Use of Microbial Biostimulants to Increase the Salinity Tolerance of Vegetable Transplants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Transplant Production
2.2. Statistics and Principal Component Analysis
3. Results
3.1. Morphophysiological Parameters of Lettuce Seedlings
3.2. Morphophysiological Parameters of Tomato Seedlings
3.3. Principal Components Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. CRC Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Plant Growth and Development under Salinity Stress. In Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. ISBN 978-1-40205-577-5. [Google Scholar]
- Läuchli, A.; Grattan, S.R. Plant Responses to Saline and Sodic Conditions. In Agricultural Salinity Assessment and Management; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 169–205. ISBN 978-0-78447-648-2. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Snapp, S.S.; Shennan, C.; Bruggen, A.H.C. Effects of salinity on severity of infection by Phytophthora parasitica Dast., ion concentrations and growth of tomato, Lycopersicon esculentum Mill. New Phytol. 1991, 119, 275–284. [Google Scholar] [CrossRef]
- Foolad, M.R. Recent Advances in Genetics of Salt Tolerance in Tomato. Plant Cell. Tissue Organ Cult. 2004, 76, 101–119. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Reddy, M.S.; Rodriguez-Kabana, R.; Kenney, D.S.; Kokalis-Burelle, N.; Martinez-Ochoa, N.; Vavrina, C.S. Application for rhizobacteria in transplant production and yield enhancement. Acta Hortic. 2004, 631, 217–229. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Esposito, A.; Miceli, A. Fertigation Management and Growth-Promoting Treatments Affect Tomato Transplant Production and Plant Growth after Transplant. Agronomy 2020, 10, 1504. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Gama, P.B.S.; Inanaga, S.; Tanaka, K.; Nakazawa, R. Physiological response of common bean (Phaseolus vulgaris L.) seedlings to salinity stress. Afr. J. Biotechnol. 2007, 6, 79–88. [Google Scholar]
- Bayuelo-Jiménez, J.S.; Debouck, D.G.; Lynch, J.P. Growth, gas exchange, water relations, and ion composition of Phaseolus species grown under saline conditions. Field Crop. Res. 2003, 80, 207–222. [Google Scholar] [CrossRef]
- Mariani, L.; Ferrante, A. Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses—Drought, Salinity, Hypoxia, and Lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, P.; Nagpal, S.; Gupta, R.K.; Sirari, A.; Nair, R.M.; Bindumadhava, H.; Singh, S. Dual Microbial Inoculation, a Game Changer?—Bacterial Biostimulants With Multifunctional Growth Promoting Traits to Mitigate Salinity Stress in Spring Mungbean. Front. Microbiol. 2021, 11, 600576. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Ashraf, M. Improving Salinity Tolerance in Cereals. CRC Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Dajic, Z. Salt stress. In Physiology and Molecular Biology of Stress Tolerance in Plants; Rao, K., Raghavendra, A., Eddy, K., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; pp. 41–99. [Google Scholar]
- Wang, Y.; Mopper, S.; Hasenstein, K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Abiri, R.; Shaharuddin, N.A.; Maziah, M.; Yusof, Z.N.B.; Atabaki, N.; Sahebi, M.; Valdiani, A.; Kalhori, N.; Azizi, P.; Hanafi, M.M. Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environ. Exp. Bot. 2017, 134, 33–44. [Google Scholar] [CrossRef]
- Vetrano, F.; Moncada, A.; Miceli, A. Use of Gibberellic Acid to Increase the Salt Tolerance of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2020, 10, 505. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Moncada, A. Effects of Foliar Application of Gibberellic Acid on the Salt Tolerance of Tomato and Sweet Pepper Transplants. Horticulturae 2020, 6, 93. [Google Scholar] [CrossRef]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French Bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Grobelak, A.; Kokot, P.; Hutchison, D.; Grosser, A.; Kacprzak, M. Plant growth-promoting rhizobacteria as an alternative to mineral fertilizers in assisted bioremediation—Sustainable land and waste management. J. Environ. Manage. 2018, 227, 1–9. [Google Scholar] [CrossRef]
- Nagargade, M.; Tyagi, V.; Singh, M. Plant Growth-Promoting Rhizobacteria: A Biological Approach toward the Production of Sustainable Agriculture Extension. In Role of Rhizospheric Microbes in Soil; Meena, V., Ed.; Springer: Singapore, 2018; Volume 1, pp. 205–223. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, G.M.; Mohamedin, A.H. Interactions between a Vesicular-Arbuscular Mycorrhizal Fungus (Glomus Intraradices) and Streptomyces Coelicolor and their Effects on Sorghum Plants Grown in Soil Amended with Chitin of Brawn Scales; Springer: Berlin, Germany, 2000; Volume 32. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Kumar, A.; Dames, J.F.; Gupta, A.; Sharma, S.; Gilbert, J.A.; Ahmad, P. Current developments in arbuscular mycorrhizal fungi research and its role in salinity stress alleviation: A biotechnological perspective. Crit. Rev. Biotechnol. 2015, 35, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Manigundan, K.; Amaresan, N. Influence of salt tolerant Trichoderma spp. on growth of maize (Zea mays) under different salinity conditions. J. Basic Microbiol. 2017, 57, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Shameer, S.; Prasad, T.N.V.K.V. Plant growth promoting rhizobacteria for sustainable agricultural practices with special reference to biotic and abiotic stresses. Plant Growth Regul. 2018, 84, 603–615. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Albdaiwi, R.N.; Khyami-Horani, H.; Ayad, J.Y.; Alananbeh, K.M.; Al-Sayaydeh, R. Isolation and characterization of halotolerant plant growth promoting rhizobacteria from durum wheat (Triticum turgidum subsp. durum) cultivated in saline areas of the dead sea region. Oxid. Med. Cell. Longev. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing Causality: Opportunities of Synthetic Communities for Plant Microbiome Research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Thomloudi, E.-E.; Tsalgatidou, P.C.; Douka, D.; Spantidos, T.-N.; Dimou, M.; Venieraki, A.; Katinakis, P. Multistrain versus single-strain plant growth promoting microbial inoculants-The compatibility issue. Hell. Plant Prot. J. 2019, 12, 61–77. [Google Scholar] [CrossRef]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; van den Boom, T.; Weber, E. Phanologische Entwicklungsstadien von Gemusepflanzen I. Zwiebel-, Wurzel-, Knollen-und Blattgemuse. Nachr. Dtsch. Pflanzenschutzd. 1995, 47, 193–205. [Google Scholar]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; van den Boom, T.; Weber, E. Phanologische Entwicklungsstadien von Gemusepflanzen II. Fruchtgemuse und Hulsenfruchte. Nachr. Dtsch. Pflanzenschutzd. 1995, 47, 217–232. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of nutrient efficient plants in improving crop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Herrera, F.; Castillo, J.E.; Chica, A.F.; López Bellido, L. Use of municipal solid waste compost (MSWC) as a growing medium in the nursery production of tomato plants. Bioresour. Technol. 2008, 99, 287–296. [Google Scholar] [CrossRef]
- Russo, V.M. Biological amendment, fertilizer rate, and irrigation frequency for organic Bll pepper transplant production. HortScience 2006, 41, 1402–1407. [Google Scholar] [CrossRef]
- McCall, D. Effect of supplementary light on tomato transplant growth, and the after-effects on yield. Sci. Hortic. 1992, 51, 65–70. [Google Scholar] [CrossRef]
- Masson, J.; Tremblay, N.; Gosselin, A. Effects of nitrogen fertilization and HPS supplementary lighting on vegetable transplant production. II. Yield. J. Am. Soc. Hortic. Sci. 1991, 116, 599–602. [Google Scholar] [CrossRef]
- Nicola, S.; Cantliffe, D.J. Increasing cell size and reducing medium compression enhance lettuce transplant quality and field production. HortScience 1996, 31, 184–189. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Caldwell, R.D. Best management practices for minimizing nitrate leaching from container-grown nurseries. Sci. World J. 2001, 1, 96–102. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M.; Al-Qurainy, F.; Harris, P.J.C. Salt Tolerance in Selected Vegetable Crops. CRC Crit. Rev. Plant Sci. 2012, 31, 303–320. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of Salt Stress by Plant Growth-Promoting Bacteria in Hydroponic Leaf Lettuce. Agronomy 2020, 10, 1523. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant salt tolerance. Agric. Salin. Assess. Manag. 2012, 2, 405–459. [Google Scholar]
- FAO Drainage Paper 61, Agricultural Drainage Water Management in Arid and Semi-Arid Areas, Annex 1. Crop Salt Tolerance Data. Available online: http://www.fao.org/3/y4263e/y4263e0e.htm (accessed on 10 September 2020).
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hortic. 1998, 78, 83–125. [Google Scholar] [CrossRef]
- Souri, M.K.; Tohidloo, G. Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem. Biol. Technol. Agric. 2019, 6, 26. [Google Scholar] [CrossRef]
- Andriolo, J.L.; da Luz, G.L.; Witter, M.H.; Godoi, R.d.S.; Barros, G.T.; Bortolotto, O.C. Growth and yield of lettuce plants under salinity. Hortic. Bras. 2005, 23, 931–934. [Google Scholar] [CrossRef]
- Ünlükara, A.; Cemek, B.; Karaman, S.; Erşahin, S. Response of lettuce (Lactuca sativa var. crispa) to salinity of irrigation water. New Zeal. J. Crop Hortic. Sci. 2008, 36, 265–273. [Google Scholar] [CrossRef]
- Rosas, J.T.F.; Junior, E.M.; Lorenzoni, R.M.; dos Santos, F.F.L.; Martins, R.N. Effect of Salinity on Germination of Lettuce Cultivars Produced in Brazil. J. Exp. Agric. Int. 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Le Rudulier, D. Osmoregulation in rhizobia: The key role of compatible solutes. Grain Legum. 2005, 42, 18–19. [Google Scholar]
- Srivastava, J.P.; Gupta, S.C.; Lal, P.; Muralia, R.N.; Kumar, A. Effect of salt stress on physiological and biochemical parameters of wheat. Annu. Arid Zo. 1988, 27, 197–204. [Google Scholar]
- Shalhevet, J. Plants under salt and water stress. In Plant Adaptation to Environmental Stress; Fowden, L., Mansfield, T., Stoddart, J., Eds.; Chapman and Hall: London, UK, 1993; p. 133. [Google Scholar]
- Shao, J.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Contribution of indole-3-acetic acid in the plant growth promotion by the rhizospheric strain Bacillus amyloliquefaciens SQR9. Biol. Fertil. Soils 2015, 51, 321–330. [Google Scholar] [CrossRef]
- Joo, G.-J.; Kim, Y.-M.; Lee, I.-J.; Song, K.-S.; Rhee, I.-K. Growth promotion of red pepper plug seedlings and the production of gibberellins by Bacillus cereus, Bacillus macroides and Bacillus pumilus. Biotechnol. Lett. 2004, 26, 487–491. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Dietel, K.; Rändler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.-J. Gibberellins producing Bacillus methylotrophicus KE2 supports plant growth and enhances nutritional metabolites and food values of lettuce. Plant Physiol. Biochem. 2016, 109, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kasozi, N.; Kaiser, H.; Wilhelmi, B. Effect of Bacillus spp. on Lettuce Growth and Root Associated Bacterial Community in a Small-Scale Aquaponics System. Agronomy 2021, 11, 947. [Google Scholar] [CrossRef]
- Mehta, P.; Walia, A.; Kulshrestha, S.; Chauhan, A.; Shirkot, C.K. Efficiency of plant growth-promoting P-solubilizing Bacillus circulans CB7 for enhancement of tomato growth under net house conditions. J. Basic Microbiol. 2015, 55, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Dhouib, H.; Zouari, I.; Ben Abdallah, D.; Belbahri, L.; Taktak, W.; Triki, M.A.; Tounsi, S. Potential of a novel endophytic Bacillus velezensis in tomato growth promotion and protection against Verticillium wilt disease. Biol. Control 2019, 139, 104092. [Google Scholar] [CrossRef]
- Walia, A.; Mehta, P.; Chauhan, A.; Shirkot, C.K. Effect of Bacillus subtilis strain CKT1 as inoculum on growth of tomato seedlings under net house conditions. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 145–155. [Google Scholar] [CrossRef]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar] [CrossRef]
- Palaniyandi, S.; Damodharan, K.; Yang, S.; Suh, J. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J. Appl. Microbiol. 2014, 117, 766–773. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Miceli, A.; Romano, C.; Moncada, A.; Piazza, G.; Torta, L.; D’Anna, F.; Vetrano, F. Yield and quality of mini-watermelon as affected bygrafting and mycorrhizal inoculum. J. Agric. Sci. Technol. 2016, 18, 505–516. [Google Scholar]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Desai, S.; Bagyaraj, D.J.; Ashwin, R. Inoculation with microbial consortium promotes growth of tomato and capsicum seedlings raised in pro trays. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 21–28. [Google Scholar] [CrossRef]
- Desai, S.; Kumar, G.P.; Amalraj, L.D.; Bagyaraj, D.J.; Ashwin, R. Exploiting PGPR and AMF Biodiversity for Plant Health Management. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 145–160. ISBN 978-8-13222-647-5. [Google Scholar]
- Kohler, J.; Caravaca, F.; Carrasco, L.; Roldan, A. Interactions between a plant growth-promoting rhizobacterium, an AM fungus and a phosphate-solubilising fungus in the rhizosphere of Lactuca sativa. Appl. Soil Ecol. 2007, 35, 480–487. [Google Scholar] [CrossRef]
- Ważny, R.; Rozpądek, P.; Jędrzejczyk, R.J.; Śliwa, M.; Stojakowska, A.; Anielska, T.; Turnau, K. Does co-inoculation of Lactuca serriola with endophytic and arbuscular mycorrhizal fungi improve plant growth in a polluted environment? Mycorrhiza 2018, 28, 235–246. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic Action of a Microbial-based Biostimulant and a Plant Derived-Protein Hydrolysate Enhances Lettuce Tolerance to Alkalinity and Salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Sellitto, V.M.; Golubkina, N.A.; Pietrantonio, L.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Florin, I.; Caruso, G. Tomato yield, quality, mineral composition and antioxidants as affected by beneficial microorganisms under soil salinity induced by balanced nutrient solutions. Agriculture 2019, 9, 110. [Google Scholar] [CrossRef]
- Woitke, M.; Junge, H.; Schnitzler, W.H. Bacillus Subtilis as Growth Promotor in Hydroponically Grown Tomatoes under Saline Conditions. Acta Hortic. 2004, 363–369. [Google Scholar] [CrossRef]
- Cortés-Jiménez, D.; Gómez-Guzmán, A.; Iturriaga, G.; Suárez, R.; Montero Alpírez, G.; Escalante, F.M.E. Microorganisms associated to tomato seedlings growing in saline culture act as osmoprotectant. Braz. J. Microbiol. 2014, 45, 613–620. [Google Scholar] [CrossRef][Green Version]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Lugtenberg, B. Use of plant growth-promoting rhizobacteria to alleviate salinity stress in plants. In Use of Microbes for the Alleviation of Soil Stresses; Springer: New York, NY, USA, 2014; Volume 1, pp. 73–96. ISBN 978-1-46149-466-9. [Google Scholar]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Jyothsna, P.; Murthy, S.D.S. A review on effect of senescence in plants and the role of phytohormones in delaying senescence. Int. J. Plant Anim. Environ. Sci. 2016, 6, 152–161. [Google Scholar]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Vanderlinde, E.M.; Harrison, J.J.; Muszyński, A.; Carlson, R.W.; Turner, R.J.; Yost, C.K. Identification of a novel ABC transporter required for desiccation tolerance, and biofilm formation in Rhizobium leguminosarum bv. viciae 3841. FEMS Microbiol. Ecol. 2010. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, F.; Li, X.; Tian, C.; Tang, C.; Rengel, Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 2002, 12, 185–190. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Hammad, R.; Rusan, M. Response of two tomato cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza 2001, 11, 43–47. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Zuccarini, P. Mycorrhizal infection ameliorates chlorophyll content and nutrient uptake of lettuce exposed to saline irrigation. Plant Soil Environ. 2008, 53, 283–289. [Google Scholar] [CrossRef]
- Hameed, A.; Dilfuza, E.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Ahmad, P. Salinity Stress and Arbuscular Mycorrhizal Symbiosis in Plants. In Use of Microbes for the Alleviation of Soil Stresses; Springer: New York, NY, USA, 2014; Volume 1, pp. 139–159. ISBN 978-1-46149-466-9. [Google Scholar]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Jahromi, F.; Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Influence of Salinity on the In Vitro Development of Glomus intraradices and on the In Vivo Physiological and Molecular Responses of Mycorrhizal Lettuce Plants. Microb. Ecol. 2008, 55, 45–53. [Google Scholar] [CrossRef]
- Porcel, R.; Azcón, R.; Ruiz-Lozano, J.M. Evaluation of the role of genes encoding for dehydrin proteins (LEA D-11) during drought stress in arbuscular mycorrhizal Glycine max and Lactuca sativa plants. J. Exp. Bot. 2005, 56, 1933–1942. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol. Plant Microbe Interact. 2012, 25, 1264–1271. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Roldán, A.; Albacete, A.; Pascual, J.A. The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry 2011, 72, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Bagyaraj, D.J. Mycorrhizal fungi. Proc. Indian Natl. Sci. Acad. 2014, 80, 415–428. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.; Anli, M.; Meddich, A.; Oufdou, K. Use of Rhizobacteria and Mycorrhizae Consortium in the Open Field as a Strategy for Improving Crop Nutrition, Productivity and Soil Fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Nayyar, H. Microbial Consortium of Plant Growth-Promoting Rhizobacteria Improves the Performance of Plants Growing in Stressed Soils: An Overview. In Phosphate Solubilizing Microorganisms; Springer International Publishing: Cham, Switzerland, 2014; pp. 257–285. ISBN 978-3-31908-216-5. [Google Scholar]

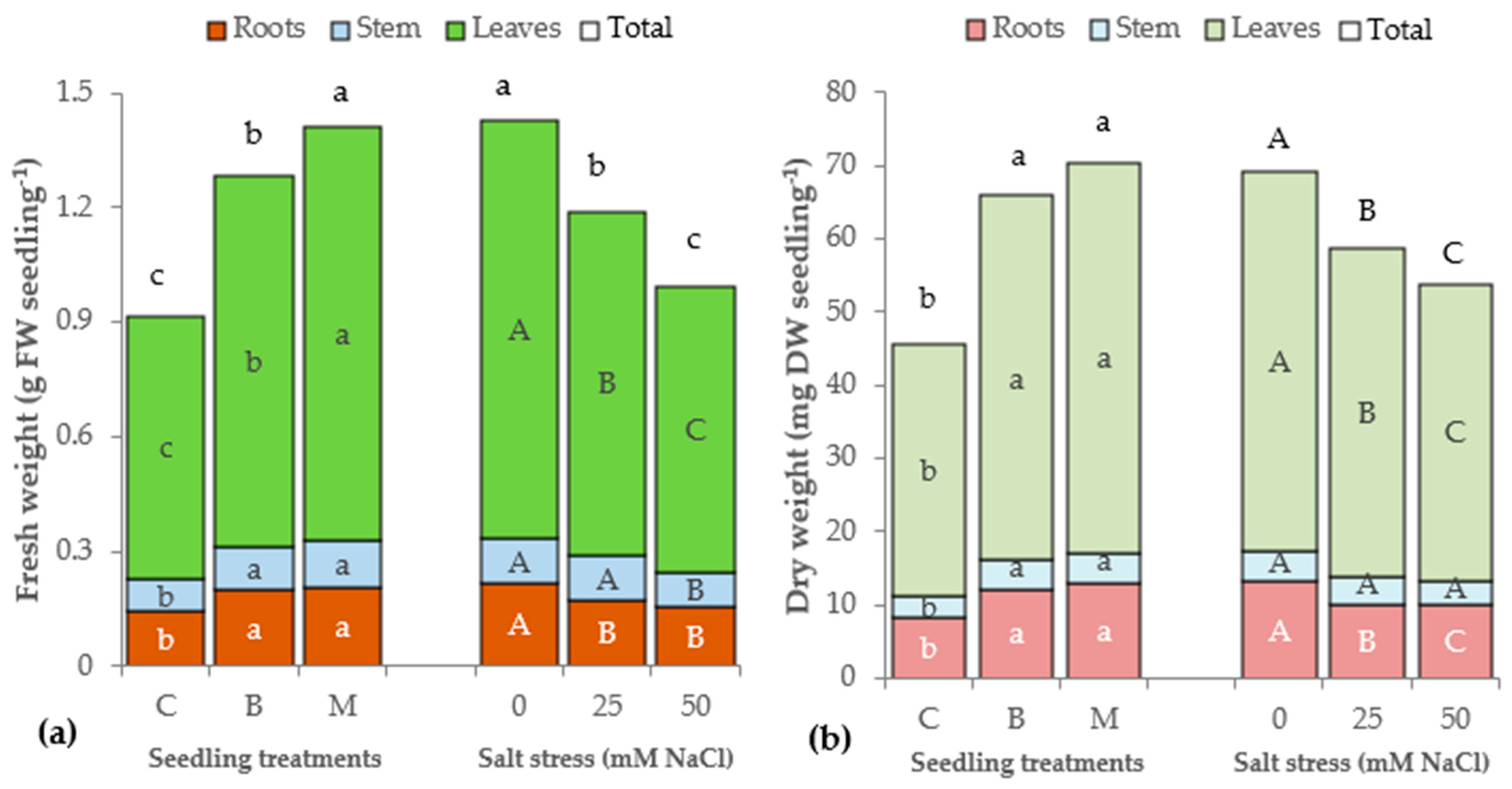
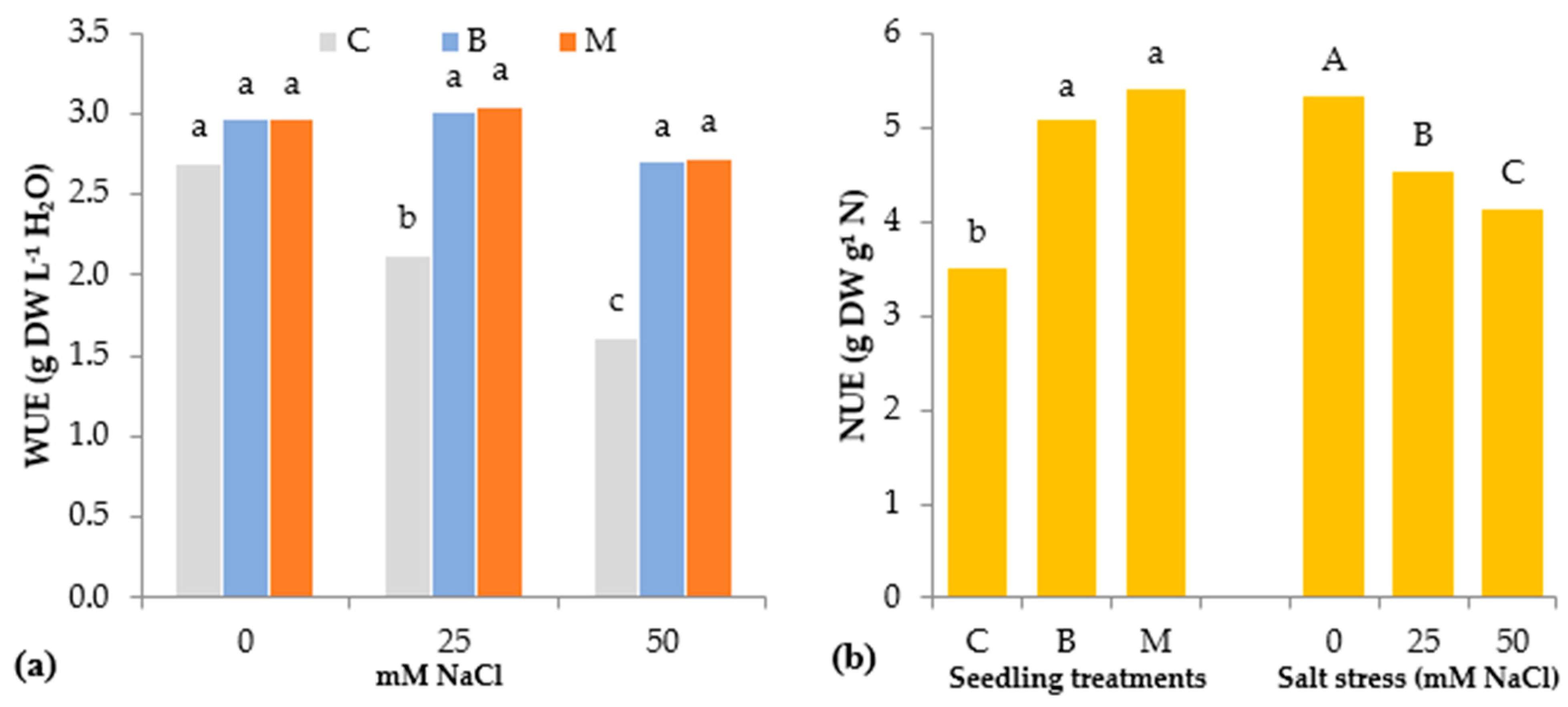

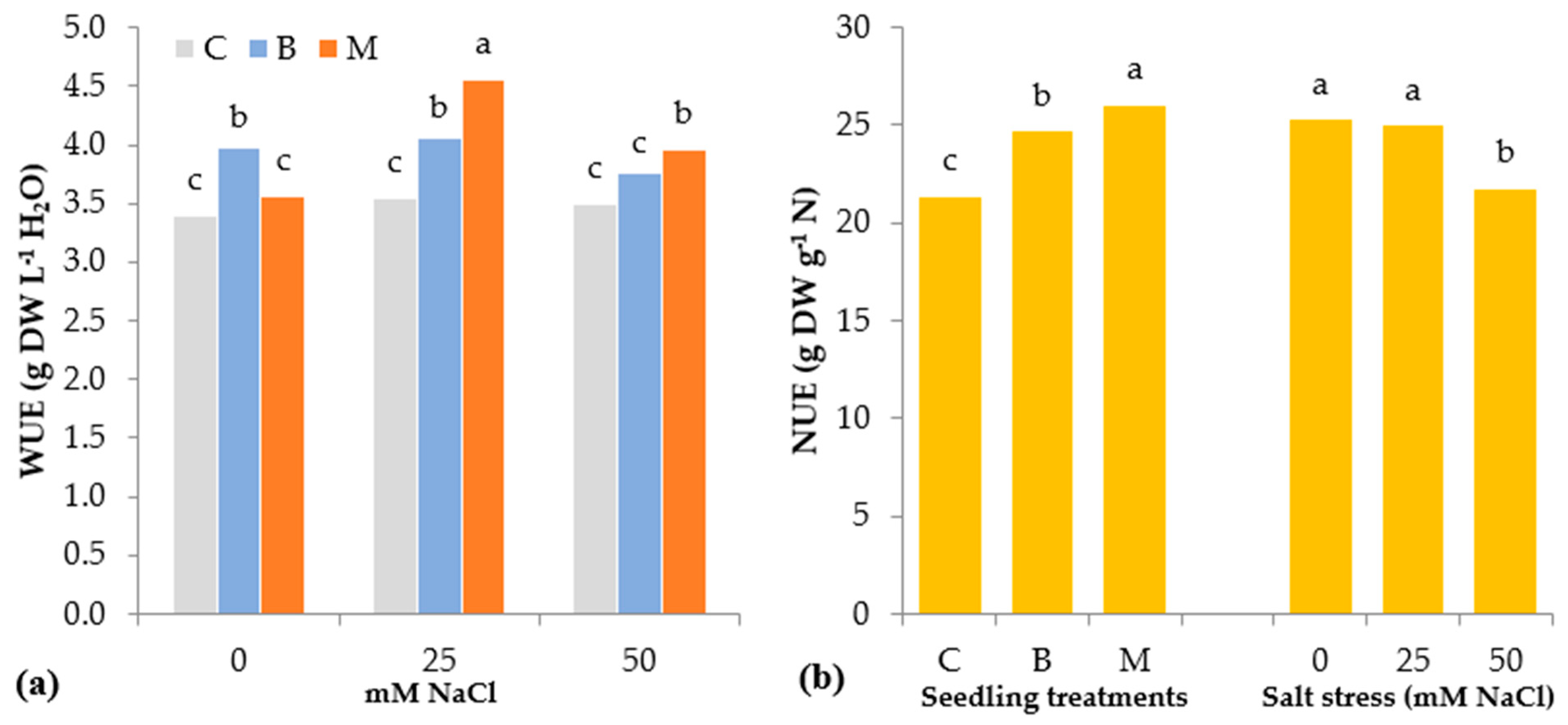
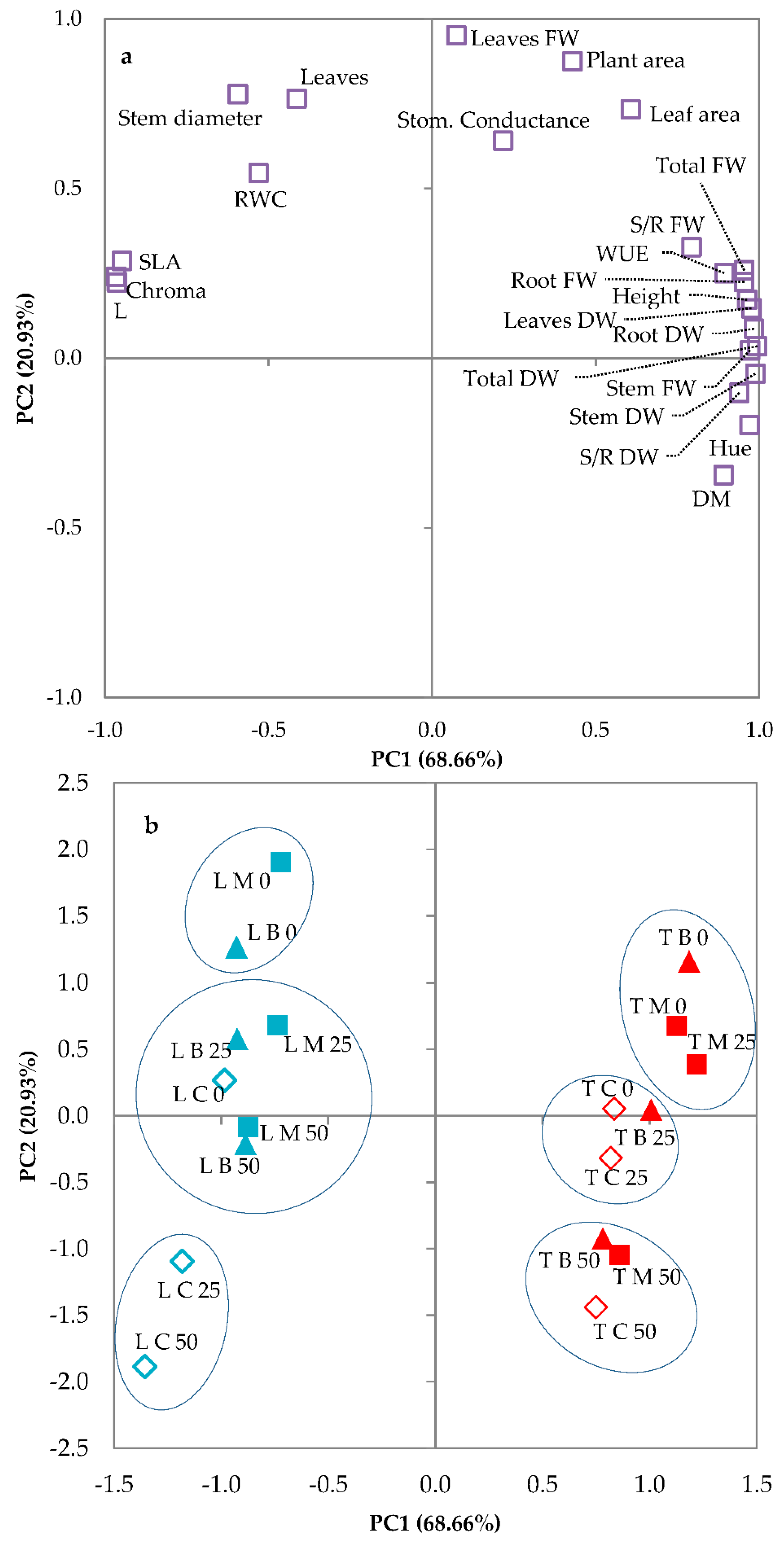
| Source of Variance | Seedling Height (cm) | StemDiameter (mm) | Seedling Fresh Weight (g FW) | Seedling Dry Weight (mg DW) | Dry Matter (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Roots | Stem | Leaves | Shoot/Root | Total | Roots | Stem | Leaves | Shoot/Root | |||||
| Treatment | ||||||||||||||
| C | z 7.5 | 2.8b | 0.92c | 0.14 | 0.09b | 0.69c | 5.4 | 45.6b | 8.2b | 3.0b | 34.3b | 4.6 | 4.9 | |
| B | 9.0 | 3.1a | 1.28b | 0.20 | 0.11a | 0.97b | 5.7 | 65.9a | 12.2a | 3.8a | 49.8a | 4.5 | 5.0 | |
| M | 9.9 | 3.1a | 1.41a | 0.20 | 0.12a | 1.09a | 6.0 | 70.3a | 12.9a | 4.2a | 53.3a | 4.6 | 4.8 | |
| NaCl (mM) | ||||||||||||||
| 0 | 10.0 | 3.2a | 1.43a | 0.22 | 0.12a | 1.10a | 5.7 | 69.3a | 13.3a | 3.9 | 52.0a | 4.3 | 4.5 | |
| 25 | 8.7 | 3.0b | 1.19b | 0.17 | 0.12a | 0.90b | 5.9 | 58.8b | 10.0b | 3.8 | 45.0b | 4.9 | 4.8 | |
| 50 | 7.8 | 2.8c | 0.99c | 0.16 | 0.09b | 0.75c | 5.4 | 53.7c | 10.0b | 3.3 | 40.4c | 4.5 | 5.3 | |
| Treatment × NaCl | ||||||||||||||
| C | 0 | 9.0bc | 3.1 | 1.13 | 0.16d | 0.10 | 0.87 | 5.9ab | 56.5 | 10.0 | 3.5 | 43.0 | 4.8 | 4.8b |
| 25 | 7.3d | 2.8 | 0.93 | 0.14de | 0.09 | 0.70 | 5.5ab | 43.3 | 7.3 | 3.0 | 33.0 | 5.0 | 4.6bc | |
| 50 | 6.2e | 2.6 | 0.69 | 0.12e | 0.07 | 0.50 | 4.7b | 36.8 | 7.3 | 2.5 | 27.0 | 4.1 | 5.2ab | |
| B | 0 | 9.4bc | 3.3 | 1.43 | 0.23b | 0.11 | 1.10 | 5.3b | 72.8 | 14.0 | 4.3 | 54.5 | 4.2 | 4.5bc |
| 25 | 9.0bc | 3.2 | 1.28 | 0.19c | 0.14 | 0.96 | 5.7ab | 66.0 | 12.0 | 4.3 | 49.8 | 4.5 | 5.0ab | |
| 50 | 8.6c | 2.9 | 1.14 | 0.17cd | 0.10 | 0.87 | 6.0ab | 58.9 | 10.7 | 3.0 | 45.3 | 4.8 | 5.4a | |
| M | 0 | 11.5a | 3.2 | 1.73 | 0.26a | 0.14 | 1.33 | 5.7ab | 78.5 | 16.0 | 4.0 | 58.5 | 4.0 | 4.2c |
| 25 | 9.7b | 3.1 | 1.36 | 0.18cd | 0.13 | 1.05 | 6.6a | 66.9 | 10.7 | 4.0 | 52.3 | 5.3 | 4.8b | |
| 50 | 8.5c | 3.1 | 1.16 | 0.18cd | 0.10 | 0.88 | 5.6ab | 65.5 | 12.0 | 4.5 | 49.0 | 4.5 | 5.5a | |
| Significance x | ||||||||||||||
| Treatment | *** | *** | *** | *** | ** | *** | * | *** | *** | * | *** | ns | ns | |
| NaCl | *** | *** | *** | *** | * | *** | ns | *** | *** | ns | *** | ns | *** | |
| Treatment × NaCl | ** | ns | ns | *** | ns | ns | * | ns | ns | ns | ns | ns | ** | |
| Source of Variance | Number of Leaves | Leaf Area (cm2 Seedling−1) | Leaf Area (cm2 Leaf−1) | SLA y (cm2 g DW−1) | Stomatal Conductance (mmol m2 s−1) | RWC (%) | L | Chroma | Hue° | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | |||||||||||
| C | z 5.1b | 31.9 | 6.3 | 926.4 | 360.0 | 94.1b | 52.6 | 41.9 | 121.6 | ||
| B | 5.6a | 46.1 | 8.2 | 928.1 | 365.1 | 95.8ab | 52.2 | 41.8 | 122.2 | ||
| M | 5.3b | 51.8 | 9.8 | 970.7 | 358.3 | 96.5a | 51.9 | 41.8 | 122.1 | ||
| NaCl (mM) | |||||||||||
| 0 | 5.5a | 51.2 | 9.3 | 982.0 | 532.9 | 96.9a | 52.7 | 42.6 | 121.5 | ||
| 25 | 5.3ab | 41.9 | 7.9 | 929.6 | 330.9 | 95.8a | 52.5 | 41.8 | 122.0 | ||
| 50 | 5.2b | 36.9 | 7.1 | 913.6 | 219.7 | 93.8b | 51.5 | 41.1 | 122.3 | ||
| Treatment × NaCl | |||||||||||
| C | 0 | 5.3 | 41.4c | 7.8c | 963.2b | 563.3a | 95.3 | 52.4ab | 41.9ab | 121.6ab | |
| 25 | 5.1 | 29.9d | 5.9d | 906.8c | 245.1c | 94.5 | 53.0a | 41.8ab | 121.8ab | ||
| 50 | 4.8 | 24.5d | 5.1d | 909.2c | 271.7c | 92.6 | 52.5ab | 42.0a | 121.2b | ||
| B | 0 | 5.8 | 50.3b | 8.6bc | 926.4bc | 526.9a | 97.3 | 52.6ab | 43.1a | 121.6ab | |
| 25 | 5.6 | 46.3bc | 8.3c | 931.5bc | 384.3b | 95.9 | 53.2a | 42.3a | 122.1ab | ||
| 50 | 5.5 | 41.8c | 7.6c | 926.5bc | 184.2c | 94.1 | 50.7b | 40.1b | 122.8a | ||
| M | 0 | 5.4 | 61.8a | 11.4a | 1056.4a | 508.5a | 98.0 | 53.2a | 43.0a | 121.4b | |
| 25 | 5.3 | 49.4b | 9.4b | 950.7bc | 363.3b | 96.9 | 51.2ab | 41.4a | 122.2ab | ||
| 50 | 5.2 | 44.3bc | 8.5bc | 905.1c | 203.2c | 94.5 | 51.4ab | 41.0ab | 122.8ab | ||
| Significance x | |||||||||||
| Treatment | *** | *** | *** | * | ns | * | ns | ns | * | ||
| NaCl | * | *** | *** | ** | *** | ** | * | *** | * | ||
| Treatment × NaCl | ns | * | ** | * | ** | ns | * | ** | * | ||
| Source of Variance | Seedling Height (cm) | Stem Diameter (mm) | Seedling Fresh Weight (g FW) | Seedling Dry Weight (mg DW) | Dry Matter (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Roots | Stem | Leaves | Shoot/Root | Total | Roots | Stem | Leaves | Shoot/Root | |||||
| Treatment | ||||||||||||||
| C | z 18.1 | 2.5 | 2.44 | 0.30b | 1.31 | 0.82 | 7.0 | 184.4c | 26.4c | 75.6c | 82.3b | 6.1b | 7.5c | |
| B | 18.3 | 2.6 | 2.63 | 0.32ab | 1.38 | 0.93 | 7.2 | 213.3b | 28.0b | 87.7b | 97.6a | 6.6a | 8.1b | |
| M | 18.9 | 2.7 | 2.64 | 0.33a | 1.41 | 0.91 | 7.0 | 224.7a | 29.3a | 93.2a | 102.2a | 6.7a | 8.6a | |
| NaCl (mM) | ||||||||||||||
| 0 | 21.0a | 2.8a | 2.93 | 0.32a | 1.64 | 0.97 | 8.1a | 218.4a | 29.8a | 92.0a | 96.6a | 6.4 | 7.2c | |
| 25 | 19.1b | 2.7a | 2.69 | 0.34a | 1.44 | 0.91 | 7.0b | 216.0a | 28.2a | 91.6a | 96.2a | 6.7 | 8.0b | |
| 50 | 15.3c | 2.4b | 2.10 | 0.29b | 1.03 | 0.78 | 6.1c | 187.9b | 25.8b | 72.8b | 89.3b | 6.3 | 9.0a | |
| Treatment × NaCl | ||||||||||||||
| C | 0 | 20.2 | 2.7 | 2.73bc | 0.31 | 1.53b | 0.89bc | 7.8 | 195.6 | 29.3 | 79.8 | 86.5 | 5.8 | 6.9 |
| 25 | 19.2 | 2.6 | 2.53c | 0.31 | 1.40bc | 0.82c | 7.1 | 188.9 | 26.7 | 81.0 | 81.3 | 6.2 | 7.3 | |
| 50 | 15.0 | 2.3 | 2.05d | 0.29 | 1.01d | 0.76c | 6.1 | 168.6 | 23.3 | 66.0 | 79.3 | 6.2 | 8.2 | |
| B | 0 | 20.9 | 2.8 | 3.11a | 0.34 | 1.72a | 1.05a | 8.2 | 228.6 | 29.3 | 97.0 | 102.3 | 6.8 | 7.2 |
| 25 | 18.6 | 2.7 | 2.66c | 0.33 | 1.39c | 0.94b | 7.0 | 216.9 | 28.7 | 90.3 | 98.0 | 6.6 | 8.1 | |
| 50 | 15.6 | 2.4 | 2.13d | 0.29 | 1.05d | 0.79c | 6.5 | 194.3 | 26.0 | 75.8 | 92.5 | 6.5 | 9.1 | |
| M | 0 | 21.9 | 2.9 | 2.96ab | 0.32 | 1.67a | 0.97b | 8.1 | 230.9 | 30.7 | 99.3 | 101.0 | 6.5 | 7.6 |
| 25 | 19.6 | 2.7 | 2.86b | 0.36 | 1.52b | 0.98b | 7.0 | 242.1 | 29.3 | 103.5 | 109.3 | 7.3 | 8.5 | |
| 50 | 15.4 | 2.4 | 2.11d | 0.31 | 1.02d | 0.78c | 5.8 | 201.0 | 28.0 | 76.8 | 96.3 | 6.2 | 9.6 | |
| Significance x | ||||||||||||||
| Treatment | ns | ns | *** | ** | ** | *** | ns | *** | * | *** | *** | * | *** | |
| NaCl | *** | ** | *** | *** | *** | *** | *** | *** | ** | *** | * | ns | *** | |
| Treatment × NaCl | ns | ns | *** | ns | * | *** | ns | ns | ns | ns | ns | ns | ns | |
| Source of Variance | Number of Leaves | Leaf Area (cm2 Seedling−1) | Leaf Area (cm2 Leaf−1) | SLA y (cm2 g DW−1) | Stomatal Conductance (mmol m2 s−1) | RWC (%) | L * | Chroma | Hue° | |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||||
| C | z 4.9b | 44.5 | 9.1b | 540.2a | 392.2 | 84.3 | 45.1 | 30.2 | 127.6 | |
| B | 5.2a | 49.2 | 9.5ab | 503.7b | 454.4 | 89.5 | 45.0 | 30.1 | 127.1 | |
| M | 5.1a | 50.4 | 9.9a | 493.5b | 358.1 | 90.3 | 45.1 | 30.4 | 127.4 | |
| NaCl (mM) | ||||||||||
| 0 | 5.2a | 52.7 | 10.2a | 547.1a | 545.4 | 92.1 | 45.4a | 30.8a | 127.2 | |
| 25 | 5.1a | 49.9 | 9.8a | 523.8b | 358.0 | 89.9 | 45.3a | 30.6a | 127.2 | |
| 50 | 4.9b | 41.4 | 8.5b | 466.4c | 301.2 | 82.1 | 44.5b | 29.3b | 127.7 | |
| Treatment × NaCl | ||||||||||
| C | 0 | 4.9 | 48.0b | 9.7 | 554.8 | 389.7b | 92.6a | 45.9 | 31.4 | 127.0 |
| 25 | 4.9 | 46.1b | 9.5 | 568.7 | 385.0b | 90.6ab | 45.3 | 30.1 | 127.6 | |
| 50 | 4.9 | 39.4c | 8.1 | 497.2 | 401.8b | 69.7c | 44.1 | 29.0 | 128.1 | |
| B | 0 | 5.4 | 55.9a | 10.5 | 548.9 | 759.8a | 90.8ab | 45.4 | 30.5 | 127.1 |
| 25 | 5.2 | 49.4b | 9.5 | 504.2 | 354.4b | 88.9b | 45.0 | 30.2 | 127.2 | |
| 50 | 5.0 | 42.4c | 8.6 | 458.1 | 249.0b | 88.8b | 44.7 | 29.6 | 127.1 | |
| M | 0 | 5.2 | 54.2a | 10.4 | 537.7 | 486.8ab | 93.0a | 45.1 | 30.4 | 127.5 |
| 25 | 5.2 | 54.4a | 10.5 | 498.6 | 334.7b | 90.1ab | 45.7 | 31.5 | 126.9 | |
| 50 | 4.8 | 42.6c | 8.9 | 444.1 | 252.8b | 87.8b | 44.5 | 29.3 | 127.8 | |
| Significance x | ||||||||||
| Treatment | ** | *** | ** | *** | ns | ** | ns | ns | ns | |
| NaCl | ** | *** | *** | *** | *** | *** | *** | ** | ns | |
| Treatment × NaCl | ns | ** | ns | ns | ** | *** | ns | ns | ns | |
| Variable | PC1 | PC2 |
|---|---|---|
| Height | 0.963 | 0.172 |
| Stem diameter | −0.594 | 0.778 |
| Total fresh weight | 0.954 | 0.259 |
| Root fresh weight | 0.954 | 0.225 |
| Stem fresh weight | 0.972 | 0.023 |
| Leaf fresh weight | 0.074 | 0.951 |
| Shoot/Root FW | 0.793 | 0.327 |
| Total dry weight | 0.994 | 0.035 |
| Root dry weight | 0.984 | 0.087 |
| Stem dry weight | 0.988 | −0.046 |
| Leaf dry weight | 0.976 | 0.147 |
| Shoot/Root DW | 0.939 | −0.102 |
| Dry matter % | 0.891 | −0.345 |
| RWC | −0.531 | 0.546 |
| WUE | 0.893 | 0.251 |
| Leaf number | −0.414 | 0.765 |
| Plant area | 0.429 | 0.875 |
| Leaf area | 0.607 | 0.733 |
| SLA | −0.948 | 0.287 |
| Stomatal conductance | 0.217 | 0.641 |
| L * | −0.963 | 0.223 |
| Chroma | −0.965 | 0.240 |
| Hue° | 0.970 | −0.197 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, A.; Moncada, A.; Vetrano, F. Use of Microbial Biostimulants to Increase the Salinity Tolerance of Vegetable Transplants. Agronomy 2021, 11, 1143. https://doi.org/10.3390/agronomy11061143
Miceli A, Moncada A, Vetrano F. Use of Microbial Biostimulants to Increase the Salinity Tolerance of Vegetable Transplants. Agronomy. 2021; 11(6):1143. https://doi.org/10.3390/agronomy11061143
Chicago/Turabian StyleMiceli, Alessandro, Alessandra Moncada, and Filippo Vetrano. 2021. "Use of Microbial Biostimulants to Increase the Salinity Tolerance of Vegetable Transplants" Agronomy 11, no. 6: 1143. https://doi.org/10.3390/agronomy11061143
APA StyleMiceli, A., Moncada, A., & Vetrano, F. (2021). Use of Microbial Biostimulants to Increase the Salinity Tolerance of Vegetable Transplants. Agronomy, 11(6), 1143. https://doi.org/10.3390/agronomy11061143






