Apple Autotetraploids—Phenotypic Characterisation and Response to Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenotypic Observations and Measurements
2.1.1. Plant Material
2.1.2. Growth Parameters
2.1.3. Leaf Characteristics
Leaf Morphological Traits
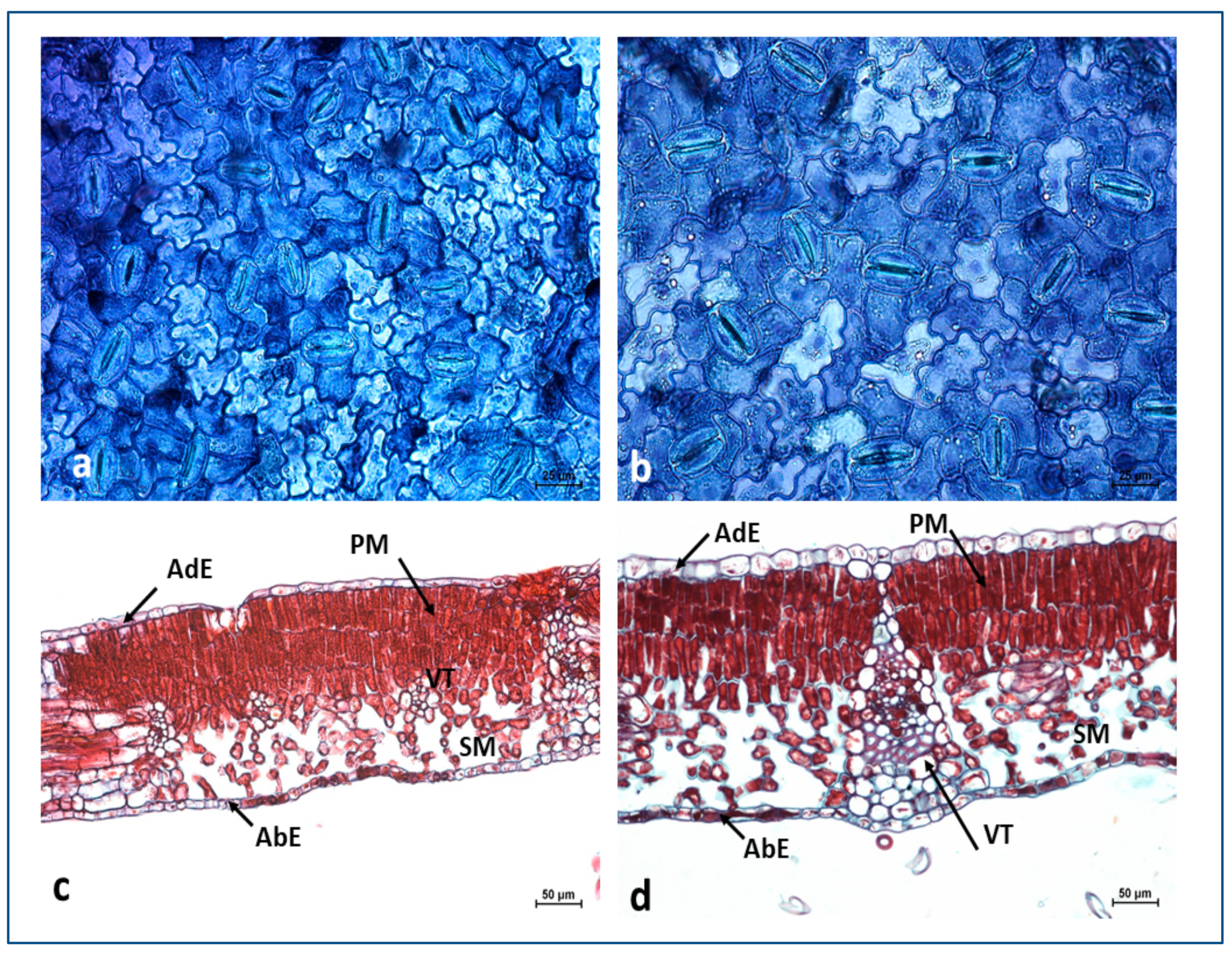
Anatomical Structure of the Leaves
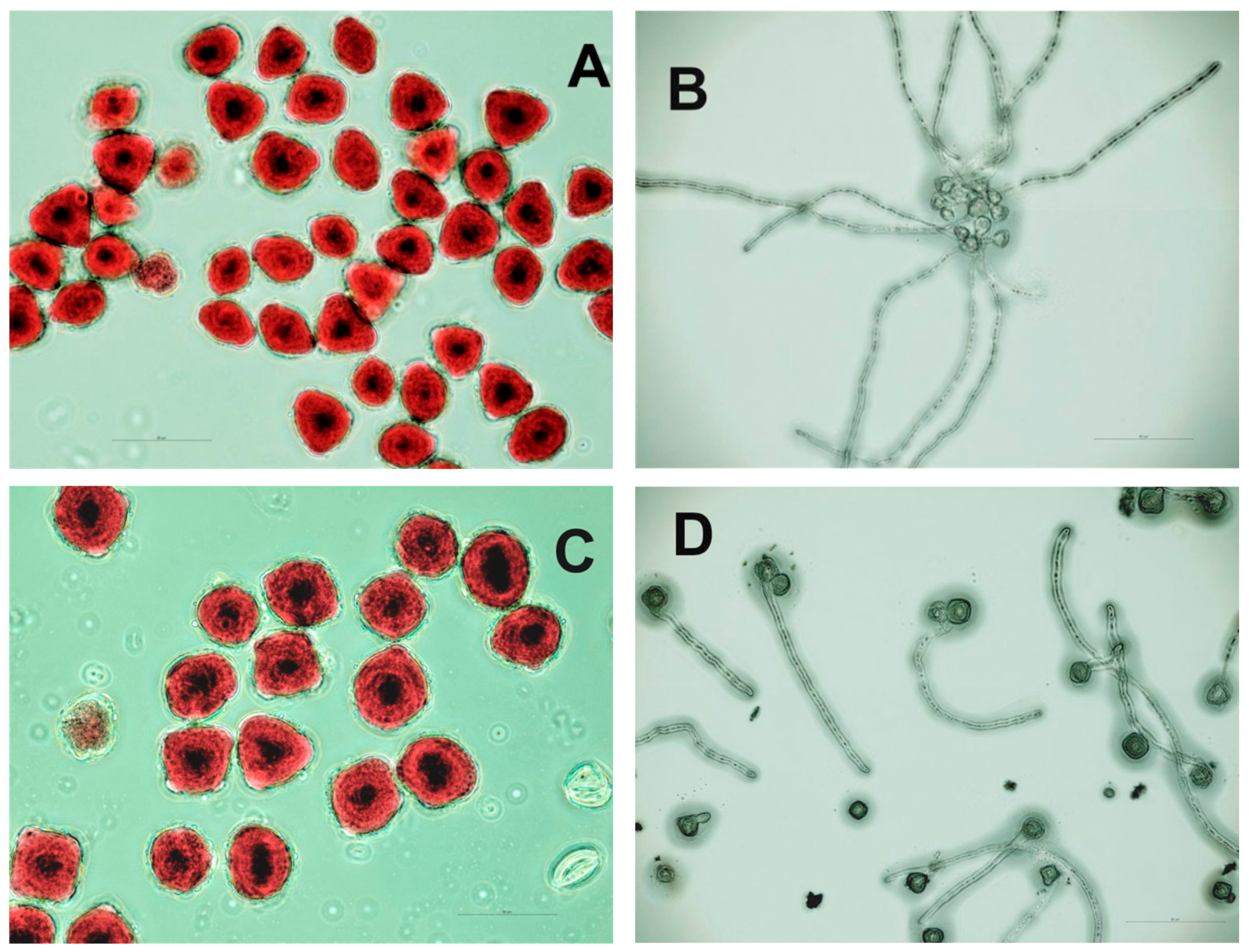
2.1.4. Pollen Characteristics
2.2. Testing for Drought Tolerance
2.2.1. Plant Material
2.2.2. Physiological Parameters
2.2.3. Growth Parameters
2.2.4. Gene Expression Analysis
2.3. Statistical Analysis
3. Results
3.1. Phenotypic Observations and Measurements
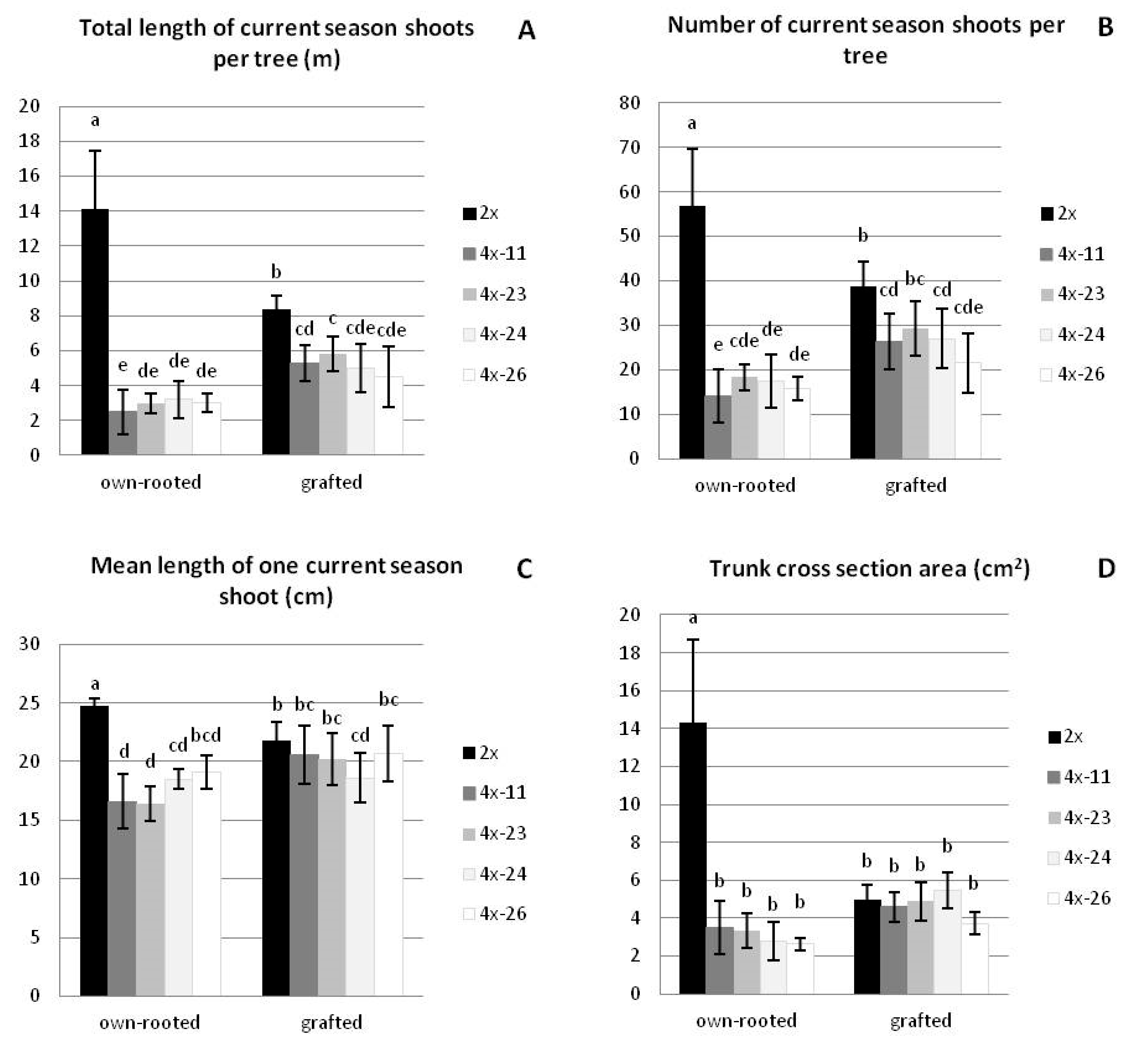
3.1.1. Growth Parameters
3.1.2. Leaf Characteristics
Leaf Morphological Traits and Chlorophyll Content
Anatomical Structure of the Leaves
3.1.3. Pollen Characteristics
3.2. Testing for Drought Tolerance
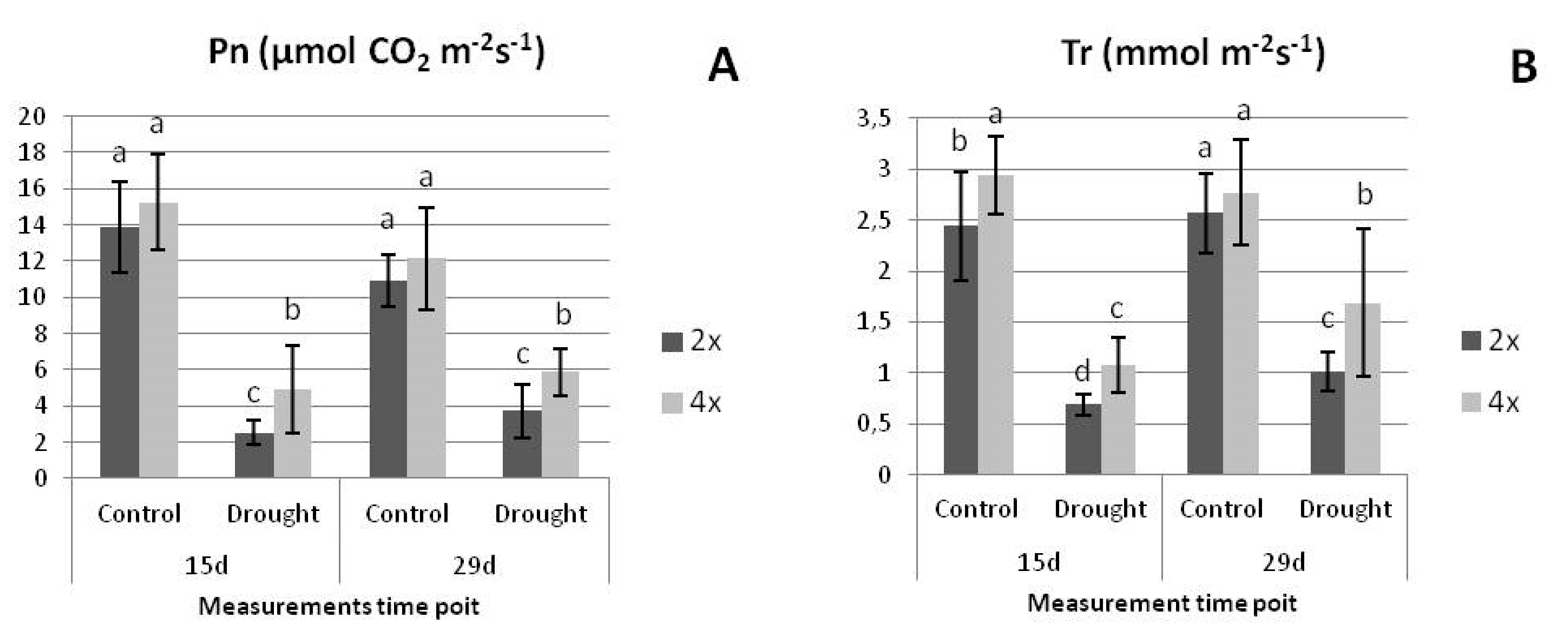
3.2.1. Physiological Parameters under Drought
3.2.2. Growth Parameters under Drought
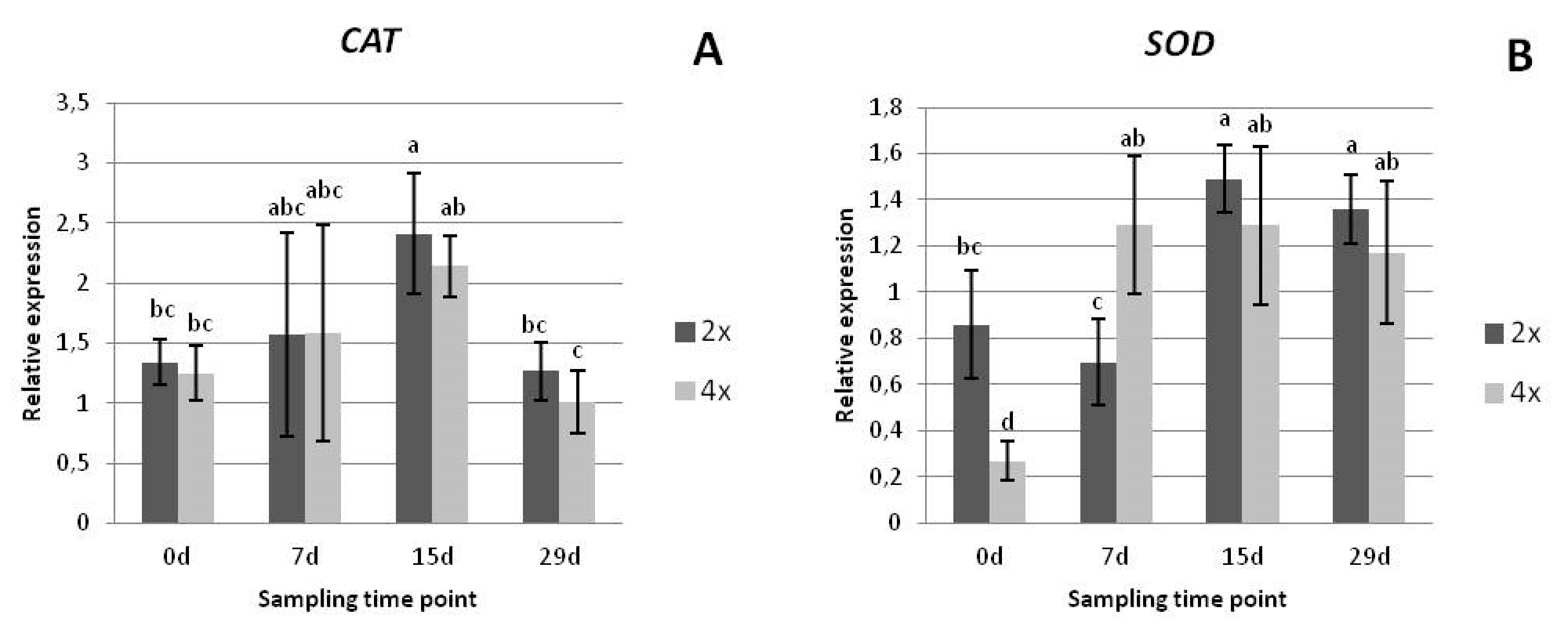
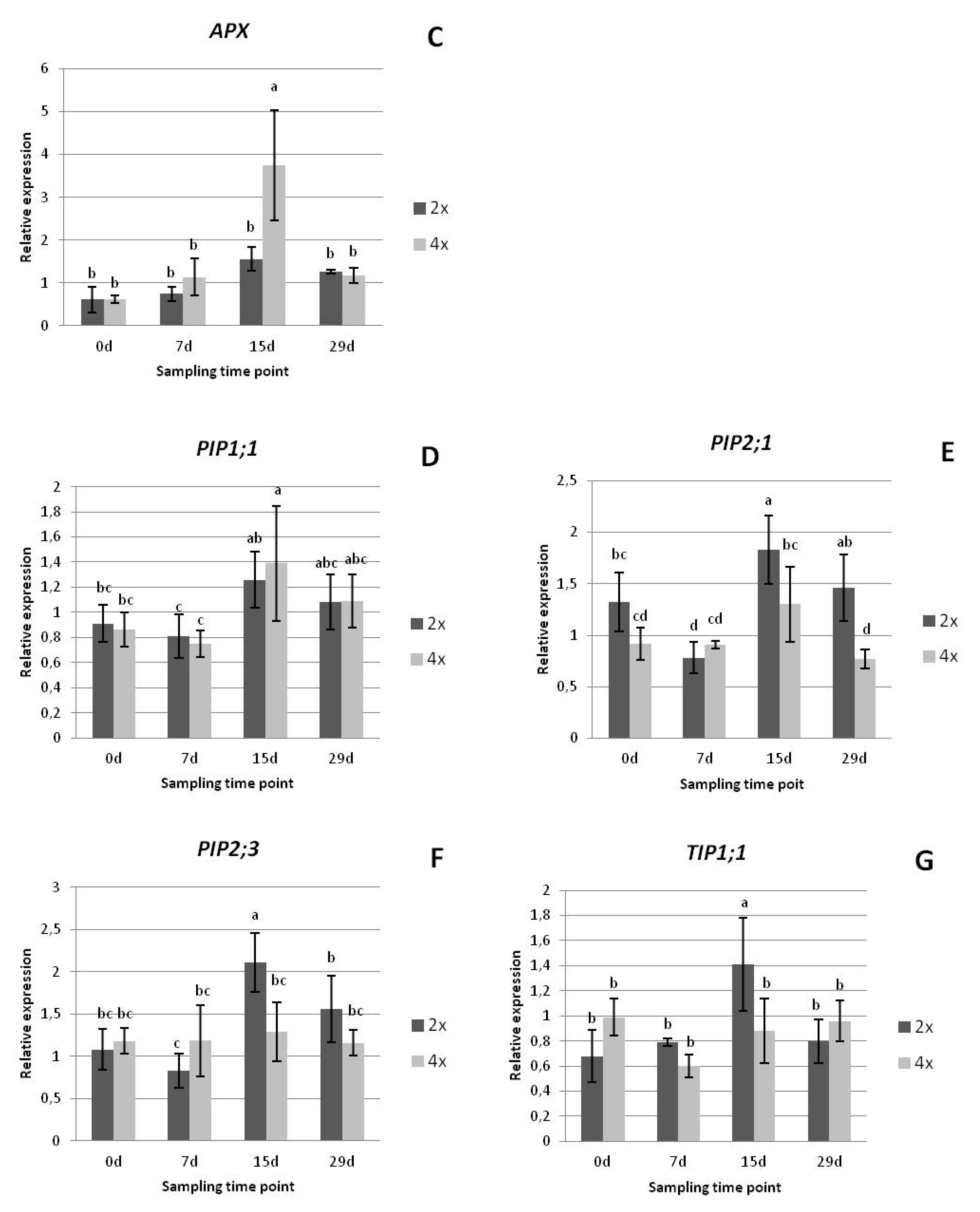
3.2.3. Gene Expression Analysis
4. Discussion
4.1. Phenotypic Characteristics of ‘Redchief’ Tetraploids
4.2. Drought Tolerance Assessment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sansavini, S.; Donati, F.; Costa, F.; Tartarini, S. Advances in apple breeding for enhanced fruit quality and resistance to biotic stresses: New varieties for the European market. J. Fruit Ornam. Plant Res. 2004, 12, 13–52. [Google Scholar]
- Peil, A.; Kellerhals, M.; Höfer, M.; Flachowsky, H. Apple breeding from the origin to genetic engineering. Fruit Veg. Cereal Sci. Biotechnol. 2011, 5, 118–138. [Google Scholar]
- Patocchi, A.; Wehrli, A.; Dubuis, P.-H.; Auwerkerken, A.; Leida, C.; Cipriani, G.; Passey, T.; Staples, M.; Didelot, F.; Philion, V.; et al. Ten years of VINQUEST: First insight for breeding new apple cultivars with durable apple scab resistance. Plant Dis. 2020, 104, 2074–2081. [Google Scholar] [CrossRef] [Green Version]
- Norelli, J.L.; Jones, A.L.; Aldwickle, H.S. Fire blight management in the twenty-first century: Using new technologies that enhance host resistance in apple. Plant Dis. 2003, 87, 756–765. [Google Scholar] [CrossRef] [Green Version]
- Nybom, H.; Mikicinski, A.; Garkava-Gustavsson, L.; Sehic, J.; Lewandowski, M.; Sobiczewski, P. Assessment of fire blight tolerance in apple based on plant inoculations with Erwinia amylovora and DNA markers. Trees 2011, 26, 199–213. [Google Scholar] [CrossRef]
- Chane, T.B.; Boyraz, N. Critical review on apple scab (Venturia inaequalis) biology, epidemiology, economic importance, management and defense mechanisms to the causal agent. J. Plant Physiol. Pathol. 2017, 5, 2. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Gao, M.; Zhang, L.; Li, B.; Han, M.; Kumar, A.; Ashraf, M. Role of exogenous glycinebetaine and humic acid in mitigating drought stress-induced adverse effects in Malus robusta seedlings. Turk. J. Bot. 2013, 37, 920–929. [Google Scholar] [CrossRef]
- Klamkowski, K.; Treder, W.; Wójcik, K. Effects of long-term water stress on leaf gas exchange, growth and yield of three strawberry cultivars. Acta Sci. Pol. Hortorum Cultus 2015, 14, 55–65. [Google Scholar]
- Soni, A.; Kumari, P.; Dhakar, S.; Kumar, N. Mechanisms and strategies for improving drought tolerance in fruit crops. Chem. Sci. Rev. Lett. 2017, 6, 1537–1543. [Google Scholar]
- Bai, T.; Li, Z.; Song, C.; Song, S.; Jiao, J.; Liu, Y.; Dong, Z.; Zheng, X. Contrasting drought tolerance in two apple cultivars associated with difference in leaf morphology and anatomy. Am. J. Plant Sci. 2019, 10, 709–722. [Google Scholar] [CrossRef] [Green Version]
- Klamkowski, K.; Treder, W. Morphological and physiological responses of strawberry plants to water stress. Agric. Conspec. Sci. 2006, 71, 159–165. Available online: https://hrcak.srce.hr/7902 (accessed on 20 November 2021).
- Mihaljević, I.; Viljevac Vuletić, M.; Šimić, D.; Tomaš, V.; Horvat, D.; Josipović, M.; Zdunić, Z.; Dugalić, K.; Vuković, D. Comparative study of drought stress effects on traditional and modern apple cultivars. Plants 2021, 10, 561. [Google Scholar] [CrossRef]
- Lei, Y.; Yin, C.; Li, C. Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol. Plant. 2006, 127, 182–191. [Google Scholar] [CrossRef]
- Nautiyal, S.; Badola, H.K.; Negi, D.S. Plant responses to water stress: Changes in growth, dry matter production, stomatal frequency and leaf anatomy. Biol. Plant. 1994, 36, 91–97. [Google Scholar] [CrossRef]
- Palliotti, A.; Cartechini, A.; Nasini, L. Grapevine adaptation to continuous water limitation during the season. Adv. Hort. Sci. 2001, 15, 39–45. [Google Scholar]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Singh, J.; Achary, V.M.M.; Reddy, M.K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Environ. Sci. 2015, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-L.; Zhang, J.-K.; Li, M.-J.; Zhou, B.-B.; Zhang, Q.; Wei, Q.-P. Genome-wide analysis of antioxidant enzyme gene families involved in drought and low-temperature responses in Apple (Malus domestica). J. Hortic. Sci. 2018, 93, 337–346. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C. Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ. 2016, 39, 1140–1160. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.K.; Yang, Z.Q.; Li, Y.X.; Liu, Q.; Han, W. Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica 2016, 54, 28–39. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Xin, M.; Ma, F.; Liu, J. Gene-wide analysis of aquaporin gene family in Malus domestica and heterologous expression of the gene MpPIP2;1 confers drought and salinity tolerance in Arabidposis thaliana. Int. J. Mol. Sci. 2019, 20, 3710. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Hu, W.; Liu, J.; Zhang, J.; Jia, C.; Miao, H.; Xu, B.; Jin, Z. A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 2014, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, Q.T.; Lei, Q.; Feng, C.; Zheng, X.; Zhou, F.; Li, L.; Liu, X.; Wang, Z.; Kong, J. Ectopically expressing MdPIP1;3, an aquaporin gene, increased fruit size and enhanced drought tolerance of transgenic tomatoes. BMC Plant Biol. 2017, 17, 246. [Google Scholar] [CrossRef] [Green Version]
- Osborn, T.C.; Pires, J.C.; Birchler, J.A.; Auger, D.L.; Chen, Z.J.; Lee, H.-S.; Comai, L.; Madlung, A.; Doerge, R.W.; Colot, V.; et al. Understanding mechanisms of novel gene expression in polyploids. Trends Genet. 2003, 19, 141–147. [Google Scholar] [CrossRef]
- Chen, Z.J. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Anssour, S.; Krugel, T.; Sharbel, T.F.; Saluz, H.P.; Bonaventure, G.; Baldwin, I.T. Phenotypic, genetic and genomic consequences of natural and synthetic polyploidization of Nicotiana attenuata and Nicotiana obtusifolia. Ann. Bot. 2009, 103, 1207–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisod, C.; Holderegger, R.; Brochmann, C. Evolutionary consequences of autopolyploidy. New Phytol. 2010, 186, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Liu, X.; Marchant, D.B.; Visger, C.J.; Soltis, D.E. Polyploidy and novelty: Gottlieb’s legacy. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balao, F.; Herrera, J.; Talavera, S. Phenotypic consequences of polyploidy and genome size at the microevolutionary scale: A multivariate morphological approach. New Phytol. 2011, 192, 256–265. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Tayalé, A.; Parisod, C. Natural pathways to polyploidy in plants and consequences for genome reorganization. Cytogenet. Genome Res. 2013, 140, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Podwyszyńska, M.; Marasek-Ciołakowska, A. Ploidy, genome size, and cytogenetics of apple. In The Apple Genome; Korban, S.S., Ed.; Springer-Nature Publ.: New York, NY, USA, 2021; pp. 47–71. [Google Scholar]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tiss. Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Sedyscheva, G.A.; Gorbacheva, N.G. Estimation of new tetraploid apple forms as donors of diploid gametes for selection on a polyploidy level. Univers. J. Plant Sci. 2013, 1, 49–54. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, F.; Zhang, Z.H.; Fu, J.F.; Wang, F.; Zhang, B.; Ma, Y. Differences in salt tolerance between diploid and autotetraploid apple seedlings exposed to salt stress. Sci. Hortic. 2015, 190, 24–30. [Google Scholar] [CrossRef]
- Zhang, F.; Xue, H.; Lu, X.; Zhang, B.; Wang, F.; Ma, Y.; Zhang, Z. Autotetraploidization enhances drought stress tolerance in two apple cultivars. Trees 2015, 29, 1773–1780. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Zhang, Z.; Fu, J.; Ma, Y. Characterization of fungi resistance in two autotetraploid apple cultivars. Sci. Hortic. 2017, 220, 27–35. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, B.; Tian, J.R.; Chen, M.M.; Zhang, Y.Y.; Zhang, Z.H.; Ma, Y. Comparison of the morphology, growth and development of diploid and autotetraploid ‘Hanfu’apple trees. Sci. Hortic. 2017, 225, 277–285. [Google Scholar] [CrossRef]
- Hias, N.; Svara, A.; Keulemans, J.W. Effect of polyploidisation on the response of apple (Malus × domestica Borkh.) to Venturia inaequalis infection. Eur. J. Plant Pathol. 2018, 151, 515–526. [Google Scholar] [CrossRef]
- Sedov, E.N.; Sedysheva, G.A.; Serova, Z.M.; Gorbacheva, N.G.; Melnik, S.A. Breeding assessment of heteroploid crosses in the development of triploid apple varieties. Russ. J. Genet. Appl. Res. 2014, 4, 52–59. [Google Scholar] [CrossRef]
- Sedov, E.N.; Sedysheva, G.A.; Makarkina, M.A.; Serova, Z.M. Development of triploid apple cultivars as a priority in selection. Russ. J. Genet. Appl. Res. 2017, 7, 773–780. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Sowik, I.; Machlańska, A.; Kruczyńska, D.; Dyki, B. In vitro tetraploid induction of Malus × domestica Borkh. using leaf or shoot explants. Sci. Hortic. 2017, 226, 379–388. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Cieślińska, M. Rooting shoots of apple varieties and their tetraploids obtained by the in vitro technique. Acta Sci. Pol. Hortorum Cultus 2018, 17, 49–62. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Markiewicz, M.; Broniarek-Niemiec, A.; Matysiak, B.; Marasek-Ciołakowska, A. Apple autotetraploids with enhanced resistance to apple scab (Venturia inaequalis) due to genome duplication—Phenotypic and genetic evaluation. Int. J. Mol. Sci. 2021, 22, 527. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Sowik, I.; Puławska, J. In vitro testing of apple tetraploids for resistance to fire blight. Acta Hortic. 2020, 1282, 343–350. [Google Scholar] [CrossRef]
- Sobiczewski, P.; Peil, A.; Mikiciński, A.; Richter, K.; Lewandowski, M.; Żurawicz, E.; Kellerhals, M. Susceptibility of apple genotypes from European genetic resources to fire blight (Erwinia amylovora). Eur. J. Plant Pathol. 2015, 141, 51–62. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Markiewicz, M.; Klamkowski, K.; Broniarek, A.; Marasek-Ciołakowska, A. The genetic background of the phenotypic variability observed in apple autotetraploids. Acta Hortic. 2021, 1307, 177–186. [Google Scholar] [CrossRef]
- Dyki, B.; Habdas, H. The method of isolation of epidermis of tomato and cucumber leaves for microscopic investigation of pathogenic fungus development. Acta Agrobot. 1996, 49, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Marasek-Ciolakowska, A.; Saniewski, M.; Dziurka, M.; Kowalska, U.; Góraj-Koniarska, J.; Ueda, J.; Miyamota, K. Formation of the secondary abscission zone induced by the interaction of methyl jasmonate and auxin in Bryophyllum calycinum: Relevance to auxin status and histology. Int. J. Mol. Sci. 2020, 21, 2784. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Niles, W.L.; Quesenberry, K.H. Pollen germination of rhizoma cv. Florigraze. Pinut Sci. 1992, 19, 105–107. [Google Scholar] [CrossRef]
- Perini, P.; Pasquali, G.; Margis-Pinheiro, M.; De Oliviera, P.R.D.; Revers, L.F. Reference genes for transcriptional analysis of flowering and fruit ripening stages in apple (Malus × domestica Borkh.). Mol. Breed. 2014, 34, 829–842. [Google Scholar] [CrossRef]
- Vergne, E.; Dugé de Bernonville, T.; Dupuis, F.; Sourice, S.; Cournol, R.; Berthelot, P.; Barny, M.A.; Brisset, M.N.; Chevreau, E. Membrane-targeted HrpNEa can modulate apple defense gene expression. Mol. Plant-Microb. Interact. 2014, 27, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Li, X.L.; Zhang, J.K.; Li, M.J.; Zhou, B.B.; Zhou, J.; Zhang, Q.; Wei, Q.P. Evaluation and gene expression analysis of different apple (Malus × domestica) dwarfing stocks on drought resistance. J. Agric. Biotechnol. 2018, 26, 401–409. [Google Scholar]
- Larionov, A.; Krause, A.; Miller, W. A standard curve based method for relative real time PCR data processing. BMC Bioinform. 2005, 6, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Xue, H.; Zhang, L.; Zhang, F.; Ou, C.; Wang, F.; Zhang, Z. Involvement of auxin and brassinosteroid in dwarfism of autotetraploid apple (Malus domestica). Sci. Rep. 2016, 6, 26719. [Google Scholar] [CrossRef] [Green Version]
- Foster, T.; McAtee, P.A.; Waite, C.N.; Boldingh, H.L.; McGhie, T.K. Apple dwarfing rootstocks exhibit an imbalance in carbohydrate allocation and reduced cell growth and metabolism. Hortic. Res. 2017, 4, 17009. [Google Scholar] [CrossRef] [Green Version]
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A.; Jiang, W.; Shi, T.; Gao, Z. An insight into dwarfing mechanism: Contribution of scion-rootstock interactions toward fruit crop improvement. Fruit Res. 2021, 1, 3. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Gabryszewska, E.; Dyki, B.; Stępowska, A.; Kowalski, A.; Jasiński, A. Phenotypic and genome size changes (variation) in synthetic tetraploids of daylily (Hemerocallis) in relation to their diploid counterparts. Euphytica 2015, 203, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.; Chen, J.; Lou, X.; Xu, Q.; Dong, R.; Tong, Z.; Huang, H.; Lin, E. Colchicine-induced polyploidy in Rhododendron fortunei Lindl. Plants 2020, 9, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Kang, X.; Li, J.; Chen, J. Induction, identification and characterization of tetraploidy in Lycium ruthenicum. Breed. Sci. 2019, 69, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Rodríguez, A.M.; Grajal-Martín, M.J. Physiological behaviour of mangos with different ploidy levels. Acta Hortic. 2013, 992, 155–158. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Baig, M.M.Q.; Quresh, A.A.; Shah, M.K.N.; Hafiz, I.A. Induction and identification of colchicine induced polyploidy in Gladiolus grandiflorus ‘White Prosperity’. Folia Hort. 2018, 30, 307–319. [Google Scholar] [CrossRef] [Green Version]
- McGoey, B.V.; Chau, K.; Dickinson, T.A. Stomata size in relation to ploidy level in North American hawthorns (Crataegus, Rosaceae). Madroño 2014, 61, 177–193. [Google Scholar] [CrossRef]




| Gene | Primer Sequence (5′-3′) | GenBank Accession No/Reference |
|---|---|---|
| CAT | Forward: GACTTCTTCTCCCACCATCCAG Reverse: TTGGTAGCGTGGCTGTGATTAG | Li et al. [56] |
| SOD | Forward: GGGAGATGGCCCAACTACTG Reverse: TTGCCAAGGTCATCAGGGTC | Li et al. [56] |
| APX | Forward: GCTTTCTTTGCTGACTATGCTGAA Reverse: ATTGGCTTCATCCCTTGTAGTTCT | XM 008346624 |
| PIP1;1 | Forward: AGCTGACGTCATGGTCGTTCTACA Reverse: TGCACTTCGACTTGGACTTGACGA | Zhang et al. [38] |
| PIP2;1 | Forward: CCTTCTACCACCAATACATTC Reverse: TGATTATCTACAATTCCATAGCC | Liu et al. [23] |
| PIP2;3 | Forward:CAAGAGGAGTGCTAGAGAC Reverse:GCCAAGTGGACAATGAAC | Liu et al. [23] |
| TIP1;1 | Forward: TCGACCGTCCAATCATCTCAACCA Reverse: ATCGAGATGAACTCGGCTAACGCA | Zhang et al. [38] |
| AC11 | Forward: GCTGTTCTTTCCCTCTACGC Reverse: GCATGGGGAAGAGCATATCC | Perini et al. [34] |
| GAPDH | Forward: GCTGCCAAGGCTGTTGGAA Reverse: CAGTCAGGTCAACAACGGAAAC | Vergne et al. [55] |
| Trait | Own-Rooted | M9-Grafted | ||
|---|---|---|---|---|
| Diploid | Tetraploid * | Diploid | Tetraploid * | |
| Total length of current season shoots per tree (m) | 14.1 ± 3.3 a ** | 2.9 ± 0.9 d | 8.4 ± 0.7 b | 5.2 ± 1.3 c |
| Number of current season shoots per tree | 56.8 ± 12.9 a | 16.3 ± 4.6 d | 38.7 ± 5.5 b | 26.0 ± 6.5 c |
| Mean length of one current season shoot (cm) | 24.7 ± 0.7 a | 17.6 ± 1.9 c | 21.8 ± 1.6 b | 20.0 ± 2.2 b |
| Trunk cross section area (cm2) | 14.3 ± 4.4 a | 3.1 ± 1.0 b | 5.0 ± 0.8 b | 4.7 ± 1.0 b |
| Trait | 2x | 4x-11 | 4x-25 | 4x-26 |
|---|---|---|---|---|
| Leaf area (cm2) | 32.1 ± 6.0 a * | 33.9 ± 5.8 a | 31.0 ± 5.1 a | 33.7 ± 4.5 a |
| Leaf length (cm) | 9.3 ± 1.5 a | 8.5 ± 1.1 ab | 8.4 ± 0.8 b | 9.0 ± 0.7 ab |
| Leaf width (cm) | 4.3 ± 0.7 b | 5.1 ± 0.8 a | 5.0 ± 0.5 a | 5.0 ± 0.6 a |
| Leaf length/width | 2.18 ± 0.29 a | 1.70 ± 0.18 b | 1.71 ± 0.16 b | 1.81 ± 0.13 b |
| Stomata length (μm) | 26.2 ± 1.2 b | 34.2 ± 1.6 a | 33.8 ± 0.6 a | 34.1 ± 0.8 a |
| Stomata frequency (no. mm−2) | 279.3 ± 10.8 a | 186.7 ± 11.1 b | 177.0 ± 5.0 b | 152.7 ± 17.6 c |
| Chlorophyll content index (CCI) | 21.2 ± 3.4 b | 27.2 ± 6.4 a | 24.4 ± 3.6 ab | 25.1 ± 5.1 a |
| Thickness (μm) | Diploid | Tetraploid |
|---|---|---|
| leaf blade | 204.1 ± 10.3 b * | 273.2 ± 14.7 a |
| spongy mesophyll | 78.0 ± 7.2 b | 116.5 ± 11.0 a |
| palisade mesophyll | 95.3 ± 10.1 b | 108.6 ± 10.7 a |
| adaxial epidermis | 10.1 ± 1.9 b | 17.0 ± 3.1 a |
| abaxial epidermis | 10.6 ± 2.4 b | 14.2 ± 2.3 a |
| Trait | Diploid | Tetraploid |
|---|---|---|
| Pollen grain length (µm) | 32.9 ± 3.2 b * | 41.2 ± 3.6 a |
| Pollen viability (%) | 91 | 48 |
| Pollen germination (%) | 77 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, D.; Marat, M.; Marasek-Ciołakowska, A.; Klamkowski, K.; Buler, Z.; Podwyszyńska, M.; Tomczyk, P.P.; Wójcik, K.; Treder, W.; Filipczak, J. Apple Autotetraploids—Phenotypic Characterisation and Response to Drought Stress. Agronomy 2022, 12, 161. https://doi.org/10.3390/agronomy12010161
Wójcik D, Marat M, Marasek-Ciołakowska A, Klamkowski K, Buler Z, Podwyszyńska M, Tomczyk PP, Wójcik K, Treder W, Filipczak J. Apple Autotetraploids—Phenotypic Characterisation and Response to Drought Stress. Agronomy. 2022; 12(1):161. https://doi.org/10.3390/agronomy12010161
Chicago/Turabian StyleWójcik, Danuta, Monika Marat, Agnieszka Marasek-Ciołakowska, Krzysztof Klamkowski, Zbigniew Buler, Małgorzata Podwyszyńska, Przemysław Piotr Tomczyk, Katarzyna Wójcik, Waldemar Treder, and Jacek Filipczak. 2022. "Apple Autotetraploids—Phenotypic Characterisation and Response to Drought Stress" Agronomy 12, no. 1: 161. https://doi.org/10.3390/agronomy12010161







