Enhancement of Agro-Industrial Waste Composting Process via the Microbial Inoculation: A Brief Review
Abstract
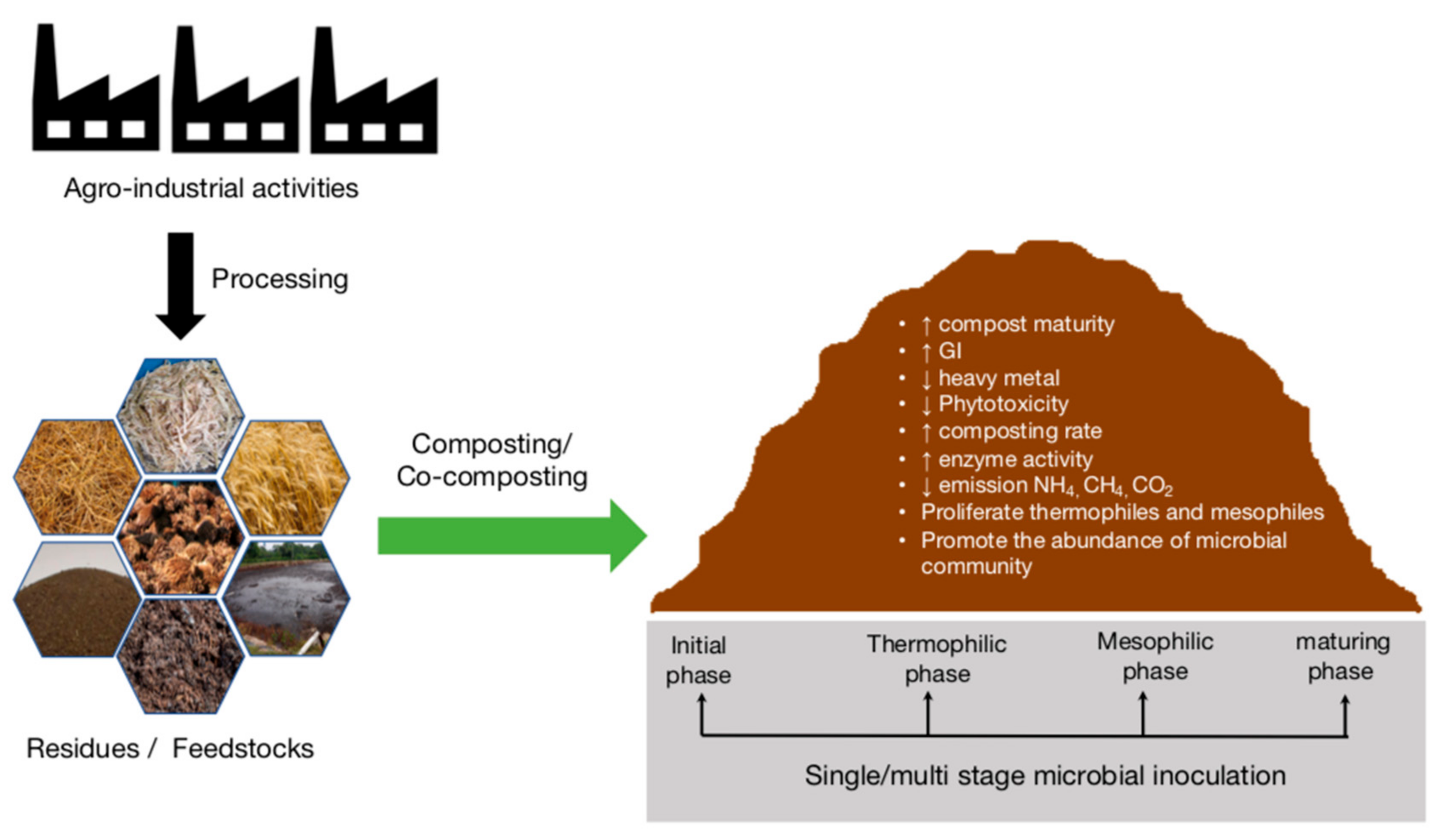
1. Introduction
2. Composting for Agro-Industrial Waste Treatment and Utilization
3. Inoculation Strategies for Enhancing the Composting Process
4. Microbial Inoculation with Additional Functions as a Means to Enhance the Composting Process
5. Future Perspectives and Recommendations
- The question of what is the appropriate technique of microbial inoculation to achieve a better composting process persists. In particular, it is difficult to establish what is the suitable concentration of a single or mixed inoculant to be added to the composting mixture. The optimum microbial concentration is still yet to be determined. In addition, the types of inoculants, functional, physiology, adaptability and stability of the microbes during the composting process also need to be considered. Therefore, research aimed at the microbial mechanism is required in order to find the most suitable microbial inoculants to be added during the composting process.
- There still exists significant knowledge gaps about the composting process enhancements through inoculation. For instance, based on the current literature search, there is limited research available dealing with the production of compost from agro-industrial waste, particularly at the semi-industrial or commercial scale. In comparison to the small or laboratory scale, it is difficult to control the composting in large-scale production since it is a self-heating and solid-state fermentation process, which hinders the replicability or reproducibility of the process. Thus, more studies on microbial inoculations at the semi-industrial or commercial scale need to be carried out for all types of composting systems such as the aerated static pile and the in-vessel system to validate the repeatability and advantages reported in small-scale studies.
- It is necessary to develop the technological procedures for the production of inoculants so that they are economically viable because growing microbes in the bioreactor submerge fermentation may be costly at the pilot or commercial scale. The solution such as the use of waste or low-cost materials such as molasses, sago and lignocellulose hydrolysate for propagating the inoculant should be developed to reduce the cost of production.
- Research in modeling and optimization for all composting systems by the engineering processing technique would be required in order to build up and sustain the overall composting process without compromising the quality of the composting product. Studies on significant statistical correlations should be carried out between the dynamics of microbial inoculants and important factors such as temperature, oxygen level, moisture and pH at different composting stages for monitoring the process. The effects of microbial inoculum on the indigenous microbial community in the composting pile should also be analyzed and recorded.
- The monitoring of microbial inoculants is also required to ensure the survival of inoculants and their impact on indigenous microbes throughout the composting process. The monitoring of microbial community structure during the composting process can be done through the use of culture-independent methods such as PCR-DGGE, high-throughput 16S rRNA sequencing.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Bank Group World Development Indicators: Structure of Output. 2020. Available online: http://wdi.worldbank.org/table/4.2 (accessed on 24 February 2020).
- Food and Agriculture Organization of the United Nations. World Crop Production. 2022. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 16 November 2021).
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Biotransformation of lignocellulosic materials into value-added products—A review. Int. J. Biol. Macromol. 2017, 98, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhaibani, N.; Selim, M.; Alderfasi, A.; El-Hendawy, S.; Selim, M. Comparative performance of integrated nutrient management between composted agricultural wastes, chemical fertilizers, and biofertilizers in improving soil quantitative and qualitative properties and crop yields under arid conditions. Agronomy 2020, 10, 1503. [Google Scholar] [CrossRef]
- Ghosh Ray, S.; Ghangrekar, M.M. Comprehensive review on treatment of high-strength distillery wastewater in advanced physico-chemical and biological degradation pathways. Int. J. Environ. Sci. Technol. 2019, 16, 527–546. [Google Scholar] [CrossRef]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Fan, Y.V.; Lee, C.T.; Ho, C.S.; Klemeš, J.J.; Wahab, R.A.; Chua, L.S.; Sarmidi, M.R. Evaluation of microbial inoculation technology for composting. Chem. Eng. Trans. 2017, 56, 433–438. [Google Scholar] [CrossRef]
- Toledo, M.; Siles, J.A.; Gutiérrez, M.C.; Martín, M.A. Monitoring of the composting process of different agroindustrial waste: Influence of the operational variables on the odorous impact. Waste Manag. 2018, 76, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Xia, J.; Chen, Y. Effect of microbial inoculation on physicochemical properties and bacterial community structure of citrus peel composting. Bioresour. Technol. 2019, 291, 121843. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Improving the sustainability of organic waste management practices in the food-energy-water nexus: A comparative review of anaerobic digestion and composting. Renew. Sustain. Energy Rev. 2018, 89, 151–167. [Google Scholar] [CrossRef]
- Huang, H.L.; Zeng, G.M.; Luo, L.; Zhang, J.C.; Yu, M.; Qin, P.F. Effect of inoculation during different phases of agricultural waste composting on spectroscopic characteristics of humic acid. J. Cent. South Univ. 2015, 22, 4177–4183. [Google Scholar] [CrossRef]
- Ngoc, Q.; Tran, M.; Mimoto, H.; Nakasaki, K. Inoculation of lactic acid bacterium accelerates organic matter degradation during composting. Int. Biodeterior. Biodegrad. 2015, 104, 377–383. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xu, Z.; Liu, Y.; Duan, H. Enhanced humification of maize straw and canola residue during composting by inoculating Phanerochaete chrysosporium in the cooling period. Bioresour. Technol. 2019, 293, 122075. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Li, J.; Liu, Z.; Xin, Y.; Wang, L.; Chen, J.; Gun, S. Effects of microbial inoculation on enzyme activity, available nitrogen content, and bacterial succession during pig manure composting. Bioresour. Technol. 2020, 306, 123–167. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Wang, X.; Cong, C.; Li, J.; Xu, Y.; Li, X.; Hou, F. Effect of inoculating microorganisms in chicken manure composting with maize straw. Bioresour. Technol. 2020, 301, 122730. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Qin, X.; Tian, X.; Zhao, Y.; Yang, T.; Huang, J. Inoculating with the microbial agents to start up the aerobic composting of mushroom residue and wood chips at low temperature. J. Environ. Chem. Eng. 2021, 9, 105294. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuge, C.; Weng, Q.; Hu, B. Additional strains acting as key microbes promoted composting process. Chemosphere 2022, 287, 132304. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Felissia, F.E.; Area, M.C. Hydrothermal treatments applied to agro- and forest-industrial waste to produce high added-value compounds. BioResources 2016, 12, 2058–2080. [Google Scholar] [CrossRef]
- Yusuf, M. Agro-industrial waste materials and their recycled value added applications: Review. In Handbook of Ecomaterials; Springer International Publishing: New York, NY, USA, 2017; pp. 1–11. ISBN 9783319482811. [Google Scholar]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Singh, R.; Das, R.; Sangwan, S.; Rohatgi, B.; Khanam, R.; Peera, S.K.P.G.; Das, S.; Lyngdoh, Y.A.; Langyan, S.; Shukla, A.; et al. Utilisation of agro-industrial waste for sustainable green production: A review. Environ. Sustain. 2021, 4, 619–636. [Google Scholar] [CrossRef]
- Hassan, M.A.; Ahmad Farid, M.A.; Shirai, Y.; Ariffin, H.; Othman, M.R.; Samsudin, M.H.; Hasan, M.Y. Oil palm biomass biorefinery for sustainable production of renewable materials. Biotechnol. J. 2019, 14, 1800394. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Bonilla, G.A.; Lopes, C.M.; Durrer, A.; Alves, P.R.L.; Passaglia, N.; Cardoso, J.B.N. Effect of phosphate-solubilizing bacteria on phosphorus dynamics and the bacterial community during composting of sugarcane industry waste. Syst. Appl. Microbiol. 2017, 40, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Tahir, P.; Liew, W.; Yih, S.; Fei, A.; Hua, S. Diversity and characterization of lignocellulolytic fungi isolated from oil palm empty fruit bunch, and identification of influencing factors of natural composting process. Waste Manag. 2020, 100, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Trisakti, B.; Mhardela, P.; Husaini, T.; Irvan; Daimon, H. Production of oil palm empty fruit bunch compost for ornamental plant cultivation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012094. [Google Scholar] [CrossRef]
- Irvan; Husaini, T.; Trisakti, B.; Batubara, F.; Daimon, H. Composting of empty fruit bunches in the tower composter-effect of air intake holes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012066. [Google Scholar] [CrossRef]
- Siddiquee, S.; Shafawati, S.N.; Naher, L. Effective composting of empty fruit bunches using potential Trichoderma strains. Biotechnol. Rep. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Zainudin, M.H.M.; Ramli, N.; Hassan, M.A.; Shirai, Y.; Tashiro, K.; Sakai, K.; Tashiro, Y. Bacterial community shift for monitoring the co-composting of oil palm empty fruit bunch and palm oil mill effluent anaerobic sludge. J. Ind. Microbiol. Biotechnol. 2017, 44, 869–877. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Susilawati, K.; Majid, N.M. Compost maturity and nitrogen availability by co-composting of paddy husk and chicken manure amended with clinoptilolite zeolite. Waste Manag. Res. 2015, 33, 322–331. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; El-din, M.N.; Refaat, B.M.; Abdel-shakour, E.H.; Ewais, E.E.; Alrefaey, H.M.A. Biotechnological application of thermotolerant cellulose-decomposing bacteria in composting of rice straw. Ann. Agric. Sci. 2016, 61, 135–143. [Google Scholar] [CrossRef]
- Gaind, S. Effect of fungal consortium and animal manure amendments on phosphorus fractions of paddy-straw compost. Int. Biodeterior. Biodegrad. 2014, 94, 90–97. [Google Scholar] [CrossRef]
- Devi, S.; Sharma, C.R.; Singh, K. Microbiological biodiversity in poultry and paddy straw wastes in composting systems. Braz. J. Microbiol. 2012, 43, 288–296. [Google Scholar] [CrossRef]
- Dadi, D.; Daba, G.; Beyene, A.; Luis, P.; Van der Bruggen, B. Composting and co-composting of coffee husk and pulp with source-separated municipal solid waste: A breakthrough in valorization of coffee waste. Int. J. Recycl. Org. Waste Agric. 2019, 8, 263–277. [Google Scholar] [CrossRef]
- Getachew, G.; Muleta, D. Optimization of compost maturity of coffee waste mixed with agricultural wastes and evaluation of their effect on growth of lettuce (Lactuca Sativa). Nat. Sci. Res. 2017, 7, 82–92. [Google Scholar]
- Calabi-floody, M.; Medina, J.; Suazo, J.; Ordiqueo, M.; Aponte, H.; Mora, D.L.L.M.; Rumpel, C. Optimization of wheat straw co-composting for carrier material development. Waste Manag. 2019, 98, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qiu, X.; Chen, L.; Zhang, C.; Ma, D.; Zhang, J. Succession of organics metabolic function of bacterial community in response to addition of earthworm casts and zeolite in maize straw composting. Bioresour. Technol. 2019, 280, 229–238. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, H.; Zhang, H.; Xun, L.; Chen, G.; Wang, L. Thermomyces lanuginosus is the dominant fungus in maize straw composts. Bioresour. Technol. 2015, 197, 266–275. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, S.; Gong, H.; Zhang, X.; Wu, C.; Li, W. Improving sewage sludge composting by addition of spent mushroom substrate and sucrose. Bioresour. Technol. 2018, 253, 197–203. [Google Scholar] [CrossRef]
- Lou, Z.; Sun, Y.; Zhou, X.; Baig, S.A.; Hu, B.; Xu, X. Composition variability of spent mushroom substrates during continuous cultivation, composting process and their effects on mineral nitrogen transformation in soil. Geoderma 2017, 307, 30–37. [Google Scholar] [CrossRef]
- Erana, F.G.; Tenkegna, T.A.; Asfaw, S.L. Effect of agro industrial wastes compost on soil health and onion yields improvemepnts: Study at field condition. Int. J. Recycl. Org. Waste Agric. 2019, 8, 161–171. [Google Scholar] [CrossRef]
- Patil, M.G.; Rathod, P.K.; Patil, V.D. Compost: A tool for managing soil borne plant pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2018, 6, 272–280. [Google Scholar]
- Mehta, C.M.; Palni, U.; Franke-Whittle, I.H.; Sharma, A.K. Compost: Its role, mechanism and impact on reducing soil-borne plant diseases. Waste Manag. 2014, 34, 607–622. [Google Scholar] [CrossRef]
- Chandini, K.R.; Kumar, R.; Prakash, O. The impact of chemical fertilizers on our environment and ecosystem. In Research Trends in Environmental Sciences; AkiNik Publications: Delhi, India, 2019; pp. 69–86. [Google Scholar]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.; Huang, Q.; Zhang, R.; Li, R.; Shen, B.; Shen, Q. Effects of organic-inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice-wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Enhancing nutrients use efficiency and grain yield of Zea mays L. Cultivated on a tropical acid soil using paddy husk compost and clinoptilolite zeolite. Bulg. J. Agric. Sci. 2017, 23, 418–428. [Google Scholar]
- Berecha, G.; Lemessa, F.; Wakjira, M. Exploring the suitability of coffee pulp compost as growth media substitute in greenhouse production. Int. J. Agric. Res. 2011, 6, 255–267. [Google Scholar] [CrossRef][Green Version]
- Yadav, K.S.; Mishra, M.M.; Kapoor, K.K. The effect of fungal inoculation on composting. Agric. Wastes 1982, 4, 329–333. [Google Scholar] [CrossRef]
- Faure, D.; Deschamps, A.M. The effect of bacterial inoculation on the initiation of composting of grape pulps. Bioresour. Technol. 1991, 37, 235–238. [Google Scholar] [CrossRef]
- Xi, B.D.; He, X.S.; Wei, Z.M.; Jiang, Y.H.; Li, M.X.; Li, D.; Li, Y.; Dang, Q.L. Effect of inoculation methods on the composting efficiency of municipal solid wastes. Chemosphere 2012, 88, 744–750. [Google Scholar] [CrossRef]
- Xi, B.; He, X.; Dang, Q.; Yang, T.; Li, M.; Wang, X.; Li, D.; Tang, J. Effect of multi-stage inoculation on the bacterial and fungal community structure during organic municipal solid wastes composting. Bioresour. Technol. 2015, 196, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.M.; Huang, H.L.; Huang, D.L.; Yuan, X.Z.; Jiang, R.Q.; Yu, M.; Yu, H.Y.; Zhang, J.C.; Wang, R.Y.; Liu, X.L. Effect of inoculating white-rot fungus during different phases on the compost maturity of agricultural wastes. Process Biochem. 2009, 44, 396–400. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Huang, Z.L.; Dong, M.; Yu, X.L.; Ning, P. A new strategy for co-composting dairy manure with rice straw: Addition of different inocula at three stages of composting. Waste Manag. 2015, 40, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, Q.; Wei, Y.; Cui, H.; Zhang, X.; Wang, X.; Shan, S.; Wei, Z. Effect of actinobacteria agent inoculation methods on cellulose degradation during composting based on redundancy analysis. Bioresour. Technol. 2016, 219, 196–203. [Google Scholar] [CrossRef]
- Zainudin, M.H.M.; Hassan, M.A.; Tokura, M.; Shirai, Y. Indigenous cellulolytic and hemicellulolytic bacteria enhanced rapid co-composting of lignocellulose oil palm empty fruit bunch with palm oil mill effluent anaerobic sludge. Bioresour. Technol. 2013, 147, 632–635. [Google Scholar] [CrossRef]
- Rastogi, M.; Nandal, M.; Khosla, B. Microbes as vital additives for solid waste composting. Heliyon 2020, 6, e03343. [Google Scholar] [CrossRef] [PubMed]
- Formowitz, B.; Elango, F.; Okumoto, S.; Müller, T.; Buerkert, A. The role of “effective microorganisms” in the composting of banana (Musa ssp.) residues. J. Plant Nutr. Soil Sci. 2007, 170, 649–656. [Google Scholar] [CrossRef]
- Cóndor-Golec, A.F.; Pérez, P.G.; Lokare, C. Effective Microorganisms: Myth or reality? Rev. Peru. Biol. 2007, 14, 315–319. [Google Scholar] [CrossRef]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.R. How effective are “Effective microorganisms® (EM)”? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- van Vliet, P.C.J.; Bloem, J.; de Goede, R.G.M. Microbial diversity, nitrogen loss and grass production after addition of Effective Micro-organisms (EM) to slurry manure. Appl. Soil Ecol. 2006, 32, 188–198. [Google Scholar] [CrossRef]
- Che Jusoh, M.L.; Abd Manaf, L.; Abdul Latiff, P. Composting of rice straw with effective microorganisms (EM) and its influence on compost quality. Iran. J. Environ. Health Sci. Eng. 2013, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.Y.; Chua, L.S.; Lee, C.T. Effects of microbial additive on the physiochemical and biological properties of oil palm empty fruit bunches compost. J. Eng. Sci. Technol. 2015, 10, 10–18. [Google Scholar]
- Wu, D.; Wei, Z.; Qu, F.; Ahmed, T.; Zhu, L.; Zhao, Y.; Jia, L. Effect of Fenton pretreatment combined with bacteria inoculation on humic substances formation during lignocellulosic biomass composting derived from rice straw. Bioresour. Technol. 2020, 303, 122849. [Google Scholar] [CrossRef]
- Yang, L.; Jie, G.; She-Qi, Z.; Long-Xiang, S.; Wei, S.; Xun, Q.; Man-li, D. Effects of adding compound microbial inoculum on microbial community diversity and enzymatic activity during co-composting. Environ. Eng. Sci. 2018, 35, 270–278. [Google Scholar] [CrossRef]
- Henry, A.B.; Chaw, E.M.H.; Kim, K.Y. Metagenomic analysis reveals enhanced biodiversity and composting efficiency of lignocellulosic waste by thermoacidophilic effective microorganism (tEM). J. Environ. Manag. 2020, 276, 111252. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Duan, W.; Qiao, C.; Shen, Q.; Li, R. Microbial community composition turnover and function in the mesophilic phase predetermine chicken manure composting efficiency. Bioresour. Technol. 2020, 313, 123658. [Google Scholar] [CrossRef]
- Cao, R.; Ben, W.; Qiang, Z.; Zhang, J. Removal of antibiotic resistance genes in pig manure composting influenced by inoculation of compound microbial agents. Bioresour. Technol. 2020, 317, 123966. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, B.; Chen, K.; Zhao, C.; Xu, L.; Yang, Z.; Sun, Q.; Chandio, F.A.; Wu, G. Rice straw addition and biological inoculation promote the maturation of aerobic compost of rice straw biogas residue. Biomass Convers. Biorefineries 2020, 34, 607–622. [Google Scholar] [CrossRef]
- Hu, T.; Wang, X.; Zhen, L.; Gu, J.; Zhang, K.; Wang, Q.; Ma, J.; Peng, H. Effects of inoculation with lignocellulose-degrading microorganisms on antibiotic resistance genes and the bacterial community during co-composting of swine manure with spent mushroom substrate. Environ. Pollut. 2019, 252, 110–118. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Li, Y.; Wu, Y.; Zeng, Z.; Xu, R.; Wang, S.; Li, H.; Zhang, J. Changes of heavy metal fractions during co-composting of agricultural waste and river sediment with inoculation of Phanerochaete chrysosporium. J. Hazard. Mater. 2019, 378, 120757. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, Z.; Li, M.; Li, Q. A compost-derived thermophilic microbial consortium enhances the humification process and alters the microbial diversity during composting. J. Environ. Manag. 2019, 243, 240–249. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Ran, F.; Han, B.; Li, J. Microbial inoculation influences bacterial community succession and physicochemical characteristics during pig manure composting with corn straw. Bioresour. Technol. 2019, 289, 121653. [Google Scholar] [CrossRef]
- Duan, M.; Zhang, Y.; Zhou, B.; Wang, Q.; Gu, J.; Liu, G.; Qin, Z.; Li, Z. Changes in antibiotic resistance genes and mobile genetic elements during cattle manure composting after inoculation with Bacillus subtilis. Bioresour. Technol. 2019, 292, 122011. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.; Xie, X.; Zhang, R.; Wei, Z. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Zhang, Z.; Wei, Y.; Wang, H.; Lu, Q.; Li, Y.; Wei, Z. Effect of thermo-tolerant actinomycetes inoculation on cellulose degradation and the formation of humic substances during composting. Waste Manag. 2017, 68, 64–73. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, Y.; Sun, Q.; Wang, X.; Cui, H.; Zhang, X.; Li, Y.; Wei, Z. A novel method for contributing to composting start-up at low temperature by inoculating cold-adapted microbial consortium. Bioresour. Technol. 2017, 238, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Gou, C.; Wang, Y.; Zhang, X.; Lou, Y.; Gao, Y. Inoculation with a psychrotrophic-thermophilic complex microbial agent accelerates onset and promotes maturity of dairy manure-rice straw composting under cold climate conditions. Bioresour. Technol. 2017, 243, 339–346. [Google Scholar] [CrossRef]
- Huang, C.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Cheng, M.; Wan, J.; Hu, L.; Zhang, Y. Effect of Phanerochaete chrysosporium inoculation on bacterial community and metal stabilization in lead-contaminated agricultural waste composting. Bioresour. Technol. 2017, 243, 294–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Chen, Y.; Lu, Q.; Li, M.; Wang, X.; Wei, Y.; Xie, X.; Wei, Z. A regulating method for reducing nitrogen loss based on enriched ammonia-oxidizing bacteria during composting. Bioresour. Technol. 2016, 221, 276–283. [Google Scholar] [CrossRef]
- Varma, V.S.; Ramu, K.; Kalamdhad, A.S. Carbon decomposition by inoculating Phanerochaete chrysosporium during drum composting of agricultural waste. Environ. Sci. Pollut. Res. 2015, 22, 7851–7858. [Google Scholar] [CrossRef]
- Xu, Z.; Li, R.; Wu, S.; He, Q.; Ling, Z.; Liu, T.; Wang, Q.; Zhang, Z.; Quan, F. Cattle manure compost humification process by inoculation ammonia-oxidizing bacteria. Bioresour. Technol. 2022, 344, 126314. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, F.B.; Zhang, L.L.; Li, J. Effects of indigenous and exogenous microbial inocula on dynamic changes of enzyme activities during composting in a bioreactor. Adv. Mater. Res. 2012, 383–390, 4017–4023. [Google Scholar] [CrossRef]
- Xu, P.; Li, J. Effects of Microbial Inoculant on Physical and Chemical Properties in Pig Manure Composting. Compost Sci. Util. 2017, 25, S37–S42. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Wei, Y.; Chen, W.; Ding, G.; Zhan, Y.; Liu, Y.; Xu, T.; Xiao, J.; Li, J. Impact of inoculation and turning for full-scale composting on core bacterial community and their co-occurrence compared by network analysis. Bioresour. Technol. 2022, 345, 126417. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Kan, Z.; Li, B.; Cao, Y.; Jin, H. Effects of two-stage microbial inoculation on organic carbon turnover and fungal community succession during co-composting of cattle manure and rice straw. Bioresour. Technol. 2021, 341, 125842. [Google Scholar] [CrossRef]
- Wang, W.K.; Liang, C.M. Enhancing the compost maturation of swine manure and rice straw by applying bioaugmentation. Sci. Rep. 2021, 11, 6103. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Li, B.; Jin, H.; Dong, Y. Increased enzyme activities and fungal degraders by Gloeophyllum trabeum inoculation improve lignocellulose degradation efficiency during manure-straw composting. Bioresour. Technol. 2021, 337, 125427. [Google Scholar] [CrossRef]
- Kianirad, M.; Muazardalan, M.; Savaghebi, G.; Farahbakhsh, M.; Mirdamadi, S. Effects of temperature treatment on corn cob composting and reducing of composting time: A comparative study. Waste Manag. Res. 2010, 28, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Zeng, G.; Dong, H.; Chen, Y.; Huang, C.; Zhu, Y.; Xu, R.; Cheng, Y.; Hou, K.; et al. Multivariate relationships between microbial communities and environmental variables during co-composting of sewage sludge and agricultural waste in the presence of PVP-AgNPs. Bioresour. Technol. 2018, 261, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, V.; Kumar Awasthi, M.; Wang, Q.; Kumar, J.; Ren, X.; Zhao, J.; Chen, H.; Wang, M.; Bhaskar, T.; Zhang, Z. Effect of composting on the thermal decomposition behavior and kinetic parameters of pig manure-derived solid waste. Bioresour. Technol. 2018, 252, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, G.; Huda, N.; Zhang, B.; Wang, M.; Luo, W. Effects of moisture and carbon/nitrogen ratio on gaseous emissions and maturity during direct composting of cornstalks used for filtration of anaerobically digested manure centrate. Bioresour. Technol. 2020, 298, 122503. [Google Scholar] [CrossRef]
- Zeng, G.; Yu, M.; Chen, Y.; Huang, D.; Zhang, J.; Huang, H.; Jiang, R.; Yu, Z. Effects of inoculation with Phanerochaete chrysosporium at various time points on enzyme activities during agricultural waste composting. Bioresour. Technol. 2010, 101, 222–227. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Akratos, C.S.; Tsihrintzis, V.A. Effect of wastewater step-feeding on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecol. Eng. 2011, 37, 431–443. [Google Scholar] [CrossRef]
- Chen, Q.L.; An, X.L.; Li, H.; Zhu, Y.G.; Su, J.Q.; Cui, L. Do manure-borne or indigenous soil microorganisms influence the spread of antibiotic resistance genes in manured soil? Soil Biol. Biochem. 2017, 114, 229–237. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Shirai, Y.; Hassan, M.A.; Baharuddin, A.S.; Raja Abdullah, N.M.; Sulaiman, A.; Busu, Z. Improved economic viability of integrated biogas energy and compost production for sustainable palm oil mill management. J. Clean. Prod. 2013, 44, 1–7. [Google Scholar] [CrossRef]
- Chin, C.F.S.; Furuya, Y.; Zainudin, M.H.M.; Ramli, N.; Hassan, M.A.; Tashiro, Y.; Sakai, K. Novel multifunctional plant growth–promoting bacteria in co-compost of palm oil industry waste. J. Biosci. Bioeng. 2017, 124, 506–513. [Google Scholar] [CrossRef] [PubMed]
| Year | |||||
|---|---|---|---|---|---|
| Crop | 2017 | 2018 | 2019 | 2020 | |
| Area harvested (million ha) | Barley | 48 | 48 | 51.0 | 51 |
| Cocoa, beans | 12 | 12 | 12.0 | 12 | |
| Coffee, green | 10 | 11 | 11 | 11 | |
| Maize | 198 | 195 | 196 | 201 | |
| Millet | 31 | 32 | 30 | 32 | |
| Oil palm fruit | 27 | 27 | 28 | 28 | |
| Oranges | 3.8 | 3.9 | 3.9 | 3.8 | |
| Rice, paddy | 164 | 165 | 161 | 164 | |
| Sugar beet | 4.9 | 4.8 | 4.6 | 4.4 | |
| Sugar cane | 26 | 26 | 26 | 26 | |
| Wheat | 218 | 214 | 215 | 219 | |
| Production (million tons) | Barley | 148 | 139 | 158 | 157 |
| Cocoa, beans | 5.2 | 5.5 | 5.6 | 5.6 | |
| Coffee, green | 9.3 | 10 | 10 | 10 | |
| Maize | 1138 | 1124 | 1141 | 1162 | |
| Millet | 29 | 32 | 28 | 30 | |
| Oil palm fruit | 406 | 409 | 415 | 418 | |
| Oranges | 73 | 73 | 75 | 75 | |
| Rice, paddy | 747 | 759 | 749 | 757 | |
| Sugar beet | 313 | 273 | 280 | 252 | |
| Sugar cane | 183 | 193 | 195 | 187 | |
| Wheat | 772 | 732 | 765 | 761 | |
| Yield (hg/ha) | Barley | 31,014 | 29,243 | 31,060 | 30,432 |
| Cocoa, beans | 4478 | 4626 | 4640 | 4674 | |
| Coffee, green | 9022 | 9756 | 9069 | 9679 | |
| Maize | 57,452 | 57,542 | 58,127 | 57,547 | |
| Millet | 9212 | 9679 | 9202 | 9485 | |
| Oil palm fruit | 150,255 | 147,556 | 146,434 | 145,614 | |
| Oranges | 188,312 | 192,285 | 193,660 | 194,251 | |
| Rice, paddy | 45,539 | 45,795 | 46,312 | 46,089 | |
| Sugar beet | 630,328 | 571,355 | 604,012 | 569,869 | |
| Sugar cane | 697,722 | 727,979 | 726,377 | 706,434 | |
| Wheat | 35,377 | 34,222 | 35,432 | 34,744 | |
| Type of Agro-Industry | Feedstock | Composting Description | Reference |
|---|---|---|---|
| Oil palm | Oil palm empty fruit bunch (OPEFB) | OPEFB was kept individually at 15 cm apart inside a sheltered building with walls, good air ventilation, at room temperature around 27–28 °C. | [26] |
| OPEFB compost was added with activated liquid organic fertilizer (ALOF) in basket composer. Sand and rice husk were added to the mixture 1:1:1, 1:3:1, 1:0:1. | [27] | ||
| OPEFB mixed with activated liquid organic fertilizer (ALOF) in tower composter. | [28] | ||
| Two effective Trichoderma strains were mixed with OPEFB in the plastic bags and tightly closed with rubber bands. | [29] | ||
| Co-composting of oil palm empty fruit bunch (OPEFB) and palm oil mill effluent (POME) anaerobic sludge with ratio 1:1. | [30] | ||
| Paddy | Paddy husk | Co-composting of paddy husk and chicken slurry with clinoptilolite zeolite and urea. | [31] |
| Paddy straw | Single-strain or mixed cultures of Bacillus licheniformis 1-1v and Bacillus sonorensis 7-1v were inoculated in composting of rice straw. | [32] | |
| Co-composting of paddy straw with cattle manure, farmyard manure and poultry manure. Inoculated with Aspergillus niger ITCC 6719, Aspergillus flavus ITCC 6720 and Trichoderma harzianum ITCC 6721. | [33] | ||
| Co-composting of poultry manure and paddy straw with five treatment combinations in plastic pots of 10 kg capacity and in composting pits of the size of 1 m × 1 m × 0.5 m for each treatment. | [34] | ||
| Sugarcane | Bagasse | Two methods are applied: in-vessel composting for five mixtures and windrow composting for two mixtures. The mixtures are composed of cow dung, dry leaves, bagasse, filter mud and furnace ash. | [23] |
| Filter cake and ash | All compost piles consist of filter cake, boiler and fly ash (2:1) with laying of chicken manure. | [25] | |
| Coffee | Coffee husk | Co-composting coffee husk and pulp with source-separated municipal solid waste in different proportions (0, 33, 50 and 100%). | [35] |
| Co-composting coffee husk with cow dung, poultry manure and Desmodium triflorum. | [36] | ||
| Wheat | Wheat straw | Different wheat straw sizes, nitrogen addition and Trichoderma harzianum were applied and the composting process was carried out with 500 g of wheat straw styrene boxes in a dark room at 30–35 °C. | [37] |
| Maize | Maize straw | Maize straw composting with a spent mushroom substrate, earthworm casts and zeolite as an additive. | [38] |
| Maize straw was placed in piles (length, 10 m; width, 3 m; height, 1.5 m). | [39] | ||
| Mushroom | Spent mushroom substrate | Sewage sludge mixed with different dosages of spent mushroom substrate. | [40] |
| The spent mushroom substrate was mixed with 2% CaSO4, 0.5% mineral salt, small amounts of lime water and conducted with a combined static, heap-turning composting process. | [41] |
| Compost Materials | Inoculum/ Microorganisms | Rate of Inoculum Addition | Composting Conditions | Impact on the Entire Composting Process | References |
|---|---|---|---|---|---|
| Mushroom residue | Paenibacillus GX 5 Paenibacillus GX 7 Paenibacillus GX 13 Brevibacillus, GX 5 Brevibacillus, GX 7 Brevibacillus, GX 13 | 2 mL 100 g−1 | C/N ratio (12), Temperature (57 °C), MC (60 to 24%), pH (8) | Improved degradation rate of lignocellulose and organic matter, prolonged thermophilic period, enhanced microbial interaction. | [19] |
| Mushroom residue and wood chips | Aspergillus, Penicillium Bacillus, Streptomyces | 0.2% (w w−1) | C/N ratio (22), Temperature (58.4 °C), MC (50%), pH (7.8) | Prolonged thermophilic stage, increased degradation efficiency of cellulose and hemicellulose, optimizing the microbial community structure. | [18] |
| Chicken manure and maize straw | Strains isolated from natural chicken manure and maize straw compost: Bacillus licheniformis, Bacillus amyloliquefaciens, Ureibacillus thermosphaericus, Bacillus megaterium, Geobacillus pallidus, Bacillus pumilus, Geobacillus sp. Paracoccus denitrificans | 200 mL with 1 × 108 CFU mL−1 cell concentration | C/N ratio (21), Temperature (68.4 °C), MC (55.6 to 42%), pH (8.7) | Increased germination index, NO3 content, prolonged thermophilic stage, reduced volatile solids contents, improved humification and compost maturity level. | [15] |
| Chicken manure and rice husk | Ureibacillus terrenus BE8 and Bacillus tequilensis BG7 | 5% (v w−1) | Total C (263 g kg−1), and Total N (34 g kg−1), Temperature (65 °C), MC (78.1%) | Enhanced germination index values, accelerated compost maturity by stimulating different key microbes at the initial stage which promotes better phytotoxicity-free compost than the control treatment. | [67] |
| Pig manure and wheat straw | Microbial agent solution consisting of photosynthetic bacteria, actinomycetes, yeasts, and lactic acid bacteria | 40 mL 10 kg−1 | Total C (41.2 ± 0.5%), Total N (1.79 ± 0.03%), Temperature (68.4 °C), MC (55%) | Changes in ARG profiles and bacterial communities have promoted the changes in the potential hosts of ARGs, thus increasing the removal of total ARGs. | [68] |
| Rice straw | Compound bacterial agent screened from rice straw composts: Aeromonas caviae sp. SD3 (KR868995.1), Shinella sp. XM2 (CP015736.1), Rhizobium sp. S8 (KF261556.1), Corynebacterium pseudotuberculosis sp. SD1 (CP020356.1) and Streptomyces clavuligerus sp. XM (CP032052.1) | 1% (w w–1) with 1 × 109 CFU mL–1 cell concentration | C/N (30), MC (65%) | Improved the degradation of organic matter and coarse fiber content by 7.58% and, 8.82% due to the enhancement of core microbial metabolism. | [64] |
| Chicken manure, rice bran and pine waste | Bacteria: Bacillus spp., Alicyclobacillus spp., Pseudomonas spp., Lactobacillus spp., Pediococcuss spp., and Actinomycetes. Fungi: Rhizomucor pusillus, Aspergillus spp. | 0.2% (w w−1) | C/N ratio (28.4), Temperature (65 °C), MC (60 to 40%), pH (8.5) | Increased microbial diversity and population, enhanced in composting rate and mineralization. | [66] |
| Rice straw biogas residue and rice straw | Aspergillus niger CICIMF0410 and P. chrysosporium AF 96007 | 1% (v w−1) with 1 × 108 CFU mL−1 cell concentration | C/N ratio (32), Temperature (68.3 °C), MC (60%) | Reduced the time required for decomposition of organic matter, removed the toxicity risk for crops and promoted the stability of the compost. | [69] |
| Swine manure and spent mushroom substrate | Microbial suspension of lignocellulose- degrading microorganism’s consortium consisting of Bacillus, Brevibacillus, Paenibacillus and Lysinibacillus genera | 10% (v w−1) | Mixture ratio (1:1), Temperature (68 °C), MC (60%), pH (7.6) | Promoted the changes of the bacterial community in the mesophilic phase and reduced the risk of ARGs in the final compost. | [70] |
| Maize straw and canola residue | Phanerochaete chrysosporium | 1 × 108 CFU mL−1 | C/N ratio (25), Temperature (60 °C), MC (52%), pH (8.17) | Improved lignin degradation during the cooling stage, enhanced compost humification. | [13] |
| River sediment, rice straw, vegetables, and bran | Phanerochaete chrysosporium | 0.5% (v w−1) | C/N ratio (30), Temperature (69 °C), MC (60%), pH (8.6) | Enhanced the passivation of copper and reduced the effect of pH on the bioavailability of heavy metals. | [71] |
| Dairy manure and sugarcane leaves | Thermophilic lignocellulolytic microbes screened from dairy and sugarcane leaves compost samples: Bacillus licheniformis (TA65), Aspergillus nidulans (GXU-1) and Aspergillus oryzae (GXU-11) | 2% (w w−1) | C/N ratio (30), Temperature (55 °C), | Improve the mineralization of organic carbon, promoted the lignocellulose degradation and the humification process. | [72] |
| Pig manure and corn stalk | Compound bacterium agent comprised of Acinetobacter pittii, Bacillus subtilis sub sp. Stercoris and Bacillus altitudinis | 1% (v w−1) with 1 × 109 CFU mL–1 cell concentration | C/N ratio (30), Temperature (67.3 °C), MC (60%), pH (8.8) | Prolonged at the thermophilic stage, decreased abundance of human disease-related functional genes, increased the numbers of biomarkers and enhanced the maturity and fertility. | [73] |
| Citrus peel. bran and lime | The bacterial consortium which was screened from citrus peel compost samples | 3% (w w−1) | C/N ratio (25), Temperature (65 °C), MC (60%), pH (8.5) | Decreased C/N, organic matter, moisture, pectin and cellulose content, and enhanced the richness and diversity of the bacterial community. | [9] |
| Cattle manure and wheat stalks | Bacillus subtilis | 0.5% (w w−1) | C/N ratio (25), MC (60%), pH (7.61) | Promoted changes in ARGs and removed a large number of pathogenic bacteria. | [74] |
| Wheat straw, rice, corn and soybean | Actinomycetes: Streptomyces sp. H1 (KX641927.1), Mycobacerium sp. G1 (KY910181.1), Micromonospora sp. G7 (LC333394.1) and Saccha-romonospora sp. T9 (NR074713.2) | 3 mL kg−1 with 1 × 109 CFU mL−1 cell concentration | C/N ratio (30), Temperature (63 °C), MC (50 to 60%), pH (9.4) and the aeration rates: 0.5 L kg−1 (dry matter) min−1 | Improved 34.3% lignocellulose degradation and 8.3% enzyme activity. | [75] |
| Pig manure and apple tree branches | Microbial inoculum: Ralstoinia sp., Penicillium sp., Penicillium aurantiogriseum, and Acremonium alternatum | 2% (v w−1) | C/N ratio (30), Temperature (77 °C), MC (60%), pH (8.1) | Enhanced cellulase, urease, and polyphenol oxidase activities and promoted the succession of the bacterial community structure. | [65] |
| Corn straw and dairy manure | Thermo-tolerant actinomycetes Streptomyces sp. H1, Streptomyces sp. G1, Streptomyces sp. G2 and Actinobacteria bacterium T9 | 2% (v w−1) with 1 × 109 CFU mL−1 cell concentration | C/N ratio (30), Temperature (57 °C), MC (60%) | Enhanced cellulase activities and increased degradation of cellulose, humic substances content. | [76] |
| Food waste and maize straw | Cold adapted microbial consortium comprised of stains Pseudomonas fragi (KY283110), Pseudomonas simiae (KY283111), Clostridium vincentii (KY283112), Pseudomonas jessenii (KY283113) and Iodobacter fluviatilis (KY283114). | 1% (v w−1) with 1 × 108 CFU mL−1 cell concentration | C/N ratio (18), Temperature (45 °C), MC (66%) | Increased organic matter degradation at low temperature and promoted the change of the bacterial community composition and succession. | [77] |
| Dairy manure and rice straw | Psychrotrophic-thermophilic complex microbial agent (PTCMA): Bacillus diminuta CB1, Flavobacterium glaciei CB23, Aspergillus niger CF5 and Penicillium commune CF8 | 10 mL kg−1 with 1 × 108 CFU mL−1 cell concentration | C/N ratio (32), Temperature (63 to 45 °C), MC (60%), pH (8.2 to 8.4) | Increasing the composting pile temperature and significantly enriched compost Maturity and proposed inoculation of PTCMA is an effective approach in cold climates. | [78] |
| Sugarcane industry waste | Phosphate-solubilizing bacteria: Pseudomonas aeruginosa, Bacillus sp., Lactobacillales, Bacillales, Pseudomonas sp., Clostridiales | 8 L mg−1 with 1 × 108 CFU mL−1 cell concentration | C/N ratio (30), Temperature (60 °C) | Enhanced bacterial growth, mainly of the order Lactobacillales, thus causing the heating of the piles during the initial phase and enriched phosphorus content at the end of composting. | [25] |
| Rice straw, soil, vegetables, and bran | Phanerochaete chrysosporium | 2% (v w–1) with 1 × 106 CFU mL–1 cell concentration | C/N ratio (30), Temperature (58 °C), MC (60%), pH (8) | Decreased the toxicity of lead and increased the diversity of bacterial community in the composting. | [79] |
| Chicken manure and rice straw | Ammonia-oxidizing bacteria | 5% (v w−1) with 1 × 106 CFU mL−1 cell concentration | C/N ratio (25), Temperature (57 °C), MC (60 to 70%), pH (7.4) aeration rate: 0.5 L/min | Decreased ammonia emission and nitrogen loss by transforming ammonium into nitrite and also enhanced the abundance of bacterial community. | [80] |
| Rice straw | Cellulase producing bacteria: Bacillus licheniformis 1-1v and Bacillus sonorensis 7-1v | 1% (v w−1) with 3.6 and 6.8 × 107 CFU mL−1 cell concentration | C/N ratio (35.8), Temperature (54 °C), MC (35%), pH (8.1) | Shortened the composting time by 40 to 43%, resulting in a higher decrease in the total organic carbon and C/N ratio and enriched compost quality. | [32] |
| Vegetable waste: cattle manure: sawdust | Phanerochaetechrysosporium (MTCC 787) | 107 to 108 spores g−1 of compost | Compost mixture ratio (5:4), Temperature (64 °C), MC (65%), pH (7.5) | Enhanced the volatile solids reduction by 1.45-fold in trial 2 (initial phase) and 1.7-fold (thermophilic phase) in trial 3 as compared to uninoculated compost treatment. | [81] |
| Rice straw and goat manure | EM: lactic acid bacteria, yeast and phototrophic bacteria. | 5% (v w−1) | C/N ratio (32.4) | Improved the mineralization in composting process. | [62] |
| Wheat straw and cattle manure | Ammonium-oxidizing bacteria: Bacillaceae (strain T-AOB-2, M-AOB-4 and MT-AOB, 2-4) | 5% (v w−1) with 1 × 108 CFU mL–1 cell concentration | C/N ratio (30), MC (65%) | Promote formation of humic substances by reducing total organic carbon and dissolved organic carbon, improving bacterial activity. | [82] |
| Chicken manure, furfural residues and bagasse | Exogenous microbes (VT) and indigenous microbes (M3T) | 0.5% (v w−1) | C/N ratio (30), Temperature (50 to 58 °C), MC (55%) | Improved rate of temperature increase, enhanced urease, protease and cellulase activity. | [83] |
| Maize straw and pig manure | Bacillus subtilis, Bacillus licheniformis, Phanerochaetechrysosporium, Trichoderma koningii, Saccharomyces cerevisiae | 0.1% (w w−1) | C/N ratio (27.7), Temperature (66 °C), MC (60%) | Improved rate of temperature increase, increased micronutrients (N, P, K), enhanced decomposition of organic carbon, improved germination index. | [84] |
| Wheat straw and dairy manure | Microbial agent: Aspergillus niger, Saccharomyces cerevisiae, Lactobacillus plantarum, Lactobacillus acidophilus, Bacillus megaterium, Streptomyces albogriseus and Bacillus subtilis | 0.2% (v w−1) | C/N ratio (16), Temperature (60 °C), MC (60%), pH (8.0) | Increased composting maturity and total organic carbon degradation, decreased abundance of potential pathogen and improved key bacterial network interaction. | [85] |
| Rice straw and cattle manure | Malbranchea cinnamonmea, Gloephyllum trabeum | 10 mL kg−1 | C/N ratio (25), Temperature (73 °C), MC (65%), pH (8.5) | Promoted cellulose, hemicellulose and lignin degradation, increased nutrients and humus carbon, increased diversity and relative abundance of lignocellulosic fungi. | [86] |
| Rice straw and swine manure | Kitasatospora phosalacinea C1, Paenibacillus glycanilyticus X1, Bacillus licheniformis S3, Brevibacillus agri E4 and Phanerochaete chrysosporium | Not mentioned | C/N ratio (27.5), Temperature (62 °C) | Improved rate of temperature increase, enhanced maturation level. | [87] |
| Wheat straw and swine manure | Gloephyllum trabeum | 1 × 108 spores kg−1 | C/N ratio (27), Temperature (73 °C), MC (60%) | Shorten maturation period, increased decomposition rate of cellulose, hemicellulose and lignin, influencing fungal community by increasing relative abundance of Aspergillus, Mycothemus and melanocapus. | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainudin, M.H.M.; Zulkarnain, A.; Azmi, A.S.; Muniandy, S.; Sakai, K.; Shirai, Y.; Hassan, M.A. Enhancement of Agro-Industrial Waste Composting Process via the Microbial Inoculation: A Brief Review. Agronomy 2022, 12, 198. https://doi.org/10.3390/agronomy12010198
Zainudin MHM, Zulkarnain A, Azmi AS, Muniandy S, Sakai K, Shirai Y, Hassan MA. Enhancement of Agro-Industrial Waste Composting Process via the Microbial Inoculation: A Brief Review. Agronomy. 2022; 12(1):198. https://doi.org/10.3390/agronomy12010198
Chicago/Turabian StyleZainudin, Mohd Huzairi Mohd, Aisyah Zulkarnain, Ain Sahira Azmi, Shalini Muniandy, Kenji Sakai, Yoshihito Shirai, and Mohd Ali Hassan. 2022. "Enhancement of Agro-Industrial Waste Composting Process via the Microbial Inoculation: A Brief Review" Agronomy 12, no. 1: 198. https://doi.org/10.3390/agronomy12010198
APA StyleZainudin, M. H. M., Zulkarnain, A., Azmi, A. S., Muniandy, S., Sakai, K., Shirai, Y., & Hassan, M. A. (2022). Enhancement of Agro-Industrial Waste Composting Process via the Microbial Inoculation: A Brief Review. Agronomy, 12(1), 198. https://doi.org/10.3390/agronomy12010198






