Farmers’ Knowledge, Attitudes, and Control Practices of Rodents in an Agricultural Area of Taiwan
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Area
2.2. Data Collection
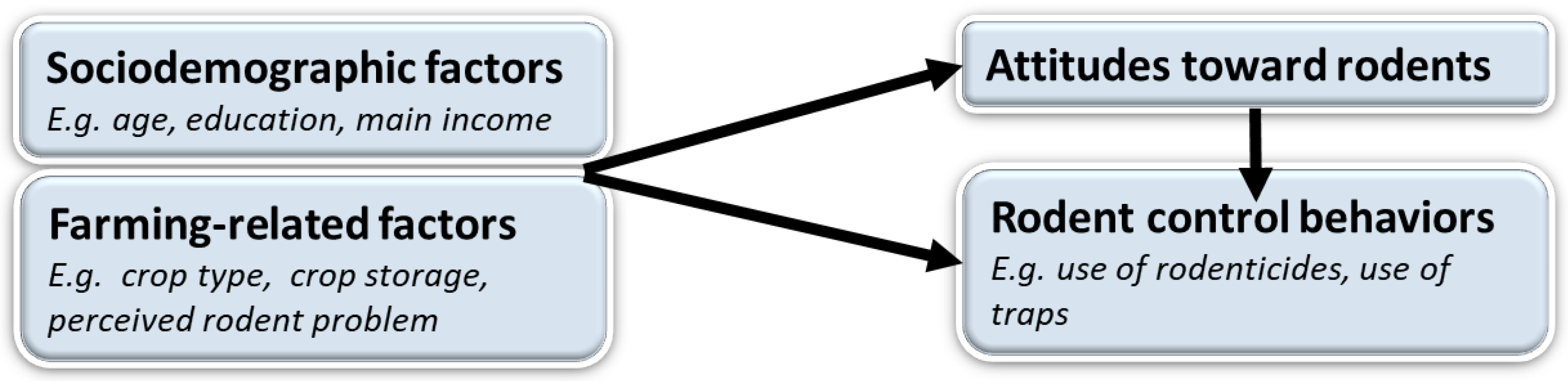
2.3. Data Analysis
3. Results
3.1. Farmer Background Information
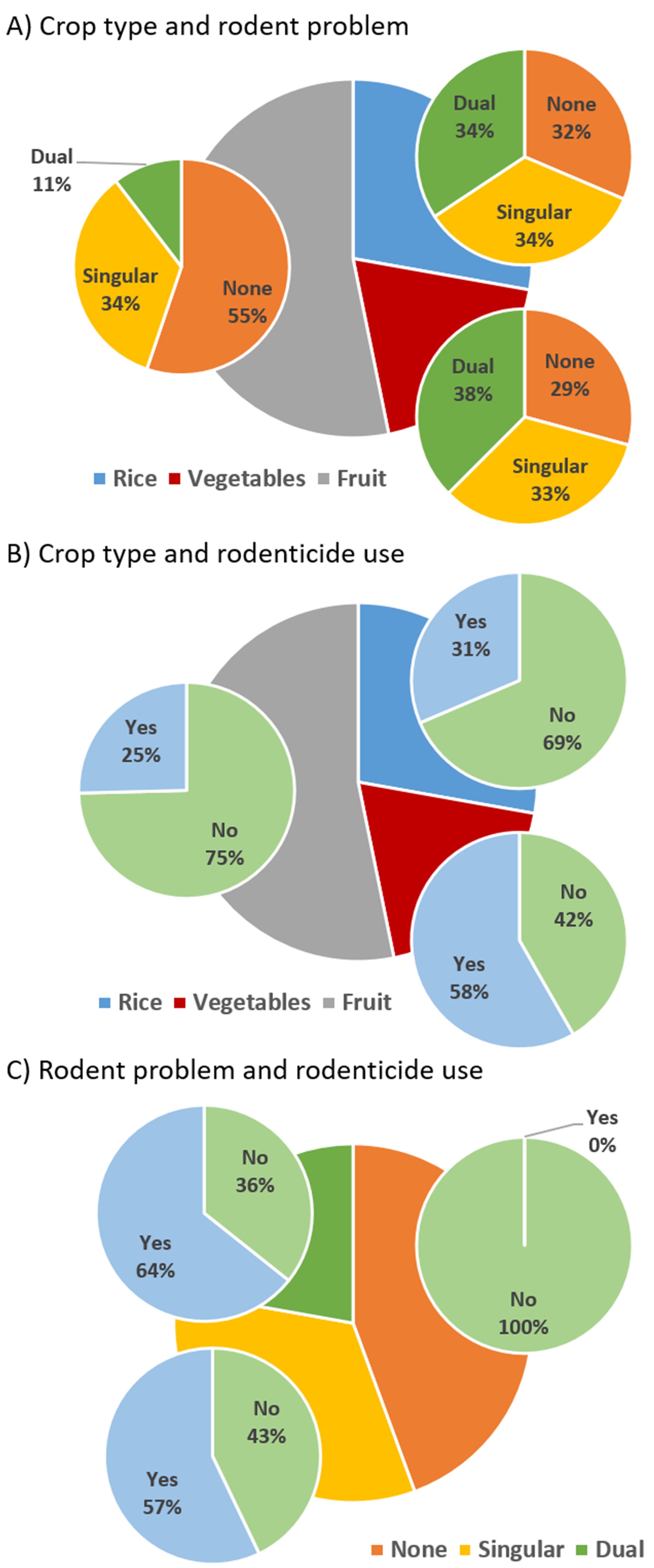
3.2. Rodent Problems and Severity on Crops
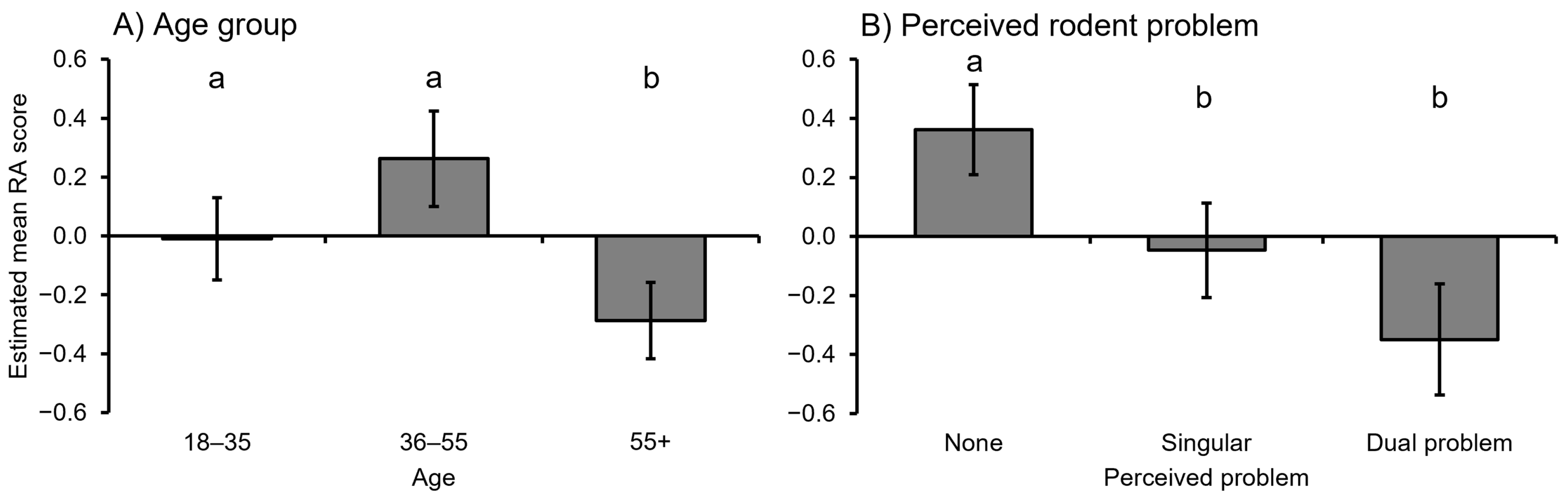
3.3. Attitudes toward Rodents
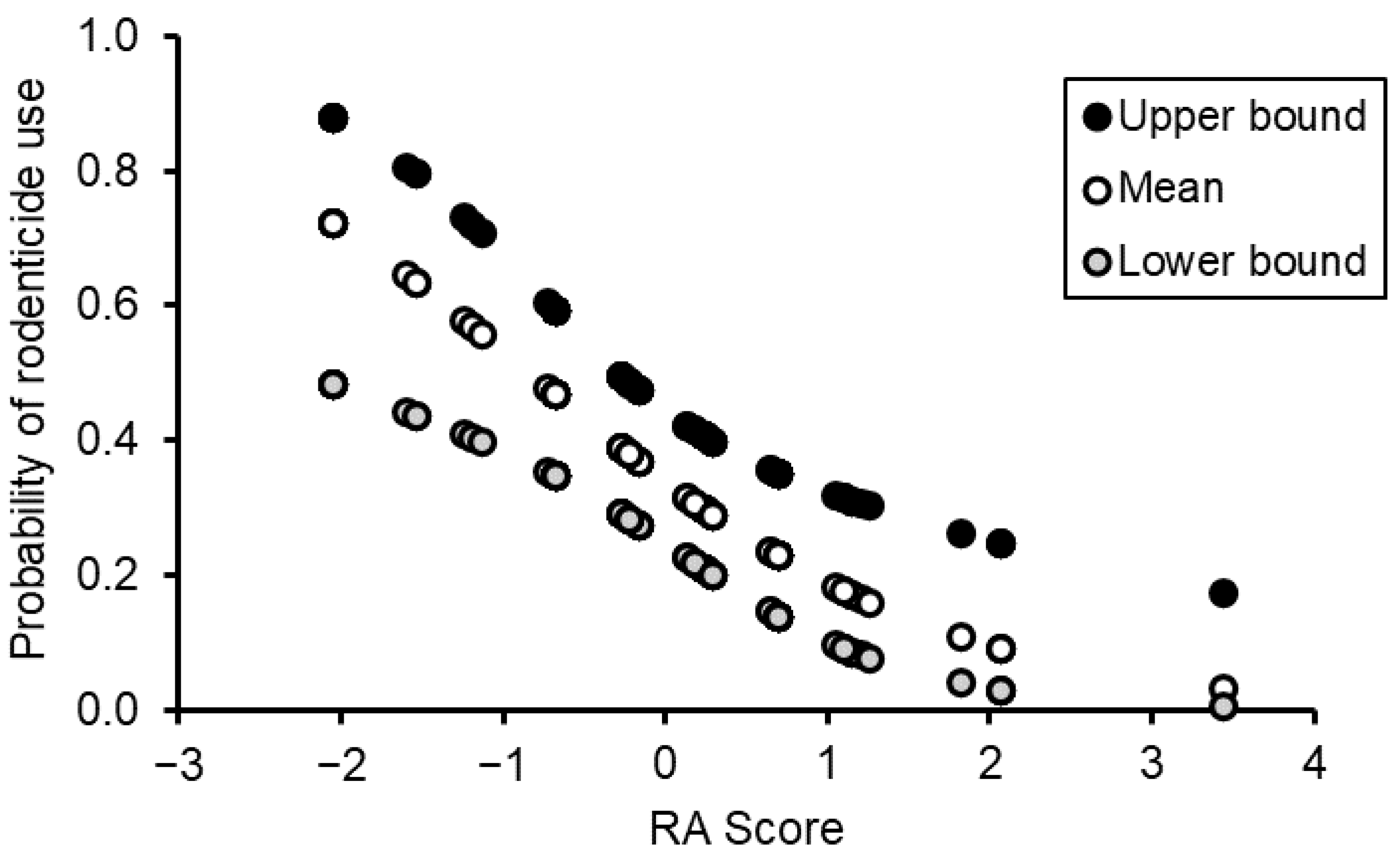
3.4. Rodent Management Behavior
4. Discussion
4.1. Rodent Problems
4.2. Predictors of Attitudes toward Rodents
4.3. Determinants of Rodent Management
4.4. Alternatives for Rodent Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FGAR | First-generation anticoagulant rodenticide |
| SGAR | Second-generation anticoagulant rodenticide |
| KAP | Knowledge, attitude, and practice |
| PCA | Principal component analysis |
| GLM | Generalized linear model |
| RA score | Rodent attitude score |
| AICc | Akaike information criterion corrected |
| LSD | Least significant difference |
| IQR | Interquartile range |
| EBRM | Ecologically based rodent management |
References
- Witmer, G.W.; Pitt, W.C.; Howald, G. Invasive Rodent Ecology, Impacts, and Management with an Emphasis on the United States. USDA National Wildlife Research Center—Staff Publications. 1785. 2014. Available online: https://digitalcommons.unl.edu/icwdm_usdanwrc/1785 (accessed on 28 April 2022).
- Wilson, D.E.; Mittermeier, R.A.; Latcher, T.E. (Eds.) Handbook of Mammals of the World–Volume 7, Rodents II; Lynx Edicons: Barcelona, Spain, 2017. [Google Scholar]
- Meehan, A.P. Rats and mice. In Their Biology and Control; Rentokil Ltd.: East Grinstead, UK, 1984. [Google Scholar]
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef] [PubMed]
- Han, B.A.; Schmidt, J.P.; Bowden, S.E.; Drake, J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 7039–7044. [Google Scholar] [CrossRef] [PubMed]
- Buckle, A.P.; Smith, R.H. Rodent Pests and Their Control, 2nd ed.; CABI: Boston, MA, USA, 2015. [Google Scholar]
- Mendoza, H.; Rubio, A.V.; García-Peña, G.E.; Suzán, G.; Simonetti, J.A. Does land-use change increase the abundance of zoonotic reservoirs? Rodents say yes. Eur. J. Wildl. Res. 2019, 66, 6. [Google Scholar] [CrossRef]
- LoGiudice, K.; Ostfeld, R.S.; Schmidt, K.A.; Keesing, F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA 2003, 100, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Huang, C.L.; Wang, H.C. Identification of potential hosts and vectors of scrub typhus and tick-borne spotted fever group rickettsiae in eastern Taiwan. Med. Vet. Entomol. 2011, 25, 169–177. [Google Scholar] [CrossRef]
- Khalil, H.; Ecke, F.; Evander, M.; Magnusson, M.; Hörnfeldt, B. Declining ecosystem health and the dilution effect. Sci. Rep. 2016, 6, 31314. [Google Scholar] [CrossRef] [PubMed]
- John, A. Rodent outbreaks and rice pre-harvest losses in Southeast Asia. Food Secur. 2014, 6, 249–260. [Google Scholar] [CrossRef]
- Singleton, G.R. Impacts of Rodents on Rice Production in Asia. Los Baños: International Rice Research Institute. 2003. Available online: http://books.irri.org/DPS45_content.pdf (accessed on 29 October 2020).
- Meerburg, B.G.; Singleton, G.R.; Leirs, H. The Year of the Rat ends-time to fight hunger! Pest Manag. Sci. 2009, 65, 351–352. [Google Scholar] [CrossRef]
- Bedoya-Pérez, M.A.; Smith, K.L.; Kevin, R.C.; Luo, J.L.; Crowther, M.S.; McGregor, I.S. Parameters That Affect Fear Responses in Rodents and How to Use Them for Management. Front. Ecol. Evol. 2019, 7, 136. [Google Scholar] [CrossRef]
- Singleton, G.R.; Petch, D.A. A Review of the Biology and Management of Rodent Pests in Southeast Asia (No. 436-2016-33804); Australian Centre for International Agricultural Research: Canberra, Australia, 1994. [Google Scholar]
- Stuart, A.; Prescott, C.V.; Singleton, G.; Joshi, R.C. Knowledge, attitudes and practices of farmers on rodent pests and their management in the lowlands of the Sierra Madre Biodiversity Corridor, Philippines. Crop Prot. 2011, 30, 147–154. [Google Scholar] [CrossRef]
- Prakash, I. Bait Shyness and Poison Aversion. In Rodent Pest Management; Taylor Francis: Abingdon, UK, 2018; pp. 321–329. [Google Scholar] [CrossRef]
- Kogan, M. Integrated Pest Management: Historical Perspectives and Contemporary Developments. Annu. Rev. Entomol. 1998, 43, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Ehler, L. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.G.; Brom, F.W.; Kijlstra, A. The ethics of rodent control. Pest Manag. Sci. 2008, 64, 1205–1211. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Morrissey, C.; Lin, H.-S.; Lin, K.-S.; Lin, W.-L.; Yao, C.-T.; Lin, T.-E.; Chan, F.-T.; Sun, Y.-H. Frequent detection of anticoagulant rodenticides in raptors sampled in Taiwan reflects government rodent control policy. Sci. Total Environ. 2019, 691, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.E.; Hindmarch, S.; Albert, C.A.; Emery, J.; Mineau, P.; Maisonneuve, F. Exposure pathways of anticoagulant rodenticides to nontarget wildlife. Environ. Monit. Assess. 2013, 186, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Rattner, B.A.; Lazarus, R.S.; Elliott, J.E.; Shore, R.F.; Brink, N.V.D. Adverse Outcome Pathway and Risks of Anticoagulant Rodenticides to Predatory Wildlife. Environ. Sci. Technol. 2014, 48, 8433–8445. [Google Scholar] [CrossRef]
- Redpath, S.M.; Bhatia, S.; Young, J. Tilting at wildlife: Reconsidering human–wildlife conflict. Oryx 2014, 49, 222–225. [Google Scholar] [CrossRef]
- Jacobs, M.H. Human Emotions Toward Wildlife. Hum. Dimens. Wildl. 2012, 17, 1–3. [Google Scholar] [CrossRef]
- Slagle, K.M.; Bruskotter, J.; Wilson, R. The Role of Affect in Public Support and Opposition to Wolf Management. Hum. Dimens. Wildl. 2012, 17, 44–57. [Google Scholar] [CrossRef]
- Larson, L.R.; Cooper, C.B.; Hauber, M.E. Emotions as Drivers of Wildlife Stewardship Behavior: Examining Citizen Science Nest Monitors’ Responses to Invasive House Sparrows. Hum. Dimens. Wildl. 2015, 21, 18–33. [Google Scholar] [CrossRef]
- Castillo-Huitrón, N.M.; Naranjo, E.J.; Fita, D.S.; Estrada-Lugo, E. The Importance of Human Emotions for Wildlife Conservation. Front. Psychol. 2020, 11, 1277. [Google Scholar] [CrossRef] [PubMed]
- Petway, J.R.; Lin, Y.-P.; Wunderlich, R.F. Analyzing Opinions on Sustainable Agriculture: Toward Increasing Farmer Knowledge of Organic Practices in Taiwan-Yuanli Township. Sustainability 2019, 11, 3843. [Google Scholar] [CrossRef]
- Bagheri, A.; Bondori, A.; Allahyari, M.S.; Damalas, C.A. Modeling farmers’ intention to use pesticides: An expanded version of the theory of planned behavior. J. Environ. Manag. 2019, 248, 109291. [Google Scholar] [CrossRef]
- Council of Agriculture (COA). Overview. 2020. Available online: https://eng.coa.gov.tw/ws.php?id=9501 (accessed on 29 October 2020).
- Hsing, Y.I.C. Rice in Taiwan. In Encyclopaedia of the History of Science, Technology, and Medicine in Non-Western Cultures; Selin, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 3769–3772. [Google Scholar]
- Lu, K.H.; Ku, T.Y.; Wang, S.C. A review of field rodent control in Taiwan. Plant Prot. Bull. Spec. Pulb. 2003, 5, 323–328. [Google Scholar]
- Hong, S.-Y.; Lin, H.-S.; Walther, B.A.; Shie, J.-E.; Sun, Y.-H. Recent Avian Poisonings Suggest a Secondary Poisoning Crisis of Black Kites During the 1980s in Taiwan. J. Raptor Res. 2018, 52, 326. [Google Scholar] [CrossRef]
- Miaoli County Government. Population and Nationality of Miaoli. 2020. Available online: https://www.miaoli.gov.tw/eng/cp.aspx?n=438 (accessed on 29 October 2020).
- Miaoli Land Use. Regional Planning. 2020. Available online: http://landev.miaoli.gov.tw/mi/p02-2.html (accessed on 14 September 2020).
- Miaoli Farmer Association. Miaoli Farmer Association Information. 2022. Available online: http://www.miaoli.org.tw/about_us.php?rec=0&rec2= (accessed on 28 April 2022).
- Adler, G.H. Habitat relations within lowland grassland rodent communities in Taiwan. J. Zool. 1995, 237, 563–576. [Google Scholar] [CrossRef]
- Tourangeau, R.; Yan, T. Sensitive questions in surveys. Psychol. Bull. 2007, 133, 859–883. [Google Scholar] [CrossRef]
- Nuno, A.; John, F.A.S. How to ask sensitive questions in conservation: A review of specialized questioning techniques. Biol. Conserv. 2015, 189, 5–15. [Google Scholar] [CrossRef]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Kaiser, H.F. Coefficient alpha for a principal component and the Kaiser-Guttman rule. Psychol. Rep. 1991, 68, 855–858. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Agresti, A. Foundations of Linear and Generalized Linear Models; John Wiley Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95364-7. [Google Scholar]
- Upton, G.J. Fisher’s exact test. J. Royal. Stat. Soc. Series A 1992, 155, 395–402. [Google Scholar] [CrossRef]
- Parshad, V. Rodent Control in India. Integr. Pest Manag. Rev. 1999, 4, 97–126. [Google Scholar] [CrossRef]
- Schiller, J.M.; Boupha, B.D.; Bounnaphol, O. Rodents in agriculture in Lao PDR—A problem with an unknown future. In Ecologically-Based Management of Rodent Pests; Singleton, R., Hinds, L.A., Leirs, H., Zhang, Z., Eds.; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 1999; pp. 372–387. [Google Scholar]
- Tuan, N.P.; Williams, S.J.; Brown, P.R.; Singleton, G.R.; Tan, T.Q.; Hue, D.T.; Ha, P.T.T.; Hoa, P.T. Farmer’s perceptions and practices in rat management in Vinh Phuc Province, northern Vietnam. In Rats, Mice and People: Rodent Biology and Management; Singleton, G.R., Hinds, L.A., Krebs, C.J., Spratt, D.M., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2003; pp. 339–402. [Google Scholar]
- Brown, P.R.; Khamphoukeo, K. Changes in farmers’ knowledge, attitudes and practices after implementation of ecologically-based rodent management in the uplands of Lao PDR. Crop Prot. 2010, 29, 577–582. [Google Scholar] [CrossRef]
- Brown, P.R.; Yee, N.; Singleton, G.; Kenney, A.J.; Htwe, N.M.; Myint, M.; Aye, T. Farmers’ knowledge, attitudes, and practices for rodent management in Myanmar. Int. J. Pest Manag. 2008, 54, 69–76. [Google Scholar] [CrossRef]
- Chin, C.; Chiueh, T.-S.; Yang, W.-C.; Yang, T.-H.; Shih, C.-M.; Lin, H.-T.; Lin, K.-C.; Lien, J.-C.; Tsai, T.F.; Ruo, S.L.; et al. Hantavirus infection in Taiwan: The experience of a geographically unique area. J. Med. Virol. 1999, 60, 237–247. [Google Scholar] [CrossRef]
- Batt, S. Human attitudes towards animals in relation to species similarity to humans: A multivariate approach. Biosci. Horiz. 2009, 2, 180–190. [Google Scholar] [CrossRef]
- Prokop, P.; Randler, C. Biological predispositions and individual differences in human attitudes toward animals. In Ethnozoology; Alves, R.R.N., Albuquerque, U.P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 447–466. [Google Scholar]
- Suryawanshi, K.R.; Bhatia, S.; Bhatnagar, Y.V.; Redpath, S.; Mishra, C. Multiscale Factors Affecting Human Attitudes toward Snow Leopards and Wolves. Conserv. Biol. 2014, 28, 1657–1666. [Google Scholar] [CrossRef]
- Best, I.; Pei, K.J.C. Factors influencing local attitudes towards the conservation of leopard cats Prionailurus bengalensis in rural Taiwan. Oryx 2020, 54, 866–872. [Google Scholar] [CrossRef]
- Greenspan, E.; Giordano, A.J.; Nielsen, C.K.; Sun, N.C.-M.; Pei, K.J.-C. Taiwanese attitudes toward the clouded leopard (Neofelis nebulosa) and its potential reintroduction. Hum. Dimens. Wildl. 2020, 25, 301–323. [Google Scholar] [CrossRef]
- Røskaft, E.; Händel, B.; Bjerke, T.; Kaltenborn, B.P. Human attitudes towards large carnivores in Norway. Wildl. Biol. 2007, 13, 172–185. [Google Scholar] [CrossRef]
- Morzillo, A.T.; Mertig, A.G. Urban resident attitudes toward rodents, rodent control products, and environmental effects. Urban Ecosyst. 2010, 14, 243–260. [Google Scholar] [CrossRef]
- Marchini, S. Who’s in conflict with whom? Human dimensions of the conflicts involving wildlife. In Applied Ecology and Human Dimensions in Biological Conservation; Verdade, L.M., Lyra-Jorge, M.C., Pina, C.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 189–209. [Google Scholar]
- Soulsbury, C.D.; White, P.C. Human–wildlife interactions in urban areas: A review of conflicts, benefits and opportunities. Wildl. Res. 2015, 42, 541–553. [Google Scholar] [CrossRef]
- Fall, M.W.; Fiedler, L.A. Rodent Control in Practice: Tropical Field Crops; Buckle, A.P., Smith, R.H., Eds.; Rodent pests and their control; CABI: Boston, MA, USA, 2015; pp. 269–294. [Google Scholar]
- Tomass, Z.; Shibru, S.; Yonas, M.; Leirs, H. Farmers’ perspectives of rodent damage and rodent management in smallholder maize cropping systems of Southern Ethiopia. Crop Prot. 2020, 136, 105232. [Google Scholar] [CrossRef]
- Jacob, J.; Buckle, A. Use of anticoagulant rodenticides in different applications around the world. In Anticoagulant Rodenticides and Wildlife; van den Brink, N.W., Elliott, J.E., Shore, R.F., Rattner, B.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 11–43. [Google Scholar]
- Lund, M. Resistance to the second-generation anticoagulant rodenticides. In Proceedings of the Vertebrate Pest Conference, Sacramento, CA, USA, 6–8 March 1984; Volume 11. [Google Scholar]
- Quy, R.J.; Cowan, D.P.; Prescott, C.V.; Gill, J.E.; Kerins, G.M.; Dunsford, G.; Jones, A.; Macnicoll, A.D. Control of a population of Norway rats resistant to anticoagulant rodenticides. Pestic. Sci. 1995, 45, 247–256. [Google Scholar] [CrossRef]
- Buckle, A. Anticoagulant resistance in the United Kingdom and a new guideline for the management of resistant infestations of Norway rats (Rattus norvegicus Berk.). Pest Manag. Sci. 2013, 69, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H. The susceptibility of Apodemus agrarius and Rattus losea to the anticoagulant rodenticide, flocoumafen. Int. Biodeterior. 1990, 26, 69–74. [Google Scholar] [CrossRef]
- Lin, W.-L.; Chen, K.-H.; Liao, C.-P.; Tseng, H.-Y. Short-term exposure of anticoagulant rodenticides leads to the toxin accumulation from prey (Rattus losea) to predator (Elanus caeruleus). Ecotoxicol. Environ. Saf. 2022, 233, 113361. [Google Scholar] [CrossRef]
- Rusiniak, K.W.; Hankins, W.G.; Garcia, J.; Brett, L.P. Flavor-illness aversions: Potentiation of odor by taste in rats. Behav. Neural Biol. 1979, 25, 1–17. [Google Scholar] [CrossRef]
- Inglis, I.; Shepherd, D.; Smith, P.; Haynes, P.; Bull, D.; Cowan, D.; Whitehead, D. Foraging behaviour of wild rats (Rattus norvegicus) towards new foods and bait containers. Appl. Anim. Behav. Sci. 1996, 47, 175–190. [Google Scholar] [CrossRef]
- Zhang, W.; Kato, E.; Bianchi, F.; Bhandary, P.; Gort, G.; van der Werf, W. Farmers’ perceptions of crop pest severity in Nigeria are associated with landscape, agronomic and socio-economic factors. Agric. Ecosyst. Environ. 2018, 259, 159–167. [Google Scholar] [CrossRef]
- Manfredo, M.J.; Dayer, A. Concepts for Exploring the Social Aspects of Human–Wildlife Conflict in a Global Context. Hum. Dimens. Wildl. 2004, 9, 1–20. [Google Scholar] [CrossRef]
- Dickman, A.J. Complexities of conflict: The importance of considering social factors for effectively resolving human-wildlife conflict. Anim. Conserv. 2010, 13, 458–466. [Google Scholar] [CrossRef]
- Krijger, I.M.; Belmain, S.R.; Singleton, G.R.; Groot Koerkamp, P.W.; Meerburg, B.G. The need to implement the landscape of fear within rodent pest management strategies. Pest Manag. Sci. 2017, 73, 2397–2402. [Google Scholar] [CrossRef]
- Brakes, C.R.; Smith, R.H. Exposure of non-target small mammals to rodenticides: Short-term effects, recovery and implications for secondary poisoning. J. Appl. Ecol. 2005, 42, 118–128. [Google Scholar] [CrossRef]
- Gabriel, M.W.; Woods, L.W.; Poppenga, R.; Sweitzer, R.A.; Thompson, C.; Matthews, S.M.; Higley, J.M.; Keller, S.; Purcell, K.; Barrett, R.H.; et al. Anticoagulant Rodenticides on our Public and Community Lands: Spatial Distribution of Exposure and Poisoning of a Rare Forest Carnivore. PLoS ONE 2012, 7, e40163. [Google Scholar] [CrossRef]
- Lohr, M.T.; Davis, R.A. Anticoagulant rodenticide use, non-target impacts and regulation: A case study from Australia. Sci. Total Environ. 2018, 634, 1372–1384. [Google Scholar] [CrossRef]
- Singleton, G.R.; Leirs, H.; Hinds, L.A.; Zhang, Z. Ecologically-based management of rodent pests–re-evaluating our approach to an old problem. In Ecologically-Based Management of Rodent Pests; Singleton, G.R., Hinds, L.A., Leirs, H., Zhang, Z., Eds.; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 1999; pp. 17–29. [Google Scholar]
- Brown, P.R.; Tuan, N.P.; Singleton, G.; Ha, P.T.T.; Hoa, P.T.; Hue, D.T.; Tan, T.Q.; Van Tuat, N.; Jacob, J.; Müller, W.J. Ecologically based management of rodents in the real world: Applied to a mixed agroecosystem in Vietnam. Ecol. Appl. 2006, 16, 2000–2010. [Google Scholar] [CrossRef]
- Constant, N.L.; Swanepoel, L.H.; Williams, S.; Soarimalala, V.; Goodman, S.M.; Massawe, A.T.; Mulungu, L.S.; Makundi, R.H.; Mdangi, M.E.; Taylor, P.J.; et al. Comparative assessment on rodent impacts and cultural perceptions of ecologically based rodent management in 3 Afro-Malagasy farming regions. Integr. Zool. 2020, 15, 578–594. [Google Scholar] [CrossRef]
- Jones, C.R.; Lorica, R.P.; Villegas, J.M.; Ramal, A.F.; Horgan, F.G.; Singleton, G.; Stuart, A.M. The stadium effect: Rodent damage patterns in rice fields explored using giving-up densities. Integr. Zool. 2017, 12, 438–445. [Google Scholar] [CrossRef]
- Hall, T.R.; Howard, W.E.; Marsh, R.E. Raptor use of artificial perches. Wildl. Soc. Bull. 1981, 9, 296–298. [Google Scholar]
- Labuschagne, L.; Swanepoel, L.H.; Taylor, P.J.; Belmain, S.R.; Keith, M. Are avian predators effective biological control agents for rodent pest management in agricultural systems? Biol. Control 2016, 101, 94–102. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lin, H.S.; Huang, Z.L.; Choi, W.S.; Wang, W.I.; Sun, Y.H. Perch-Mounted Camera Traps Record Predatory Birds in Farmland. J. Raptor Res. 2022, 56, 116–124. [Google Scholar] [CrossRef]
- Singleton, G.; Brown, P.; Jacob, J. Ecologically-based rodent management: Its effectiveness in cropping systems in South-East Asia. NJAS Wagening. J. Life Sci. 2004, 52, 163–171. [Google Scholar] [CrossRef][Green Version]
- Jacob, J.; Singleton, G.R.; Herawati, N.A.; Brown, P.R. Ecologically based management of rodents in lowland irrigated rice fields in Indonesia. Wildl. Res. 2010, 37, 418–427. [Google Scholar] [CrossRef]
- Palis, F.G.; Singleton, G.R.; Brown, P.R.; Huan, N.H.; Umali, C.; Nga, N.T.D. Can humans outsmart rodents? Learning to work collectively and strategically. Wildl. Res. 2011, 38, 568–578. [Google Scholar] [CrossRef]
| Predictor | Definition | Type of Variable and Subgroups |
|---|---|---|
| Age | Age of participant | Ordinal. 1 = 18–35, 2 = 36–55, 3 = 55+ |
| Gender | Gender of participant | Nominal (binary). 1 = Male, 2 = Female |
| Education | Highest level of education participant has completed | Ordinal. 1 = Elementary school, 2 = High school, 3 = University/College, 4 = Graduate studies |
| Main income | The main income of the participant | Nominal (binary). 1 = Farming, 2 = Other |
| Own farm | Whether a farmer owns their farmland or not | Nominal (binary). 0 = No, 1 = Yes |
| Years farming a | The number of years a participant has been farming | Ordinal. 1 = 1–10, 2 = 11–20, 3 = 21–30, 4 = 30+ |
| Pets | Whether a participant has pets (dogs and/or cats) | Nominal (binary). 0 = No, 1 = Yes |
| Farm animals | Whether a participant has farm animals | Nominal (binary). 0 = No, 1 = Yes |
| Crop type | The main crop grown by farmers grouped into categories | Nominal. 1 = Rice, 2 = Vegetables, 3 = Fruit |
| Crop storage | Whether a farmer stores their crops before distribution/sale | Nominal (binary). 0 = No, 1 = Yes |
| Rodent problem | The extent of problems caused by rodents | Ordinal. 0 = None, 1 = Singular (only problematic during growing or storing, not both), 2 = Dual (Problematic during both growing and storing) |
| Use of traps a | Whether farmers use any sort of trap for rodents, e.g., live or lethal | Nominal (binary). 0 = No, 1 = Yes |
| Original Statement | Rodent Attitude Loading Score | Mean Likert Score (1–5) a | % of Farmers | ||
|---|---|---|---|---|---|
| Agree | Neutral | Disagree | |||
| Rodents are a major pest for my house | 0.777 | 2.62 | 43.6 | 42.6 | 13.9 |
| Rodents are a major pest for farming in my community | 0.915 | 2.57 | 45.5 | 43.6 | 10.9 |
| Rodents are a risk to people | 0.795 | 2.29 | 64.4 | 29.7 | 5.9 |
| Model | Predictor Variables | K | Log-Like | AICc | ∆AICc | wi |
|---|---|---|---|---|---|---|
| 9 | RA score ~ Age * + Rodent problem ** | 6 | −93.95 | 201.09 | 0.00 | 0.60 |
| 8 | RA score ~ Age * + Crop type + Rodent problem * | 8 | −92.36 | 202.80 | 1.71 | 0.25 |
| 7 | RA score ~ Age ** + Education + Crop type + Rodent problem * | 11 | −89.61 | 205.21 | 4.12 | 0.076 |
| 6 | RA score ~ Age ** + Education + Own farm + Crop type + Rodent problem * | 12 | −88.71 | 206.21 | 5.12 | 0.046 |
| 5 | RA score ~ Age ** + Education + Own farm + Crop type + Crop storage + Rodent problem * | 13 | −88.27 | 208.22 | 7.13 | 0.017 |
| 4 | RA score ~ Age ** + Education + Own farm + Farm animal + Crop type + Crop storage + Rodent problem * | 14 | −87.72 | 210.10 | 9.01 | 0.0066 |
| 3 | RA score ~ Age ** + Education + Own farm + Pets + Farm animal + Crop type + Crop storage + Rodent problem * | 15 | −87.30 | 212.35 | 11.26 | 0.0021 |
| 2 | RA score ~ Age ** + Education + Main income + Own farm + Pets + Farm animal + Crop type + Crop storage + Rodent problem * | 16 | −87.30 | 215.51 | 14.42 | 0.00044 |
| 1 | RA score ~ Age ** + Education + Gender + Main income + Own farm + Pets + Farm animals + Crop type + Crop storage + Rodent problem * | 17 | −87.30 | 218.79 | 17.70 | 0.000086 |
| Variable | n | χ2 | df | p |
|---|---|---|---|---|
| Age | 120 a | 3.79 | 2 | 0.151 |
| Gender | 107 a | 0.15 | 1 | 0.696 |
| Education | 123 a | 2.82 | 3 | 0.429 |
| Main income | 126 | 1.34 | 1 | 0.247 |
| Ownership | 124 a | 0.40 | 1 | 0.528 |
| Years farming | 117 a | 4.83 | 3 | 0.184 |
| Pets | 125 a | 0.15 | 1 | 0.701 |
| Farm animals | 124 a | 0.00 | 1 | 0.989 |
| Crop type | 126 | 8.72 | 2 | <0.05 |
| Crop storage | 126 | 1.55 | 1 | 0.213 |
| Use of traps | 117 a | 3.17 | 1 | 0.075 |
| Rodent problem | 126 | 50.79 | 2 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Best, I.N.; Shaner, P.-J.L.; Pei, K.J.-C.; Kuo, C.-C. Farmers’ Knowledge, Attitudes, and Control Practices of Rodents in an Agricultural Area of Taiwan. Agronomy 2022, 12, 1169. https://doi.org/10.3390/agronomy12051169
Best IN, Shaner P-JL, Pei KJ-C, Kuo C-C. Farmers’ Knowledge, Attitudes, and Control Practices of Rodents in an Agricultural Area of Taiwan. Agronomy. 2022; 12(5):1169. https://doi.org/10.3390/agronomy12051169
Chicago/Turabian StyleBest, Ian Nicholas, Pei-Jen Lee Shaner, Kurtis Jai-Chyi Pei, and Chi-Chien Kuo. 2022. "Farmers’ Knowledge, Attitudes, and Control Practices of Rodents in an Agricultural Area of Taiwan" Agronomy 12, no. 5: 1169. https://doi.org/10.3390/agronomy12051169
APA StyleBest, I. N., Shaner, P.-J. L., Pei, K. J.-C., & Kuo, C.-C. (2022). Farmers’ Knowledge, Attitudes, and Control Practices of Rodents in an Agricultural Area of Taiwan. Agronomy, 12(5), 1169. https://doi.org/10.3390/agronomy12051169






