Effect of Meiotic Polyploidisation on Selected Morphological and Anatomical Traits in Interspecific Hybrids of Brassica oleracea × B. napus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Assessment of the Ploidy Level
2.2.1. Flow Cytometry Analysis (FCM)
2.2.2. Chromosome Count
2.3. Analysis of the Morphological Characteristics
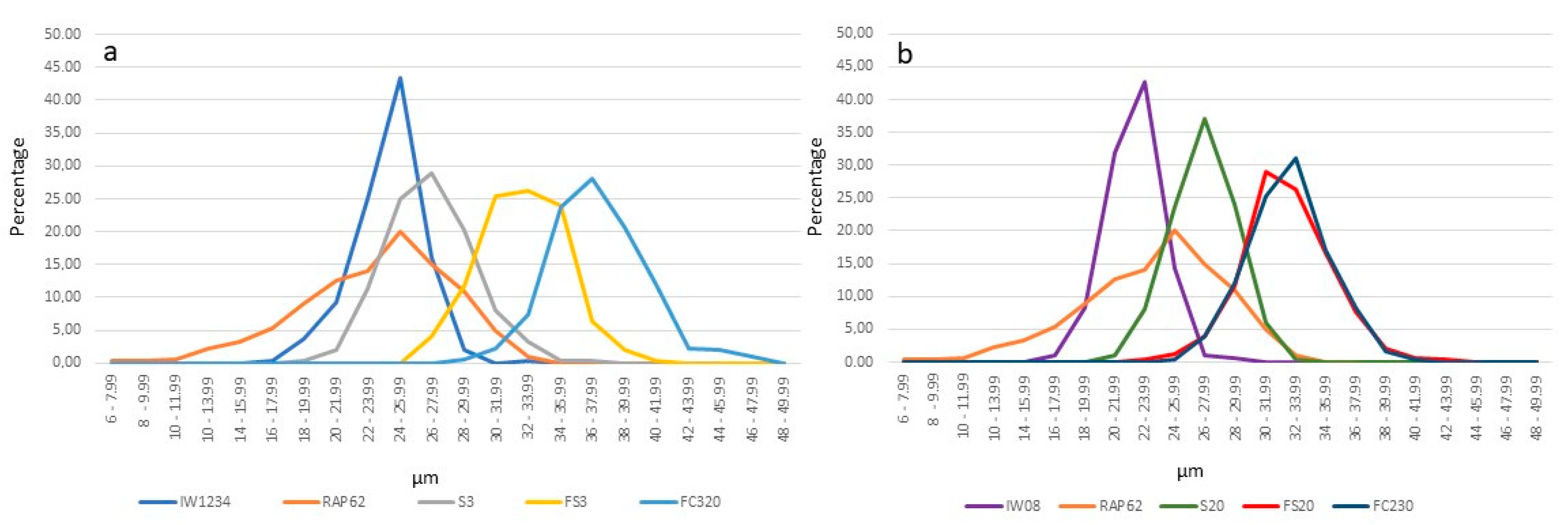
2.4. Pollen Grain Diameter and Viability
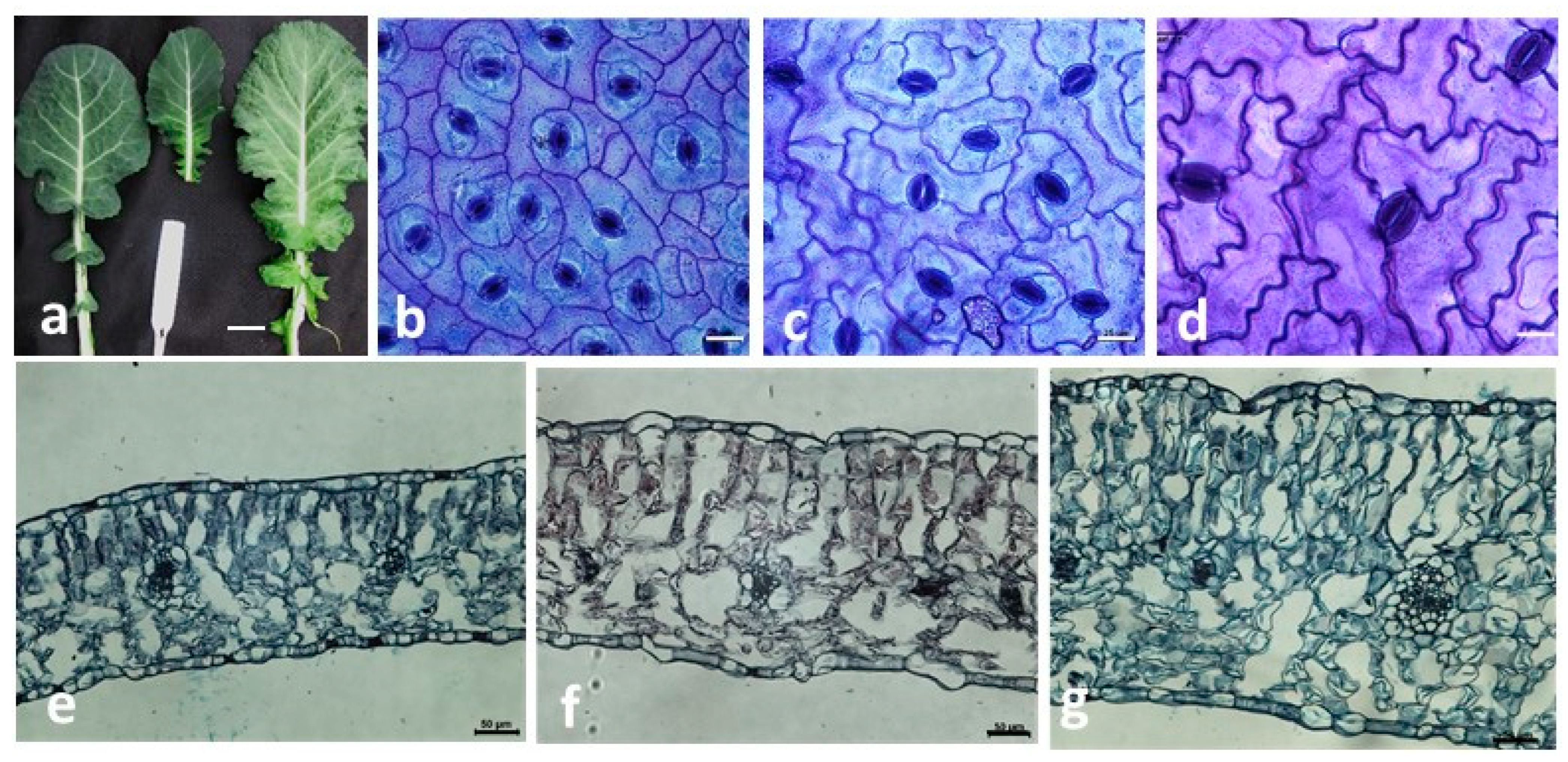
2.5. Leaf Anatomy
2.6. Stomata Length and Density
2.7. Statistical Analyses
3. Results
3.1. Genome Size Determination by FCM and Somatic Chromosome Count
3.2. Characteristics of Parental Genotypes and Interspecific Hybrids of the F1 and F2 Generations in the Generative Stage
3.3. Characteristics of Morphological and Anatomical Traits of Leaves of Parental Genotypes and the Interspecific Hybrids of the F1 and F2 Generations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masterson, J. Stomatal size in fossil plants: Evidence for polyploidy in majority of Angiosperms. Science 1994, 264, 421–424. [Google Scholar] [CrossRef]
- Casacuberta, J.M.; Jackson, S.; Panaud, O.; Purugganan, M.; Wendel, J. Evolution of plant phenotypes, from genomes to traits. G3 Genes Genomes Genet. 2016, 6, 775–778. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The ploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Sochacki, D.; Marciniak, P. Breeding aspects of selected ornamental bulbous crops. Agronomy 2021, 11, 1709. [Google Scholar] [CrossRef]
- Kreiner, J.M.; Kron, P.; Husband, B.C. Frequency and maintenance of unreduced gametes in natural plant populations: Associations with reproductive mode, life history and genome size. New Phytol. 2017, 214, 879–889. [Google Scholar] [CrossRef]
- Ramsey, J.; Schemske, D.W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef]
- Pecinka, A.; Fang, W.; Rehmsmeier, M.; Levy, A.A.; Scheid, O.M. Polyploidization increases meiotic recombination frequency in Arabidopsis. BMC Biol. 2011, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Dar, J.A.; Beigh, Z.A.; Wani, A.A. Polyploidy: Evolution and crop improvement. In Chromosome Structure and Aberration; Chapter 10; Bhat, T.A., Wani, A.A., Eds.; Springer: New Delhi, India, 2017; pp. 201–217. [Google Scholar]
- Podwyszyńska, M.; Marasek-Ciolakowska, A. Ploidy, Genome Size, and Cytogenetics of Apple. In The Apple Genome, Compendium of Plant Genomes; Korban, S.S., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Lage, J.; Trethowan, R.M. CIMMYT’s use of synthetic hexaploid wheat in breeding for adaptation to rainfed environments globally. Aus. J. Agri. Res. 2008, 59, 461–469. [Google Scholar] [CrossRef]
- Yin, L.; Zhu, Z.; Luo, X.; Huang, L.; Li, Y.; Mason, A.S.; Yang, J.; Ge, X.; Long, Y.; Wang, J.; et al. Genome-Wide duplication of allotetraploid Brassica napus produces novel characteristics and extensive ploidy variation in self-pollinated progeny. G3 Genes Genomes Genet. 2020, 10, 3687–3699. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, J.; Wang, X. Genome triplication drove the diversification of Brassica plants. Hortic. Res. 2014, 1, 14024. [Google Scholar] [CrossRef]
- Katche, E.; Quezada-Martinez, D.; Katche, E.I.; Vasquez-Teuber, P.; Mason, A.S. Interspecific hybridization for Brassica crop improvement. Crop Breed. Genet. Genom. 2019, 1, e190007. [Google Scholar] [CrossRef]
- Nagaharu, U. Genome analysis in Brassica with special reference to the experimental formation of Brassica napus and peculiar mode of fertilization. Jap. J. Bot. 1935, 7, 389–453. [Google Scholar]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef]
- Roy, N.N. Interspecific tranfer of Brassica juncea-type high blackleg resistance to Brassica napus. Euphytica 1984, 33, 295–303. [Google Scholar] [CrossRef]
- Gerdemann-Knorck, M.; Nielen, S.; Tzscheetzsch, C.; Iglisch, J.; Schieder, O. Transfer of disease resistance within the genus Brassica through asymmetric somatic hybridization. Euphytica 1995, 85, 247–253. [Google Scholar] [CrossRef]
- Sharma, B.B.; Kalia, P.; Singh, D.; Sharma, T.R. Introgression of Black Rot Resistance from Brassica carinata to Cauliflower (Brassica oleracea botrytis Group) through Embryo Rescue. Front. Plant Sci. 2017, 8, 1255. [Google Scholar] [CrossRef]
- Tonguc, M.; Griffiths, P.D. Transfer of powdery mildew resistance from Brassica carinata to Brassica oleracea through embryo rescue. Plant Breed. 2008, 123, 587–589. [Google Scholar] [CrossRef]
- Mafakheri, M.; Kordrostami, M. Biotechnological Approach for Enhancing Capability of Brassica oleracea var. italica Against Stresses Under Changing Climate. In The Plant Family Brassicaceae; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Seepaul, R.; Kumar, S.; Iboyi, J.E.; Bashyal, M.; Stansly, T.L.; Bennett, R.; Boote, K.J.; Mulvaney, M.J.; Small, I.M.; George, S.; et al. Brassica carinata: Biology and agronomy as a biofuel crop. GCB Bioenergy 2021, 13, 582–599. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y. Cytogenetics and germplasm enrichment in Brassica allopolyploids in China. J. Integr. Agric. 2017, 16, 2698–2708. [Google Scholar] [CrossRef]
- Wang, G.-X.; Tang, Y.; Yan, H.; Sheng, X.-G.; Hao, W.-W.; Zhang, L.; Lu, K.; Liu, F. Production and characterization of interspecific somatic hybrids between Brassica oleracea var. botrytis and B. nigra and their progenies for the selection of advanced pre-breeding materials. Plant Cell Rep. 2011, 30, 1811–1821. [Google Scholar] [CrossRef]
- Gaebelein, R.; Alnajar, D.; Koopmann, B.; Mason, A.S. Hybrids between Brassica napus and B. nigra show frequent pairing between the B and A/C genomes and resistance to blackleg. Chromosome Res. 2019, 27, 221–236. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, M.; Qiao, H.; Li, F.; Zhang, S.; Zhang, S.; Zhang, H. Characterization of interspecific hybrid between flowering Chinese cabbage and broccoli. Scientia Hortic. 2018, 240, 552–557. [Google Scholar] [CrossRef]
- Lee, S.S.; Lee, S.A.; Yang, J.; Kim, J. Developing stable progenies of ×Brassicoraphanus, an intergeneric allopolyploid between Brassica rapa and Raphanus sativus, through induced mutation using microspore culture. Theor. Appl. Genet. 2011, 122, 885–891. [Google Scholar] [CrossRef]
- Dixon, G.R. Vegetable Brassicas and Related Crucifers; CAB International: Wallingford, UK, 2007; 327p. [Google Scholar]
- Starzycki, M.; Starzycka, E.; Pszczoła, J. Development of Alloplasmic Rape. Adv. Bot. Res. 2007, 45, 313–335. [Google Scholar]
- Kamiński, P.; Podwyszyńska, M.; Starzycki, M.; Starzycka-Korbas, E. Interspecific hybridisation of cytoplasmic male-sterile rapeseed with Ogura cytoplasm and Brassica rapa var. pekinensis as a method to obtain male-sterile Chinese cabbage inbred lines. Euphytica 2016, 208, 519–534. [Google Scholar] [CrossRef]
- Kamiński, P.; Marasek-Ciolakowska, A.; Podwyszyńska, M.; Starzycki, M.; Starszycka-Korbas, E.; Nowak, K. Development and characteristics of interspecific hybrids between Brassica oleracea L. and B. napus L. Agronomy 2020, 10, 1339. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Trzewik, A.; Marasek-Ciołakowska, A. In vitro polyploidisation of tulips (Tulipa gesneriana L.)—Phenotype assessment of tetraploids. Sci. Hortic. 2018, 242, 155–163. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; He, H.; Bijman, P.; Ramanna, M.S.; Arens, P.; van Tuyl, J.M. Assessment of intergenomic recombination through GISH analysis of F1, BC1 and BC2 progenies of Tulipa gesneriana and T. fosteriana. Plant Syst. Evol. 2012, 298, 887–899. [Google Scholar] [CrossRef]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain. Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Saniewski, M.; Dziurka, M.; Kowalska, U.; Góraj-Koniarska, J.; Ueda, J.; Miyamota, K. Formation of the secondary abscission zone Induced by the interaction of methyl jasmonate and auxin in Bryophyllum calycinum: Relevance to auxin status and histology. Int. J. Mol. Sci. 2020, 21, 2784. [Google Scholar] [CrossRef] [PubMed]
- Dyki, B.; Habdas, H. Metoda izolowania epidermy liści pomidora i ogórka dla mikroskopowej oceny rozwoju grzybów patogenicznych. (The method of isolation of epidermis of tomato and cucumber leaves for microscopic investigation of pathogenic fungus development). Acta Agrobot. 1996, 49, 123–129. (In Polish) [Google Scholar] [CrossRef][Green Version]
- Pradhan, A.; Plummer, J.A.; Neslon, M.N.; Cowling, W.A.; Yan, G. Successful induction of trigenomic hexaploid Brassica from a triploid hybrid of B. napus L. and B. nigra (L.) Koch. Euphytica 2010, 176, 87–98. [Google Scholar] [CrossRef]
- Meng, J.L.; Shi, S.; Li, G.; Li, Z.; Qu, X. The production of yellow-seeded Brassica napus (AACC) through crossing interspecific hybrids of B. campestris (AA) and B. carianata (BBCC) with B. napus. Euphytica 1998, 103, 329–333. [Google Scholar] [CrossRef]
- Zhang, K.; Mason, A.S.; Fariiq, M.A.; Islam, F.; Quezada-Martinez, D.; Hu, D.; Yang, S.; Zou, J.; Zhou, W. Challenges and prospects for a potential allohexaploid Brassica crop. Theor. App. Genet. 2021, 134, 2711–2726. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.S.; Yan, G.; Cowling, W.A.; Nelson, M.N. A new method for producing allohexaploid Brassica through unreduced gametes. Euphytica 2012, 186, 277–287. [Google Scholar] [CrossRef]
- Silkova, O.G.; Shchapova, A.I.; Shumny, V.K. Patterns of meiosis in ABDR amphihaploids depend on the specific type of univalent chromosome division. Euphytica 2011, 178, 415–426. [Google Scholar] [CrossRef]
- Xiong, Z.Y.; Gaeta, R.T.; Pires, J.C. Homoeologous shufing and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus. Proc. Natl. Acad. Sci. USA 2011, 108, 7908–7913. [Google Scholar] [CrossRef]
- Bhatia, R.; Sharma, K.; Parkash, C.; Pramanik, A.; Singh, D.; Singh, S.; Kumar, R.; Dey, S.S. Microspore derived population developed from an inter-specific hybrid (Brassica oleracea × B. carinata) through a modifed protocol provides insight into B genome derived black rot resistance and inter-genomic interaction. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 145, 417–434. [Google Scholar] [CrossRef]
- He, D.; Jing, J.; Snowdon, R.J.; Mason, A.S.; Shen, J.; Meng, J.; Zou, J. Exploring the gene pool of Brassica napus by genomics-based approaches. Plant Biotech. J. 2021, 19, 1693–1712. [Google Scholar]
- Yuan, S.-x.; Liu, Y.-m.; Fang, Z.-y.; Yang, L.-m.; Zhuang, M.; Zhang, Y.-y.; Sun, P.-t. Study on the relationship between the ploidy level of microspore-derived plants and the number of chloroplasts in stomatal guard cells in Brassica oleracea. Agr. Sci. China 2009, 8, 939–946. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Oh, Y.-J.; Lee, K.-R.; Ko, H.-C.; Cho, H.-S.; Lee, Y.-H.; Chang, A. Characteristics analysis of F1 hybrids between genetically modified Brassica napus and B. rapa. PLoS ONE 2016, 11, e0162103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dewitte, A.; Van Laere, K.; Van Huylenbroeck, J. Use of 2n gametes in plant breeding. In Plant Breeding; Abdurakhmonov, I.Y., Ed.; Intech.: Rijeka, Croatia, 2011; pp. 59–86. [Google Scholar]
- Padoan, D.; Mossad, A.; Chiancone, B.; Germana, M.A.; Kjan, P.S.S.V. Ploidy levels in Citrus clementine affects leaf morphology, stomatal density and water content. Theor. Exp. Plant. Physiol. 2013, 25, 283–290. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Markiewicz, M.; Broniarek-Niemiec, A.; Matysiak, B.; Marasek-Ciolakowska, A. Apple autotetraploids with enhanced resistance to apple scab (Venturia inaequalis) due to genome duplication-phenotypic and genetic evaluation. Int. J. Mol. Sci. 2021, 22, 527. [Google Scholar] [CrossRef]
- Brownlee, C. The long and short of stomata density signals. Trends Plant Sci. 2001, 6, 441–442. [Google Scholar] [CrossRef]
- Li, M.; Qian, W.; Meng, J.; Li, Z. Construction of novel Brassica napus genotypes through chromosomal substitution and elimination using interploid species hybridization. Chromosome Res. 2004, 12, 417–426. [Google Scholar] [CrossRef]
- Seki, K. Leaf-morphology-assisted selection for resistance to two-spotted spider mite Tetranychus uriticae Koch (Acari: Tetranychidae) in carnations (Dianthus caryophyllus L.). Pest Manag. Sci. 2016, 72, 1926–1933. [Google Scholar] [CrossRef]
- Goiana, E.S.S.; Dias-Pini, N.S.; Muniz, C.R.; Soares, A.A.; Alves, J.C.; Vidal-Neto, F.C.; Da Silva, C.S.B. Dwarf-cashew resistance to whitefly (Aleurodicus cocois) linked to morphological and histochemical characteristics of leaves. Pest Manag. Sci. 2020, 76, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Marasek-Ciolakowska, A.; Soika, G.; Warabieda, W.; Kowalska, U.; Rybczyński, D. Investigation on the relationship between morphological and anatomical characteristic of savoy cabbage and kale leaves and infestation by cabbage whitefly (Aleyrodes proletella L.). Agronomy 2021, 11, 275. [Google Scholar] [CrossRef]
- Malek, M.A.; Rahman, L.; Das, M.L.; Hassan, L.; Rafii, M.Y.; Ismail, M.R. Development of hexaploid Brassica (AABBCC) from hybrids (ABC) of Brassica carinata (BBCC) × B. rapa (AA). Aust. J. Crop Sci. 2013, 7, 1375–1382. [Google Scholar]
- Davis, A.R.; Fowke, L.C.; Sawhney, V.K.; Low, N.H. Floral nectar secretion and ploidy in Brassica rapa and B. napus (Brassicaceae). II. Quantified variability of nectary structure and function in rapid-cycling lines. Ann. Bot. 1996, 77, 223–234. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.; Zhao, M.; Ma, J.; Yu, J.; Huang, S.; et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef] [PubMed]

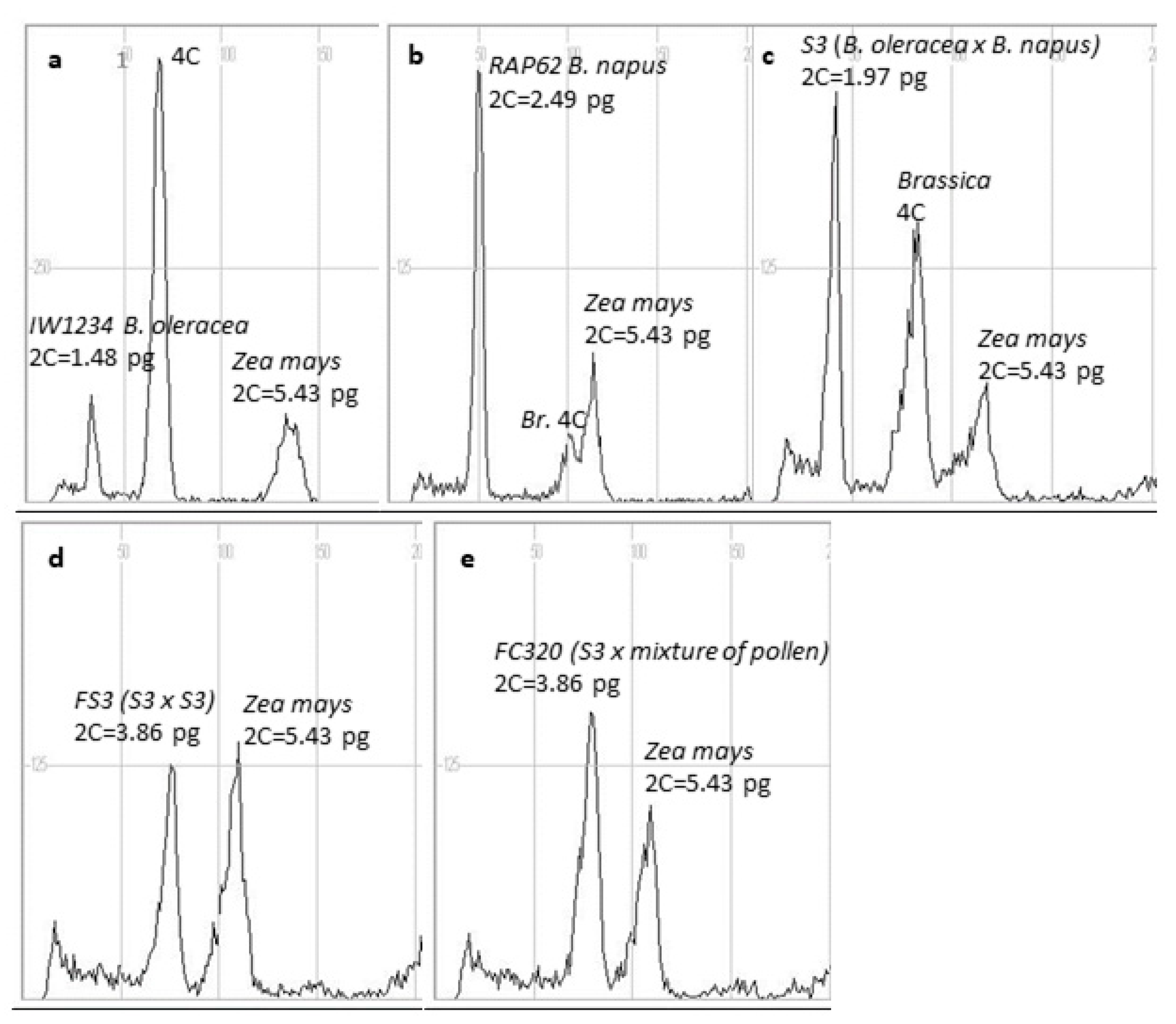



| Genotype | Genome | DNA Content (pg) | Std. dev. | Chromosome Number |
|---|---|---|---|---|
IW1234  head cabbage head cabbage | CC | 1.48 d * | 0.021 | 18 |
IW08  kale kale | CC | 1.51 d | 0.022 | 18 |
RAP62  rapeseed rapeseed | AACC | 2.49 b | 0.036 | 38 |
| Interspecific hybrids of the F1 generation | ||||
| S3 (head cabbage × rapeseed) | ACC | 1.97 c | 0.030 | 28 |
| S20 (kale × rapeseed) | ACC | 1.99 c | 0.017 | 28 |
| Interspecific hybrids of the F2 generation | ||||
| FS3 (S3 × S3) | AACCCC | 3.86 a | 0.034 | 56 |
| FS20 (S20 × S20) | AACCCC | 3.81 a | 0.102 | 55 |
| FC320 (S3 × mixture of pollen) | AACCCC | 3.86 a | 0.141 | 56 |
| FC230 (S20 × mixture of pollen | AACCCC | 3.95 a | 0.091 | 55 |
 maternal line;
maternal line;  pollen donor.
pollen donor.| Genotype | Bud Length (mm) | Flower Diameter (mm) | Pistil Length (mm) | Stamen Length (µm) | Anther Length (mm) | Median Nectary Length (µm) | Lateral Nectary Length (µm) | Pollen Diameter (µm) | Pollen Viability % | Pod Length (cm) | Seed Number/pod |
|---|---|---|---|---|---|---|---|---|---|---|---|
IW1234  head cabbage head cabbage | 8.00 bc * ±0.10 | 24.72 c ±0.17 | 11.83 b ±0.43 | 11.82 bc ±0.67 | 3.35 bc ±0.20 | 738.638 b ±86.02 | 517.64 e ±73.76 | 25.79 c ±1.86 | 89.50 a ±2.93 | 7.34 ab ±0.44 | 2.66 c ±0.331 |
IW08  kale kale | 8.22 bc ±0.13 | 24.60 c ±0.21 | 15.65 a ±0.75 | 16.40 a ±0.40 | 3.17 bc ±0.17 | 1095.78 ab ±113.40 | 651.31 cd ±67.70 | 24.54 c ±1.34 | 85.82 a ±8.12 | 7.50 ab ±0.75 | 5.18 ab ±1.01 |
RAP62  rapeseed rapeseed | 8.56 bc ±0.14 | 23.40 d ±0.26 | 9.37 d ±0.47 | 10.97 bc ±0.45 | 3.51 bc ±0.13 | 978.29 ab ±44.35 | 578.51 e ±63.93 | 29.86 b ±1.50 | 99.37 a ±1.09 | 8.20 a ±0.47 | 6.91 a ±0.62 |
| Interspecific hybrids of the F1 generation | |||||||||||
| S3 (head cabbage × rapeseed) | 7.41 c ±0.11 | 22.04 e ±0.36 | 9.92 d ±0.15 | 10.06 b ±0.43 | 2.71 b ±0.11 | 932.03 ab ±40.28 | 624.40 cd ±35.35 | 33.74 ab ±4.16 | 6.765 b ±2.77 | 2.46 de ±0.15 | 0.00 d ±0.00 |
| S20 (kale × rapeseed) | 9.22 ab ±0.06 | 23.00 de ±0.07 | 11.66 bc ±0.13 | 11.64 bc ±0.39 | 3.11 bc ±0.046 | 1154.26 a ±52.06 | 688.85 b–d ±50.53 | 33.04 ab ±3.78 | 13.46 b ±9.29 | 2.22 e ±0.13 | 0.03 d ±0.07 |
| Interspecific hybrids of the F2 generation | |||||||||||
| FS3 (S3 × S3) | 9.46 ab ±0.23 | 24.60 c ±0.17 | 11.53 bc ±0.50 | 11.51 bc ±0.34 | 4.03 a,c ±0.15 | 1014.29 ab ±79.48 | 717.99 a–d ±39.95 | 33.63 ab ±1.95 | 75.38 a ±4.96 | 4.46 ce ±0.50 | 0.07 d ±0.15 |
| FS20 (S20 × S20) | 10.42 a ±0.13 | 27.80 a ±0.22 | 11.66 bc ±0.65 | 13.30 a,c ±0.32 | 4.24 a,c ±0.15 | 1076.63 ab ±60.01 | 729.12 a–d ±81.35 | 34.04 a ±2.06 | 85.79 a ±3.09 | 4.96 cd ±0.65 | 0.47 cd ±0.15 |
| FC320 (S3 × mixture of pollen) | 10.19 a ±0.19 | 26.60 b ±0.20 | 11.72 bc ±0.49 | 13.39 a,c ±1.09 | 4.86 a ±0.25 | 1102.10 ab ±63.37 | 839.01 a ±47.96 | 33.81 a ±2.24 | 88.24 a ±4.03 | 6.26 ac ±0.49 | 1.67 cd ±0.49 |
| FC230 (S20 × mixture of pollen) | 10.57 a ±0.13 | 25.20 c ±0.42 | 10.47 cd ±0.59 | 12.69 bc ±1.15 | 4.71 a ±0.47 | 1120.84 a ±123.23 | 803.28 ab ±85.51 | 32.71 ab ±2.20 | 78.71 a ±2.52 | 4.28 ce ±0.59 | 2.76 bc ±0.38 |
 maternal line;
maternal line;  pollen donor.
pollen donor.| Genotype | Leaf Surface (cm2) | Leaf Thickness (µm) | Palisade Mesophyll Thickness (µm) | Spongy Mesophyll Thickness (µm) | Abaxial Epidermis (µm) | Adaxial Epidermis (µm) | Stomata Length (µm) | Stomata Density (No./mm2) |
|---|---|---|---|---|---|---|---|---|
IW1234  head cabbage head cabbage | 164.50 c * ±9.43 | 264.60 cd ±19.63 | 103.3 bc ±15.56 | 113.9 c–e ±16.48 | 20.42 bc ±0.65 | 15.53 bd ±2.65 | 24.38 b ±0.07 | 114.11 ce ±6.26 |
IW08  kale kale | 85.24 c ±8.61 | 234.30 d ±14.33 | 93.6 c ±13.61 | 103.5 c–f ±15.93 | 15.43 d ±1.47 | 13.24 d ±1.68 | 22.34 b ±0.54 | 271.22 a ±13.80 |
RAP62  rapeseed rapeseed | 137.54 c ±12.76 | 232.32 d ±21.08 | 91.96 c ±12.30 | 92.6 f ±15.68 | 20.05 c ±2.90 | 15.86 bd ±1.73 | 23.54 b ±4.12 | 181.33 b ±18.28 |
| Interspecific hybrids of the F1 generation | ||||||||
| S3 (head cabbage × rapeseed) | 165.58 c ±18.91 | 249.14 cd ±17.17 | 114.1 bc ±15.65 | 94 ef ±16.28 | 21.26 bc ±2.05 | 15.16 cd ±1.82 | 26.89 b ±1.28 | 140.78 bc ±10.58 |
| S20 (kale × rapeseed) | 133.20 c ±26.13 | 294.83 bc ±16.88 | 129.6 bc ±15.31 | 123.6 bd ±15.54 | 19.23 cd ±1.38 | 14.99 cd ±1.65 | 26.87 b ±0.66 | 120.89 cd ±5.83 |
| Interspecific hybrids of the F2 generation | ||||||||
| FS3 (S3 × S3) | 346.50 b ±9.24 | 327.91 b ±12.73 | 138.3 b ±18.66 | 138 b ±14.38 | 26.05 a ±4.08 | 22.47 a ±2.82 | 32.66 a ±0.39 | 87.88 ce ±1.39 |
| FS20 (S20 × S20) | 364.06 b ±12.09 | 291. 76 bd ±18.53 | 120.4 bc ±15.62 | 120.3 b–d ±15.20 | 24.41 ab ±2.55 | 19.74 ab ±2.74 | 32.45 a ±0.97 | 67.00 de ±7.36 |
| FC320 (S3 × mixture of pollen) | 603.72 a ±13.03 | 409.60 a ±28.72 | 183 a ±26.25 | 180.1 a ±24.76 | 17.84 cd ±2.40 | 17.50 bd ±2.80 | 32.50 a ±1.33 | 104.00 ce ±3.34 |
| FC230 (S20 × mixture of pollen) | 372.34 b ±20.35 | 294.76 bc ±14.07 | 119.6 bc ±10.91 | 129.9 bc ±14.33 | 24.24 ab ±1.31 | 19.32 ac ±2.98 | 37.40 a ±1.29 | 55.13 e ±3.29 |
 maternal line;
maternal line;  pollen donor.
pollen donor.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasek-Ciolakowska, A.; Kamiński, P.; Podwyszyńska, M.; Kowalska, U.; Starzycki, M.; Starzycka-Korbas, E. Effect of Meiotic Polyploidisation on Selected Morphological and Anatomical Traits in Interspecific Hybrids of Brassica oleracea × B. napus. Agronomy 2022, 12, 26. https://doi.org/10.3390/agronomy12010026
Marasek-Ciolakowska A, Kamiński P, Podwyszyńska M, Kowalska U, Starzycki M, Starzycka-Korbas E. Effect of Meiotic Polyploidisation on Selected Morphological and Anatomical Traits in Interspecific Hybrids of Brassica oleracea × B. napus. Agronomy. 2022; 12(1):26. https://doi.org/10.3390/agronomy12010026
Chicago/Turabian StyleMarasek-Ciolakowska, Agnieszka, Piotr Kamiński, Małgorzata Podwyszyńska, Urszula Kowalska, Michał Starzycki, and Elżbieta Starzycka-Korbas. 2022. "Effect of Meiotic Polyploidisation on Selected Morphological and Anatomical Traits in Interspecific Hybrids of Brassica oleracea × B. napus" Agronomy 12, no. 1: 26. https://doi.org/10.3390/agronomy12010026
APA StyleMarasek-Ciolakowska, A., Kamiński, P., Podwyszyńska, M., Kowalska, U., Starzycki, M., & Starzycka-Korbas, E. (2022). Effect of Meiotic Polyploidisation on Selected Morphological and Anatomical Traits in Interspecific Hybrids of Brassica oleracea × B. napus. Agronomy, 12(1), 26. https://doi.org/10.3390/agronomy12010026







