Genetic Gain for Grain Micronutrients and Their Association with Phenology in Historical Wheat Cultivars Released between 1911 and 2016 in Pakistan
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Trials
2.2. Phenotyping
2.3. Genotyping Using KASP Markers and GBTS
2.4. Statistical Analysis
3. Results
3.1. Variation in Micronutrients and Morphological Traits
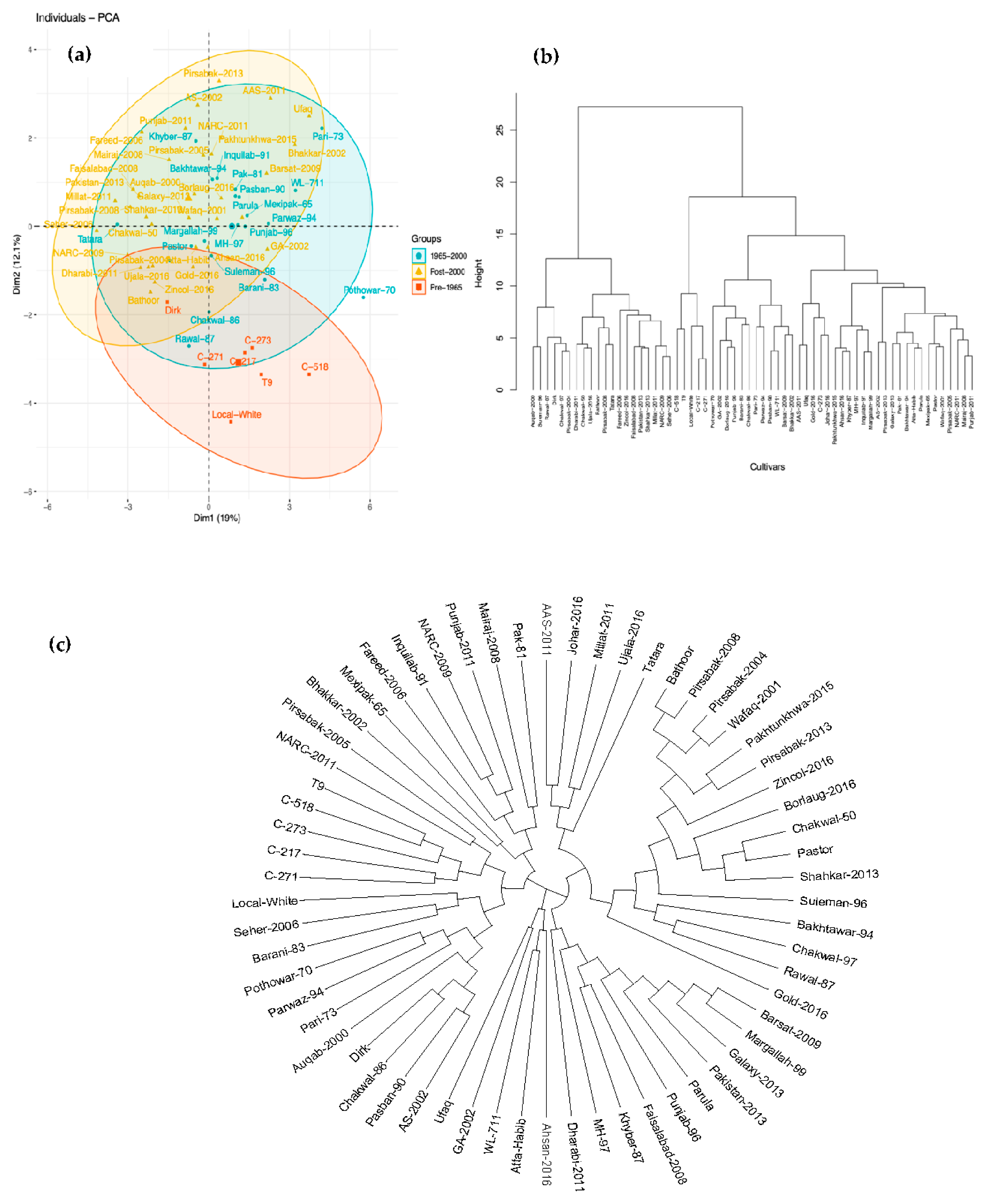
3.2. Correlation between Traits and Multivariate Analysis
3.3. Genetic Gain for Micronutrients and Morphological Traits
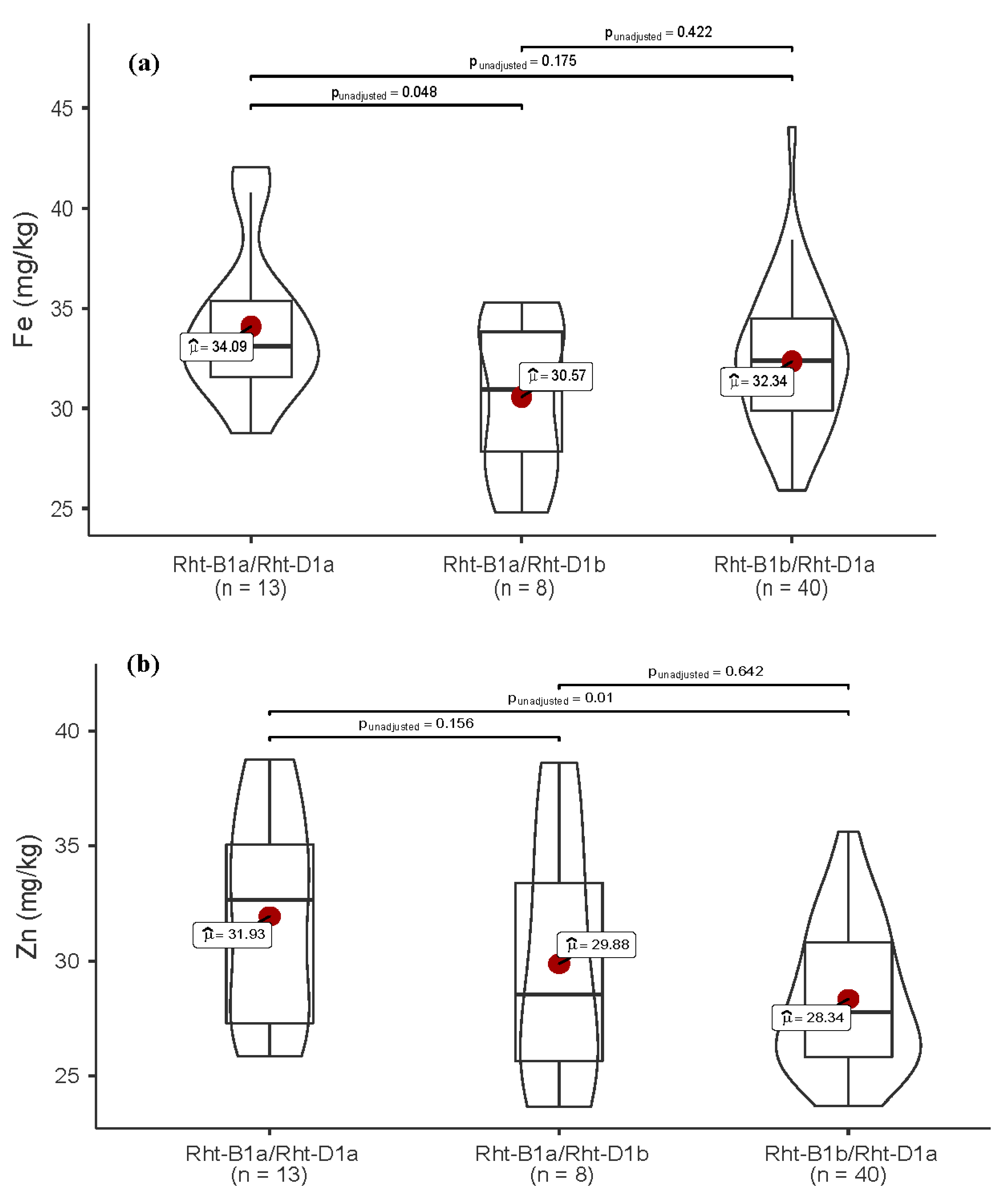
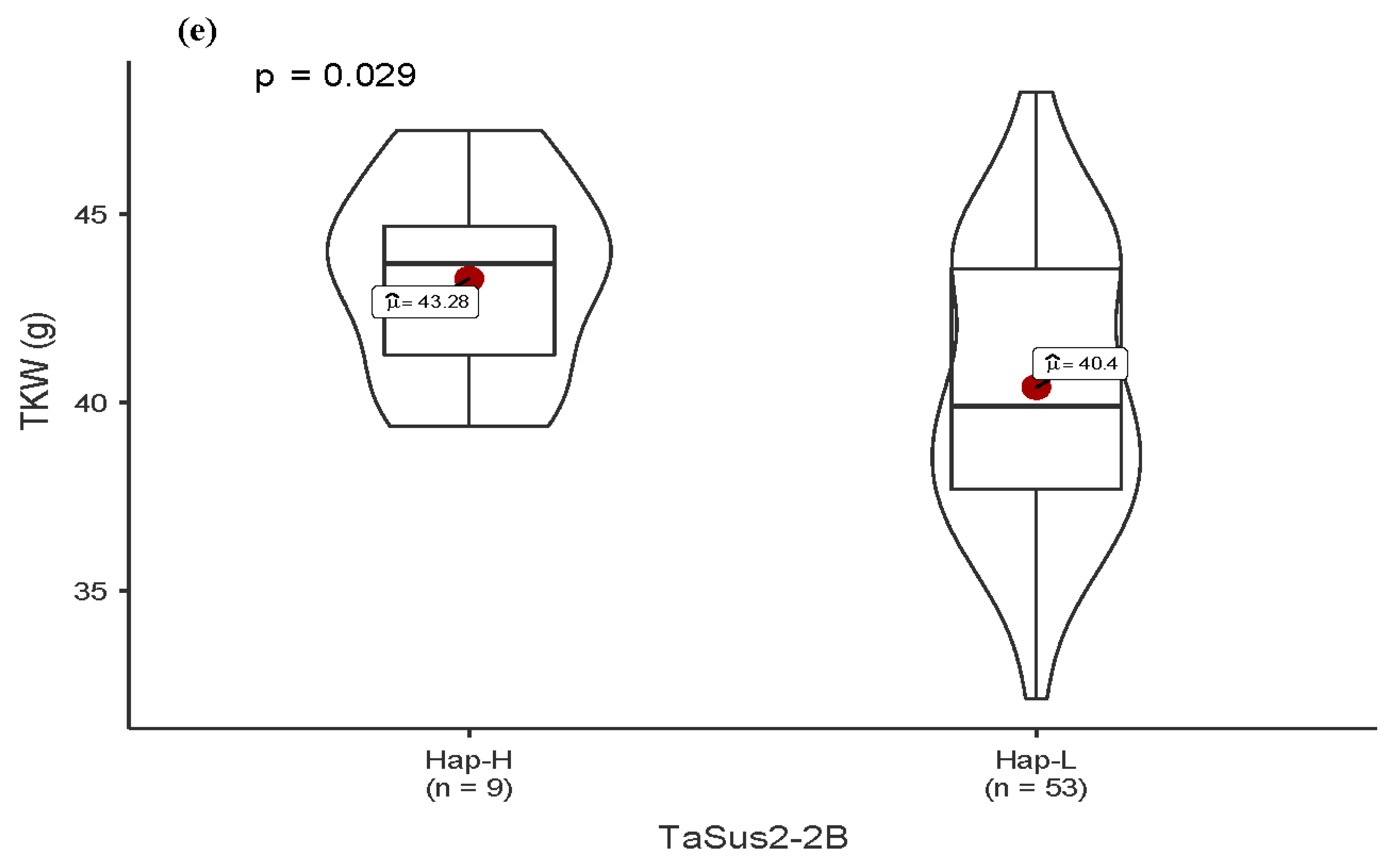
3.4. Allelic Variation in Functional Genes and Association with Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition. 2018. Available online: http://www.fao.org/3/i9553en/i9553en.pdf (accessed on 12 May 2021).
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. 2017. Available online: http://www.fao.org/faostat/en/ (accessed on 12 May 2021).
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- Cakmak, I.; Torun, B.; Erenoğlu, B.; Öztürk, L.; Marschner, H.; Kalayci, M.; Ekiz, H.; Yilmaz, A. Morphological and physiological differences in the response of cereals to zinc deficiency (Reprinted from Wheat: Prospects for global improvement, 1998). Euphytica 1998, 100, 349–357. [Google Scholar] [CrossRef]
- Ali, M.W.; Borrill, P. Applying genomic resources to accelerate wheat biofortification. Heredity 2020, 125, 386–395. [Google Scholar] [CrossRef]
- Fan, M.-S.; Zhao, F.-J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.P.; Huerta, J.; Guzman, C. Genetic impact of Rht dwarfing genes on grain micronutrients concentration in wheat. Field Crops Res. 2017, 214, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Reeves, P.G.; Jones, S.S. Relationship between yield and mineral nutrient concentrations in historical and modern spring wheat cultivars. Euphytica 2008, 163, 381–390. [Google Scholar] [CrossRef]
- Singh, R.; Govindan, V.; Andersson, M.S. Zinc-Biofortified Wheat: Harnessing Genetic Diversity for Improved Nutritional Quality; Science Brief; HarvestPlus: Washington, DC, USA, 2017. [Google Scholar]
- Randhawa, H.S.; Asif, M.; Pozniak, C.; Clarke, J.M.; Graf, R.; Fox, S.L.; Humphreys, D.G.; Knox, R.E.; Depauw, R.M.; Singh, A.K.; et al. Application of molecular markers to wheat breeding in Canada. Plant Breed. 2013, 132, 458–471. [Google Scholar] [CrossRef]
- Paltridge, N.G.; Milham, P.J.; Ortiz-Monasterio, J.I.; Velu, G.; Yasmin, Z.; Palmer, L.J.; Guild, G.E.; Stangoulis, J. Energy-dispersive X-ray fluorescence spectrometry as a tool for zinc, iron and selenium analysis in whole grain wheat. Plant Soil 2012, 361, 261–269. [Google Scholar] [CrossRef]
- Ain, Q.-U.; Rasheed, A.; Anwar, A.; Mahmood, T.; Imtiaz, M.; Xia, X.; He, Z.; Quraishi, U.M. Genome-wide association for grain yield under rainfed conditions in historical wheat cultivars from Pakistan. Front. Plant Sci. 2015, 6, 743. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Wen, W.; Gao, F.; Zhai, S.; Jin, H.; Liu, J.; Guo, Q.; Zhang, Y.; Dreisigacker, S.; Xia, X.; et al. Development and validation of KASP assays for functional genes underpinning key economic traits in wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar] [CrossRef]
- Cormier, F.; Throude, M.; Ravel, C.; Le Gouis, J.; Leveugle, M.; Lafarge, S.; Exbrayat-Vinson, F.; Duranton, N.; Praud, S. Detection of NAM-A1 Natural Variants in Bread Wheat Reveals Differences in Haplotype Distribution between a Worldwide Core Collection and European Elite Germplasm. Agronomy 2015, 5, 143–151. [Google Scholar] [CrossRef]
- Gao, F.; Ma, D.; Yin, G.; Rasheed, A.; Dong, Y.; Xiao, Y.; Xia, X.; Wu, X.; He, Z. Genetic Progress in Grain Yield and Physiological Traits in Chinese Wheat Cultivars of Southern Yellow and Huai Valley since 1950. Crop Sci. 2017, 57, 760–773. [Google Scholar] [CrossRef]
- Calderini, D.; Slafer, G. Has yield stability changed with genetic improvement of wheat yield? Euphytica 1999, 107, 51–59. [Google Scholar] [CrossRef]
- Morgounov, A.I.; Belan, I.; Zelenskiy, Y.; Roseeva, L.; Tömösközi, S.; Békés, F.; Abugalieva, A.; Cakmak, I.; Vargas, M.; Crossa, J. Historical changes in grain yield and quality of spring wheat varieties cultivated in Siberia from 1900 to 2010. Can. J. Plant Sci. 2013, 93, 425–433. [Google Scholar] [CrossRef]
- Garvin, D.F.; Welch, R.M.; Finley, J.W. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J. Sci. Food Agric. 2006, 86, 2213–2220. [Google Scholar] [CrossRef]
- Jobson, E.M.; Martin, J.M.; Schneider, T.M.; Giroux, M.J. The impact of the Rht-B1b, Rht-D1b, and Rht-8 wheat semi-dwarfing genes on flour milling, baking, and micronutrients. Cereal Chem. 2018, 95, 770–778. [Google Scholar] [CrossRef]
- Velu, G.; Crossa, J.; Singh, R.P.; Hao, Y.; Dreisigacker, S.; Perez-Rodriguez, P.; Joshi, A.K.; Chatrath, R.; Gupta, V.; Balasubramaniam, A.; et al. Genomic prediction for grain zinc and iron concentrations in spring wheat. Theor. Appl. Genet. 2016, 129, 1595–1605. [Google Scholar] [CrossRef]
- Morgounov, A.; Gómez-Becerra, H.F.; Abugalieva, A.; Dzhunusova, M.; Yessimbekova, M.; Muminjanov, H.; Zelenskiy, Y.; Ozturk, L.; Cakmak, I. Iron and zinc grain density in common wheat grown in Central Asia. Euphytica 2007, 155, 193–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Q.; Yan, J.; Tang, J.; Zhao, R.; Zhang, Y.; He, Z.; Zou, C.; Ortiz-Monasterio, I. Mineral element concentrations in grains of Chinese wheat cultivars. Euphytica 2010, 174, 303–313. [Google Scholar] [CrossRef]
- Guttieri, M.J.; Baenziger, P.S.; Frels, K.; Carver, B.; Arnall, B.; Waters, B.M. Variation for Grain Mineral Concentration in a Diversity Panel of Current and Historical Great Plains Hard Winter Wheat Germplasm. Crop Sci. 2015, 55, 1035–1052. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef]
- Zhao, F.; Su, Y.; Dunham, S.; Rakszegi, M.; Bedo, Z.; McGrath, S.; Shewry, P. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 2009, 49, 290–295. [Google Scholar] [CrossRef]
- McDonald, G.K.; Genc, Y.; Graham, R.D. A simple method to evaluate genetic variation in grain zinc concentration by correcting for differences in grain yield. Plant Soil 2008, 306, 49–55. [Google Scholar] [CrossRef]
- Peleg, Z.; Cakmak, I.; Ozturk, L.; Yazici, A.; Jun, Y.; Budak, H.; Korol, A.B.; Fahima, T.; Saranga, Y. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat × wild emmer wheat RIL population. Theor. Appl. Genet. 2009, 119, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Calderini, D.F.; Ortiz-Monasterio, I. Are synthetic hexaploids a means of increasing grain element concentrations in wheat? Euphytica 2003, 134, 169–178. [Google Scholar] [CrossRef]
- Ortiz, R.; Braun, H.-J.; Crossa, J.; Crouch, J.H.; Davenport, G.; Dixon, J.; Dreisigacker, S.; Duveiller, E.; He, Z.; Huerta, J.; et al. Wheat genetic resources enhancement by the International Maize and Wheat Improvement Center (CIMMYT). Genet. Resour. Crop Evol. 2008, 55, 1095–1140. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, J.; Palacios-Rojas, N.; Meng, E.; Pixley, K.; Trethowan, R.; Peña, R. Enhancing the mineral and vitamin content of wheat and maize through plant breeding. J. Cereal Sci. 2007, 46, 293–307. [Google Scholar] [CrossRef]
- Manickavelu, A.; Hattori, T.; Yamaoka, S.; Yoshimura, K.; Kondou, Y.; Onogi, A.; Matsui, M.; Iwata, H.; Ban, T. Genetic Nature of Elemental Contents in Wheat Grains and Its Genomic Prediction: Toward the Effective Use of Wheat Landraces from Afghanistan. PLoS ONE 2017, 12, e0169416. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.; Balasubramaniam, A.; Mishra, V.K.; Chand, R.; Tiwari, C.; Joshi, A.; Virk, P.; Cherian, B.; Pfeiffer, W. Reaching out to Farmers with High Zinc Wheat Varieties through Public-Private Partnerships—An Experience from Eastern-Gangetic Plains of India. Adv. Food Technol. Nutr. Sci. 2015, 1, 73–75. [Google Scholar] [CrossRef]
- Lyons, G.; Ortiz-Monasterio, I.; Stangoulis, J.; Graham, R. Selenium concentration in wheat grain: Is there sufficient genotypic variation to use in breeding? Plant Soil 2005, 269, 369–380. [Google Scholar] [CrossRef]
- AlOmari, D.Z.; Eggert, K.; Von Wirén, N.; Pillen, K.; Röder, M.S. Genome-Wide Association Study of Calcium Accumulation in Grains of European Wheat Cultivars. Front. Plant Sci. 2017, 8, 1797. [Google Scholar] [CrossRef] [PubMed]




| Cultivar | Release Year | Pedigree |
|---|---|---|
| T9 | 1911 | Nil |
| C-518 | 1933 | T9/8A |
| C-217 | 1944 | C516/C591 |
| C-271 | 1957 | C230/IP165 |
| C-273 | 1957 | C209/C591 |
| Dirk | 1958 | FORD//DUNDEE/BOBIN or FORD/DONDEE (I) |
| Mexipak-65 | 1965 | PJ/GB55 or PJ62/GB55 |
| Pothowar-70 | 1970 | BURT/KENYA//QUETA(L)/3/NAD63 |
| Pari-73 | 1973 | CNO67//SN64/KLRE/3/8156 |
| Parula | 1973 | Nil |
| WL-711 | 1978 | S308/CHRIS//KAL |
| Pak-81 | 1981 | KVZ/BUHO//KAL/BB |
| Barani-83 | 1983 | BB/GLL/3/GTO/7C//BB/CNO67 |
| Chakwal-86 | 1986 | FORLANI/ACC//ANA or Fln/ACS//ANA |
| Khyber-87 | 1987 | KVZ/TRM//PTM/ANA |
| Rawal-87 | 1987 | MAYA/MON//KVZ/TRM |
| Inquilab-91 | 1991 | WL 711/CROW “S” |
| Pasban-90 | 1991 | INIA F66/TH.DISTICHUM//INIAF66/3/GENARO T81 |
| Pastor | 1993 | PFAU/SERI-82//BOBWHITE |
| Bakhtawar-94 | 1994 | AU/UP301//GLL/SX/3/PEW/4/MAI/MAYA//PEW |
| Parwaz-94 | 1995 | V.5648/PARULA |
| Punjab-96 | 1996 | SA42*2/4/CC/INIA//BB/3/INIA/HD832 |
| Suleman-96 | 1996 | F6.74/BUN//SIS/3/VEE#7 or F6-74/BUN//SIS/3/VEE#7 |
| Tatara | 1996 | JUP/ALD’S’//KLT’S’ |
| Chakwal-97 | 1997 | BUC’S’/FCT’S’ |
| MH-97 | 1997 | NORD-DESPREZ(ND)/VG-9144//KALYANSONA/BLUEBIRD/3/YACO/4/VEERY-5 |
| Margallah-99 | 1999 | OPATA/BOW’S’ |
| Auqab-2000 | 2000 | CROW’S’/NAC//BOW’S’ |
| Wafaq-2001 | 2001 | OPATA/RAYON//KAUZ |
| AS-2002 | 2002 | KHP/D31708//CM74A370/3/CNO79/4/RL6043/4*NAC |
| Bhakkar-2002 | 2002 | P20102/PIMA/SKA/3/TTR’S’/BOW’S’ |
| GA-2002 | 2002 | DWL5023/SNB//SNB |
| Ufaq | 2002 | V.84133/V83150 |
| Pirsabak-2004 | 2004 | KAUZ/STAR |
| Pirsabak-2005 | 2005 | MUNIA/CHTO//AMSEL |
| Fareed-2006 | 2006 | PT’S’/3/TOB/LFN//BB/4/BB/HD-832-5//ON/5/G-V/ALD’S’//HPO |
| Seher-2006 | 2006 | CHILL/2* STAR/4/BOW//BUC/PVN/3/2*VEE#10 |
| Bathoor | 2008 | URES/JUN//KAUZ |
| Chakwal-50 | 2008 | ATTILA/3/HUI/CARC//CHEN/CHTO/4/ATTILA |
| Faisalabad-2008 | 2008 | PBW65/2*Pastor |
| Mairaj-2008 | 2008 | SPARROW/INIA//V.7394/WL711/13/BAUS |
| Pirsabak-2008 | 2008 | KAUZ/PASTOR |
| NARC-2009 | 2009 | INQALAB 91*2/TUKURU |
| Atta-Habib | 2010 | INQALAB 91*2/TUKURU |
| Barsat-2009 | 2010 | FRET2 |
| AAS-2011 | 2011 | PRL/PASTOR//2236(V6550/SUTLEH-86) |
| Dharabi-2011 | 2011 | HXL-7573/2*BAGULA//PASTOR |
| Millat-2011 | 2011 | CHENAB2000/INQ-91 |
| NARC-2011 | 2011 | OASIS/SKAUZ//4*BCN/3/2*PASTOR |
| Punjab-2011 | 2011 | AMSEL/ATTILA//INQ-91/PEW’S’ |
| Galaxy-2013 | 2013 | PUNJAB-96/V-87094//MH-97 |
| Pakistan-2013 | 2013 | MEX94.27.1.20/3/Sokoll//Attila/3*BCN |
| Pirsabak-2013 | 2013 | CS/TH.SC//3*PVN/3/MIRLO/BUC/4/MILAN/5/TILHI |
| Shahkar-2013 | 2013 | CMH84.3379/CMH78.578//MILAN |
| Pakhtunkhwa-2015 | 2015 | WBLL1*2/4/YACO/PBW65/3/KAUZ*2/TRAP//KAUZ |
| Ujala-2016 | 2015 | KIRITATI/4/2*WEAVER/TSC//WEAVER/3/WEAVER |
| Ahsan-2016 | 2016 | Pastor/3/Altar 84/Ae. squarrosa//Opata |
| Borlaug-2016 | 2016 | Sokoll/3/Pastor//HXL7573/2*BAU |
| Gold-2016 | 2016 | Nil |
| Johar-2016 | 2016 | KAUZ/PASTOR//V.3009 |
| Zincol-2016 | 2016 | OASIS/ SKAUZ//4*BCN/3/2*PASTOR/4/T.SPELTA PI348449/5/BACEU#1/6/ WBLL1*2/CHAPIO |
| Local-White | - | Nil |
| Pre-1965 (n = 7) | 1965–2000 (n = 21) | Post-2000 (n = 34) | Overall (n = 62) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Traits | Min | Mean | Mean | CV(%) | Min | Max | Mean | CV(%) | Min | Max | Mean | CV(%) | Min | Max | Mean | CV (%) |
| Fe.EDXRF (mg/kg) | 31 | 42 | 35.5 | 12.34 | 27.6 | 38.4 | 31.9 | 8.9 | 24.8 | 44 | 32.2 | 12.39 | 24.8 | 44 | 32.5 | 11.66 |
| Zn.EDXRF (mg/kg) | 24 | 38.4 | 33.1 | 14.14 | 24.1 | 38.8 | 29.3 | 14.2 | 23.6 | 36 | 28.5 | 11.72 | 23.6 | 38.8 | 29.3 | 13.62 |
| Fe.ICPOES (mg/kg) | 30.5 | 38.6 | 32.7 | 8.96 | 26.9 | 37.3 | 32.8 | 6.83 | 26.8 | 37.6 | 33.7 | 6.5 | 26.8 | 38.6 | 33.3 | 6.91 |
| Zn.ICPOES (mg/kg) | 18.8 | 33.1 | 28.8 | 16.63 | 20.4 | 40.8 | 29.8 | 14.7 | 23.9 | 37.2 | 28.5 | 11.82 | 18.8 | 40.8 | 28.9 | 13.43 |
| Al (mg/kg) | 2.69 | 4.78 | 3.87 | 21.06 | 2.44 | 7.78 | 4.48 | 23.21 | 2.06 | 4.97 | 3.58 | 18.91 | 2.06 | 7.78 | 3.92 | 23.37 |
| Ca (mg/kg) | 453 | 635 | 547 | 11.99 | 476 | 701 | 588 | 9.73 | 415 | 720 | 563 | 11.26 | 415 | 720 | 570 | 10.91 |
| Cu (mg/kg) | 3.81 | 5.28 | 4.68 | 10.51 | 3.25 | 5.69 | 4.16 | 17 | 3.06 | 5.47 | 4.08 | 14.24 | 3.06 | 5.69 | 4.18 | 15.22 |
| K (mg/kg) | 3591 | 4118 | 3892 | 4.83 | 3762 | 4891 | 4385 | 6.07 | 3732 | 5344 | 4279 | 8.72 | 3591 | 5344 | 4271 | 8.19 |
| Mg (mg/kg) | 984 | 1288 | 1181 | 8.64 | 923 | 1318 | 1134 | 8.06 | 970 | 1293 | 1130 | 7.22 | 923 | 1318 | 1137 | 7.69 |
| Mn (mg/kg) | 24.4 | 37.4 | 32.2 | 13.45 | 25.6 | 37.8 | 32 | 9.53 | 26.3 | 38.2 | 32.7 | 9.88 | 24.4 | 38.2 | 32.4 | 10.06 |
| Na (mg/kg) | 8.75 | 47.3 | 28.7 | 55.4 | 10 | 159 | 43.1 | 87.01 | 8.94 | 79.9 | 32.1 | 59.5 | 8.75 | 159 | 35.4 | 75.42 |
| P (mg/kg) | 2752 | 3349 | 3108 | 7.53 | 2862 | 3918 | 3361 | 7.94 | 2719 | 3657 | 3150 | 7.71 | 2719 | 3918 | 3217 | 8.33 |
| Se (mg/kg) | 0.199 | 0.258 | 0.232 | 8.84 | 0.132 | 0.24 | 0.199 | 18.69 | 0.135 | 0.26 | 0.212 | 14.29 | 0.132 | 0.26 | 0.21 | 15.71 |
| TPP | 3.13 | 4.25 | 3.58 | 12.04 | 2.83 | 4.34 | 3.53 | 12.32 | 2.44 | 4.08 | 3.23 | 11.8 | 2.44 | 4.34 | 3.37 | 12.76 |
| PH (cm) | 104 | 121 | 111 | 5.6 | 77.1 | 107 | 97.6 | 9.54 | 81.4 | 107 | 93.7 | 11.11 | 77.1 | 121 | 97 | 8.85 |
| SL | 12.8 | 18.5 | 15.8 | 12.72 | 15.1 | 22.2 | 17.2 | 9.42 | 14.1 | 19.2 | 16.9 | 6.98 | 12.8 | 22.2 | 16.9 | 8.70 |
| SNPS | 18.1 | 20.3 | 19.3 | 4.13 | 18.1 | 20.9 | 19.7 | 3.88 | 17.6 | 21.1 | 19.2 | 4.29 | 17.6 | 21.1 | 19.4 | 4.27 |
| GPS | 39.3 | 52.3 | 45.9 | 11.18 | 48.6 | 61.3 | 53.3 | 6.42 | 44.1 | 64 | 54.2 | 8.03 | 39.3 | 64 | 53 | 9.11 |
| GY (t/ha) | 0.975 | 1.84 | 1.31 | 20.76 | 1.3 | 2.82 | 2.09 | 21.77 | 1.87 | 3.22 | 2.44 | 12.05 | 0.975 | 3.22 | 2.19 | 22.74 |
| TKW (g) | 39.4 | 47.2 | 43.4 | 6.66 | 32.1 | 46.7 | 39.1 | 9.26 | 33.9 | 48.2 | 41.3 | 8.45 | 32.1 | 48.2 | 40.8 | 9.02 |
| GL (mm) | 1.11 | 1.36 | 1.25 | 7.01 | 1.16 | 2.04 | 1.46 | 14.11 | 1.16 | 2.23 | 1.38 | 16.09 | 1.11 | 2.23 | 1.39 | 15.32 |
| GW (mm) | 1.97 | 2.44 | 2.11 | 7.91 | 2.15 | 2.73 | 2.35 | 5.62 | 2.02 | 2.52 | 2.27 | 5.55 | 1.97 | 2.73 | 2.27 | 6.56 |
| GD (mm) | 5.86 | 6.65 | 6.16 | 4.69 | 5.89 | 6.65 | 6.36 | 3.25 | 5.77 | 6.8 | 6.38 | 3.65 | 5.77 | 6.8 | 6.34 | 3.77 |
| Replication | Genotype (G) | Year (Y) | G x Y Interaction | ||
|---|---|---|---|---|---|
| df | 2 | 61 | 1 | 61 | |
| Traits | Means Squares | Heritability | |||
| TPP | 1.162 * | 0.7386 *** | 0.0413 ns | 0.9095 *** | 0.35 |
| PH | 203.1443 ns | 465.0146 *** | 256.8787 ns | 156.766 | 0.67 |
| SL | 1.2842 ns | 8.6695 *** | 13.518 * | 5.5964 ** | 0.49 |
| SNPS | 222.2401 *** | 4.5472 *** | 141.0344 *** | 1.6726 ns | 0.48 |
| GNPS | 675.4824 *** | 93.4753 *** | 598.6102 *** | 51.7566 ns | 0.52 |
| GY | 0.1032 *** | 0.9954 *** | 2.3278 *** | 0.1575 ** | 0.68 |
| TKW | 0.3556 ns | 54.2871 *** | 0.0429 ns | 19.7962 ns | 0.66 |
| GL | 7.9117 *** | 0.2239 *** | 0.0032 ns | 0.0451 ns | 0.80 |
| GW | 8.6088 *** | 0.1296 *** | 0.0147 ns | 0.0455 ns | 0.65 |
| GD | 8.329 *** | 0.2752 *** | 0.0022 ns | 0.0579 ns | 0.78 |
| Al | 68.6896 *** | 3.1559 *** | 0.127 ns | 0.3507 ns | 0.52 |
| Ca | 35,341.2148 *** | 11,898.9395 *** | 81.7116 ns | 75.8745 ns | 0.86 |
| Cu | 8.2654 *** | 1.1161 *** | 0.5172 ns | 0.066 ns | 0.72 |
| Fe.EDXRF | 4.5506 ** | 40.2081 *** | 0.1301 ns | 37.163 *** | 0.51 |
| Fe.ICPOES | 14.5627 *** | 15.3527 *** | 120.9392 *** | 8.7979 *** | 0.62 |
| K | 112,509.1797 * | 455,115.7812 *** | 756,099.6875 *** | 113,190.0078 *** | 0.79 |
| Mg | 6691.2295 * | 25,110.6953 *** | 52,088.6211 *** | 8330.1592 *** | 0.73 |
| Mn | 5.0568 * | 35.1404 *** | 5.8521 * | 11.7127 * | 0.74 |
| Na | 23.8786 ns | 2031.3976 *** | 6190.4634 *** | 2507.9097 *** | 0.43 |
| P | 49,623.0234 * | 205,982.9375 *** | 390,194.75 *** | 120,418.9297 *** | 0.61 |
| Zn.EDXRF | 11.2164 ** | 39.5774 *** | 11.9913 * | 34.2843 *** | 0.52 |
| Zn.ICPOES | 8.4438 ** | 41.8175 *** | 594.0781 *** | 18.7137 *** | 0.68 |
| Se | 7.3049 *** | 0.045 ns | 0.0319 ns | 0.0518 ns | 0.0057 |
| Traits | Fe.ICPOES (mg/kg) | Zn.ICPOES (mg/kg) | Al (mg/kg) | Ca (mg/kg) | Cu (mg/kg) | K (mg/kg) | Mg (mg/kg) | Mn (mg/kg) | Na (mg/kg) | P (mg/kg) | Se (mg/kg) | TPP | PH (cm) | SL (cm) | SNPS | GPS | GY (t/ha) | TKW (g) | GL (cm) | GW (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn.ICPOES (mg/kg) | 0.313 * | — | ||||||||||||||||||
| Al (mg/kg) | −0.041 | 0.166 | — | |||||||||||||||||
| Ca (mg/kg) | 0.278 * | 0.613 *** | 0.234 | — | ||||||||||||||||
| Cu (mg/kg) | 0.197 | 0.601 *** | 0.359 ** | 0.378 ** | — | |||||||||||||||
| K (mg/kg) | 0.149 | 0.399 ** | 0.13 | 0.551 *** | 0.188 | — | ||||||||||||||
| Mg (mg/kg) | 0.354 ** | 0.747 *** | 0.058 | 0.521 *** | 0.536 *** | 0.278 * | — | |||||||||||||
| Mn (mg/kg) | 0.299 * | 0.24 | −0.121 | 0.226 | 0.088 | 0.042 | 0.412 *** | — | ||||||||||||
| Na (mg/kg) | −0.01 | 0.299 * | 0.286 * | 0.253 * | 0.331 ** | 0.38 ** | 0.321 * | −0.171 | — | |||||||||||
| P (mg/kg) | 0.126 | 0.637 *** | 0.195 | 0.498 *** | 0.417 *** | 0.555 *** | 0.667 *** | 0.353 ** | 0.279 * | — | ||||||||||
| Se (mg/kg) | 0.26 * | 0.373 ** | 0.132 | 0.402 ** | 0.42 *** | 0.174 | 0.536 *** | 0.296 * | 0.302 * | 0.101 | — | |||||||||
| TPP | −0.134 | 0.11 | 0.355 ** | −0.083 | 0.315 * | 0.027 | 0.043 | −0.071 | 0.281 * | 0.203 | −0.024 | — | ||||||||
| PH (cm) | −0.093 | 0.114 | −0.004 | −0.094 | 0.291 * | −0.317 * | 0.114 | 0.053 | 0.051 | 0.004 | 0.131 | 0.296 * | — | |||||||
| SL (cm) | 0.156 | 0.191 | −0.019 | 0.187 | −0.018 | 0.118 | 0.09 | 0.084 | −0.02 | 0.282 * | −0.127 | 0.181 | 0.252 * | — | ||||||
| SNPS | −0.041 | 0.153 | 0.207 | 0.078 | 0.114 | 0.019 | −0.07 | −0.055 | 0.059 | 0.148 | −0.059 | 0.359 ** | 0.344 ** | 0.459 *** | — | |||||
| GPS | 0.113 | 0.012 | −0.046 | 0.02 | −0.065 | 0.211 | −0.137 | 0.103 | −0.069 | 0.123 | −0.147 | −0.111 | −0.09 | 0.282 * | 0.457 *** | — | ||||
| GY (t/ha) | 0.2 | −0.149 | −0.354 ** | −0.176 | −0.462 *** | 0.07 | −0.227 | 0.17 | −0.169 | −0.108 | −0.248 | −0.329 ** | −0.216 | 0.187 | −0.073 | 0.393 ** | — | |||
| TKW (g) | −0.005 | −0.084 | −0.152 | −0.103 | −0.013 | −0.093 | 0.124 | 0.111 | −0.042 | −0.117 | 0.324 * | −0.157 | 0.175 | −0.015 | −0.257 * | −0.115 | 0.011 | — | ||
| GL (cm) | 0.205 | 0.072 | 0.053 | 0.141 | −0.166 | 0.108 | 0.113 | −0.145 | −0.03 | 0.162 | −0.07 | −0.108 | −0.027 | 0.195 | −0.023 | 0.061 | 0.166 | −0.128 | — | |
| GW (cm) | 0.19 | 0.129 | 0.211 | 0.331 * | 0.17 | 0.503 *** | 0.067 | −0.238 | 0.301 * | 0.229 | 0.11 | −0.026 | −0.185 | 0.173 | 0.077 | 0.232 | 0.086 | −0.124 | 0.37 ** | — |
| GD (cm) | −0.059 | −0.488 *** | −0.081 | −0.372 *** | −0.404 ** | −0.027 | −0.309 | −0.044 | −0.004 | −0.179 | −0.246 | 0.045 | 0.026 | 0.179 | −0.073 | 0.259 * | 0.359 ** | 0.304 * | 0.215 | 0.278 * |
| Cultivar Release Era | |||||
|---|---|---|---|---|---|
| Genes | Alleles | Pre-1965 | 1965–2000 | Post-2000 | Overall |
| Rht-8 | Rht8 | 8.1 | 30.6 | 50.0 | 88.7 |
| rht-8 | 3.2 | 3.2 | 4.8 | 11.3 | |
| Rht-1 | Rht-B1a/Rht-D1a | 9.7 | 6.5 | 4.8 | 21.0 |
| Rht-B1a/Rht-D1b | 0.0 | 4.8 | 8.1 | 12.9 | |
| Rht-B1b/Rht-D1a | 0.0 | 24.2 | 41.9 | 66.1 | |
| TaSus2-2B | Hap-H | 11.3 | 1.6 | 1.6 | 14.5 |
| Hap-L | 0.0 | 32.3 | 53.2 | 85.5 | |
| TaGW2-6B | Hap-I | 1.6 | 16.1 | 14.5 | 32.3 |
| Hap-II | 9.7 | 17.7 | 40.3 | 67.7 | |
| TaGW2-6A | Hap-6A-A | 11.3 | 24.2 | 45.2 | 80.6 |
| Hap-6A-G | 0.0 | 9.7 | 9.7 | 19.4 | |
| NAM-A1 | NAM-A1b | 4.8 | 11.3 | 24.2 | 40.3 |
| NAM-A1d | 6.5 | 22.6 | 30.6 | 59.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaukat, M.; Sun, M.; Ali, M.; Mahmood, T.; Naseer, S.; Maqbool, S.; Rehman, S.; Mahmood, Z.; Hao, Y.; Xia, X.; et al. Genetic Gain for Grain Micronutrients and Their Association with Phenology in Historical Wheat Cultivars Released between 1911 and 2016 in Pakistan. Agronomy 2021, 11, 1247. https://doi.org/10.3390/agronomy11061247
Shaukat M, Sun M, Ali M, Mahmood T, Naseer S, Maqbool S, Rehman S, Mahmood Z, Hao Y, Xia X, et al. Genetic Gain for Grain Micronutrients and Their Association with Phenology in Historical Wheat Cultivars Released between 1911 and 2016 in Pakistan. Agronomy. 2021; 11(6):1247. https://doi.org/10.3390/agronomy11061247
Chicago/Turabian StyleShaukat, Muzzafar, Mengjing Sun, Mohsin Ali, Tariq Mahmood, Samar Naseer, Saman Maqbool, Shoaib Rehman, Zahid Mahmood, Yuanfeng Hao, Xianchun Xia, and et al. 2021. "Genetic Gain for Grain Micronutrients and Their Association with Phenology in Historical Wheat Cultivars Released between 1911 and 2016 in Pakistan" Agronomy 11, no. 6: 1247. https://doi.org/10.3390/agronomy11061247
APA StyleShaukat, M., Sun, M., Ali, M., Mahmood, T., Naseer, S., Maqbool, S., Rehman, S., Mahmood, Z., Hao, Y., Xia, X., Rasheed, A., & He, Z. (2021). Genetic Gain for Grain Micronutrients and Their Association with Phenology in Historical Wheat Cultivars Released between 1911 and 2016 in Pakistan. Agronomy, 11(6), 1247. https://doi.org/10.3390/agronomy11061247









