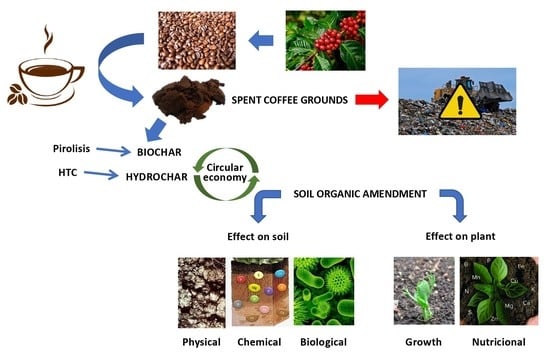
Why Should We Be Concerned with the Use of Spent Coffee Grounds as an Organic Amendment of Soils? A Narrative Review
Abstract
1. Introduction
2. Potential Applications of Spent Coffee Grounds
3. Spent Coffee Grounds as a Soil Organic Amendment
3.1. Soil Fertility
3.1.1. Chemical Properties
3.1.2. Physical Properties
3.1.3. Biological Properties
3.2. Effects of Spent Coffee Grounds on the Growth and Mineral Nutrition of Plants
3.2.1. Effects on Plant Growth
3.2.2. Effects on Mineral Content and Other Compounds
3.3. Use of By-Products Derived from Spent Coffee Grounds in Agriculture
3.3.1. Effects on Soil Fertility
3.3.2. Effects on Plants
3.4. Comparison with the Effect of Other Organic Amendments
3.5. Therefore, Why Should We Be Concerned with the Use of Spent Coffee Grounds as an Organic Amendment of Soils?
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ICO. Historia del Café. Available online: https://www.ico.org/ES/coffee_storyc.asp (accessed on 11 September 2022).
- Hoffman, J. The World Atlas of Coffee: From Beans to Brewing-Coffees Explored, Explained and Enjoyed, 1st ed.; Firefly Books: Richmon Hill, ON, Canada, 2014. [Google Scholar]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.F.; Oliveira, M.P.P. State of the Art in Coffee Processing By-Products. In Handbook of Coffee Processing By-Products: Sustainable Applications, 1st ed.; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–22. [Google Scholar]
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Res. Conserv. Recyc. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Kasongo, R.K.; Verdoodt, A.; Kanyankagote, P.; Baert, G.; Van Ranst, E. Coffee waste as an alternative fertilizer with soil improving properties for sandy soils in humid tropical environments. Soil Use Manag. 2011, 27, 94–102. [Google Scholar] [CrossRef]
- Kasongo, R.K.; Verdoodt, A.; Kanyankogote, P.; Baert, G.; Van Ranst, E. Response of Italian ryegrass (Lolium multiflorum Lam.) to coffee waste application on a humid tropical sandy soil. Soil Use Manag. 2013, 29, 22–29. [Google Scholar] [CrossRef]
- Shemekite, F.; Gomez-Brandon, M.; Franke-Whittle, I.H.; Praehauser, B.; Insam, H.; Assefa, F. Coffee husk composting: An investigation of the process using molecular and nonmolecular tools. Waste Manag. 2014, 34, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; Guerra-Hernández, E.; García-Villanova, B. Maillard reaction in enteral formula processing: Furosine, loss of o-phthaldialdehyde reactivity, and fluorescence. Food Res. Int. 2002, 35, 527–533. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Guerra-Hernández, E.; García-Villanova, B. Colour measurement as indicator for controlling the manufacture and storage of enteral formulas. Food Cont. 2006, 17, 489–493. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carb. Pol. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products, prebiotic, antimicrobial and antioxidant properties. LWT Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Petracco, M. Technology IV—Beverage Preparation: Brewing Trends for the New Millennium. In Coffee: Recent Developments, 1st ed.; Clarke, R.J., Vitzthum, O.G., Eds.; Blackwell Science Ltd.: Oxford, MS, USA, 2001; pp. 140–164. [Google Scholar]
- Kamil, M.; Ramadan, K.M.; Awad, O.I.; Ibrahim, T.K.; Inayat, A.; Ma, X. Environmental impacts of biodiesel production from waste spent coffee grounds and its implementation in a compression ignition engine. Sci. Total Environ. 2019, 675, 13–30. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q.S. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Pina, G.; Vergara-Castaneda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Pastoriza, S.; Rufián-Henares, J.A. Contribution of melanoidins to the antioxidant capacity of the Spanish diet. Food Chem. 2014, 164, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; García-Villanova, B.; Guerra-Hernández, E. Generation of furosine and color in infant/enteral formula-resembling systems. J. Agric. Food Chem. 2004, 52, 5354–5358. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; García-Villanova, B.; Guerra-Hernández, E. Occurrence of furosine and hydroxymethylfurfural as markers of thermal damage in dehydrated vegetables. Eur. Food Res. Technol. 2008, 228, 249–256. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Rufián-Henares, J.A.; Martínez, R.G.; Miralles, B.; Berguillos, T.; Navarro-Alarcón, M.; Jauregi, P. Antioxidant, ACE-inhibitory and antimicrobial activity of fermented goat milk: Activity and physicochemical property relationship of the peptide components. Food Funct. 2017, 8, 2783–2791. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Morales, F.J. Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities. Food Res. Int. 2007, 40, 995–1002. [Google Scholar] [CrossRef]
- Moreira, A.S.P.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903. [Google Scholar] [CrossRef]
- Pastoriza, S.; Rufián-Henares, J.A.; Morales, F.J. Reactivity of acrylamide with coffee melanoidins in model systems. LWT-Food Sci. Technol. 2012, 45, 198–203. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Rufián-Henares, J.A. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem. 2020, 316, 126309. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; de la Cueva, S.P. Antimicrobial activity of coffee melanoidins-a study of their metal-chelating properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; Morales, F.J. Angiotensin-I converting enzyme inhibitory activity of coffee melanoidins. J. Agric. Food Chem. 2007, 54, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Atabani, A.E.; Al-Muhtaseb, A.H.; Kumar, G.; Saratale, G.D.; Aslam, M.; Khan, H.A.; Said, Z.; Mahmoud, E. Valorization of spent coffee grounds into biofuels and value-added products: Pathway towards integrated bio-refinery. Fuel 2019, 254, 115640. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Van Zwieten, L.; Collins, L.; Pitt, W.; Armstrong, R.; Tavakkoli, E. Additive effects of organic and inorganic amendments can significantly improve structural stability of a sodic dispersive subsoil. Geoderma 2021, 404, 115281. [Google Scholar] [CrossRef]
- Garbuz, S.; Mackay, S.; Camps-Arbestain, M.; DeVantier, B.; Minor, M. Biochar amendment improves soil physico-chemical properties and alters root biomass and the soil food web in grazed pastures. Agric. Ecosys. Environ. 2021, 319, 107517. [Google Scholar] [CrossRef]
- Anli, M.; Boutasknit, A.; Ben-Laouane, R.; Ait-el-Mokhtar, M.; Ait-Rahou, Y.; Raho, O.; Fakhech, A.; Meddich, A. Evaluating the performance of lactuca sativa under four different organic fertilizers and subsequent impact on the soil health. J. Basic Appl. Res. Int. 2021, 27, 53–66. [Google Scholar]
- Sulok, K.M.T.; Ahmed, O.H.; Khew, C.Y.; Zehnder, J.A.M.; Jalloh, M.B.; Musah, A.A.; Abdu, A. Chemical and Biological Characteristics of Organic Amendments Produced from Selected Agro-Wastes with Potential for Sustaining Soil Health: A Laboratory Assessment. Sustainability 2021, 13, 4919. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Sarkar, S. Effect of organic amendments on the growth, yield and nutrient status of cowpea (Vigna unguiculata (L.) Walp.). Plant Physiol. Rep. 2021, 26, 535–540. [Google Scholar] [CrossRef]
- Apori, S.O.; Byalebeka, J.; Murongo, M.; Ssekandi, J.; Noel, G.L. Effect of co-applied corncob biochar with farmyard manure and NPK fertilizer on tropical soil. Res. Environ. Sustain. 2021, 5, 1000034. [Google Scholar] [CrossRef]
- Ramos, S.J.; Pinto, D.A.; Guedes, R.S.; Dias, Y.N.; Caldeira, C.F.; Gastauer, M.; Souza-Filho, P.W.; Fernandes, A.R. Açaí Biochar and Compost Affect the Phosphorus Sorption, Nutrient Availability, and Growth of Dioclea apurensis in Iron Mining Soil. Minerals 2021, 11, 674. [Google Scholar] [CrossRef]
- Maselesele, D.; Ogola, J.B.O.; Murovhi, R.N. Macadamia Husk Compost Improved Physical and Chemical Properties of a Sandy Loam Soil. Sustainability 2021, 13, 6997. [Google Scholar] [CrossRef]
- Bouajila, K.; Chibani, R.; Mechri, M.; Moussa, M.; Ben Jeddi, F. Carbon and nitrogen mineralization dynamics in tow amended soils collected from the semi-arid and arid regions of Tunisia. Arab. J. Geosci. 2021, 14, 1005. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of Organic Wastes through Composting: Process Performance and Compost Application in Agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Eden, M.; Gerke, H.H.; Houot, S. Organic waste recycling in agriculture and related effects on soil water retention and plant available water: A review. Agron. Sustain. Dev. 2017, 37, 11. [Google Scholar] [CrossRef]
- Reeve, J.R.; Hoagland, L.A.; Villalba, J.J.; Carr, P.M.; Atucha, A.; Cambardella, C.; Davis, D.R.; Delate, K. Organic Farming, Soil Health, and Food Quality: Considering Possible Links. Adv. Agron. 2016, 137, 319–367. [Google Scholar] [CrossRef]
- Liu, K.; Price, G.W. Evaluation of three composting systems for the management of spent coffee grounds. Biores. Technol. 2011, 102, 7966–7974. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of different rates of spent coffee grounds (SCG) on composting process, gaseous emissions and quality of end-product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef]
- Ronga, D.; Pane, C.; Zaccardelli, M.; Pecchioni, N. Use of Spent Coffee Ground Compost in Peat-Based Growing Media for the Production of Basil and Tomato Potting Plants. Commun. Soil Sci. Plant Anal. 2016, 47, 356–368. [Google Scholar] [CrossRef]
- Kopeć, M.; Baran, A.; Mierzwa-Hersztek, M.; Gondek, K.; Chmiel, M.J. Effect of the Addition of Biochar and Coffee Grounds on the Biological Properties and Ecotoxicity of Composts. Waste Biomass Valor. 2018, 9, 1389–1398. [Google Scholar] [CrossRef]
- Dominguez, J.; Gomez-Brandon, M. Vermicomposting: Composting with Earthworms to Recycle Organic Wastes. In Management of Organic Waste, 1st ed.; Kumar, S., Bharti, A., Eds.; InTech Open Science: London, UK, 2012; pp. 29–48. [Google Scholar]
- González-Moreno, M.A.; García Gracianteparaluceta, B.; Marcelino Sádaba, S.; Zaratiegui Urdin, J.; Robles Domínguez, E.; Pérez Ezcurdia, M.A.; Seco Meneses, A. Feasibility of Vermicomposting of Spent Coffee Grounds and Silverskin from Coffee Industrie: A Laboratory Study. Agronomy 2020, 10, 1125. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Domínguez, J. Vermicompost Derived from Spent Coffee Grounds: Assessing the Potential for Enzymatic Bioremediation. In Handbook of Coffee Processing By-Products: Sustainable Applications, 1st ed.; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 369–398. [Google Scholar]
- Yamane, K.; Kono, M.; Fukunaga, T.; Iwai, K.; Sekine, R. Field Evaluation of Coffee Grounds Application for Crop Growth Enhancement, Weed Control, and Soil Improvement. Plant Prod. Sci. 2014, 17, 93–102. [Google Scholar] [CrossRef]
- Comino, F.; Cervera-Mata, A.; Aranda, V.; Martín-García, J.M.; Delgado, G. Short-term impact of spent coffee grounds over soil organic matter composition and stability in two contrasted Mediterranean agricultural soils. J. Soils Sedim. 2020, 20, 1182–1198. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent coffee grounds by-products and their influence on soil C-N dynamics. J. Environ. Manag. 2022, 302, 114075. [Google Scholar] [CrossRef]
- Hardgrove, S.J.; Livesley, S.J. Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban For. Urban Green. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Kitou, M.; Yoshida, S. Effect of coffee residue on the growth of several crop species. J. Weed Sci. Technol. 1997, 42, 25–30. [Google Scholar] [CrossRef]
- Cruz, R.; Mendes, E.; Torrinha, Á.; Morais, S.; Pereira, J.A.; Baptista, P.; Casal, S. Revalorization of spent coffee residues by a direct agronomic approach. Food Res. Int. 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Cruz, R.; Gomes, T.; Ferreira, A.; Mendes, E.; Baptista, P.; Cunha, S.; Pereira, J.A.; Ramalhosa, E.; Casal, S. Antioxidant activity and bioactive compounds of lettuce improved by espresso coffee residues. Food Chem. 2014, 145, 95–101. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M. Recycling coffee and tea wastes to increase plant available Fe in alkaline soils. Plant Soil 2008, 304, 249–255. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Fernández-Arteaga, A.; Navarro-Alarcón, M.; Hinojosa, D.; Pastoriza, S.; Delgado, G.; Rufián-Henares, J.A. Spent coffee grounds as a source of smart biochelates to increase Fe and Zn levels in lettuces. J. Clean. Prod. 2021, 328, 129548. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Aranda, V.; Ontiveros-Ortega, A.; Comino, F.; Martín-García, J.M.; Vela-Cano, M.; Delgado, G. Hydrophobicity and surface free energy to assess spent coffee grounds as soil amendment. Relationships with soil quality. Catena 2021, 165, 104826. [Google Scholar] [CrossRef]
- Turek, M.E.; Freitas, K.S.; Armindo, R.A. Spent coffee grounds as organic amendment modify hydraulic properties in a sandy loam Brazilian soil. Agric. Water Manag. 2019, 222, 313–321. [Google Scholar] [CrossRef]
- Vela-Cano, M.; Gómez-Brandón, M.; Pesciaroli, C.; Insam, H.; González-López, J. Study of total bacteria and ammonia-oxidizing bacteria and ammonia-oxidizing archaea in response to irrigation with sewage sludge compost tea in agricultural soil. Compos. Sci. Util. 2018, 26, 145–155. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Delgado, G.; Pastoriza, S.; Montilla-Gómez, J.; Llopis, J.; Sánchez-González, C.; Rufián-Henares, J.A. Spent coffee grounds improve the nutritional value in elements of lettuce (Lactuca sativa L.) and are an ecological alternative to inorganic fertilizers. Food Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Rufián-Henares, J.A.; Pastoriza, S.; Montilla-Gómez, J.; Delgado, G. Phytotoxicity and chelating capacity of spent coffee grounds: Two contrasting faces in its use as soil organic amendment. Sci. Total Environ. 2020, 717, 137247. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Xylia, P.; Petropoulos, S.; Tzortzakis, N. The use of spent coffee grounds in growing media for the production of Brassica seedlings in nurseries. Environ. Sci. Pol. Res. 2021, 28, 24279–24290. [Google Scholar] [CrossRef]
- Cruz, R.; Baptista, P.; Cunha, S.; Pereira, J.A.; Casal, S. Carotenoids of lettuce (Lactuca sativa L.) grown on soil enriched with spent coffee grounds. Molecules 2012, 17, 1535–1547. [Google Scholar] [CrossRef]
- Cruz, R.; Morais, S.; Mendes, E.; Pereira, J.A.; Baptista, P.; Casal, S. Improvement of vegetables elemental quality by espresso coffee residues. Food Chem. 2014, 148, 294–299. [Google Scholar] [CrossRef]
- Cruz, S.; Cordovil, C.S.C. Espresso coffee residues as a nitrogen amendment for small-scale vegetable. J. Sci. Food Agric. 2015, 95, 3059–3066. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Vicente, E.D.; Gomes, A.P.; Nunes, M.I.; Alves, C.; Tarelho, L.A.C. Effect of industrial and domestic ash from biomass combustion, and spent coffee grounds, on soil fertility and plant growth: Experiments at field conditions. Environ. Sci. Pol. Res. 2017, 24, 15270–15277. [Google Scholar] [CrossRef] [PubMed]
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete utilization of spent coffee grounds to produce biodiesel, bio-oil, and biochar. ACS Sust. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- Caliskan, S.; Ozok, N.; Makineci, E. Utilization of Spent Coffee Grounds as Media for Stone Pine (Pinus pinea) Seedlings. J. Soil Sci. Plant Nutr. 2020, 20, 2014–2024. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Poluszyńska, J.; Sławińska, I. Acute toxicity of experimental fertilizers made of blood meal, spent coffee ground and biomass ash. J. Water Land Develop. 2017, 34, 95–102. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Lara, L.; Fernández-Arteaga, A.; Rufián-Henares, J.A.; Delgado, G. Washed hydrochar from spent coffee grounds: A second generation of coffee residues. Evaluation as organic amendment. Waste Manag. 2021, 120, 322–329. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Martín-García, J.; Delgado, R.; Sánchez-Marañón, M.; Delgado, G. Short-term effects of spent coffee grounds on the physical properties of two Mediterranean agricultural soils. Int. Agrophys. 2019, 33, 205–216. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Mondini, C.; Martín-García, J.M.; Delgado, G. Effects of the addition of spent coffee grounds combined with a nitrogen fertilizer on the soil-plant system. Agrochimica 2021, 63, 261–277. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.R.M.; Ferreira, I.M. Changes in macrominerals, trace elements and pigments content during lettuce (Lactuca sativa L.) growth: Influence of soil composition. Food Chem. 2014, 152, 603–611. [Google Scholar] [CrossRef]
- Morra, L.; Bilotto, M.; Baldantoni, D.; Alfani, A.; Biano, S. A seven-year experiment in a vegetable crops sequence: Effects of replacing mineral fertilizers with Biowaste compost on crop productivity, soil organic carbon and nitrates concentrations. Scientia Hortic. 2021, 290, 110534. [Google Scholar] [CrossRef]
- Hussain, N.; Abbasi, S.A. Efficacy of the Vermicomposts of Different Organic Wastes as “Clean” Fertilizers: State-of-the-Art. Sustainability 2018, 10, 1205. [Google Scholar] [CrossRef]
| Property | Effect | Organic Amendment | Reference |
|---|---|---|---|
| SOC | + | Biowaste compost | [11] |
| C stabilization | + | ||
| N-NO3 release | - | ||
| Microbial biomass C | + | Sorghum stubble, sugarcane bagasse, sugarcane mill mud | [29] |
| SOC | + | ||
| Soil aggregation | + | ||
| BD | + | Biochar | [30] |
| Microbial biomass C | + | ||
| Soil available N | + | Compost and vermicompost | [31] |
| BD | + | Agro-wastes | [32] |
| Porosity | + | Fermented plant juice | |
| CEC | + | Fermented plant juice with biochar | |
| C/N | + | ||
| Exchangeable K and Ca | + | ||
| Soil respiration | + | ||
| Soil microorganisms count | + | ||
| Fresh and dry weight | + | Bagasse, bio-slurry, kitchen waste compost | [33] |
| pH | + | Biochar + compost + NPK | [34] |
| TN | + | ||
| P available | + | ||
| CEC | + | ||
| K and Ca | + | ||
| Nutrient availability | + | Biochar from Euterpe oleracea seeds | [35] |
| Plant growth | - | ||
| BD | + | Macadamia husk compost | [36] |
| W33 | + | ||
| TOC | - | Crop residues | [37] |
| Net nitrogen mineralization | - | ||
| Soil structure | + | Biochar | [38] |
| Nutrient use efficiency | + | ||
| Aeration | + | ||
| Porosity | + | ||
| W33 | + | ||
| Aggregate stability | + | Compost | [39] |
| BD | + | ||
| W33 | + | ||
| Nutrients level | + | ||
| Plant available water content | + | Organic waste | [40] |
| Organic matter quality | + | ||
| Biologically available SOM | + | Organic farming | [41] |
| Soil microbe | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Burillo, S.; Cervera-Mata, A.; Fernández-Arteaga, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Delgado, G. Why Should We Be Concerned with the Use of Spent Coffee Grounds as an Organic Amendment of Soils? A Narrative Review. Agronomy 2022, 12, 2771. https://doi.org/10.3390/agronomy12112771
Pérez-Burillo S, Cervera-Mata A, Fernández-Arteaga A, Pastoriza S, Rufián-Henares JÁ, Delgado G. Why Should We Be Concerned with the Use of Spent Coffee Grounds as an Organic Amendment of Soils? A Narrative Review. Agronomy. 2022; 12(11):2771. https://doi.org/10.3390/agronomy12112771
Chicago/Turabian StylePérez-Burillo, Sergio, Ana Cervera-Mata, Alejandro Fernández-Arteaga, Silvia Pastoriza, José Ángel Rufián-Henares, and Gabriel Delgado. 2022. "Why Should We Be Concerned with the Use of Spent Coffee Grounds as an Organic Amendment of Soils? A Narrative Review" Agronomy 12, no. 11: 2771. https://doi.org/10.3390/agronomy12112771
APA StylePérez-Burillo, S., Cervera-Mata, A., Fernández-Arteaga, A., Pastoriza, S., Rufián-Henares, J. Á., & Delgado, G. (2022). Why Should We Be Concerned with the Use of Spent Coffee Grounds as an Organic Amendment of Soils? A Narrative Review. Agronomy, 12(11), 2771. https://doi.org/10.3390/agronomy12112771