ± 1.35 a |
| Shoot | 12.68 ± 1.01 a | 13.47 ± 0.98 a | 13.05 ± 1.11 a | |
| Root | 13.72 ± 1.23 a | 14.32 ± 1.49 a | 13.66 ± 1.07 a | |
| P | Leaf | 1.86 ± 0.13 a | 1.99 ± 0.17 a | 1.81 ± 0.21 a |
| Shoot | 0.77 ± 0.06 a | 1.04 ± 0.06 a | 0.95 ± 0.05 a | |
| Root | 1.45 ± 0.14 b | 2.12 ± 0.16 a | 1.76 ± 0.19 ab | |
| K+ | Leaf | 36.15 ± 2.90 a | 18.74 ± 1.61 b | 17.15 ± 1.94 b |
| Shoot | 7.82 ± 0.87 a | 6.19 ± 0.55 b | 4.07 ± 0.73 c | |
| Root | 13.96 ± 1.15 a | 9.81 ± 0.98 b | 7.69 ± 0.76 c | |
| Ca2+ | Leaf | 16.68 ± 1.54 a | 4.32 ± 0.53 b | 4.39 ± 0.39 b |
| Shoot | 10.83 ± 1.03 a | 2.62 ± 0.24 b | 2.05 ± 0.22 b | |
| Root | 3.93 ± 0.38 a | 1.12 ± 0.08 b | 0.85 ± 0.12 b | |
| Mg2+ | Leaf | 4.36 ± 0.49 a | 1.01 ± 0.08 b | 0.89 ± 0.09 b |
| Shoot | 5.28 ± 0.82 a | 0.93 ± 0.18 b | 0.62 ± 0.12 b | |
| Root | 2.58 ± 0.45 a | 0.53 ± 0.12 b | 0.34 ± 0.08 b | |
| Cl− | Leaf | 12.11 ± 0.21 c | 33.56 ± 2.98 b | 53.30 ± 5.19 a |
| Shoot | 29.49 ± 1.87 b | 43.33 ± 3.51 a | 45.76 ± 4.61 a | |
| Root | 5.21 ± 0.81 c | 15.96 ± 1.81 b | 27.46 ± 4.89 a | |
| Na+ | Leaf | 2.80 ± 0.29 c | 4.38 ± 0.68 b | 9.47 ± 1.06 a |
| Shoot | 17.08 ± 1.41 a | 14.84 ± 1.33 b | 14.67 ± 0.98 b | |
| Root | 2.63 ± 0.18 c | 5.20 ± 0.79 b | 8.16 ± 0.73 a | |
| K+/Na+ | Leaf | 12.91 ± 1.11 a | 4.28 ± 0.18 b | 1.81 ± 0.26 c |
| Shoot | 0.46 ± 0.03 a | 0.42 ± 0.04 a | 0.28 ± 0.03 b | |
| Root | 5.31 ± 0.73 a | 1.89 ± 0.20 b | 0.94 ± 0.14 c |
| Measurements | Control | SL3 | SL5 |
|---|---|---|---|
| CE1:2 (dS m−1) | 1.75 ± 0.21c | 2.52 ± 0.26b | 3.27 ± 0.29a |
| pH | 5.42 ± 0.52a | 5.22 ± 0.47a | 5.66 ± 0.39a |
| Na+ (mg g−1) | 11.5 ± 1.02c | 16.9 ± 1.39b | 21.7 ± 2.02a |
| Cl− (mg g−1) | 14.7 ± 1.23c | 29.5 ± 2.15b | 34.6 ± 2.98a |
| K+ (mg g−1) | 1.8 ± 0.17a | 1.5 ± 0.16ab | 1.3 ± 0.11b |
| NO3− (mg g−1) | 4.7 ± 0.37a | 3.3 ± 0.29b | 1.4 ± 0.14c |
| Ca2+ (mg g−1) | 11.2 ± 1.09a | 8.6 ± 0.99b | 7.9 ± 0.76b |
| SO42− (mg g−1) | 14.5 ± 1.87a | 9.1 ± 1.02b | 5.3 ± 0.42c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bañón, D.; Alarcón, J.J.; Sánchez-Blanco, M.J.; Ortuño, M.F.; Bañón, S.; Lorente, B.; Ochoa, J. Response of Potted Hebe andersonii to Salinity under an Efficient Irrigation Management. Agronomy 2022, 12, 1696. https://doi.org/10.3390/agronomy12071696
Bañón D, Alarcón JJ, Sánchez-Blanco MJ, Ortuño MF, Bañón S, Lorente B, Ochoa J. Response of Potted Hebe andersonii to Salinity under an Efficient Irrigation Management. Agronomy. 2022; 12(7):1696. https://doi.org/10.3390/agronomy12071696
Chicago/Turabian StyleBañón, Daniel, Juan José Alarcón, María Jesús Sánchez-Blanco, María Fernanda Ortuño, Sebastián Bañón, Beatriz Lorente, and Jesús Ochoa. 2022. "Response of Potted Hebe andersonii to Salinity under an Efficient Irrigation Management" Agronomy 12, no. 7: 1696. https://doi.org/10.3390/agronomy12071696
APA StyleBañón, D., Alarcón, J. J., Sánchez-Blanco, M. J., Ortuño, M. F., Bañón, S., Lorente, B., & Ochoa, J. (2022). Response of Potted Hebe andersonii to Salinity under an Efficient Irrigation Management. Agronomy, 12(7), 1696. https://doi.org/10.3390/agronomy12071696






