Application of Light-Emitting Diodes with Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhiza Fungi for Tomato Seedling Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Investigation of the Optimum LED Light Condition
2.3. Determination of the Tomato Seedling Growth
2.4. Detection of H2O2 Accumulation and Antioxidant Enzyme Activities
2.5. Gene Reference Selection and Primers Design
2.6. Preparation of Plant Sample for RNA Extraction and Gene Expressions Analysis
2.7. Effect of PGPR Inoculation on Seedling Growth
2.8. Effect of PGPR Inoculation on Tomatoes under Different Light Conditions
2.9. Effect of AMF on Tomato Seedling Growth
2.10. Evaluation of Tomato Fruit Yield Production
2.11. Statistical Analysis
3. Results
3.1. Effect of Different Light Intensity on Tomato Seedling Growth
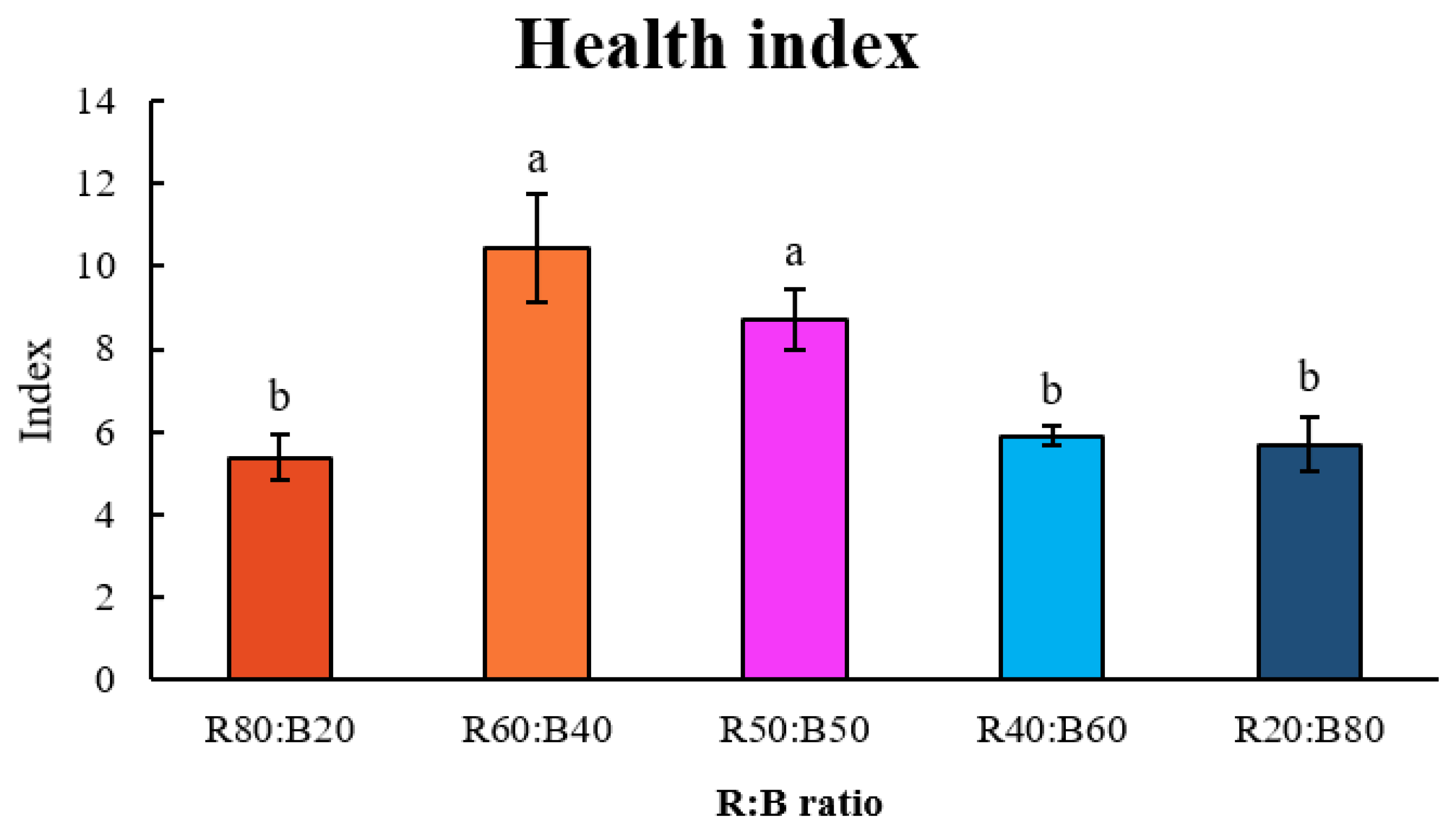
3.2. The Effect of Red (R) and Blue (B) Light Ratios on the Tomato Seedling Growth
3.3. Effects of Light Photoperiod on Tomato Seedlings Growth
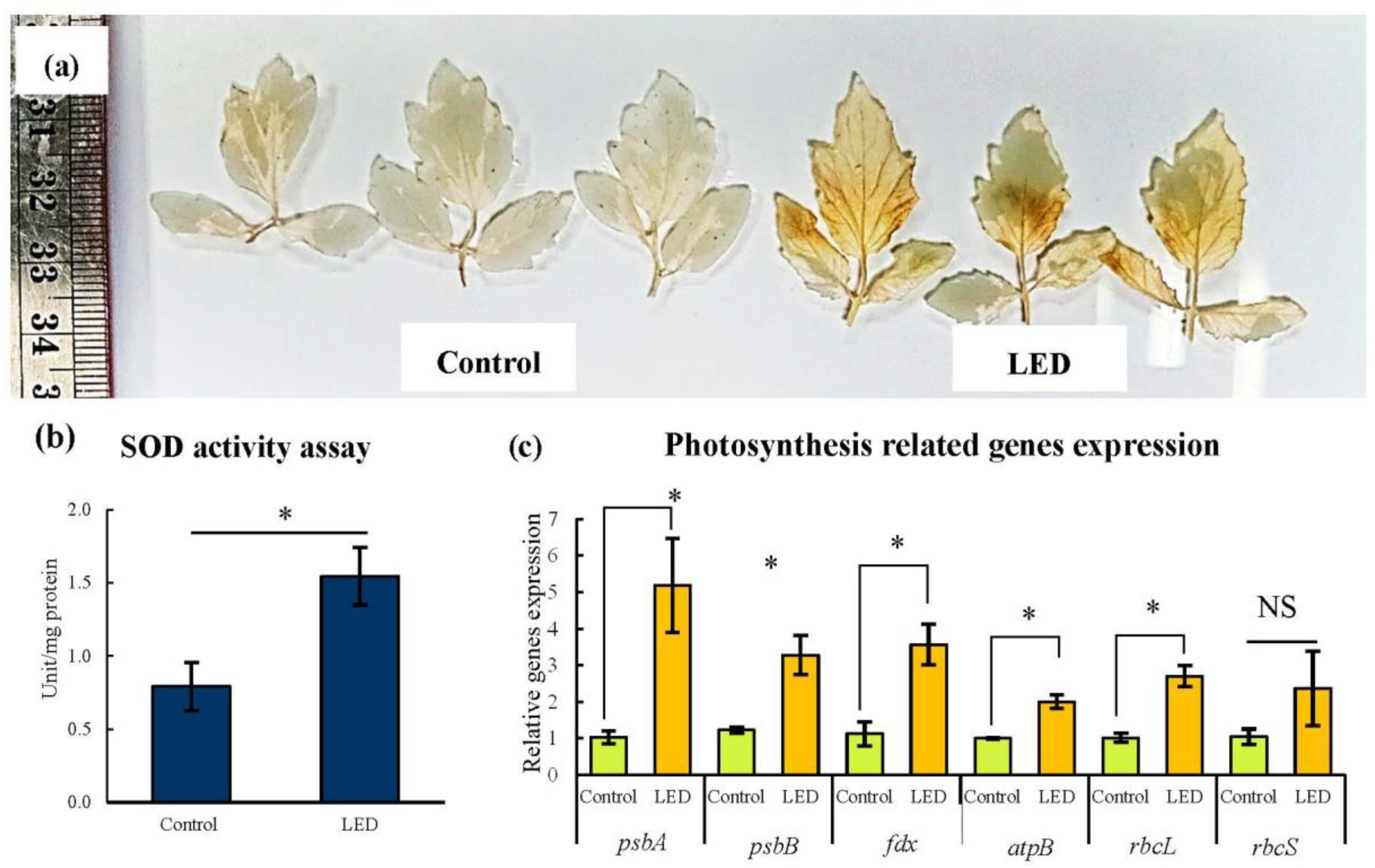
3.4. H2O2 Accumulation, SOD Activity Assay, and Photosynthetic Gene Expression in Leaves
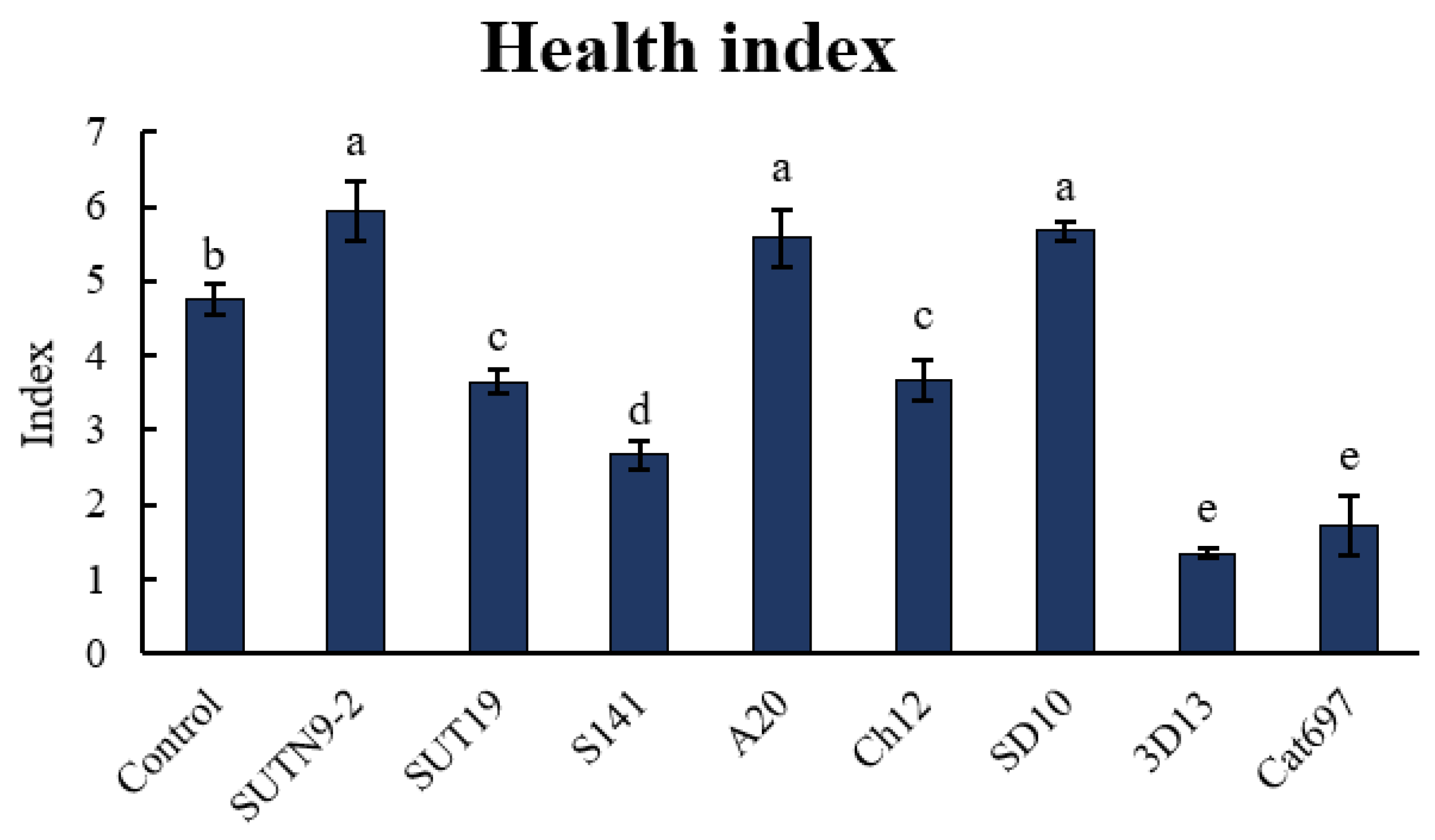
3.5. The Effect of PGPR on the Tomato Seedling Growth
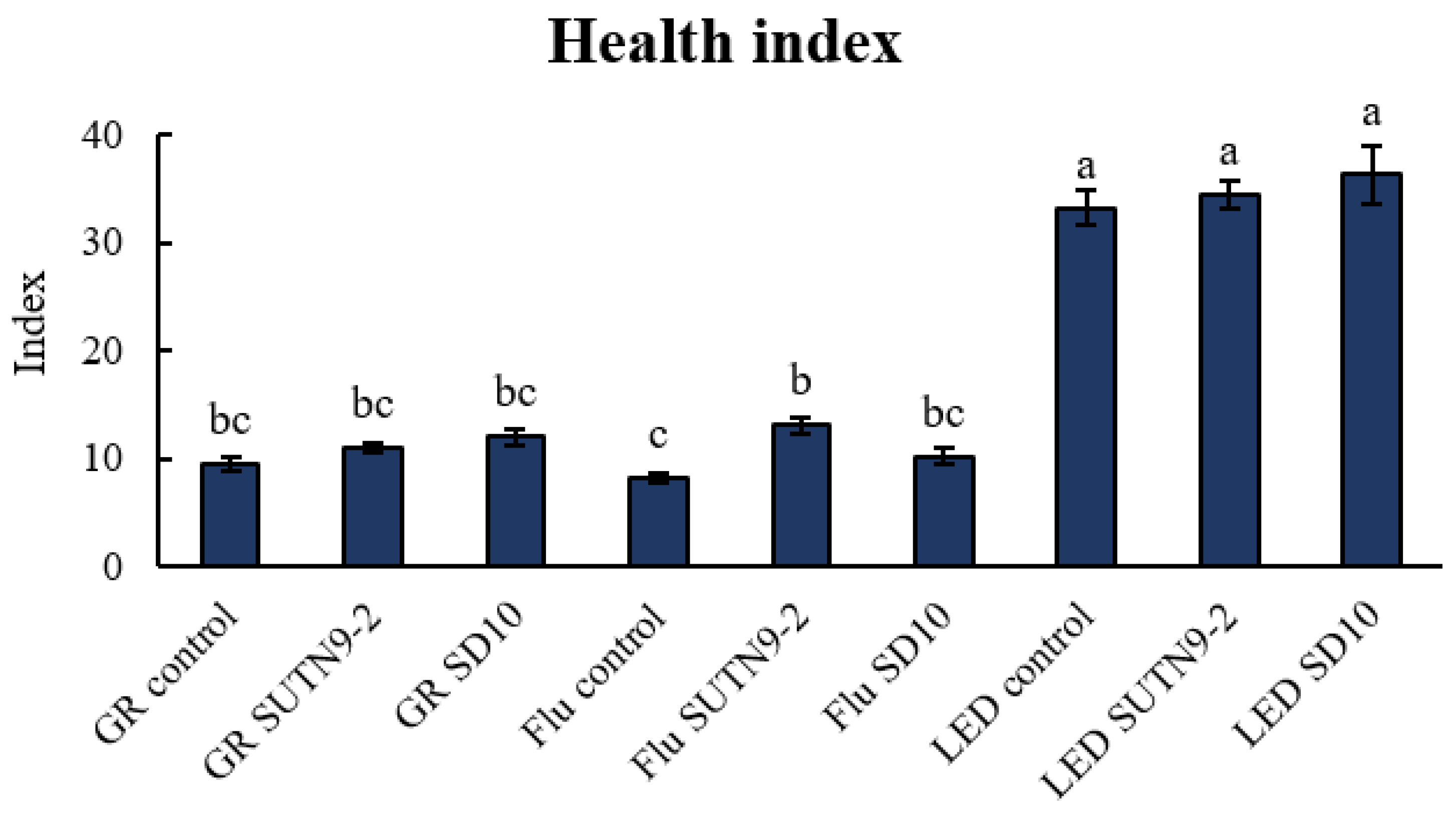
3.6. Effect of PGPR on Tomato Seedlings under Different Lighting Conditions
3.7. Effects of PGPR Inoculation on LED-illuminated Tomato Seedlings and AMF on Tomato Seedling Growth under Greenhouse Conditions
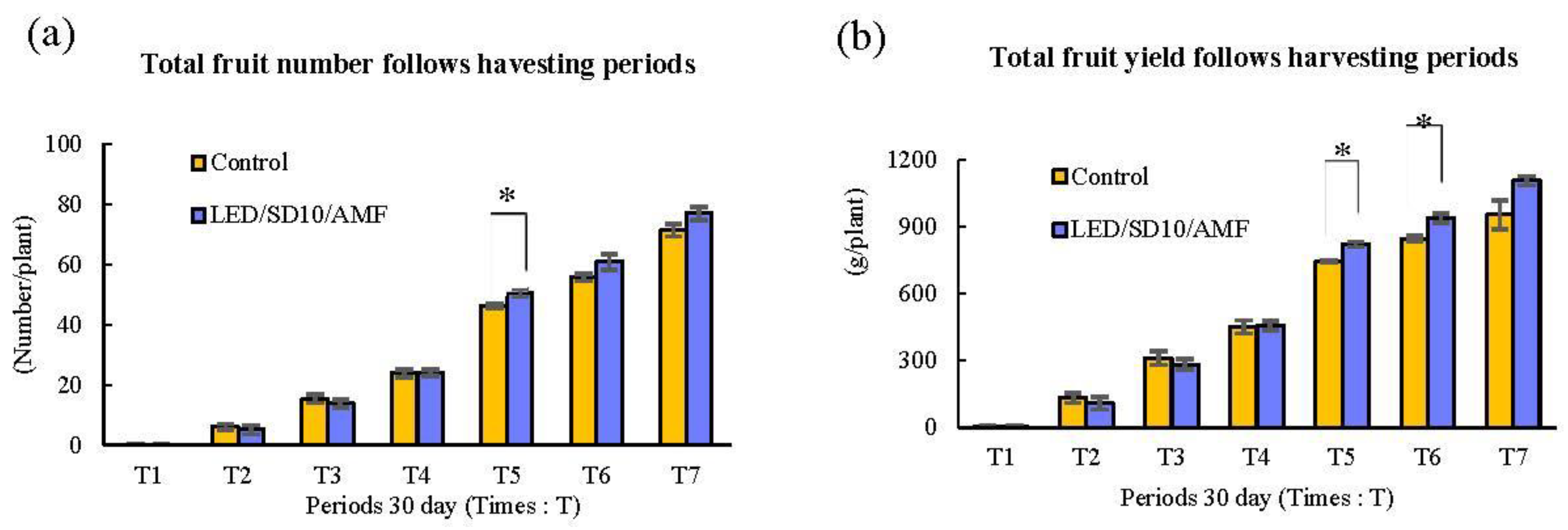
3.8. Evaluation of Tomato Fruit Yield Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal Red:Blue Ratio in Led Lighting for Nutraceutical Indoor Horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Song, W.; Wang, L.; Guo, W.; Xue, X. Growth and Nutritional Properties of Lettuce Affected by Different Alternating Intervals of Red and Blue LED Irradiation. Sci. Hortic. 2017, 223, 44–52. [Google Scholar] [CrossRef]
- Yao, X.; Liu, X.; Xu, Z.; Jiao, X. Effects of Light Intensity on Leaf Microstructure and Growth of Rape Seedlings Cultivated under a Combination of Red and Blue LEDs. J. Integr. Agric. 2017, 16, 97–105. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Długosz-Grochowska, O.; Kołton, A.; Żupnik, M. Effects of LED Supplemental Lighting on Yield and Some Quality Parameters of Lamb’s Lettuce Grown in Two Winter Cycles. Sci. Hortic. 2015, 187, 80–86. [Google Scholar] [CrossRef]
- Xu, H.; Fu, Y.; Li, T.; Wang, R. Effects of Different LED Light Wavelengths on the Resistance of Tomato against Botrytis Cinerea and the Corresponding Physiological Mechanisms. J. Integr. Agric. 2017, 16, 106–114. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue Light-Emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth after Transplanting in Red Leaf Lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Alori, E.T.; Babalola, O.O. Microbial Inoculants for Improving Crop Quality and Human Health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef]
- Trivedi, P.; Schenk, P.M.; Wallenstein, M.D.; Singh, B.K. Tiny Microbes, Big Yields: Enhancing Food Crop Production with Biological Solutions. Microb. Biotechnol. 2017, 10, 999–1003. [Google Scholar] [CrossRef]
- Alori, E.T.; Babalola, O.O.; Prigent-Combaret, C. Impacts of Microbial Inoculants on the Growth and Yield of Maize Plant. Open Agric. J. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol 2016, 6, 1559. [Google Scholar] [CrossRef]
- Commatteo, J.; Consolo, V.F.; Barbieri, P.A.; Covacevich, F. Publicación: Indigenous arbuscular mycorrhiza and Trichoderma from systems with soybean predominance can improve tomato growth. Soil Environ. 2019, 38, 151–161. [Google Scholar] [CrossRef]
- Chliyeh, M.; Chahdi, A.O.; Selmaoui, K.; Ouazzani, A.; Maltouf, A.F.; Modafar, C.E.; Moukhli, A.; Oukabli, A.; Benkirane, R.; Douira, A. Effect of Trichoderma harzianum and arbuscular mycorrhizal fungi against verticillium wilt of tomato. Int. J. Recent Sci. Res. 2014, 5, 449–459. [Google Scholar]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X. Effects of Light Intensity on the Growth and Leaf Development of Young Tomato Plants Grown under a Combination of Red and Blue Light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical Detection of Superoxide and H2O2 Accumulation in Brassica Juncea Seedlings. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- Muneer, S.; Ko, C.H.; Wei, H.; Chen, Y.; Jeong, B.R. Physiological and Proteomic Investigations to Study the Response of Tomato Graft Unions under Temperature Stress. PLoS ONE 2016, 11, e0157439. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Shen, W.; Cui, J. Analyzing Photosynthetic Activity and Growth of Solanum Lycopersicum Seedlings Exposed to Different Light Qualities. Acta Physiol. Plant 2014, 36, 1411–1420. [Google Scholar] [CrossRef]
- Guo, X.; Xie, Q.; Li, B.; Su, H. Molecular Characterization and Transcription Analysis of DNA Methyltransferase Genes in Tomato (Solanum Lycopersicum). Genet. Mol. Biol. 2020, 43, e20180295. [Google Scholar] [CrossRef]
- Greetatorn, T.; Hashimoto, S.; Maeda, T.; Fukudome, M.; Piromyou, P.; Teamtisong, K.; Tittabutr, P.; Boonkerd, N.; Kawaguchi, M.; Uchiumi, T.; et al. Mechanisms of Rice Endophytic Bradyrhizobial Cell Differentiation and Its Role in Nitrogen Fixation. Microbes Environ. 2020, 35, ME20049. [Google Scholar] [CrossRef]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.; Teaumroong, N. Co-Inoculation of Bacillus Velezensis Strain S141 and Bradyrhizobium Strains Promotes Nodule Growth and Nitrogen Fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Venkateswar Rao, L. Isolation and Characterization of Drought-Tolerant ACC Deaminase and Exopolysaccharide-Producing Fluorescent Pseudomonas sp. Ann. Microbiol. 2014, 64, 493–502. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, X.; Wang, J.; Ding, X. Phosphate Solubilization and Gene Expression of Phosphate-Solubilizing Bacterium Burkholderia multivorans WS-FJ9 under Different Levels of Soluble Phosphate. J. Microbiol. Biotechnol. 2017, 27, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Yuttavanichakul, W.; Lawongsa, P.; Wongkaew, S.; Teaumroong, N.; Boonkerd, N.; Nomura, N.; Tittabutr, P. Improvement of Peanut Rhizobial Inoculant by Incorporation of Plant Growth Promoting Rhizobacteria (PGPR) as Biocontrol against the Seed Borne Fungus, Aspergillus niger. Biol. Control 2012, 63, 87–97. [Google Scholar] [CrossRef]
- Kaur, H.; Sharda, R.; Sharma, P. Effect of hoagland solution for growing tomato hydroponi-cally in greenhouse. HortFlora Res. Spectr. 2016, 5, 310–315. [Google Scholar]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V.; Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de Méthode d’estimation Ayant Une Signification Fonctionnelle. In Proceedings of the European symposium on mycorrhizae, Paris, France, 1–5 July 1985. [Google Scholar]
- Feng, L.; Raza, M.A.; Li, Z.; Chen, Y.; Khalid, M.H.B.; Du, J.; Liu, W.; Wu, X.; Song, C.; Yu, L.; et al. The Influence of Light Intensity and Leaf Movement on Photosynthesis Characteristics and Carbon Balance of Soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef]
- Modarelli, G.C.; Paradiso, R.; Arena, C.; De Pascale, S.; Van Labeke, M.-C. High Light Intensity from Blue-Red LEDs Enhance Photosynthetic Performance, Plant Growth, and Optical Properties of Red Lettuce in Controlled Environment. Horticulturae 2022, 8, 114. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Guiducci, M.; Falcinelli, B.; Del Buono, D.; Benincasa, P. Blue: Red LED Light Proportion Affects Vegetative Parameters, Pigment Content, and Oxidative Status of Einkorn (Triticum monococcum L. ssp. Monococcum) Wheatgrass. J. Agric. Food Chem. 2020, 68, 8757–8763. [Google Scholar] [CrossRef]
- Ying, Q.; Jones-Baumgardt, C.; Zheng, Y.; Bozzo, G. The Proportion of Blue Light from Light-Emitting Diodes Alters Microgreen Phytochemical Profiles in a Species-Specific Manner. HortScience 2021, 56, 13–20. [Google Scholar] [CrossRef]
- Kang, J.; Sugumaran, K.; Atulba, S.L.; Jeong, B.R.; Hwang, S. Light Intensity and Photoperiod Influence the Growth and Development of Hydroponically Grown Leaf Lettuce in a Closed-Type Plant Factory System. Horticulture 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED Photoperiods and Light Qualities on in Vitro Growth and Chlorophyll Fluorescence of Cunninghamia Lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Dieleman, J.; Visser, P.; Meinen, E.; Grit, J.; Dueck, T. Integrating Morphological and Physiological Responses of Tomato Plants to Light Quality to the Crop Level by 3D Modeling. Front. Plant Sci. 2019, 10, 839. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Klejnot, J.; Lin, C. The Cryptochrome Blue Light Receptors. Arab. Book 2010, 8, e0135. [Google Scholar] [CrossRef] [PubMed]
- Ologundudu, A.F.; Adelusi, A.A.; Adekoya, K.P. Effect of Light Stress on Germination and Growth Parameters of Corchorus olitoriusi, Celosia argentea, Amaranthus cruentus, Abelmoschus esculentus and Delonix regia. Not. Sci. Biol. 2013, 5, 468–475. [Google Scholar] [CrossRef][Green Version]
- Naznin, M.T.; Lefsrud, M.G. Impact of LED Irradiance on Plant Photosynthesis and Action Spectrum of Plantlet; Awwal, A.A.S., Iftekharuddin, K.M., Matin, M.A., Márquez, A., Eds.; SPIE: Bellingham, WA, USA, 2014; p. 921602. [Google Scholar]
- Lu, T.; Yu, H.; Li, Q.; Chai, L.; Jiang, W. Improving Plant Growth and Alleviating Photosynthetic Inhibition and Oxidative Stress From Low-Light Stress With Exogenous GR24 in Tomato (Solanum Lycopersicum L.) Seedlings. Front. Plant Sci. 2019, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, S.; Zhang, M.; Jiang, S.; Xiao, Y. Light Intensity Affects Growth, Photosynthetic Capability, and Total Flavonoid Accumulation of Anoectochilus Plants. HortScience 2010, 45, 863–867. [Google Scholar] [CrossRef]
- Mil Thorpe, F.L.; Newton, P. Studies on the Expansion of the Leaf Surface: III. The influence of radiation on cell division and leaf expansion. J. Exp. Bot. 1963, 14, 483–495. [Google Scholar] [CrossRef]
- OuYang, F.; Mao, J.-F.; Wang, J.; Zhang, S.; Li, Y. Transcriptome Analysis Reveals That Red and Blue Light Regulate Growth and Phytohormone Metabolism in Norway Spruce [Picea Abies (L.) Karst.]. PLoS ONE 2015, 10, e0127896. [Google Scholar]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving Spinach, Radish, and Lettuce Growth under Red Light-Emitting Diodes (LEDs) with Blue Light Supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.; He, S.; Shi, L.; Song, Y.; Lou, X.; He, D. LED-Supplied Red and Blue Light Alters the Growth, Antioxidant Status, and Photochemical Potential of in Vitro-Grown Gerbera jamesonii Plantlets. Hortic. Sci. Technol. 2019, 37, 473–489. [Google Scholar]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of Red and Blue Light on Leaf Anatomy, CO2 Assimilation and the Photosynthetic Electron Transport Capacity of Sweet Pepper (Capsicum Annuum L.) Seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod Control of Plant Growth: Flowering Time Genes Beyond Flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef]
- Huang, J.J.; D’Souza, C.; Zhou, W. Light-Time-Biomass Response Model for Predicting the Growth of Choy Sum (Brassica Rapa var. Parachinensis) in Soil-Based LED-Constructed Indoor Plant Factory for Efficient Seedling Production. Front. Plant Sci. 2021, 12, 623682. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, J.; Hu, J.; Jeong, B.R. Critical Photoperiod and Optimal Quality of Night Interruption Light for Runner Induction in June-Bearing Strawberries. Agronomy 2021, 11, 1996. [Google Scholar] [CrossRef]
- Lee, J.-B.; Kim, S.-H.; Lee, S.-C.; Kim, H.-G.; Ahn, H.-G.; Li, Z.; Yoon, K.C. Blue Light-Induced Oxidative Stress in Human Corneal Epithelial Cells: Protective Effects of Ethanol Extracts of Various Medicinal Plant Mixtures. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4119–4127. [Google Scholar] [CrossRef] [PubMed]
- Rossa, M.M.; de Oliveira, M.C.; Okamoto, O.K.; Lopes, P.F.; Colepicolo, P. Effect of Visible Light on Superoxide Dismutase (SOD) Activity in the Red Alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). J. Appl. Phycol. 2002, 14, 151–157. [Google Scholar] [CrossRef]
- Luciński, R.; Jackowski, G. The Structure, Functions and Degradation of Pigment-Binding Proteins of Photosystem II. Acta Biochim. Pol. 2006, 53, 693–708. [Google Scholar] [CrossRef]
- De Weerd, F.L.; van Stokkum, I.H.M.; van Amerongen, H.; Dekker, J.P.; van Grondelle, R. Pathways for Energy Transfer in the Core Light-Harvesting Complexes CP43 and CP47 of Photosystem II. Biophys. J. 2002, 82, 1586–1597. [Google Scholar] [CrossRef]
- Kim, N.N.; Shin, H.S.; Park, H.G.; Lee, J.; Kil, G.-S.; Choi, C.Y. Profiles of Photosynthetic Pigment Accumulation and Expression of Photosynthesis-Related Genes in the Marine Cyanobacteria Synechococcus sp.: Effects of LED Wavelengths. Biotechnol. Bioprocess Eng. 2014, 19, 250–256. [Google Scholar] [CrossRef]
- Singh, M. Turnover of D1 Protein Encoded by PsbA Gene in Higher Plants and Cyanobacteria Sustains Photosynthetic Efficiency to Maintain Plant Productivity Under Photoinhibitory Irradiance. Photosynthetica 2000, 38, 161–169. [Google Scholar] [CrossRef]
- Ahmad, N.; Zaidi, S.S.-A.; Mansoor, S. Alternative Routes to Improving Photosynthesis in Field Crops. Trends Plant Sci. 2020, 25, 958–960. [Google Scholar] [CrossRef]
- Kale, R.; Hebert, A.E.; Frankel, L.K.; Sallans, L.; Bricker, T.M.; Pospíšil, P. Amino Acid Oxidation of the D1 and D2 Proteins by Oxygen Radicals during Photoinhibition of Photosystem II. Proc. Natl. Acad. Sci. USA 2017, 114, 2988–2993. [Google Scholar] [CrossRef]
- Pfannschmidt, T.; Nilsson, A.; Tullberg, A.; Link, G.; Allen, J. Direct Transcriptional Control of the Chloroplast Genes PsbA and PsaAB Adjusts Photosynthesis to Light Energy Distribution in Plants. IUBMB Life 1999, 48, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Khan, R.; Gao, H.; Liu, H.; Zhang, J.; Ma, X. Low Light Alters the Photosynthesis Process in Cigar Tobacco via Modulation of the Chlorophyll Content, Chlorophyll Fluorescence, and Gene Expression. Agriculture 2021, 11, 755. [Google Scholar] [CrossRef]
- Chotewutmontri, P.; Barkan, A. Light-Induced PsbA Translation in Plants Is Triggered by Photosystem II Damage via an Assembly-Linked Autoregulatory Circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 21775–21784. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, D.; Yang, X.; Zhao, Y.; Dai, L.; Zeng, D.; Wang, Q.; Gao, L.; Li, S. ZmFdC2 Encoding a Ferredoxin Protein With C-Terminus Extension Is Indispensable for Maize Growth. Front. Plant Sci. 2021, 12, 646359. [Google Scholar] [CrossRef]
- Kozuleva, M.; Goss, T.; Twachtmann, M.; Rudi, K.; Trapka, J.; Selinski, J.; Ivanov, B.; Garapati, P.; Steinhoff, H.-J.; Hase, T.; et al. Ferredoxin:NADP(H) Oxidoreductase Abundance and Location Influences Redox Poise and Stress Tolerance1. Plant Physiol. 2016, 172, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, X.; Jeong, B.R. Manipulating the Difference between the Day and Night Temperatures Can Enhance the Quality of Astragalus membranaceus and Codonopsis lanceolata Plug Seedlings. Agronomy 2019, 9, 654. [Google Scholar] [CrossRef]
- Bovy, A.; Van Den Berg, C.; De Vrieze, G.; Thompson, W.F.; Weisbeek, P.; Smeekens, S. Light-Regulated Expression of the Arabidopsis thaliana Ferredoxin Gene Requires Sequences Upstream and Downstream of the Transcription Initiation Site. Plant Mol. Biol. 1995, 27, 27–39. [Google Scholar] [CrossRef]
- Gallo-Meagher, M.; Sowinski, D.A.; Thompson, W.F. The Pea Ferredoxin I Gene Exhibits Different Light Responses in Pea and Tobacco. Plant Cell 1992, 4, 383–388. [Google Scholar]
- Ren, X.; Liu, Y.; Jeong, H.K.; Jeong, B.R. Supplementary Light Source Affects the Growth and Development of Codonopsis lanceolata Seedlings. Int. J. Mol. Sci. 2018, 19, 3074. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J.; Li, Y.; Sun, H.; Ma, T.; Huai, J.; Yang, W.; Zhang, W.; Lin, R. The CDC48 Complex Mediates Ubiquitin-Dependent Degradation of Intra-Chloroplast Proteins in Plants. Cell Rep. 2022, 39, 110664. [Google Scholar] [CrossRef]
- Valle, K.C.; Nymark, M.; Aamot, I.; Hancke, K.; Winge, P.; Andresen, K.; Johnsen, G.; Brembu, T.; Bones, A.M. System Responses to Equal Doses of Photosynthetically Usable Radiation of Blue, Green, and Red Light in the Marine Diatom Phaeodactylum tricornutum. PLoS ONE 2014, 9, e114211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, N.; Li, X.; Sun, G.; Shi, G. Photosynthetic Function and the Photoprotective Mechanism of Leaves of Morus Alba L. Seedlings under NaCl and NaHCO3 Stress Revealed by Proteomics. Preprints 2019, 2019050167. [Google Scholar]
- Manzara, T.; Gruissem, W. Organization and Expression of the Genes Encoding Ribulose-1,5-Bisphosphate Carboxylase in Higher Plants. Photosynth. Res. 1988, 16, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Davydov, I.I.; Besnard, G.; Salamin, N. Duplication History and Molecular Evolution of the RbcS Multigene Family in Angiosperms. J. Exp. Bot. 2019, 70, 6127–6139. [Google Scholar] [CrossRef] [PubMed]
- Inamine, G.; Nash, B.; Weissbach, H.; Brot, N. Light Regulation of the Synthesis of the Large Subunit of Ribulose-1,5-Bisphosphate Carboxylase in Peas: Evidence for Translational Control. Proc. Natl. Acad. Sci. USA 1985, 82, 5690–5694. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ishiye, M.; Sakihama, T.; Kamikubo, T. Light-Induced Increase of MRNA Activity Coding for the Small Subunit of Ribulose-1,5-Bisphosphate Carboxylase. J. Biol. Chem. 1981, 256, 2315–2320. [Google Scholar] [CrossRef]
- Matusova, R.; Rani, K.; Verstappen, F.W.A.; Franssen, M.C.R.; Beale, M.H.; Bouwmeester, H.J. The Strigolactone Germination Stimulants of the Plant-Parasitic Striga and Orobanche spp. Are Derived from the Carotenoid Pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef]
- Nazir, N.; Kamili, A.N.; Zargar, M.Y.; Khan, I.; Shah, D.; Tyub, S. Effect of Root Exudates on Rhizosphere Soil Microbial Communities. J. Res. Dev. 2016, 16, 9. [Google Scholar]
- Williams, A.; de Vries, F.T. Plant Root Exudation under Drought: Implications for Ecosystem Functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef]
- Yang, L. Root Exudation Pattern of Sugar Beet (Beta Vulgaris L.) as Influenced by Light Intensity and P Deficiency. Ph.D. Thesis, Georg-August-University of Göttingen, Lower Saxony, Germany, 2016. Available online: https://ediss.uni-goettingen.de/handle/11858/00-1735-0000-0028-87DC-8 (accessed on 29 June 2022).
- Zhou, C.; Zhang, Y.; Liu, W.; Zha, L.; Shao, M.; Li, B. Light Quality Affected the Growth and Root Organic Carbon and Autotoxin Secretions of Hydroponic Lettuce. Plants 2020, 9, 1542. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-Inoculation of Arbuscular Mycorrhizal Fungi and the Plant Growth-Promoting Rhizobacteria Improve Growth and Photosynthesis in Tobacco Under Drought Stress by Up-Regulating Antioxidant and Mineral Nutrition Metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, H.; Zhang, W.; Liu, K.; Liu, M.; Shao, X. Cooperation between Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Bacteria and Their Effects on Plant Growth and Soil Quality. PeerJ 2022, 10, e13080. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Luque, A.; Tille, S.; Johnson, I.; Pascual-Pardo, D.; Ton, J.; Cameron, D.D. The Interactive Effects of Arbuscular Mycorrhiza and Plant Growth-Promoting Rhizobacteria Synergistically Enhance Host Plant Defences against Pathogens. Sci. Rep. 2017, 7, 16409. [Google Scholar] [CrossRef] [PubMed]
- Son, K.-H.; Kim, E.-Y.; Oh, M.-M. Growth and Development of Cherry Tomato Seedlings Grown under Various Combined Ratios of Red to Blue LED Lights and Fruit Yield and Quality after Transplanting. Prot. Hortic. Plant Fact. 2018, 27, 54–63. [Google Scholar] [CrossRef]
- Young, T.; Cameron, D.D.; Phoenix, G.K. Using AMF Inoculum to Improve the Nutritional Status of Prunella Vulgaris Plants in Green Roof Substrate during Establishment. Urban For. Urban Green 2015, 14, 959–967. [Google Scholar] [CrossRef]
- Salvioli, A.; Zouari, I.; Chalot, M.; Bonfante, P. The Arbuscular Mycorrhizal Status Has an Impact on the Transcriptome Profile and Amino Acid Composition of Tomato Fruit. BMC Plant Biol. 2012, 12, 44. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.; Anli, M.; Meddich, A.; Oufdou, K. Use of Rhizobacteria and Mycorrhizae Consortium in the Open Field as a Strategy for Improving Crop Nutrition, Productivity and Soil Fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef]
- Nadimi, M.; Sun, D.-W.; Paliwal, J. Recent Applications of Novel Laser Techniques for Enhancing Agricultural Production. Laser Phys. 2021, 31, 053001. [Google Scholar] [CrossRef]



| Primer | Sequence (5′-3′) | Reference |
|---|---|---|
| Actin | F: GAAATAGCATAAGATGGCAGACG R: ATACCCACCATCACACCAGTAT | [17] |
| rbcL | F: CTGCGAATCCCTCCTGCTTA R: CCAACAGGGGACGACCATAC | [16] |
| rbcS | F: TGAGACTGA GCACGGATTTG R: TTTAGCCTCTTGAACCT CAGC | |
| pabA | F: CCGTAAAGTAGAGACCCTGAAAC R: TGGATG GTTTGGTGTTTTGATG | |
| pabB | F: CCTATTCCATCTTAGCGTCCG R: TTGCC GAACCATACCACATAG | |
| atpB | F: TGGGCGGTTTCGTAATGTTC R: GTACCCGCAGACGATTTGAC | This study |
| fdx | F: GTGTGATTCATACTCACCAGG R: CACCTGACCATTCTCAATTACAG |
| Light Intensity (µmol/m2/s) | Shoot Height (cm) | Root Length (cm) | Stem Diameter (mm) | Total Fresh Weight (g) | Total Dry Weight (mg) | Chlorophyll Content (SPAD Unit) | Leaves Area (cm2) | Health Index |
|---|---|---|---|---|---|---|---|---|
| Control | 7.30 ± 0.06 a | 6.11 ± 0.17 bc | 1.33 ± 0.09 a | 0.31 ± 0.04 a | 20.76 ± 2.98 ab | 23.9 ± 0.6 e | 6.96 ± 0.89 a | 3.87 ± 0.84 b |
| Fluorescent | 4.18 ± 0.18 b | 5.64 ± 0.32 c | 1.06 ± 0.09 b c | 0.19 ± 0.00 c | 13.40 ± 0.30 cd | 28.6 ± 1.0 d | 6.911.04 a | 3.38 ± 0.22 b |
| 50 | 3.68 ± 0.05 c | 2.90 ± 0.11 d | 0.98 ± 0.07 c | 0.11 ± 0.00 d | 7.92 ± 0.78 d | 27.5 ± 1.2 de | 3.560.08 b | 2.14 ± 0.35 b |
| 100 | 4.07 ± 0.05 b | 6.10 ± 0.63 bc | 1.23 ± 0.02 abc | 0.24 ± 0.01 bc | 16.38 ± 1.56 bc | 30.8 ± 2.3 d | 7.730.63 a | 4.96 ± 0.56 b |
| 200 | 4.14 ± 0.13 b | 8.03 ± 0.77 ab | 1.43 ± 0.06 a | 0.33 ± 0.01 a | 26.93 ± 0.96 a | 39.2 ± 2.2 c | 8.88 ± 0.82 a | 9.29 ± 0.57 a |
| 300 | 3.89 ± 0.10 bc | 8.94 ± 0.61 a | 1.45 ± 0.09 a | 0.33 ± 0.04 a | 26.70 ± 3.61 a | 40.0 ± 1.3 bc | 8.78 ± 1.14 a | 9.85 ± 1.21 a |
| 400 | 3.31 ± 0.05 d | 8.46 ± 1.21 a | 1.32 ± 0.04 abc | 0.26 ± 0.02 abc | 27.53 ± 2.91 a | 44.0 ± 1.2 ab | 6.66 ± 0.64 a | 10.97 ± 1.33 a |
| 500 | 3.23 ± 0.09 d | 7.71 ± 0.12 a b | 1.24 ± 0.11 ab | 0.27 ± 00 abc | 27.01 ± 1.33 a | 46.3 ± 0.9 a | 6.49 ± 0.78 a | 10.42 ± 1.25 a |
| Treatment | Shoot Height (cm) | Stem Diameter (mm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Total Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | Total Dry Weight (g) | Root Colonization (%) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 43.6 ± 1.86 d | 4.6 ± 0.15 e | 17.0 ± 1.65 d | 5.3 ± 0.59 c | 22.4 ± 2.21 d | 2.2 ± 0.27 c | 0.43 ± 0.05 c | 2.6 ± 0.32 a | 0.0 ± 0.00 c |
| Con/SD10 | 67.2 ± 0.58 b | 5.3 ± 0.21 bc | 32.2 ± 1.91 b | 9.3 ± 1.17 ab | 41.5 ± 3.00 b | 4.6 ± 0.21 b | 0.60 ± 0.12 c | 5.2 ± 0.12 bc | 0.0 ± 0.00 c |
| Con/AMF | 56.4 ± 1.91 c | 4.7 ± 0.13 de | 23.5 ± 1.30 c | 7.4 ± 0.60 bc | 31.0 ± 1.69 c | 2.8 ± 0.17 c | 0.53 ± 0.04 c | 3.3 ± 0.20 d | 11.3 ± 0.95 a |
| Con/SD10/AMF | 70.0 ± 2.28 b | 4.9 ± 0.08 cde | 29.4 ± 2.9 b | 7.8 ± 0.93 bc | 37.4 ± 3.33 b c | 4.2 ± 0.38 b | 0.67 ± 0.08 bc | 4.8 ± 0.42 bc | 1.5 ± 0.53 c |
| LED | 67.4 ± 1.07 b | 5.2 ± 0.10 bcd | 32.4 ± 1.96 b | 8.0 ± 0.87 bc | 40.1 ± 2.46 b | 4.4 ± 0.22 b | 0.69 ± 0.10 bc | 5.1 ± 0.29 bc | 0.0 ± 0.00 c |
| LED/SD10 | 66.4 ± 1.80 b | 5.5 ± 0.18 ab | 33.7 ± 2.20 b | 11.3 ± 1.31 a | 45.1 ± 3.33 b | 4.8 ± 0.46 b | 0.90 ± 0.12 ab | 5.7 ± 0.41 b | 0.0 ± 0.00 c |
| LED/AMF | 65.4 ± 1.63 b | 5.6 ± 0.12 ab | 32.1 ± 0.73 b | 8.2 ± 0.19 b c | 40.2 ± 0.87 b | 3.9 ± 0.11 b | 0.61 ± 0.02 bc | 4.5 ± 0.11 c | 10 ± 0.83 a |
| LED/SD10/AMF | 75.0 ± 1.44 a | 6.0 ± 0.29 a | 40.2 ± 1.14 a | 12.3 ± 1.71 a | 52.5 ± 2.48 a | 6.0 ± 0.22 a | 1.08 ± 0.13 a | 7.0 ± 0.32 a | 7.4 ± 1.19 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Songsaeng, A.; Tittabutr, P.; Umnajkitikorn, K.; Boonkerd, N.; Wongdee, J.; Songwattana, P.; Piromyou, P.; Greetatorn, T.; Girdthai, T.; Teaumroong, N. Application of Light-Emitting Diodes with Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhiza Fungi for Tomato Seedling Production. Agronomy 2022, 12, 2458. https://doi.org/10.3390/agronomy12102458
Songsaeng A, Tittabutr P, Umnajkitikorn K, Boonkerd N, Wongdee J, Songwattana P, Piromyou P, Greetatorn T, Girdthai T, Teaumroong N. Application of Light-Emitting Diodes with Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhiza Fungi for Tomato Seedling Production. Agronomy. 2022; 12(10):2458. https://doi.org/10.3390/agronomy12102458
Chicago/Turabian StyleSongsaeng, Apisit, Panlada Tittabutr, Kamolchanok Umnajkitikorn, Nantakorn Boonkerd, Jenjira Wongdee, Pongpan Songwattana, Pongdet Piromyou, Teerana Greetatorn, Teerayoot Girdthai, and Neung Teaumroong. 2022. "Application of Light-Emitting Diodes with Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhiza Fungi for Tomato Seedling Production" Agronomy 12, no. 10: 2458. https://doi.org/10.3390/agronomy12102458
APA StyleSongsaeng, A., Tittabutr, P., Umnajkitikorn, K., Boonkerd, N., Wongdee, J., Songwattana, P., Piromyou, P., Greetatorn, T., Girdthai, T., & Teaumroong, N. (2022). Application of Light-Emitting Diodes with Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhiza Fungi for Tomato Seedling Production. Agronomy, 12(10), 2458. https://doi.org/10.3390/agronomy12102458





