Impacts of House Mice on Sustainable Fodder Storage in Australia
Abstract
1. Introduction
2. Potential Impacts of Rodents on Fodder Storage
2.1. Disease Risk
- Botulism is a disease caused by the botulinum toxin, which is produced by the bacterium Clostridium botulinum [19]. C. botulinum spores are common in the soil, and also in the gut of healthy normal cattle and other animals. Spores are the dormant form of the organism. Only the actively growing or “vegetative” C. botulinum bacteria produce botulinum toxin, and it is the toxin that produces the disease. C. botulinum spores will only germinate and grow under anaerobic conditions. Botulism outbreaks can occur in intensively fed beef and dairy cattle when the feed is contaminated by the botulism bacteria growing in rotting animals (e.g., dead mice) or vegetable material in the stored feed.
- Rodents are carriers of spirochetes of the genus Leptospira throughout the world and are important reservoirs of infection for humans and domestic animals [14]. It can lead to foetal abortion and stillbirths in livestock. Two of these strains (L. hardjo and L. pomona) are known to cause abortion in cattle, and there is a vaccine available to prevent this [20]. Humans acquire infection through consumption of food or water that is contaminated by rodents or by contact through skin or mucous membranes with soil or water (or contaminated hay or fodder) that is contaminated by rodent urine. Handling of dead infected rodents may also form a source of infection [14].
- Another significant disease is lymphocytic choriomeningitis virus (LCMV). It is a rodent-borne virus that can cause lymphocytic choriomeningitis (LCM) in humans, and causes flu-like symptoms through to meningitis and encephalitis [14]. It can also cause intrauterine infections leading to foetal death. Infections have occurred in Australia, and there was a report of a farmer that contracted the disease during the 2021 mouse plague in NSW (ABC news: https://www.abc.net.au/news/2021-04-04/mouse-plague-farmer-contracts-lymphocytic-choriomeningitis/100037256, accessed 16 November 2021). LCM is not a notifiable disease in Australia, so the full extent is unknown. It is likely the disease is spread through breathing air (dust from contaminated hay) that is contaminated with mouse excrements.
2.2. Physical Damage
3. Mouse Control Option
3.1. Rodenticides
3.2. Physical Management
- Robust plastic coverings: There are a range of silage tarps and hay harvest rolls on the market. Large tarps could be used to cover large stacks of hay or fodder, and individual rolls could also be covered. It is critical that these are waterproof and airtight. This would protect the bales, but importantly, an airtight covering would hopefully mean that mice do not smell the hay or fodder inside and gnaw through the plastic. Heavy-duty plastic is needed to provide a robust defence against mice because current silage wraps provide little defence against infiltration from mice.
- Burying bales: Many growers are familiar with the practice of burying silage. The advantage of this approach is that they could then be covered with large tarps or buried under soil to make it difficult for mice to dig down to the fodder. Subsequent use of the buried fodder can be logistically problematic. Fodder buried in pits needs to be removed from the pit as soon as the pit is opened. Uncovered fodder in pits is at risk of weather damage associated with significant rainfall events.
- Mouse-proof fencing around storage areas: Small metal fences could be used as a physical barrier around fodder storages. The barriers would need to be 30–50 cm high with a folded lip at the top to prevent mice from climbing over the fence. The fence would need to be set into a concrete strip or buried and back-filled with compressed road base or blue stone (out to 30–50 cm) so that mice could not dig burrows. This might be feasible in areas where hay or fodder is stored on a permanent basis. The weak link in this system is the construction of a gate, or similar, to allow access by heavy machinery, which needs to be mouse-proof.
- Build mouse-proof structures: Mouse-proof structures can be constructed with collars around the base to prevent mice climbing up onto the structure. A major limitation of this is that mice are excellent climbers, and there would be a substantial amount of engineering required to build structures over a large area.
- Shipping containers: Store hay and fodder in mouse-proof shipping containers; however, the capacity and expense of containers are limiting factors.
- Move hay/fodder to areas not affected by mice: Place hay and fodder on trucks and move it to areas where mice are not a problem to be stored, or sell it, so that other hay or fodder could be bought when needed.
3.3. Other Strategies
- Trapping: There are many types of traps on the market, from single-capture spring traps to gas-fired piston multiple-capture traps, through to various homemade traps. Many traps are required in order to have an effect on the mouse population and appear to be ineffective when mouse populations are extremely high. In reality, these traps capture only a small proportion of the mice, especially during a mouse plague, and so are not really practical or effective in this situation. Trapping does provide an indication of a change in mouse activity, so can be a useful method for monitoring mouse populations.
- Fumigants: Might be of use in sealed or covered bales/rolls but need to be tested. As mice would die inside bales or rolls, there is still an issue of carcass disposal, because they still present a disease risk.
- Ultrasonic/magnetic devices: These might be of use only in and around hay sheds or other sheds. There are several ultrasonic and/or magnetic devices that emit electrical pulses at a range of frequencies. They might have a short-term effect on the rodents, and probably only shift the animals to another location. There are no published studies demonstrating the long-term benefit of these machines.
- Cats: Cats are not recommended because of the high risk of Toxoplasmosis transfer to livestock. Cats might prey on rodents, increasing predation risk (meaning rodents are less likely to move away from cover/shelter), but the disease transfer risk to livestock is simply too great.
4. Caste Study—Survey of Mouse Damage to Fodder Storage
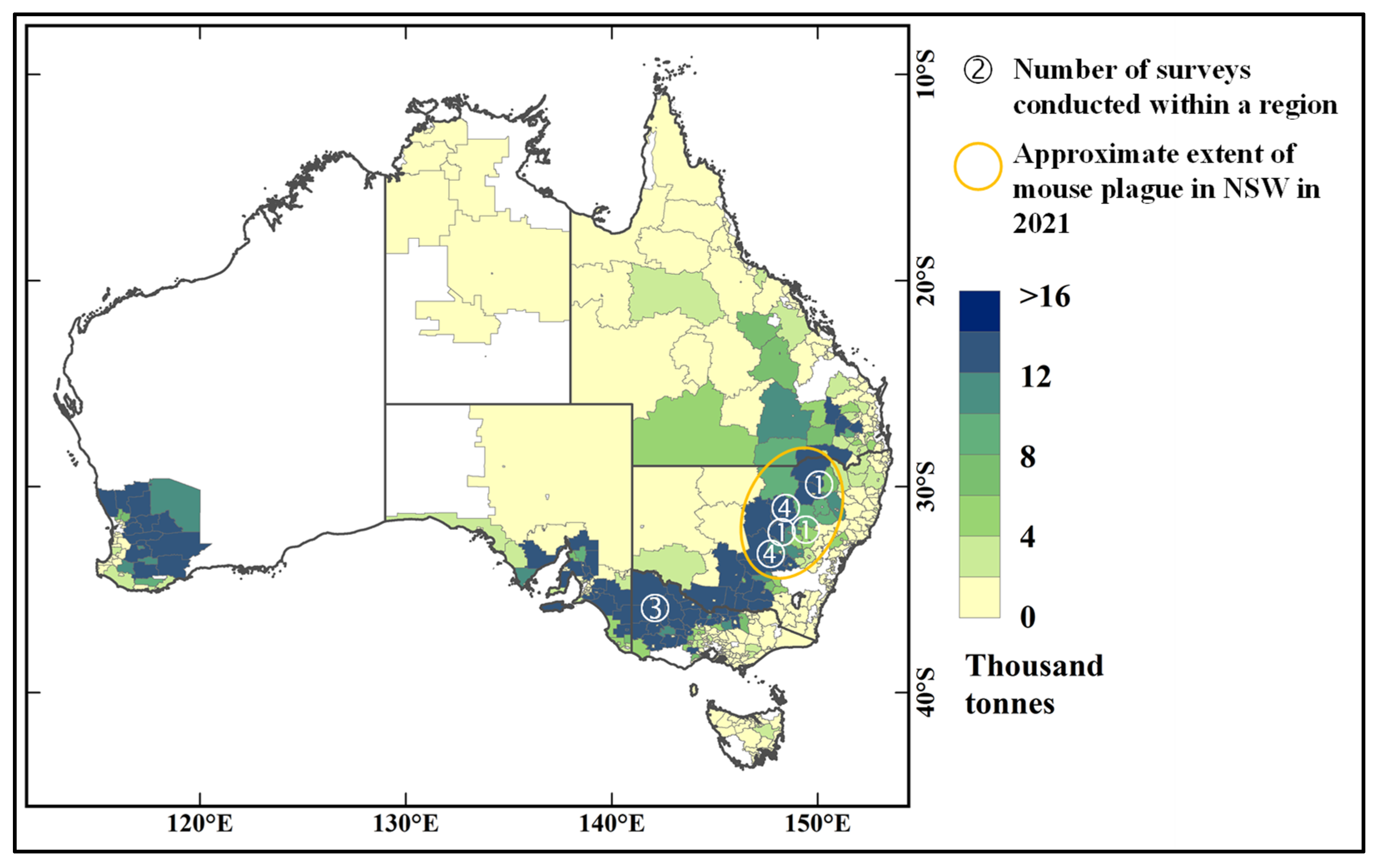
4.1. Design of Survey
4.2. Nature of Mouse Damage
4.3. Social and Economic Impacts
- Direct loss to fodder: The magnitude and cost of the impact was not well quantified. Some growers had very high levels of damage (mean loss = ~35%, up to 70% in some cases, Table 2). Bales of hay/fodder were estimated to be worth AUD 50 or AUD 60 a bale, and up to AUD 400/bale. Most growers do not plan, or were not planning, on selling their hay at the time of the survey, so it is difficult to determine the economic loss associated with this. Additional costs will be incurred when they run out of hay/fodder and need to purchase more. Bales were falling apart because mice were infesting them, and they were not able to move them. Most hay was expected to be used within three years, but because of the mice, they must be used straight away.
- Poor efficiency of hay: Some growers were doling out twice as much hay as normal for their cattle and sheep as they are fussy eaters avoiding hay contaminated by mice, meaning they will go through their reserves twice as fast.
- Direct losses to other parts of the farming enterprise: One respondent was concerned that mice were causing all sorts of problems with his enterprise, in sheds, in the house, and in his machinery. He said he had to clean the steering wheel each time he sits in his tractor (concerned about disease) and mice had infested his seat. Another respondent recently lost an AUD 300,000 tractor to rats and mice (fire started after gnawing on electrical wires). There was also additional damage by mice to bags of pasture seed, which took extra time and labour to clean, and needed to be replaced. There were also damages to sheds and other machinery. Mice were the major problem in the grain-production component of their enterprises, and all growers were expending considerable resources in managing the mouse problem in their crops.
- Cost of mouse control: Estimates of the cost of mouse control were not well quantified. Some growers spent a few hundred dollars on managing mice through to several thousand. The main cost was for the purchase of mouse baits (principally zinc phosphide baits) (mean cost of rodenticide baits = AUD 11,250 per respondent, Table 2). Additional costs were the time required to manage the mouse problem, which ranged from one day a month to one or two days a week (average cost of ~AUD 3000 over a 6-month period, Table 2). Some growers were worried about the amount of time they were spending on managing the mouse problem when they could be doing other things around the farm that had direct economic benefit.
4.4. Concerns about Disease and Contamination
4.5. Management Practices Undertaken
5. General Discussion
5.1. Economic Damage
5.2. Disease Risk
5.3. Managing Mice in Fodder
- What mouse control strategies should be conducted in low mouse years (normal phase)? What routine practices should be recommended each year, especially when numbers are not high? Is there any benefit of controlling mice in “low” years so that their impact is not as great in “high” years?
- What mouse control strategies should be conducted when mouse populations are increasing or in “high” mouse years (consideration of preventative measures versus emergency response)?
- How effective are a range of different management practices? What are the benefits of covering bales or fodder rolls, burying, using other physical barriers, baiting, or burning fodder or hay bales?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, P.R.; Singleton, G.R. Impacts of house mice on crops in Australia—Costs and damage. In Human Conflicts with Wildlife: Economic Considerations; Clark, L., Hone, J., Shivik, J.A., Watkins, R.A., VerCauteren, K.C., Yoder, J.K., Eds.; National Wildlife Research Center: Fort Collins, CO, USA, 2002; pp. 48–58. [Google Scholar]
- Caughley, J.; Monamy, V.; Heiden, K. Impact of the 1993 Mouse Plague; GRDC: Canberra, Australia, 1994. [Google Scholar]
- Mutze, G.J. The 1993 strychnine baiting program for mouse control in South Australian grain crops. I. Efficacy. Wildl. Res. 1998, 25, 533–546. [Google Scholar] [CrossRef]
- Singleton, G.R.; Brown, P.R.; Jacob, J.; Aplin, K.P.; Sudarmaji. Unwanted and unintended effects of culling: A case for ecologically-based rodent management. Integr. Zool. 2007, 2, 247–259. [Google Scholar] [CrossRef]
- Singleton, G.R.; Brown, P.R.; Pech, R.P.; Jacob, J.; Mutze, G.J.; Krebs, C.J. One hundred years of eruptions of house mice in Australia—A natural biological curio. Biol. J. Linn. Soc. 2005, 84, 617–627. [Google Scholar] [CrossRef]
- Singleton, G.R.; Redhead, T.D. House mouse plagues. In Mediterranean Landscapes in Australia: Mallee Ecosystems and Their Management; Noble, J.C., Bradstock, R.A., Eds.; CSIRO: Melbourne, Australia, 1989; pp. 418–433. [Google Scholar]
- Gabriel, S.I.; Stevens, M.I.; Mathias, M.d.L.; Searle, J.B. Of mice and ‘convicts’: Origin of the Australian house mouse, Mus musculus. PLoS ONE 2011, 6, e28622. [Google Scholar] [CrossRef][Green Version]
- Brown, P.R.; Singleton, G.R.; Pech, R.P.; Hinds, L.A.; Krebs, C.J. Rodent outbreaks in Australia: Mouse plagues in cereal crops. In Rodent Outbreaks: Ecology and Impacts; Singleton, G.R., Belmain, S.R., Brown, P.R., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2010; pp. 225–238. [Google Scholar]
- Singleton, G.R.; Krebs, C.J. The secret world of wild mice. In The Mouse in Biomedical Research, 2nd ed.; Fox, J.G., Davisson, M.T., Quimby, F.W., Barthold, S.W., Newcomer, C.E., Smith, A.L., Eds.; Academic Press: Burlington, NJ, USA, 2007; pp. 25–51. [Google Scholar]
- Buckle, A.P.; Pelz, H.J. Rodent control in practice: Temperate field crops and forestry. In Rodent Pests and Their Control, 2nd ed.; Buckle, A.P., Smith, R.H., Eds.; CAB International: Oxford, UK, 2015; pp. 247–268. [Google Scholar]
- NSW Farmers. Urgent Action Required as Mouse Plague Spreads. Available online: https://www.nswfarmers.org.au/NSWFA/Posts/Media_Releases/mr.39.21.aspx?WebsiteKey=da5d907f-f3f9-47eb-9495-689ec91aac80 (accessed on 16 November 2021).
- NSW Government. Help for Regional Communities Impacted by the Mouse Plague. Available online: https://www.nsw.gov.au/initiative/mouse-control-support-program (accessed on 16 November 2021).
- Buckle, A.P.; Smith, R.H. Rodent Pests and Their Control, 2nd ed.; CAB International: Oxford, UK, 2015. [Google Scholar]
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef]
- Meat and Livestock Australia. State of the Industry Report 2020. Available online: https://www.mla.com.au/globalassets/mla-corporate/prices--markets/documents/trends--analysis/soti-report/mla-state-of-industry-report-2020.pdf (accessed on 16 November 2021).
- Australian Fodder Industry Association. About Fodder. Available online: https://afia.org.au/about-fodder/#fodder-industry (accessed on 16 November 2021).
- Daniels, M.J.; Hutchings, M.R. The response of cattle and sheep to feed contaminated with rodent faeces. Vet. J. 2001, 162, 211–218. [Google Scholar] [CrossRef]
- NSW Health. Q Fever Fact Sheet. Available online: https://www.health.nsw.gov.au/Infectious/factsheets/Pages/q-fever.aspx (accessed on 16 November 2021).
- Queensland Government. Pest, Diseases and Disorders of Animals: Botulism. Available online: https://www.business.qld.gov.au/industries/farms-fishing-forestry/agriculture/livestock/animal-welfare/pests-diseases-disorders/botulism (accessed on 16 November 2021).
- Kelly, J. Mice Contaminated hay—What’s the Risk? Available online: https://www.lls.nsw.gov.au/news-and-events/news/cw-news/2021/mice-contaminated-hay (accessed on 16 November 2021).
- Singleton, G.R.; Smith, A.L.; Krebs, C.J. The prevalence of viral antibodies during a large population fluctuation of house mice in Australia. Epidemiol. Infect. 2000, 125, 719–727. [Google Scholar] [CrossRef]
- Singleton, G.R.; Smith, A.L.; Shellam, G.R.; Fitzgerald, N.; Muller, W.J. Prevalence of viral antibodies and helminths in field populations of house mice (Mus domesticus) in southeastern Australia. Epidemiol. Infect. 1993, 110, 399–417. [Google Scholar] [CrossRef]
- Singleton, G.R.; Smythe, L.; Smith, G.; Spratt, D.M.; Aplin, K.; Smith, A.L. Rodent diseases in Southeast Asia and Australia: Inventory of recent surveys. In Rats, Mice and People: Rodent Biology and Management; Singleton, G.R., Hinds, L.A., Krebs, C.J., Spratt, D.M., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2003; Volume ACIAR Monograph 96, pp. 25–30. [Google Scholar]
- Singleton, G.R. Population dynamics of Mus musculus and its parasites in Mallee Wheatlands in Victoria during and after a drought. Aust. Wildl. Res. 1985, 12, 437–445. [Google Scholar] [CrossRef]
- Smith, A.L.; Singleton, G.R.; Hansen, G.M.; Shellam, G.R. A serological survey for viruses and Mycoplasma pulmonis among wild house mice (Mus domesticus) in southeastern Australia. J. Wildl. Dis. 1993, 29, 219–229. [Google Scholar] [CrossRef]
- Beck, M.A.; Levander, O.A. Host nutritional status and its effect on a viral pathogen. J. Infect. Dis. 2000, 182, S93–S96. [Google Scholar] [CrossRef] [PubMed]
- Boillat, M.; Hammoudi, P.-M.; Dogga, S.K.; Pagès, S.; Goubran, M.; Rodriguez, I.; Soldati-Favre, D. Neuroinflammation-associated aspecific manipulation of mouse predator fear by Toxoplasma gondii. Cell Rep. 2020, 30, 320–334.e326. [Google Scholar] [CrossRef]
- Murray, D.L.; Cary, J.R.; Keith, L.B. Interactive effects of sublethal nematodes and nutritional status on snowshoe hare vulnerability to predation. J. Anim. Ecol. 1997, 66, 250–264. [Google Scholar] [CrossRef]
- Chang, W.-S.; Eden, J.-S.; Hartley, W.J.; Shi, M.; Rose, K.; Holmes, E.C. Metagenomic discovery and co-infection of diverse wobbly possum disease viruses and a novel hepacivirus in Australian brushtail possums. One Health Outlook 2019, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Jenckel, M.; Hall, R.N.; Strive, T. First description of hepatitis E virus in Australian rabbits. Aust. Vet. J. 2021, 99, 356–358. [Google Scholar] [CrossRef]
- Mahar, J.E.; Hall, R.N.; Shi, M.; Mourant, R.; Huang, N.; Strive, T.; Holmes, E.C. The discovery of three new hare lagoviruses reveals unexplored viral diversity in this genus. Virus Evol. 2019, 5, vez005. [Google Scholar] [CrossRef]
- Newsome, A.E. The ecology of house-mice in cereal haystacks. J. Anim. Ecol. 1971, 40, 1–16. [Google Scholar] [CrossRef]
- Newsome, A.E.; Crowcroft, P. Outbreaks of house-mice in South Australia in 1965. CSIRO Wildl. Res. 1971, 16, 41–47. [Google Scholar] [CrossRef]
- Singleton, G.R. A demographic and genetic study of house mice, Mus musculus, colonising pasture haystacks on a cereal farm. Aust. J. Zool. 1985, 33, 437–450. [Google Scholar] [CrossRef]
- Chambers, L.K.; Singleton, G.R.; Krebs, C.J. Movements and social organization of wild house mice (Mus domesticus) in the wheatlands of Northwestern Victoria, Australia. J. Mammal. 2000, 81, 59–69. [Google Scholar] [CrossRef]
- Krebs, C.J.; Kenney, A.J.; Singleton, G.R. Movements of feral house mice in agricultural landscapes. Aust. J. Zool. 1995, 43, 293–302. [Google Scholar] [CrossRef]
- Meerburg, B.G.; Bonde, M.; Brom, F.W.A.; Endepols, S.; Jensen, A.N.; Leirs, H.; Lodal, J.; Singleton, G.R.; Pelz, H.-J.; Rodenburg, T.B.; et al. Towards sustainable management of rodents in organic animal husbandry. Neth. J. Agric. Sci. 2004, 52, 195–205. [Google Scholar] [CrossRef][Green Version]
- Brown, P.R.; Chambers, L.K.; Singleton, G.R. Pre-sowing control of house mice (Mus domesticus) using zinc phosphide: Efficacy and potential non-target effects. Wildl. Res. 2002, 29, 27–37. [Google Scholar] [CrossRef]
- Mutze, G.J.; Sinclair, R. Efficacy of zinc phosphide, strychnine and chlorpyrifos as rodenticides for the control of house mice in South Australian cereal crops. Wildl. Res. 2004, 31, 249–257. [Google Scholar] [CrossRef]
- Lohr, M.T.; Davis, R.A. Anticoagulant rodenticide use, non-target impacts and regulation: A case study from Australia. Sci. Total Environ. 2018, 634, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Tosh, D.G.; Shore, R.F.; Jess, S.; Withers, A.; Bearhop, S.; Montgomery, W.I.; McDonald, R.A. User behaviour, best practice and the risks of non-target exposure associated with anticoagulant rodenticide use. J. Environ. Manag. 2011, 92, 1503–1508. [Google Scholar] [CrossRef]
- Ylönen, H.; Jacob, J.; Davies, M.J.; Singleton, G.R. Predation risk and habitat selection of Australian house mice Mus domesticus during an incipient plague: Desperate behaviour due to food depletion. Oikos 2002, 99, 284–289. [Google Scholar] [CrossRef]
- Arthur, A.D.; Pech, R.P.; Dickman, C.R. Effects of predation and habitat structure on the population dynamics of house mice in large outdoor enclosures. Oikos 2005, 108, 562–572. [Google Scholar] [CrossRef]
- Brown, P.R. Short- and long-term demographic changes in house mouse populations after control in dryland farming systems in Australia. Wildl. Res. 2006, 33, 457–466. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Agricultural Commodities—Australia, States and Territories and ASGS Regions—2015–2016; Australian Bureau of Statistics: Canberra, Australia, 2017. Available online: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/7121.02015-16?OpenDocument (accessed on 16 November 2021).

| Taxon/Category | Species |
|---|---|
| Nematodes (Roundworms) | Aspiculuris tetraptera |
| Calodium hepaticum | |
| Gallegostrongylus australis | |
| Heligmosomoides polygyrus | |
| Heterakis spumosa | |
| Muspicea borreli | |
| Protospirura muris | |
| Syphacia obvelata | |
| Trichosomoides crassicauda | |
| Trichuris muris | |
| Cestodes (Tapeworms) | Taenia taeniaeformis |
| Rodentolepis diminuta | |
| Rodentolepis fraternal | |
| Rodentolepis microstoma | |
| Trematodes (Tapeworms) | Brachylaima cribbi |
| Acarina (Ticks and mites) | Cheleytus sp. |
| Echinonyssus butantanensis | |
| Eulaelaps sp. (stabularis ?) | |
| Kleemania lumosa | |
| Mesolaelaps australiensis | |
| Myobia murismusculi | |
| Myocoptes musculinus | |
| Ornithonyssus bacoti | |
| Paraspeleognathopsis bakeri | |
| Radfordia affinis | |
| Trichoecius rombousti | |
| Siphonaptera (Fleas) | Nosopsyllus fasciatus |
| Nosopsyllus londiniensis | |
| Bacteria | Escherichia coli (E. coli) |
| Salmonella sp. (Salmonella) | |
| Streptobacillus moniliformis | |
| Leptospira sp. (Leptospirosis) | |
| Clostridium botulinum (Botulism) | |
| Viruses | Epizootic diarrhoea of infant mice virus (rotavirus) (EDIM) |
| Lymphocytic choriomeningitis virus (LCMV) | |
| Mouse adenovirus (MAdV) | |
| Minute virus of mice (MVM) | |
| Mouse hepatitis virus (MHV) | |
| Mouse parvovirus (MPV) | |
| Murine cytomegalovirus (MCMV) | |
| Pneumonia virus of mice (PVM) | |
| Reovirus serotype 3 (Reo 3) | |
| Theiler’s mouse encephalomyelitis virus (TMEV) |
| Value of Bales | Estimated Loss by Mice | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grower | State & Postcode | No. Bales | AUD/ Bale | Value (AUD) | Estimated Loss (%) | Lost Value (AUD) | Cost of Baits (AUD) | Labour | Labour Cost 1 (AUD) | Other (AUD) | Total Loss (AUD) | Overall Loss (%) |
| #1 | NSW, 2870 | 600 | 60 | 36,000 | 70% | −25,200 | −1000 | 1 day/month | −1020 | 0 | −27,220 | −75.6% |
| #2 | NSW, 2870 | 370 | 50 | 18,500 | 20% | −3700 | −5000 | 1 day/week | −4080 | 0 (−300,000) 2 | −12,780 (−312,780) | −69.1% (−1690.7%) |
| #3 | NSW, 2870 | 2300 | 100 | 230,000 | 30% | −69,000 | −7000 | 1 day/week | −4080 | 0 | −80,080 | −34.8% |
| #4 | NSW, 2870 | 700 | 60 | 42,000 | 33% | −13,860 | −500 | 1 day/month | −1020 | 0 | −15,380 | −36.6% |
| #5 | Vic, 3390 | 500 | 200 | 100,000 | 0% | 0 | −14,000 | 0 | 0 | 0 | −14,000 | −14.0% |
| #6 | Vic, 3395 | 2000 | 200 | 400,000 | 10% | −40,000 | −10,000 | 0 | 0 | 0 | −50,000 | −12.5% |
| #7 | Vic, 3392 | 2500 | 200 | 500,000 | 0% | 0 | −7000 | 0 | 0 | 0 | −7000 | −1.4% |
| #8 | NSW, 2820 | 3500 | 250 | 875,000 | 30% | −262,500 | −25,000 | 1 day/week | −4080 | 0 | −291,580 | −33.3% |
| #9 | NSW, 2829 | 0 | NA | 0 | 80% | 0 | −40,000 | 1 day/week | −4080 | −5000 | −49,080 | NA |
| #10 | NSW, 2829 | 400 | 400 | 160,000 | 13% | −20,000 | −20,000 | 1 day/week | −4080 | 0 | −44,080 | −27.6% |
| #11 | NSW, 2827 | 9000 | 190 | 1,710,000 | 25% | −427,500 | 0 | 1 day/week | −4080 | −30,000 | −461,580 | −27.0% |
| #12 | NSW, 2828 | 2500 | 250 | 625,000 | 50% | −312,500 | −20,000 | 2 days/week | −8160 | −4000 | −296,660 | −47.5% |
| #13 | NSW, 2829 | 2300 | 150 | 345,000 | 70% | −241,500 | −7000 | 2 days/week | −8160 | 0 | −242,660 | −70.3% |
| #14 | NSW, 2827 | 3000 | 200 | 600,000 | 50% | −300,000 | −1000 | 1 day/month | −1020 | 0 | −300,020 | −50.0% |
| Mean | 2119 | 178 | 402,964 | 34% | −122,554 | −11,250 | −3133 | 139,723 (−161,151) | −34.7% (−40.0%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, P.R.; Henry, S. Impacts of House Mice on Sustainable Fodder Storage in Australia. Agronomy 2022, 12, 254. https://doi.org/10.3390/agronomy12020254
Brown PR, Henry S. Impacts of House Mice on Sustainable Fodder Storage in Australia. Agronomy. 2022; 12(2):254. https://doi.org/10.3390/agronomy12020254
Chicago/Turabian StyleBrown, Peter R., and Steve Henry. 2022. "Impacts of House Mice on Sustainable Fodder Storage in Australia" Agronomy 12, no. 2: 254. https://doi.org/10.3390/agronomy12020254
APA StyleBrown, P. R., & Henry, S. (2022). Impacts of House Mice on Sustainable Fodder Storage in Australia. Agronomy, 12(2), 254. https://doi.org/10.3390/agronomy12020254







