Fertilizer Deep Placement Significantly Affected Yield, Rice Quality, 2-AP Biosynthesis and Physiological Characteristics of the Fragrant Rice Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Details
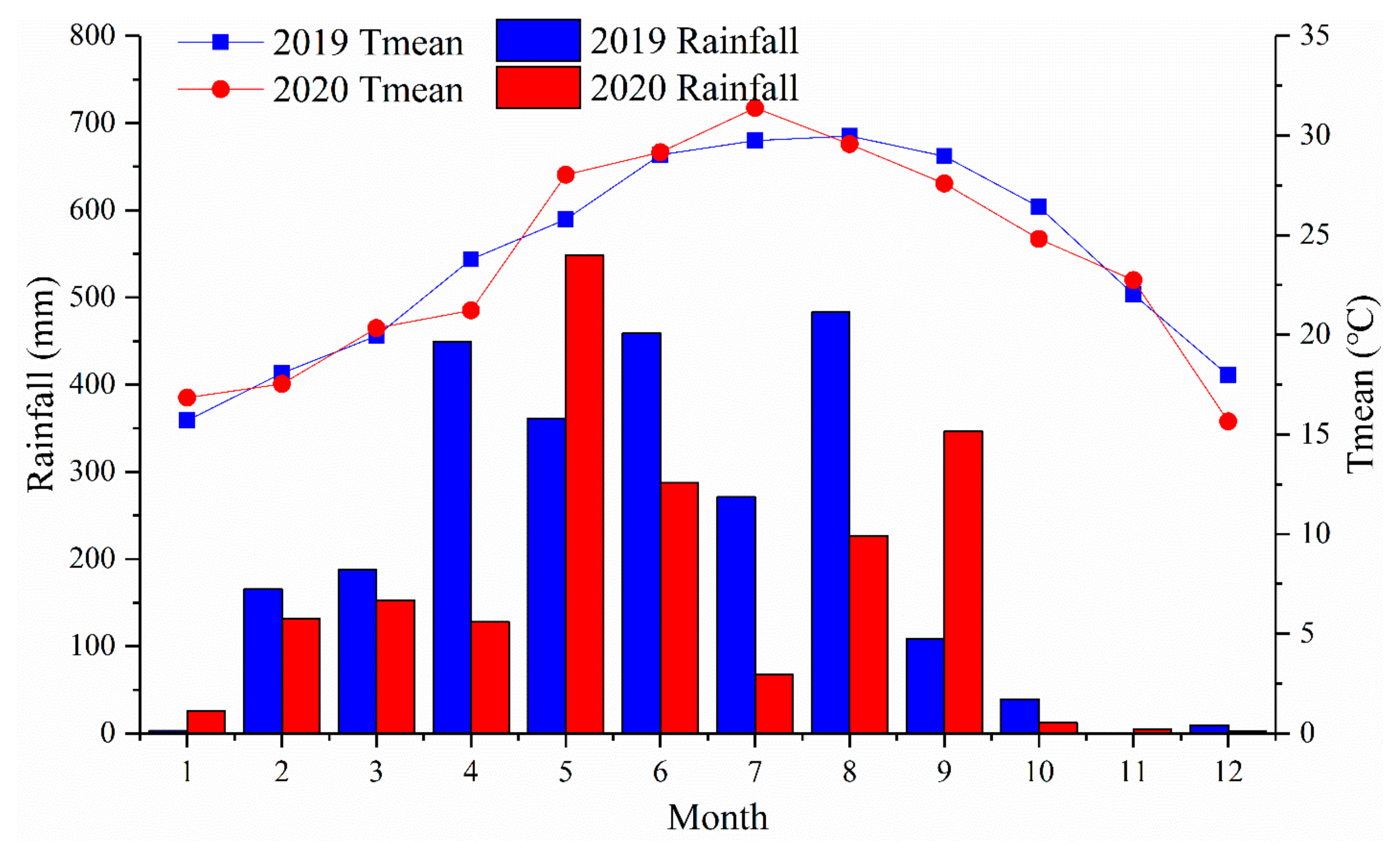
2.2. Experimental Designs and Treatments
2.3. Sampling and Data Collection
2.4. GC-MS Analysis for the Determination of 2-Acetyl-1-pyrroline
2.5. Determination of Elongation of Cooked Rice and Percentage of Amylose Content
2.6. Determination of Fragrance-Related Enzymes (Proline, Δ 1Pyrroline-5-carboxylate Synthetase (P5CS), Δ 1-Pyrroline-5-carboxylate (P5C))
2.7. Rice Quality
2.8. Statistical Analysis
3. Results
3.1. Effects of Fertilizer Placement on the Yield of Fragrant Rice and Its Components
3.2. Rice Quality and Its Components
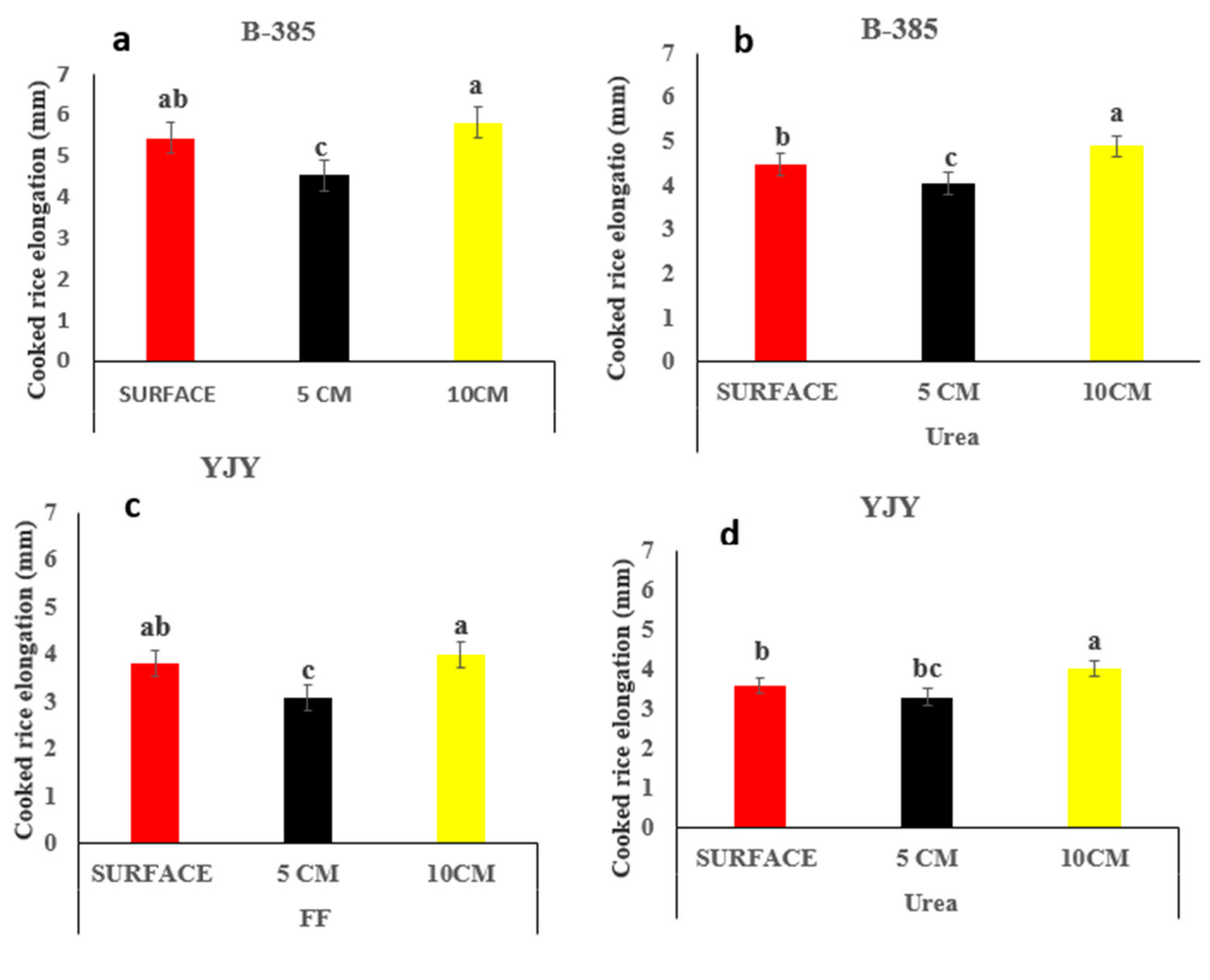
3.3. Effects of Fertilizer Application on Cooked Rice Elongation
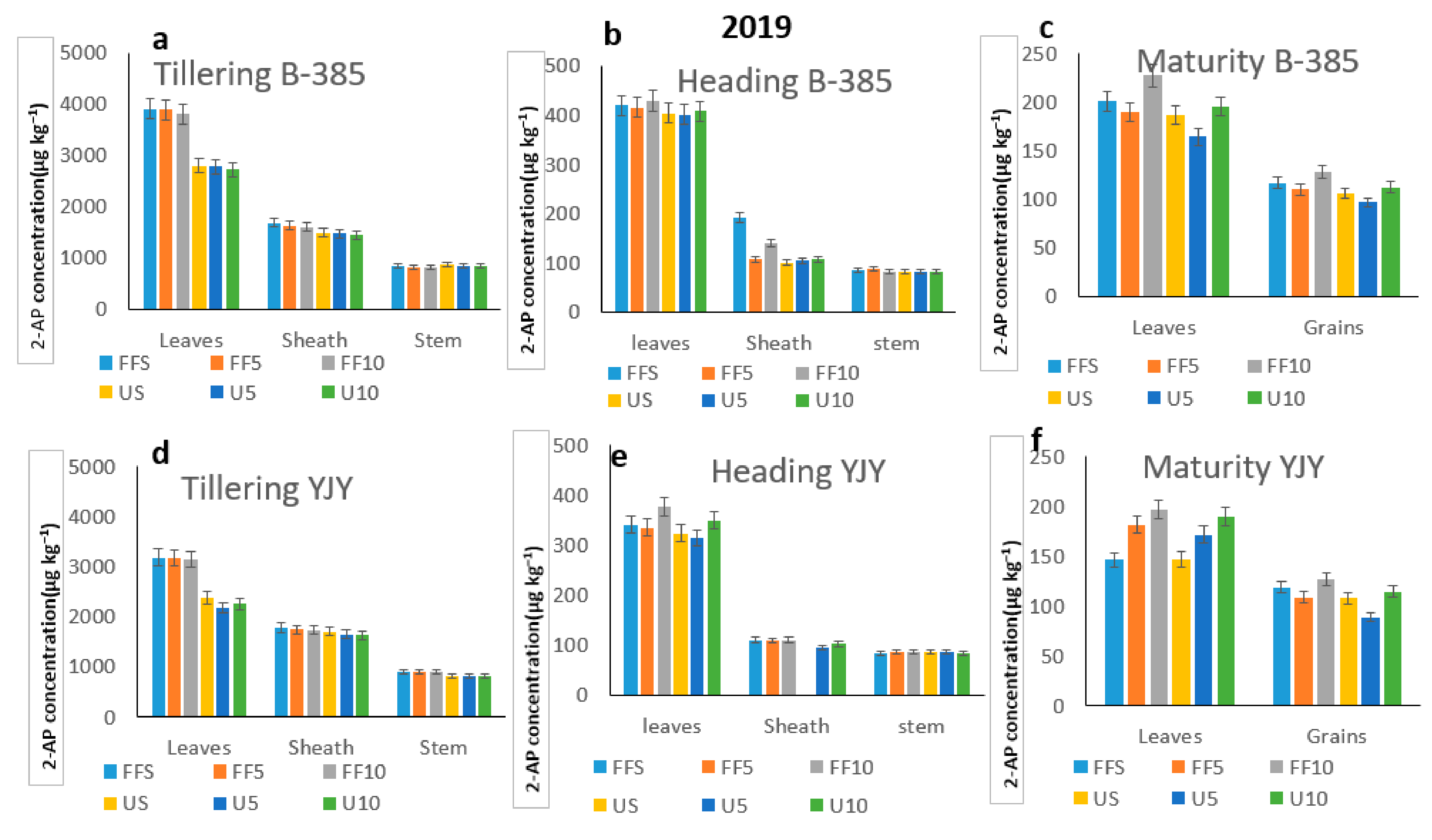
3.4. Application of 2AP and Fertilizers
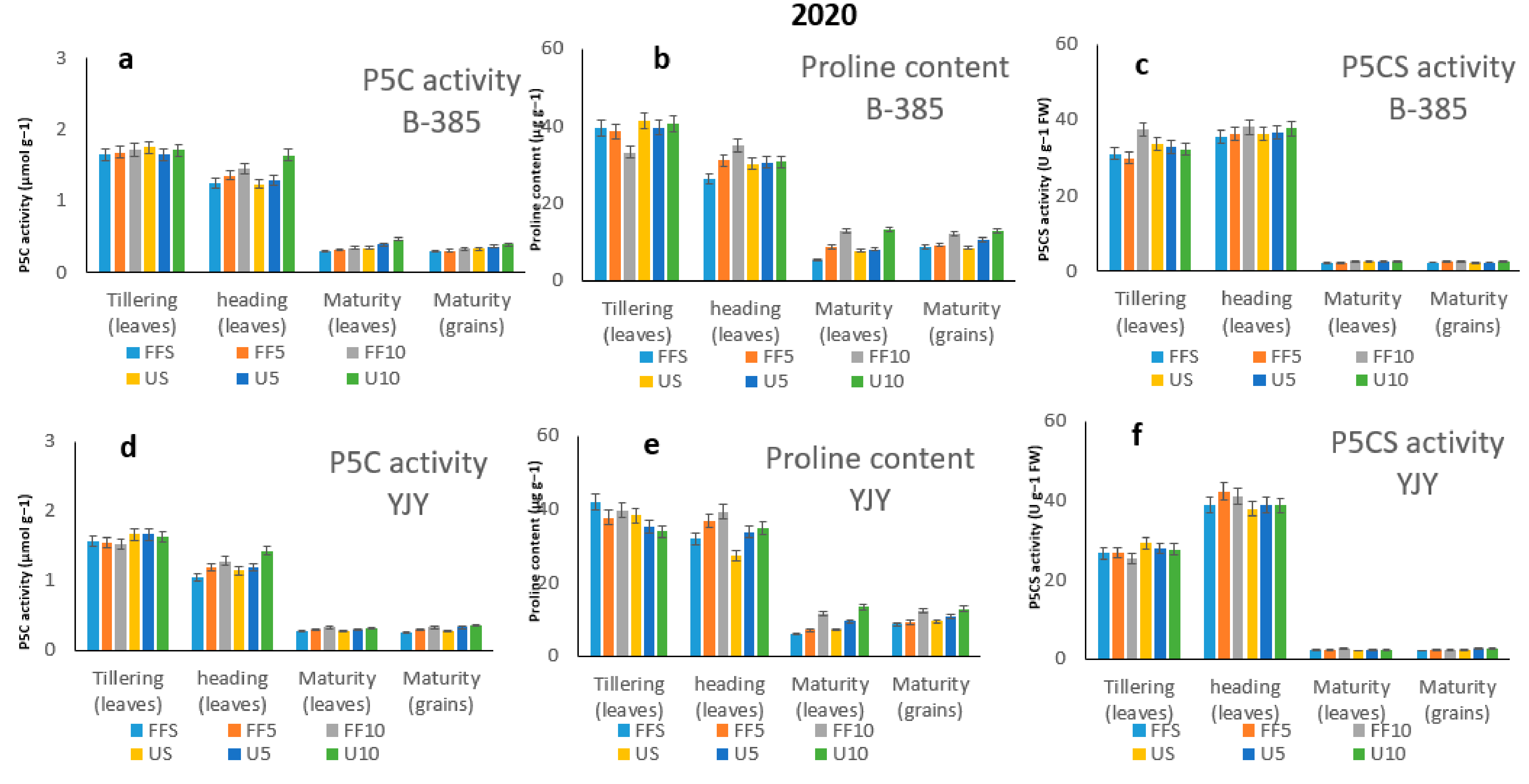
3.5. Aroma-Related Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Global Review of Agricultural Census Methodologies and Results (2006–2015); World Programme for the Census of Agriculture 2010 FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Wu, M.L.; Chou, K.L.; Wu, C.R.; Chen, J.K.; Huang, T.C. Characterization and the possible formation fechanism of 2-acetyl-1-pyrroline in aromatic vegeTable soybean (Glycine max L.). J. Food Sci. 2009, 74, 192–197. [Google Scholar] [CrossRef]
- Arouna, A.; Soullier, G.; Del Villar, P.M.; Demont, M. Policy options for mitigating impacts of COVID-19 on domestic rice value chains and food security in West Africa. Glob. Food Sec. 2020, 26, 100405. [Google Scholar] [CrossRef]
- Butardo, V.M.; Sreenivasulu, N.; Juliano, B.O. Improving Rice Grain Quality: State-of-the-Art and Future Prospects; Humana Press: New York, NY, USA, 2019; Volume 1892, ISBN 9781493989140. [Google Scholar]
- Hashemi, F.S.G.; Rafii, M.Y.; Ismail, M.R.; Mahmud, T.M.M.; Rahim, H.A.; Asfaliza, R.; Malek, M.A.; Latif, M.A. Biochemical, Genetic and Molecular Advances of Fragrance Characteristics in Rice. CRC Crit. Rev. Plant Sci. 2013, 32, 445–457. [Google Scholar] [CrossRef]
- Stuart, D.; Schewe, R.L.; McDermott, M. Reducing nitrogen fertilizer application as a climate change mitigation strategy: Understanding farmer decision-making and potential barriers to change in the US. Land Use Policy 2014, 36, 210–218. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, G.; Cheng, W.; Zhang, H.; Lu, C.; Zhang, H.; Shen, Y.; Wang, B.; Shi, W. Rice nitrogen use efficiency does not link to ammonia volatilization in paddy fields. Sci. Total Environ. 2020, 741, 140433. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, H.; Fu, Y.; Liu, Y.; Yang, C.; Zhang, T.; Wang, T.; Rong, L.; Zhang, S.; Wu, Z.; et al. Effects of different fertilization modes on rice yield and quality under a rice-crab culture system. PLoS ONE 2020, 15, e0230600. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, H.; Wang, L.; Bi, S.; Zhang, Z.; Meng, S.; Zhang, Y.; Wang, H.; Song, C.; Ma, F. The effects of magnetic treatment on nitrogen absorption and distribution in seedlings of Populus× euramericana ‘Neva’ under NaCl stress. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Sakakibara, H.; Takei, K.; Hirose, N. Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci. 2006, 11, 440–448. [Google Scholar] [CrossRef]
- Chen, P.; Nie, T.; Chen, S.; Zhang, Z.; Qi, Z.; Liu, W. Recovery efficiency and loss of 15N-labelled urea in a rice-soil system under water saving irrigation in the Songnen Plain of Northeast China. Agric. Water Manag. 2019, 222, 139–153. [Google Scholar] [CrossRef]
- Ashraf, U.; Anjum, S.A.; Khan, I.; Tanveer, M. Planting geometry-induced alteration in weed infestation, growth and yield of puddled rice. Pak. J. Weed Sci. Res. 2014, 20, 77–89. [Google Scholar]
- Pan, S.; Wen, X.; Wang, Z.; Ashraf, U.; Tian, H.; Duan, M.; Mo, Z.; Fan, P.; Tang, X. Benefits of mechanized deep placement of nitrogen fertilizer in direct-seeded rice in South China. Field Crop. Res. 2017, 203, 139–149. [Google Scholar] [CrossRef]
- Kapoor, V.; Singh, U.; Patil, S.K.; Magre, H.; Shrivastava, L.K.; Mishra, V.N.; Das, R.O.; Samadhiya, V.K.; Sanabria, J.; Diamond, R. Rice growth, grain yield, and floodwater nutrient dynamics as affected by nutrient placement method and rate. Agron. J. 2008, 100, 526–536. [Google Scholar] [CrossRef]
- Bandaogo, A.; Bidjokazo, F.; Youl, S.; Safo, E.; Abaidoo, R.; Andrews, O. Effect of fertilizer deep placement with urea supergranule on nitrogen use efficiency of irrigated rice in Sourou Valley (Burkina Faso). Nutr. Cycl. Agroecosystems 2015, 102, 79–89. [Google Scholar] [CrossRef]
- Mo, Z.; Tang, Y.; Ashraf, U.; Pan, S.; Duan, M.; Tian, H.; Wang, S.; Tang, X. Regulations in 2-acetyl-1-pyrroline contents in fragrant rice are associated with water-nitrogen dynamics and plant nutrient contents. J. Cereal Sci. 2019, 88, 96–102. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Ren, T.; Hussain, S.; Guo, C. Effects of nitrogen and tiller type on grain yield and physiological responses in rice. AoB Plants 2017, 9, plx012. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, J.; Lin, W.; Li, S.; Li, H.; Zhou, J.; Ni, P.; Dong, W.; Hu, S.; Zeng, C.; et al. The genomes of Oryza sativa: A history of duplications. PLoS Biol. 2005, 3, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.J.; He, M.R.; Wang, Z.L.; Chen, W.F.; Hou, J.; Qiu, X.K.; Zhang, J.W. Effects of new coated release fertilizer on the growth of maize. J. Soil Sci. Plant Nutr. 2016, 16, 637–649. [Google Scholar] [CrossRef][Green Version]
- Potcho, P.M.; Okpala, N.E.; Korohou, T.; Imran, M.; Kamara, N.; Zhang, J.; Aloryi, K.D.; Tang, X. Nitrogen sources affected the biosynthesis of 2-acetyl-1-pyrroline, cooked rice elongation and amylose content in rice. PLoS ONE 2021, 16, e0254182. [Google Scholar] [CrossRef]
- Park, S.; Park, H.; Baek, M.; Jeong, J.; Cho, Y.; Lee, G.; Lee, C.; Suh, J.; Kim, C.; Kim, S. Improving the Glossiness of Cooked Rice, an Important Component of Visual Rice Grain Quality. Rice 2019, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhong, C.; Zhu, C.; Zhu, L.; Zhang, J.; Wu, L.; Jin, Q. Ammonium uptake and metabolism alleviate PEG-induced water stress in rice seedlings. Plant Physiol. Biochem. 2018, 132, 128–137. [Google Scholar] [CrossRef]
- Che, S.G.; Zhao, B.Q.; Li, Y.T.; Yuan, L.; LI, W.; Lin, Z.A.; Hu, S.W.; Shen, B. Review grain yield and nitrogen use efficiency in rice production regions in China. J. Integr. Agric. 2015, 14, 2456–2466. [Google Scholar] [CrossRef]
- Poonlaphdecha, J.; Maraval, I.; Roques, S.; Audebert, A.; Boulanger, R.; Bry, X.; Gunata, Z.; Qualisud, U.M.R.; Ii, U.D.M.; Bataillon, E.; et al. Effect of Timing and Duration of Salt Treatment during Growth of a Fragrant Rice Variety on Yield and 2-Acetyl-1-pyrroline, Proline, and GABA Levels. J. Agric. Food Chem. 2012, 60, 3824–3830. [Google Scholar] [CrossRef]
- Yang, S.; Zou, Y.; Liang, Y.; Xia, B.; Liu, S.; Ibrahim, M.; Li, D.; Li, Y.; Chen, L.; Zeng, Y.; et al. Role of soil total nitrogen in aroma synthesis of traditional regional aromatic rice in China. Field Crop. Res. 2012, 125, 151–160. [Google Scholar] [CrossRef]
- Itani, T.; Tamaki, M.; Hayata, Y.; Fushimi, T.; Itani, T.; Tamaki, M.; Hayata, Y.; Fushimi, T.; Hashizume, K. Variation of 2-Acetyl-1-Pyrroline Concentration in Aromatic Rice Grains Collected in the Same Region in Japan and Factors Affecting Its Concentration. Plant Prod. Sci. 2015, 7, 178–183. [Google Scholar] [CrossRef]
- Mo, Z.; Huang, J.; Xiao, D.; Ashraf, U.; Duan, M.; Pan, S.; Tian, H.; Xiao, L.; Zhong, K.; Tang, X. Supplementation of 2-Ap, Zn and La improves 2-acetyl-1-pyrroline concentrations in detached aromatic rice panicles in vitro. PLoS ONE 2016, 11, e0149523. [Google Scholar] [CrossRef] [PubMed]
- Okpala, N.E.; Potcho, M.P.; An, T.; Ahator, S.D.; Duan, L.; Tang, X. Low temperature increased the biosynthesis of 2-AP, cooked rice elongation percentage and amylose content percentage in rice. J. Cereal Sci. 2020, 93, 102980. [Google Scholar] [CrossRef]
- Gao, C.; Wang, C.; Zheng, L.; Wang, L.; Wang, Y. A LEA gene regulates cadmium tolerance by mediating physiological responses. Int. J. Mol. Sci. 2012, 13, 5468–5481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Lu, Q.; Verma, D.P.S. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J. Biol. Chem. 1995, 270, 20491–20496. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Monza, J. Identification of Δ1-pyrroline 5-carboxylate synthase (P5CS) genes involved in the synthesis of proline in Lotus japonicus. Plant Signal. Behav. 2017, 12, e1367464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tong, T.; Potcho, P.M.; Huang, S.; Ma, L.; Tang, X. Nitrogen effects on yield, quality and physiological characteristics of giant rice. Agronomy 2020, 10, 1816. [Google Scholar] [CrossRef]
- Gaihre, Y.K.; Singh, U.; Bible, W.D.; Fugice, J.; Sanabria, J. Mitigating N2O and NO Emissions from Direct-Seeded Rice with Nitrification Inhibitor and Urea Deep Placement. Rice Sci. 2020, 27, 434–444. [Google Scholar] [CrossRef]
- Mo, Z.; Li, Y.; Nie, J.; He, L.; Pan, S.; Duan, M.; Tian, H.; Xiao, L.; Zhong, K.; Tang, X. Correction to: Nitrogen application and different water regimes at booting stage improved yield and 2-acetyl-1-pyrroline (2AP) formation in fragrant rice. Rice 2019, 12, 74. [Google Scholar] [CrossRef]
- Yatazawa, M.; Furuhashi, K. Nitrogen sources for the growth of rice callus tissue. Soil Sci. Plant Nutr. 1968, 14, 73–78. [Google Scholar] [CrossRef]
- Pan, S.; Liu, H.; Mo, Z.; Patterson, B.; Duan, M.; Tian, H.; Hu, S.; Tang, X. Effects of Nitrogen and Shading on Root Morphologies, Nutrient Accumulation, and Photosynthetic Parameters in Different Rice Genotypes. Sci. Rep. 2016, 6, 32148. [Google Scholar] [CrossRef]
- Li, L.; Tian, H.; Zhang, M.; Fan, P.; Ashraf, U.; Liu, H.; Chen, X.; Duan, M.; Tang, X.; Wang, Z.; et al. Deep placement of nitrogen fertilizer increases rice yield and nitrogen use efficiency with fewer greenhouse gas emissions in a mechanical direct-seeded cropping system. Crop J. 2021, 9, 1386–1396. [Google Scholar] [CrossRef]
- Khalofah, A.; Khan, M.I.; Arif, M.; Hussain, A.; Ullah, R.; Irfan, M.; Mahpara, S.; Shah, R.U.; Ansari, M.J.; Kintl, A.; et al. Deep placement of nitrogen fertilizer improves yield, nitrogen use efficiency and economic returns of transplanted fine rice. PLoS ONE 2021, 16, e0247529. [Google Scholar] [CrossRef]
- Liu, T.Q.; Fan, D.J.; Zhang, X.X.; Chen, J.; Li, C.F.; Cao, C.G. Deep placement of nitrogen fertilizers reduces ammonia volatilization and increases nitrogen utilization efficiency in no-tillage paddy fields in central China. Field Crop. Res. 2015, 184, 80–90. [Google Scholar] [CrossRef]
- Wu, M.; Li, G.; Li, W.; Liu, J.; Liu, M.; Jiang, C.; Li, Z. Nitrogen fertilizer deep placement for increased grain yield and nitrogen recovery efficiency in rice grown in subtropical China. Front. Plant Sci. 2017, 8, 1227. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ashraf, U.; He, L.X.; Mo, Z.W.; Wang, F.; Wan, X.C.; Kong, H.; Ran, X.L.; Tang, X.R. Irrigation and nitrogen management practices affect grain yield and 2-acetyl-1-pyrroline content in aromatic rice. Appl. Ecol. Environ. Res. 2017, 15, 1447–1460. [Google Scholar] [CrossRef]
- Jian, Z.; Wang, F.; Li, Z.; Chen, Y.; Ma, X.; Nie, L.; Cui, K.; Peng, S.; Lin, Y.; Song, H.; et al. Field Crops Research Grain yield and nitrogen use efficiency responses to N application in Bt (Cry1Ab/Ac) transgenic two-line hybrid rice. Field Crop. Res. 2014, 155, 184–191. [Google Scholar] [CrossRef]
- Mo, Z.; Ashraf, U.; Tang, Y.; Li, W.; Pan, S.; Duan, M.; Tian, H.; Tang, X. Nitrogen application at the booting stage affects 2-acetyl-1-pyrroline, proline, and total nitrogen contents in aromatic rice. Chil. J. Agric. Res. 2018, 78, 165–172. [Google Scholar] [CrossRef]
- Bao, G.; Ashraf, U.; Wang, C.; He, L.; Wei, X.; Zheng, A.; Mo, Z.; Tang, X. Molecular basis for increased 2-acetyl-1-pyrroline contents under alternate wetting and drying (AWD) conditions in fragrant rice. Plant Physiol. Biochem. 2018, 133, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Jiang, G.H.; Shen, L.H.; Wang, L.Q.; He, Y.Q. Mapping quantitative trait loci underlying the cooking and eating quality of rice using a DH population. Mol. Breed. 2005, 15, 117–124. [Google Scholar] [CrossRef]
- Okpala, N.E.; Duan, L.; Shen, G.; Zhang, G.; Qi, X. Identification of putative metabolic biomarker underlying cooked rice elongation. Plant Omics 2017, 10, 164–168. [Google Scholar] [CrossRef]
- Meng, W.U.; Ming, L.I.U.; Jia, L.I.U.; Li, W.T.; Jiang, C.Y.; Li, Z.P. Optimize nitrogen fertilization location in root-growing zone to increase grain yield and nitrogen use efficiency of transplanted rice in subtropical China. J. Integr. Agric. 2017, 16, 2073–2081. [Google Scholar] [CrossRef]
- Bufogle, A.; Bollich, P.K.; Kovar, J.L.; Lindau, C.W.; Macchiavellid, R.E. Comparison of ammonium sulfate and urea as nitrogen sources in rice production. J. Plant Nutr. 1998, 21, 1601–1614. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, T.; Yang, Z.; Wang, T.; Fu, Y.; Chen, Y.; Hu, B.; Ren, W. Morphophysiological mechanism of rice yield increase in response to optimized nitrogen management. Sci. Rep. 2017, 7, 17226. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Wang, Y.; He, Q.; Shu, F.; Liu, H.; Wang, J.; Wang, J.; Yuan, L.; Deng, H. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J. Integr. Plant Biol. 2016, 58, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Miah, M.A.M.; Gaihre, Y.K.; Hunter, G.; Singh, U.; Hossain, S.A. Fertilizer deep placement increases rice production: Evidence from farmers’ fields in southern Bangladesh. Agron. J. 2016, 108, 805–812. [Google Scholar] [CrossRef]
- Henry, R. 33 Years of 2-Acetyl-1-Pyrroline, a Principle Basmati Aroma Compound in Scented Rice (Oryza sativa L.): A status review. J. Sci. Food Agric. 2017, 97, 384–395. [Google Scholar]
- Dias, L.G.; Duarte, G.H.B.; Mariutti, L.R.B.; Bragagnolo, N. Aroma profile of rice varieties by a novel SPME method able to maximize 2-acetyl-1-pyrroline and minimize hexanal extraction. Food Res. Int. 2019, 123, 550–558. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Turnbaugh, J.G.; Juliano, B.O. Cooked Rice Aroma and 2-Acetyl- 1-pyrroline. J. Agric. Food Chem. 1983, 31, 823–826. [Google Scholar] [CrossRef]
- Maraval, I.; Sen, K.; Agrebi, A.; Menut, C.; Morere, A.; Boulanger, R.; Gay, F.; Mestres, C.; Gunata, Z. Quantification of 2-acetyl-1-pyrroline in rice by sTable isotope dilution assay through headspace solid-phase microextraction coupled to gas chromatography-tandem mass spectrometry. Anal. Chim. Acta 2010, 675, 148–155. [Google Scholar] [CrossRef]
- Li, M.; Ashraf, U.; Tian, H.; Mo, Z.; Pan, S.; Anjum, S.A.; Duan, M.; Tang, X. Manganese-induced regulations in growth, yield formation, quality characters, rice aroma and enzyme involved in 2-acetyl-1-pyrroline biosynthesis in fragrant rice. Plant Physiol. Biochem. 2016, 103, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yoshihashi, T.; Huong, N.T.T.; Inatomi, H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem. 2002, 50, 2001–2004. [Google Scholar] [CrossRef]
- Xu, C.M.; Wang, D.Y.; Chen, S.; Chen, L.P.; Zhang, X.F. Effects of Aeration on Root Physiology and Nitrogen Metabolism in Rice. Rice Sci. 2013, 20, 148–153. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Guo, R.; Sarwar, M.; Ren, X.; Krstic, D.; Aslam, Z.; Zulifqar, U.; Rauf, A.; Hano, C.; et al. Carbon Sequestration to Avoid Soil Degradation: A Review on the Role of Conservation Tillage. Plants 2021, 10, 2001. [Google Scholar] [CrossRef]


| Year | Organic Content g·kg−1 | Total N g·kg−1 | Total P g·kg−1 | Total K g·kg−1 | Available N mg·kg−1 | Available P mg·kg−1 | Available K mg·kg−1 | pH |
|---|---|---|---|---|---|---|---|---|
| 2019 | 21.52 | 2.96 | 14.12 | 16.22 | 364.32 | 34.23 | 43.21 | 5.74 |
| 2020 | 22.68 | 3.41 | 14.10 | 16.88 | 371.33 | 32.98 | 41.95 | 5.70 |
| Growing Season | Variety | Treatment | Number of Filled Grains per Panicle | Effective Panicle Number (pot−1) | 1000-Grain Weight (g) | Grain-Filling (%) | Yield pot−1 (g) |
|---|---|---|---|---|---|---|---|
| 2019 | B-385 | FFS | 128.22 a | 25 a | 24.10 a | 49.84 b | 25.18 b |
| FF5 | 114.32 b | 23 b | 19.46 b | 41.39 ab | 22.28 ab | ||
| FF10 | 122.28 a | 24 a | 23.42 ab | 54.07 a | 28.71 a | ||
| US | 102.03 b | 26 ab | 20.82 ab | 56.05 a | 27.19 b | ||
| U5 | 117.31 b | 24 b | 19.50 b | 45.70 b | 25.37 c | ||
| U10 | 132.79 a | 28 a | 24.78 a | 54.69 a | 33.11 a | ||
| YJY | FFS | 113.25 b | 27 b | 22.57 a | 55.26 a | 27.70 ab | |
| FF5 | 111.10 b | 27 b | 19.64 b | 50.94 b | 23.03 b | ||
| FF10 | 130.07 a | 28 a | 24.57 a | 58.05 a | 33.31 a | ||
| US | 127.38 b | 28 b | 23.36 b | 61.87 b | 32.35 ab | ||
| U5 | 123.43 b | 27 c | 21.85 c | 58.94 b | 27.20 b | ||
| U10 | 133.04 a | 30 a | 24.33 a | 76.54 a | 36.35 a | ||
| 2020 | B-385 | FFS | 124.22 b | 27 b | 24.10 b | 59.84 b | 29.18 a |
| FF5 | 119.32 c | 27 b | 20.46 c | 53.39 c | 22.28 b | ||
| FF10 | 129.28 a | 28 a | 27.42 a | 71.07 a | 31.71 a | ||
| US | 122.03 a | 28 ab | 22.82 ab | 68.05 b | 33.19 ab | ||
| U5 | 112.31 b | 27 b | 20.50 b | 59.70 cb | 29.37 b | ||
| U10 | 122.79 a | 29 a | 26.78 a | 75.69 a | 36.89 a | ||
| YJY | FFS | 114.25 b | 27 b | 20.57 b | 53.26 ab | 29.70 a | |
| FF5 | 112.10 b | 27 b | 19.64 b | 50.94 a | 23.03 b | ||
| FF10 | 131.07 a | 28 a | 22.57 a | 55.05 a | 30.31 a | ||
| US | 127.38 ab | 28 b | 22.36 b | 61.87 b | 26.35 b | ||
| U5 | 123.43 c | 28 b | 20.85 cb | 50.94 c | 21.20 c | ||
| U10 | 133.04 a | 31 a | 24.83 a | 76.54 a | 31.35 a |
| F Value | Number of Filled Grains per Panicle | Effective Panicle Number (pot−1) | 1000-Grain Weight (g) | Grain-Filling (%) | Yield (pot−1) | |
|---|---|---|---|---|---|---|
| 2019 | Variety | NS | ** | ** | ** | ** |
| Fertilizer Placement | NS | NS | ** | NS | NS | |
| Variety X Fertilizer Placement | NS | NS | ** | ** | ** | |
| 2020 | Variety | NS | ** | ** | ** | * |
| Fertilizer Placement | NS | NS | ** | * | NS | |
| Variety X Fertilizer Placement | NS | NS | ** | * | * | |
| Growing Season | Variety | Treatment | Brown Rice Rate (%) | Milled Rice Rate (%) | Head Rice Rate (%) | Green Grain Rate (%) | Protein Content (%) | Amylose Content (%) | Chalk White | Chalkiness |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | B-385 | FFS | 83.15 a | 71.82 a | 52.52 ab | 14.35 b | 8.50 ab | 15.87 b | 15.31 a | 7.18 bc |
| FF5 | 82.72 a | 70.90 a | 49.25 b | 26.57 a | 8.20 b | 14.90 c | 10.30 b | 5.32 c | ||
| FF10 | 85.34 a | 70.53 a | 57.91 a | 14.82 b | 8.80 a | 17.97 a | 13.35 ab | 13.33 a | ||
| US | 84.78 a | 71.91 a | 56.56 b | 25.41 b | 8.60 b | 16.70 b | 14.7 ab | 10.25 a | ||
| U5 | 83.72 a | 69.57 a | 53.74 bc | 31.62 a | 8.27 c | 16.22 c | 14.55 b | 5.48 b | ||
| U10 | 85.68 a | 71.71 a | 59.54 a | 12.51 c | 8.90 a | 17.09 a | 19.82 a | 9.96 ab | ||
| YJY | FFS | 74.64 a | 68.70 b | 58.22 a | 17.26 b | 8.43 ab | 16.43 b | 22.30 a | 11.66 ab | |
| FF5 | 73.49 a | 65.09 c | 56.70 a | 42.55 a | 8.37 b | 16.20 b | 21.31 a | 9.94 c | ||
| FF10 | 78.63 a | 69.90 a | 47.57 b | 7.91 c | 8.50 a | 17.87 a | 20.11 ab | 13.83 a | ||
| US | 76.26 a | 69.87 ab | 48.61 b | 24.94 b | 8.03 b | 16.43 b | 18.72 ab | 6.30 c | ||
| U5 | 72.55 a | 65.36 c | 56.93 a | 37.72 a | 7.53 c | 16.27 bc | 22.13 a | 13.07 a | ||
| U10 | 79.27 a | 70.08 a | 58.58 a | 20.72 c | 8.40 a | 16.93 a | 18.71 b | 9.67 b | ||
| 2020 | B-385 | FFS | 82.74 a | 67.70 ab | 53.76 a | 15.74 b | 7.37 b | 17.80 b | 8.36 b | 2.57 b |
| FF5 | 80.87 a | 63.29 b | 53.36 a | 20.54 a | 7.33 b | 17.23 bc | 9.83 b | 3.76 b | ||
| FF10 | 85.39 a | 71.63 a | 55.88 a | 10.48 c | 8.83 a | 18.57 a | 12.16 a | 4.00 a | ||
| US | 84.97 a | 68.79 ab | 58.27 a | 9.86 b | 8.07 b | 17.07 c | 14.69 a | 7.22 ab | ||
| U5 | 81.42 a | 64.70 c | 46.89 b | 15.36 a | 7.43 c | 15.07 b | 12.05 a | 5.28 b | ||
| U10 | 86.85 a | 72.69 a | 48.40 b | 7.53 c | 8.93 a | 17.53 a | 19.31 a | 7.49 a | ||
| YJY | FFS | 74.61 a | 67.92 ab | 48.77 ab | 12.93 b | 6.23 b | 16.07 b | 16.04 a | 7.59 b | |
| FF5 | 75.03 a | 65.66 c | 54.29 a | 31.19 a | 6.00 c | 15.40 c | 19.44 a | 7.86 b | ||
| FF10 | 77.57 a | 70.75 a | 52.01 b | 8.11 c | 7.00 a | 17.77 a | 6.52 b | 9.34 a | ||
| US | 77.06 a | 68.56 ab | 60.43 ab | 20.53 a | 6.83 b | 16.07 b | 6.91 b | 3.07 b | ||
| U5 | 75.68 a | 65.75 b | 57.11 ab | 22.17 a | 6.17 c | 15.67 c | 11.95 a | 4.76 a | ||
| U10 | 79.04 a | 73.53 a | 64.20 a | 18.52 b | 7.33 a | 16.27 b | 12.05 a | 4.75 a |
| F Value | Brown Rice Rate (%) | Milled Rice Rate (%) | Head Rice Rate (%) | Green Grain Rate (%) | Protein Content (%) | Amylose Content (%) | Chalk White | Chalkiness | |
|---|---|---|---|---|---|---|---|---|---|
| 2019 | Variety | NS | ** | NS | ** | ** | ** | ** | NS |
| Fertilizer Placement | * | NS | ** | ** | ** | ** | * | ** | |
| Variety X Fertilizer Placement | NS | * | NS | ** | ** | ** | ** | ** | |
| 2020 | Variety | NS | NS | ** | ** | ** | ** | ** | ** |
| Fertilizer Placement | NS | ** | * | ** | ** | ** | ** | * | |
| Variety X Fertilizer Placement | NS | NS | NS | NS | ** | ** | NS | NS | |
| F Value | Tillering | Heading | Maturity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Sheath | Stem | Leaves | Sheath | Stem | Leaves | Grains | ||
| 2019 | Variety | * | * | NS | * | ** | NS | ** | ** |
| Fertilizer Placement | * | ** | NS | ** | * | NS | * | ** | |
| Variety X Fertilizer Placement | * | ** | NS | * | * | NS | * | ** | |
| 2020 | Variety | * | * | NS | * | ** | NS | ** | ** |
| Fertilizer Placement | * | ** | NS | ** | * | NS | ** | ** | |
| Variety X Fertilizer Placement | ** | ** | NS | ** | * | NS | ** | * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potcho, P.M.; Imran, M.; Korohou, T.; Kamara, N.; Tang, X. Fertilizer Deep Placement Significantly Affected Yield, Rice Quality, 2-AP Biosynthesis and Physiological Characteristics of the Fragrant Rice Cultivars. Agronomy 2022, 12, 162. https://doi.org/10.3390/agronomy12010162
Potcho PM, Imran M, Korohou T, Kamara N, Tang X. Fertilizer Deep Placement Significantly Affected Yield, Rice Quality, 2-AP Biosynthesis and Physiological Characteristics of the Fragrant Rice Cultivars. Agronomy. 2022; 12(1):162. https://doi.org/10.3390/agronomy12010162
Chicago/Turabian StylePotcho, Pouwedeou Mouloumdema, Muhammad Imran, Tchalla Korohou, Nabieu Kamara, and Xiangru Tang. 2022. "Fertilizer Deep Placement Significantly Affected Yield, Rice Quality, 2-AP Biosynthesis and Physiological Characteristics of the Fragrant Rice Cultivars" Agronomy 12, no. 1: 162. https://doi.org/10.3390/agronomy12010162
APA StylePotcho, P. M., Imran, M., Korohou, T., Kamara, N., & Tang, X. (2022). Fertilizer Deep Placement Significantly Affected Yield, Rice Quality, 2-AP Biosynthesis and Physiological Characteristics of the Fragrant Rice Cultivars. Agronomy, 12(1), 162. https://doi.org/10.3390/agronomy12010162








