Investigating the Impact of Root-Lesion Nematodes (Pratylenchus thornei) and Crown Rot (Fusarium pseudograminearum) on Diverse Cereal Cultivars in a Conservation Farming System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiments
2.2. Experiments
2.3. Soil Sampling
2.4. Soil Water
2.5. Pratylenchus Thornei, other Nematode Species and Fusarium Pseudograminearum
2.6. Soil Chemical Properties
2.7. Crop Measurements
2.8. Plant Chemical Analysis
2.9. Statistical Analyses
2.10. Rainfall
3. Results
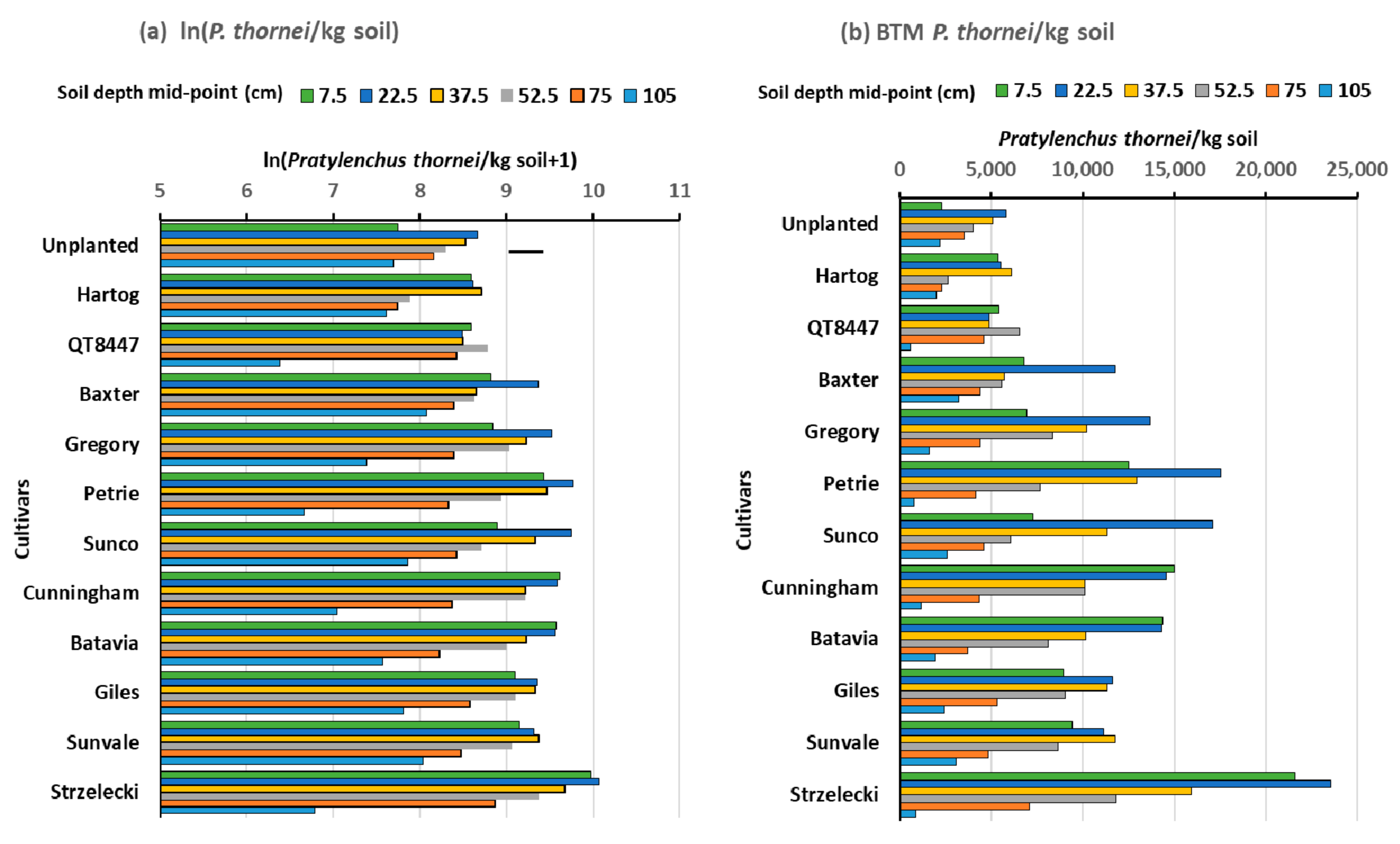
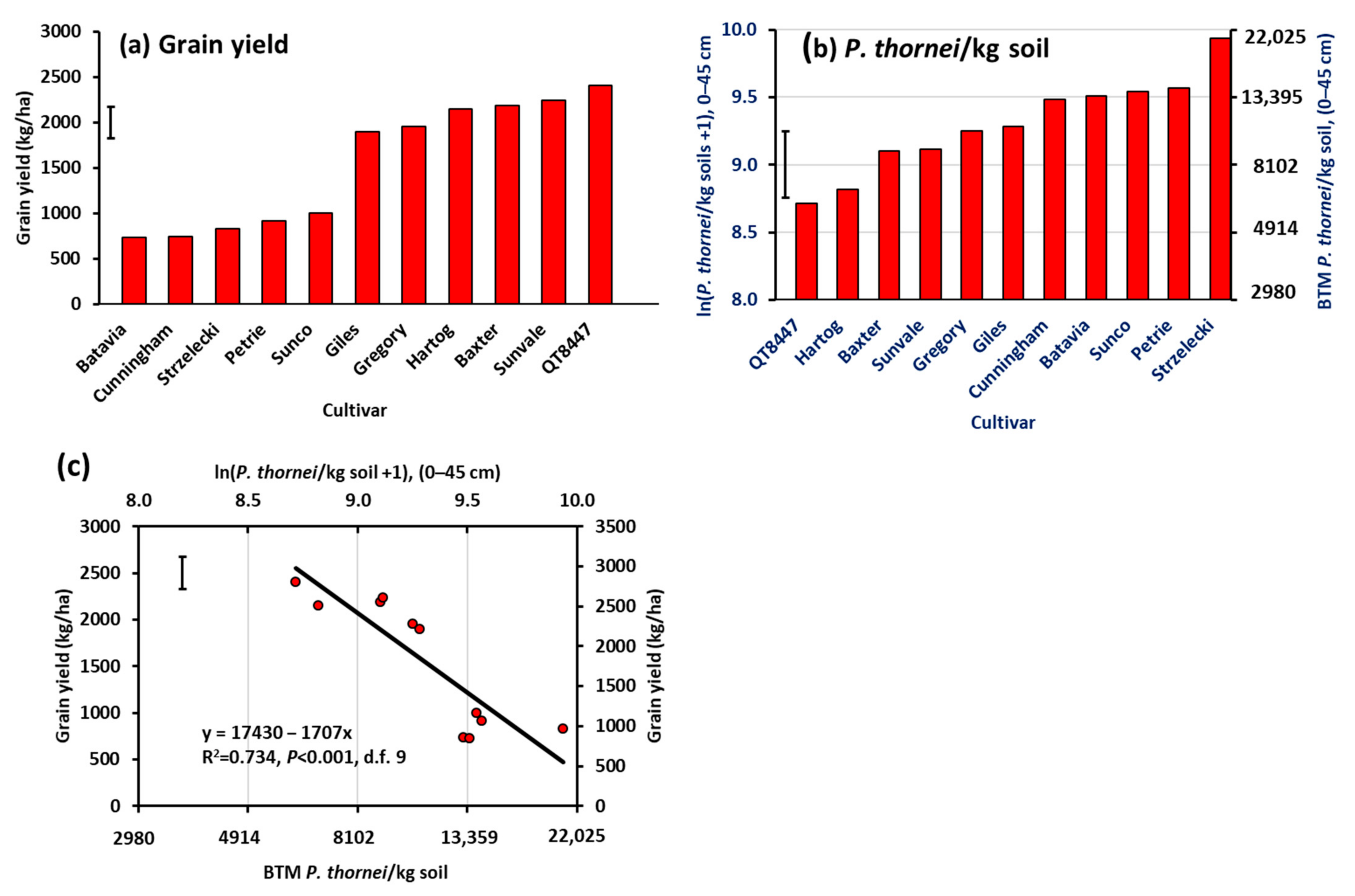
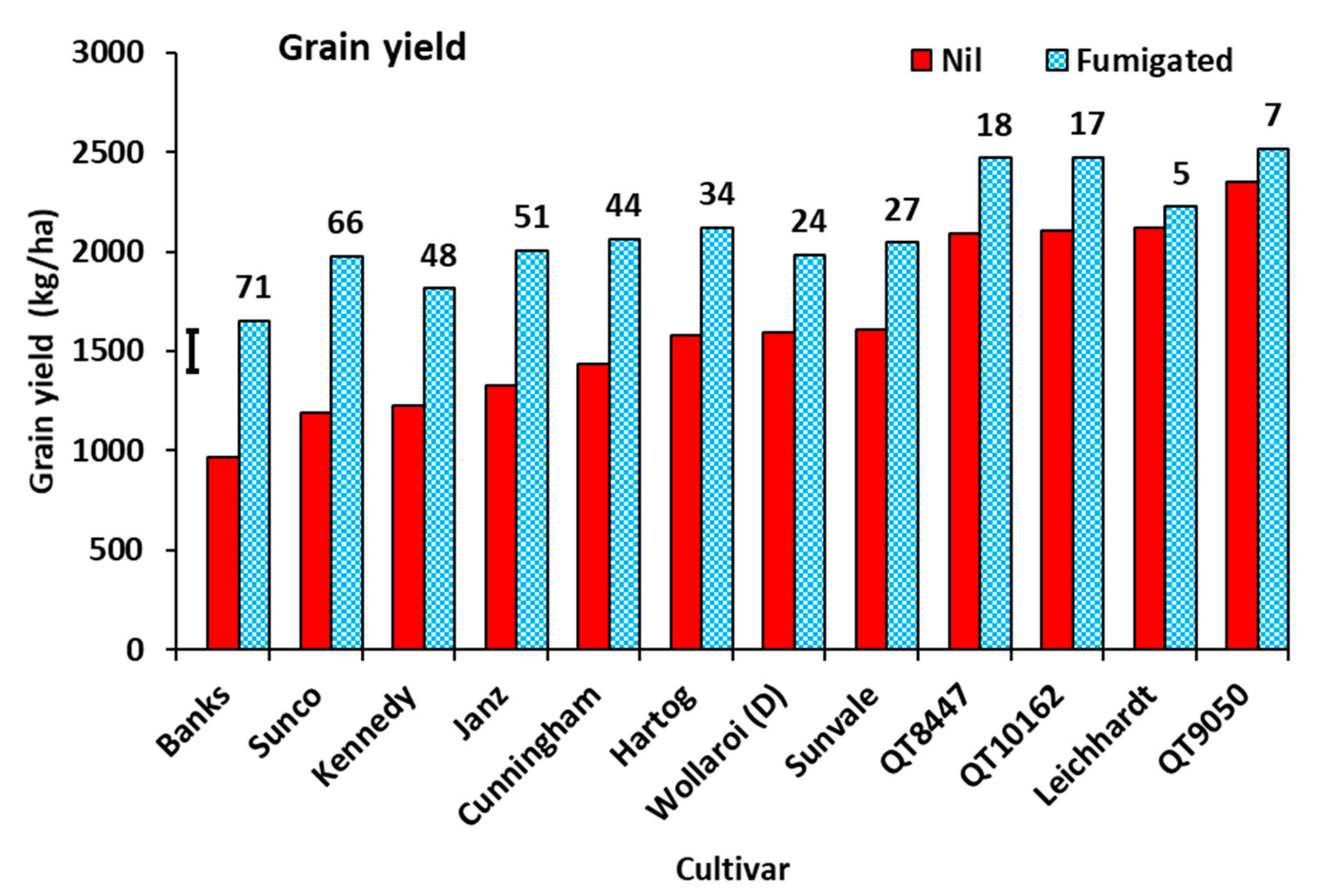
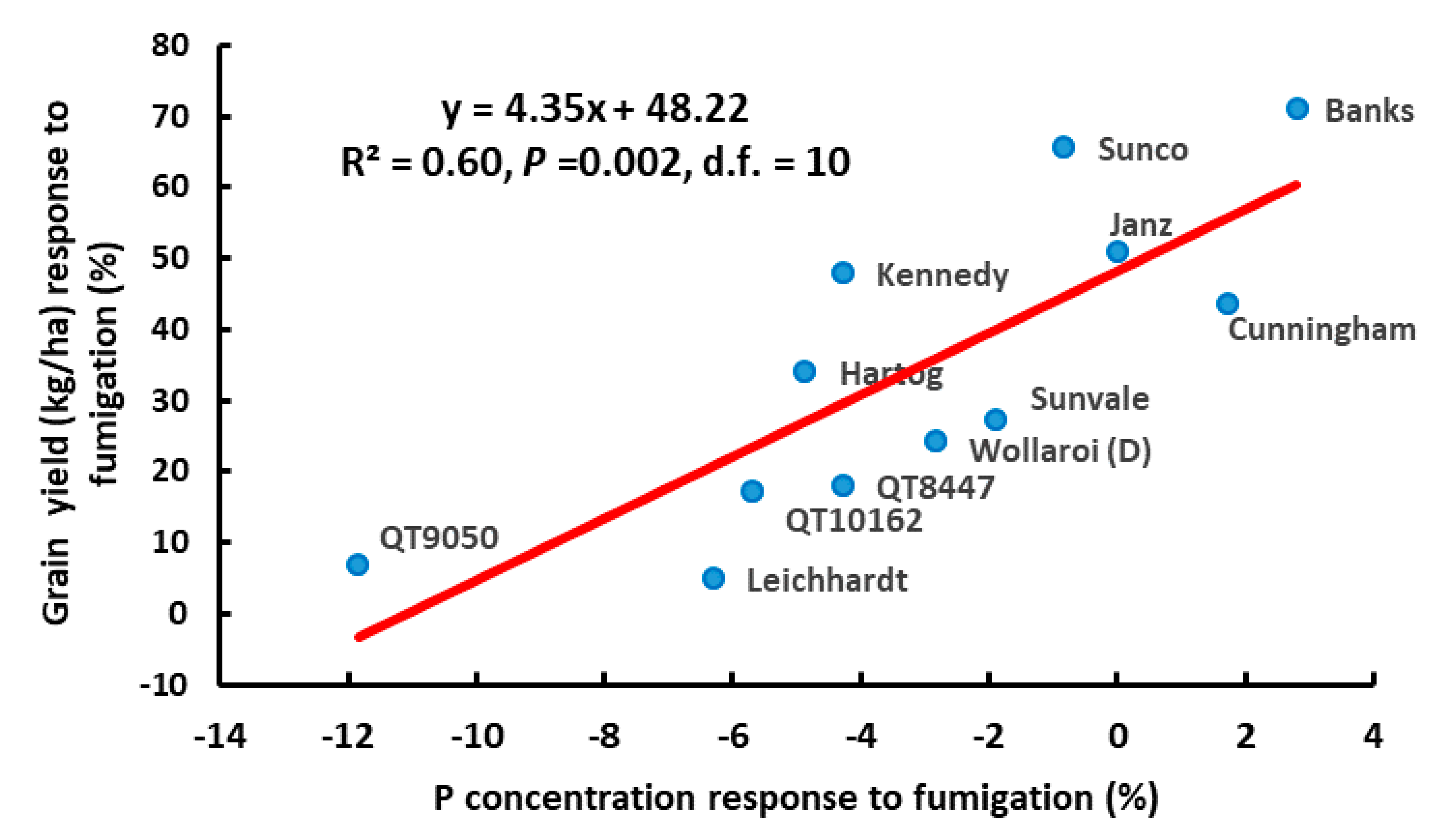
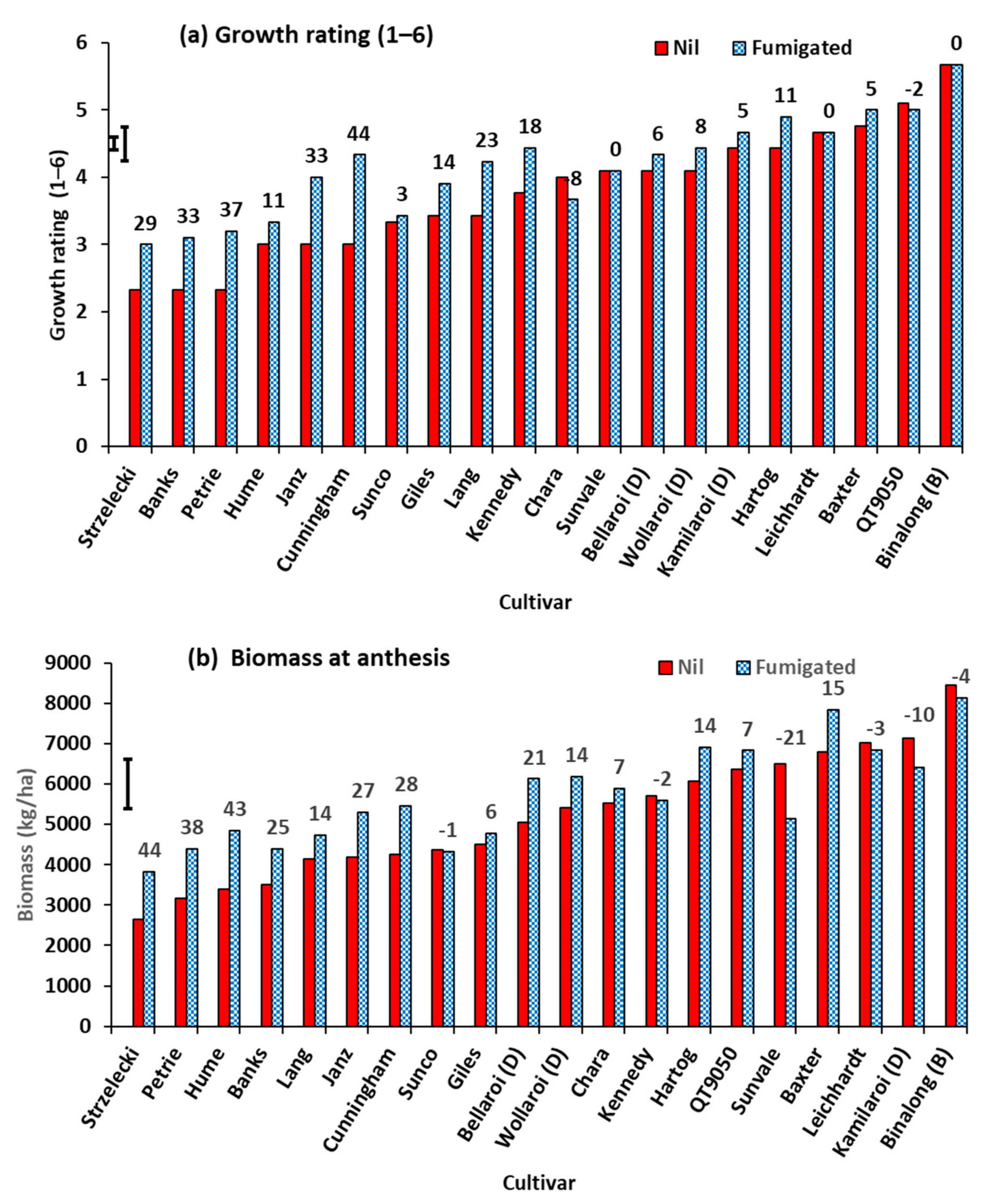
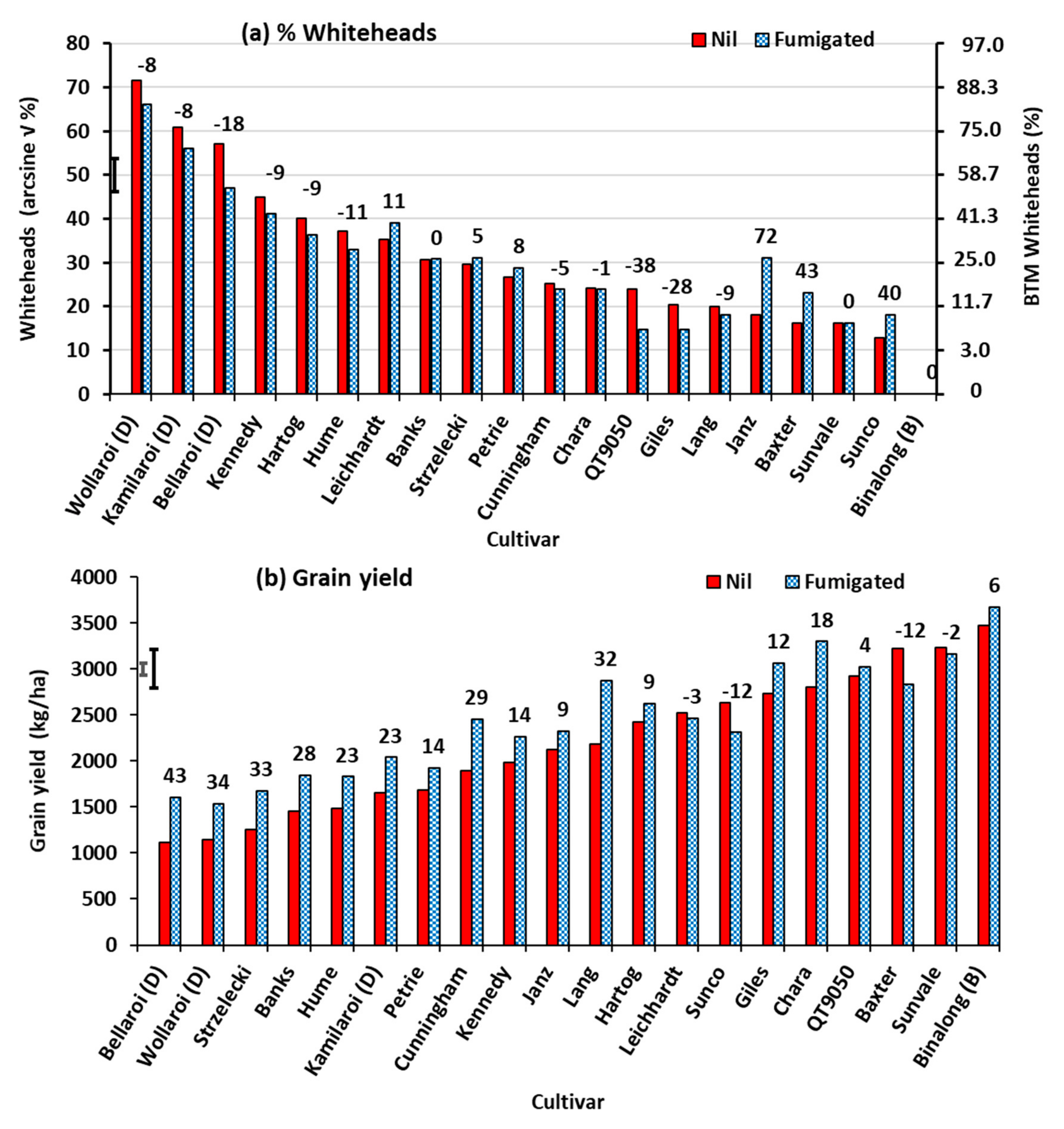
3.1. Experiment 1
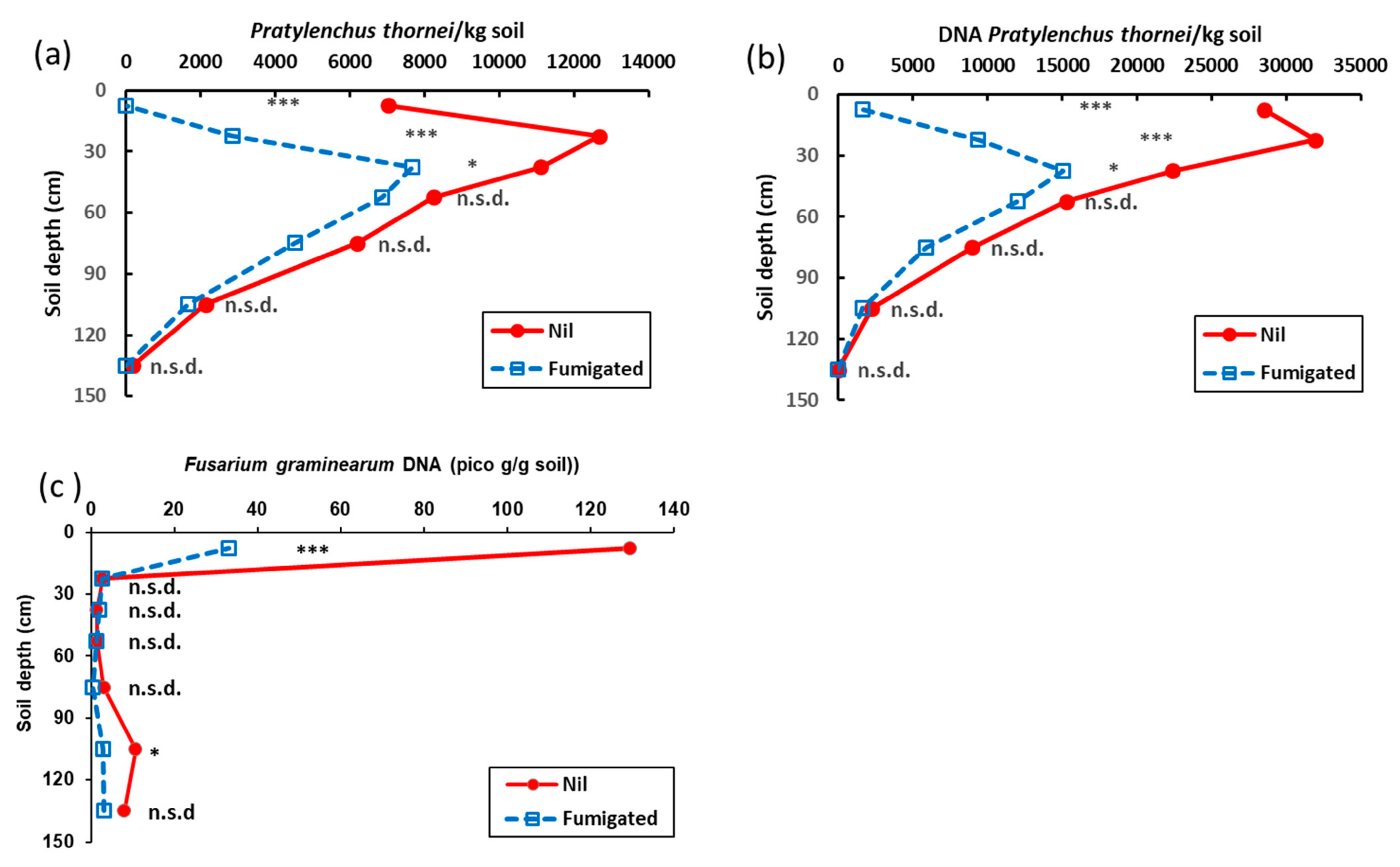
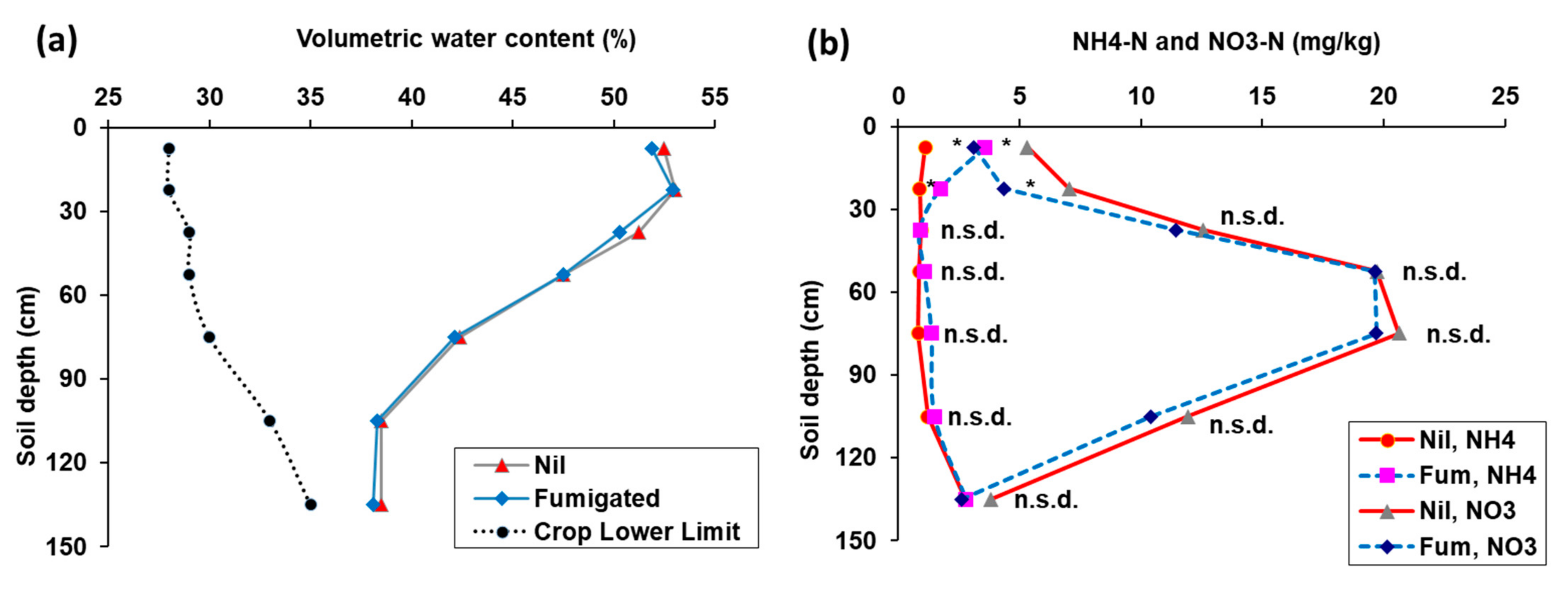
3.2. Experiment 2
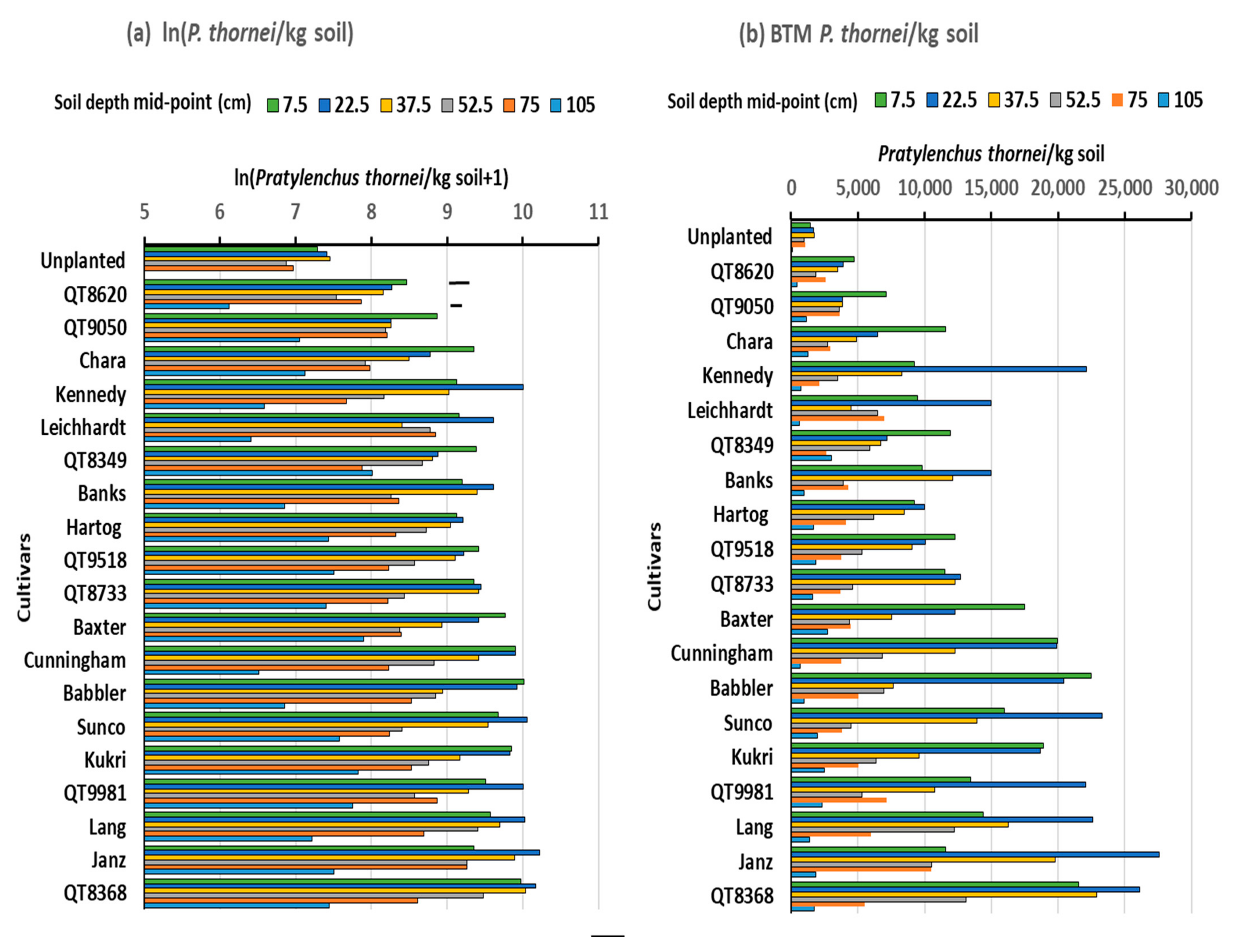
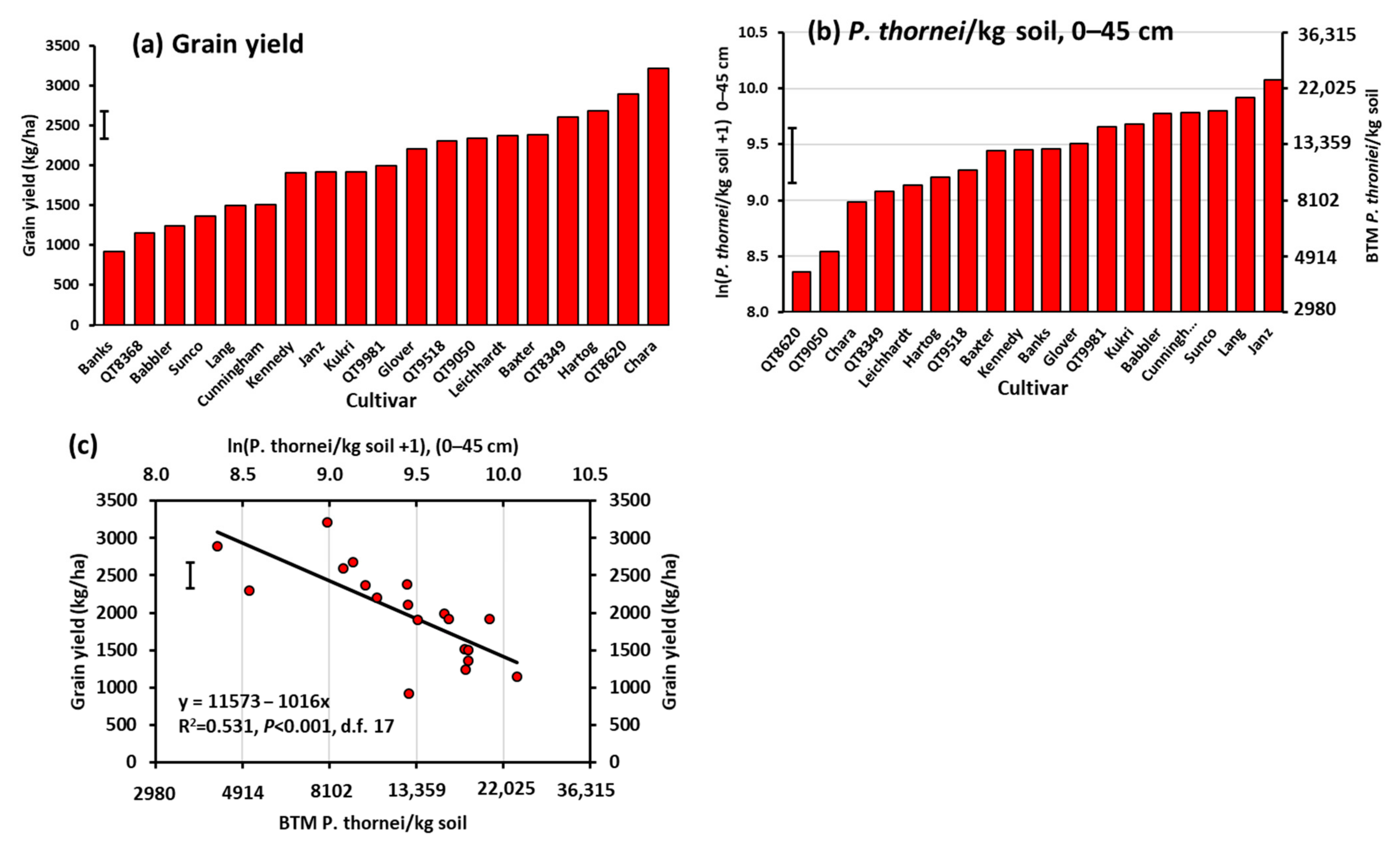
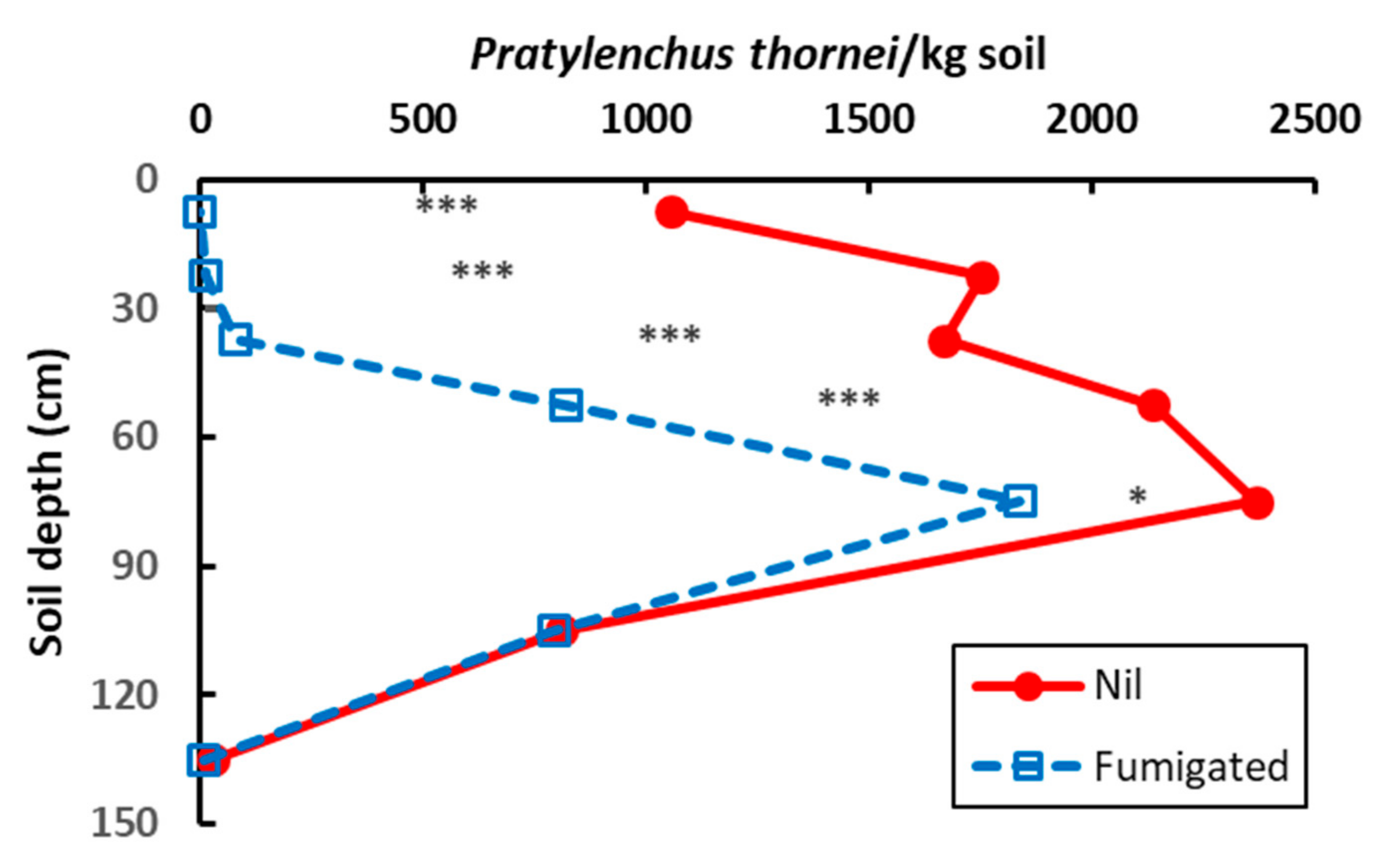
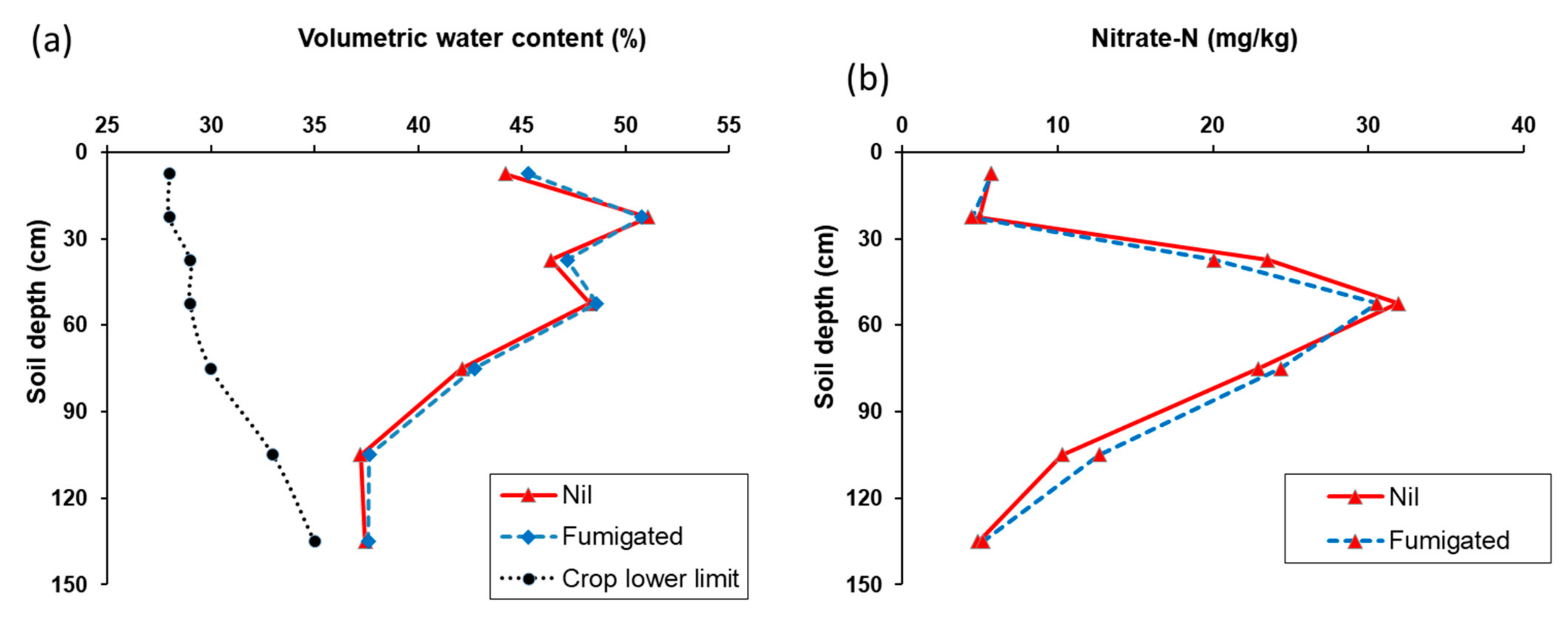
3.3. Experiment 3.
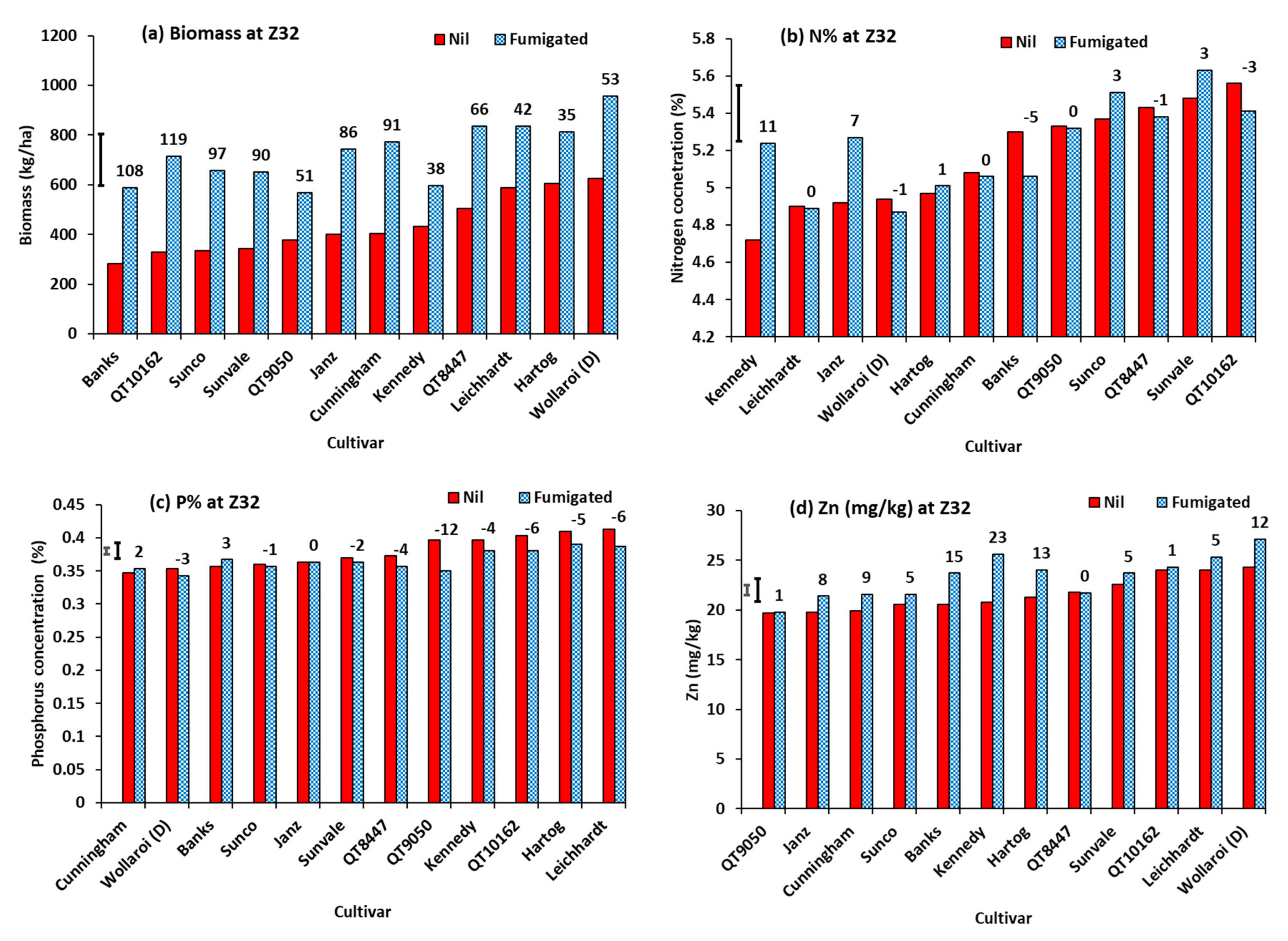
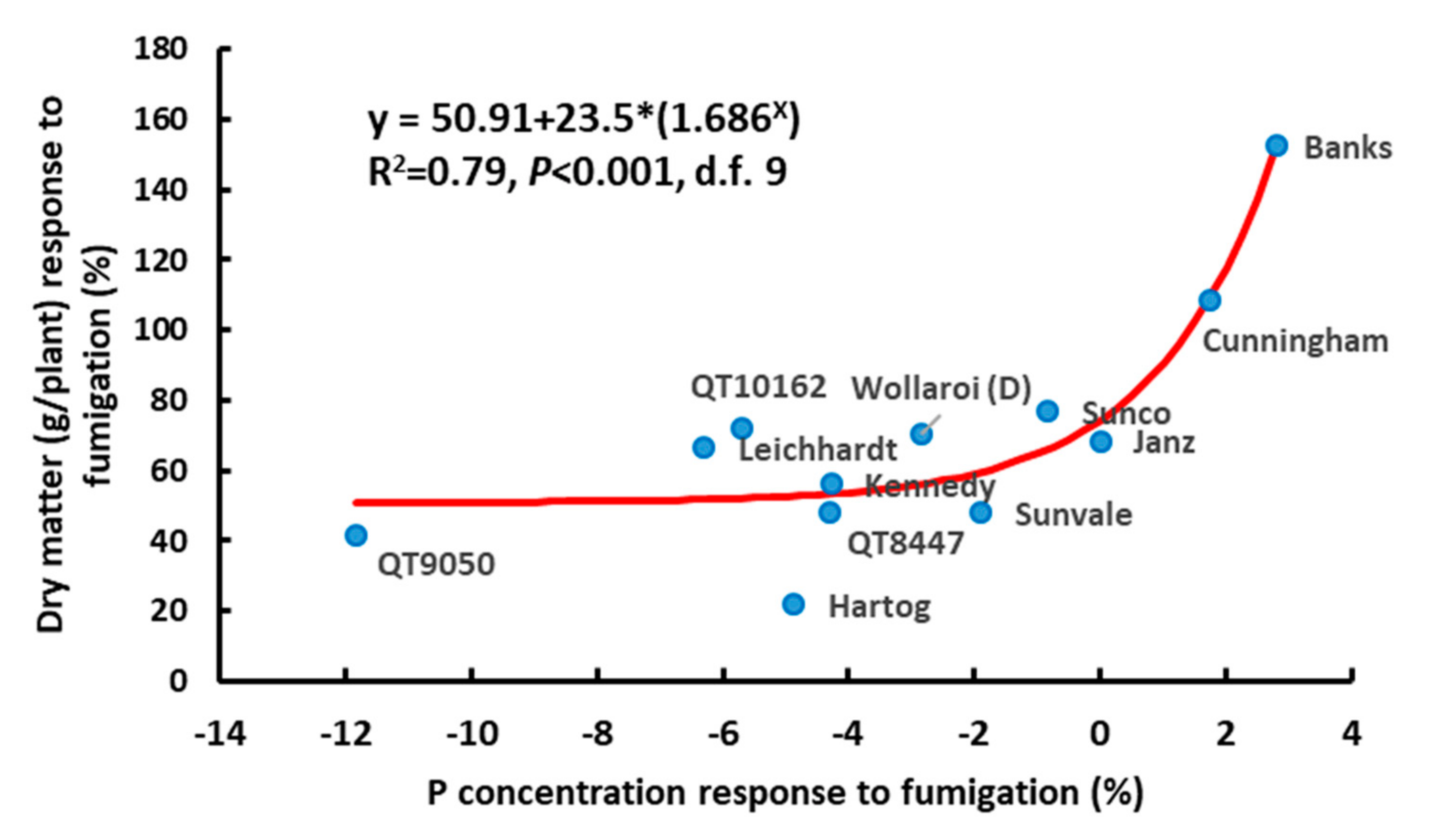
3.4. Experiment 4.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, J.P.; Owen, K.J.; Stirling, G.R.; Bell, M.J. Root-lesion nematodes (Pratylenchus thornei and P. neglectus): A review of recent progress in managing a significant pest of grain crops in northern Australia. Australas. Plant Pathol. 2008, 37, 235–242. [Google Scholar] [CrossRef]
- Vanstone, V.A.; Hollaway, G.J.; Stirling, G.R. Managing nematode pests in the southern and western regions of the Australian cereal industry: Continuing progress in a challenging environment. Australas. Plant Pathol. 2008, 37, 220–234. [Google Scholar] [CrossRef]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian wheat industry. Australas. Plant Pathol. 2009, 38, 558–570. [Google Scholar] [CrossRef]
- Mokrini, A.; Viaene, N.; Waeyenberge, L.; Dababat, A.A.; Moens, M. Root-lesion nematodes in cereal fields: Importance, distribution, identification, and management strategies. J. Plant Dis. Prot. 2019, 126, 1–11. [Google Scholar] [CrossRef]
- Smiley, R.W.; Nicol, J.M. Nematodes which challenge global wheat production. In Wheat Science and Trade; Carver, B.F., Ed.; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 171–187. [Google Scholar]
- Thompson, J.P.; Clewett, T.G.; Sheedy, J.G.; Reen, R.A.; O’Reilly, M.M.; Bell, K.L. Occurrence of root-lesion nematodes (Pratylenchus thornei and P. neglectus) and stunt nematode (Merlinius brevidens) in the northern grain region of Australia. Australas. Plant Pathol. 2010, 39, 254–264. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources. World Soil Resource Report No.84; FAO: Rome, Italy, 1998. [Google Scholar]
- Webb, A.A.; Grundy, M.J.; Powell, B.; Littleboy, M. The Australian subtropical cereal belt: Soils, climate and agriculture. In Sustainable Crop Production in the Sub-Tropics—An Australian Perspective; Clarke, A.L., Wylie, P.B., Eds.; Queensland Department of Primary Industries: Brisbane, Australia, 1997; pp. 8–23. [Google Scholar]
- Owen, K.J.; Clewett, T.G.; Bell, K.L.; Thompson, J.P. Wheat biomass and yield increased when populations of the root-lesion nematode (Pratylenchus thornei) were reduced through sequential rotation of partially resistant winter and summer crops. Crop. Pasture Sci. 2014, 65, 227–241. [Google Scholar] [CrossRef]
- Thomas, G.A.; Titmarsh, G.W.; Freebairn, D.M.; Radford, B.J. No-tillage and conservation farming practices in grain growing areas of Queensland—A review of 40 years of development. Aust. J. Exp. Agric. 2007, 47, 887–898. [Google Scholar] [CrossRef]
- Silburn, D.M.; Freebairn, D.M. Soil conservation in Australia’s semi-arid tropics: Pathways to success, and new challenges. In Conserving Soil and Water for Society: Sharing Solutions. Proceedings of the 13th International Soil Conservation Organisation Conference; Raine, S.R., Biggs, A.J.W., Menzies, N.W., Freebairn, D.M., Tolmie, P.E., Eds.; Australian Society of Soil Science Incorporated/International Erosion Control Association: Brisbane, Australia, 2004; Paper No. 416. [Google Scholar]
- Li, Y.X.; Tullberg, J.N.; Freebairn, D.M.; Radford, B.J. Wheel traffic and tillage effects and crop yields. Soil Tillage Res. 2007, 97, 282–292. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Roper, M.M.; Thompson, J.P. Harnessing the benefits of soil biology in conservation agriculture. In Australian Agriculture in 2020: From Conservation to Automation; Pratley, J., Kirkegaard, J., Eds.; Agronomy Australia and Charles Sturt University: Wagga, Australia, 2019; pp. 241–258. [Google Scholar]
- Thompson, J. Soil biotic and biochemical factors in a long-term tillage and stubble management experiment on a vertisol. 2. Nitrogen deficiency with zero tillage and stubble retention. Soil Tillage Res. 1992, 22, 339–361. [Google Scholar] [CrossRef]
- Thompson, J.; MacKenzie, J.; Amos, R. Root-lesion nematode (Pratylenchus thornei) limits response of wheat but not barley to stored soil moisture in the Hermitage long-term tillage experiment. Aust. J. Exp. Agric. 1995, 35, 1049–1055. [Google Scholar] [CrossRef]
- Page, K.L.; Dang, Y.P.; Dalal, R.C.; Reeves, S.; Thomas, G.; Wang, W.; Thompson, J.P. Changes in soil water storage with no-tillage and crop residue retention on a Vertisol: Impact on productivity and profitability over a 50 year period. Soil Tillage Res. 2019, 194, 104319. [Google Scholar] [CrossRef]
- Li, Y.; Stirling, G.R.; Seymour, N.P. The effect of organic amendment input and crop management practices on the nematode community and suppression of root-lesion nematode (Pratylenchus thornei) in a grain-growing soil. Australas. Plant Pathol. 2017, 46, 463–472. [Google Scholar] [CrossRef]
- Barker, K.R. Resistance/tolerance and related concepts/terminology in plant nematology. Plant Dis. 1993, 77, 111–113. [Google Scholar]
- Fanning, J.; Linsell, K.; McKay, A.; Gogel, B.; Santa, I.M.; Davey, R.; Hollaway, G. Resistance to the root lesion nematodes Pratylenchus thornei and P. neglectus in cereals: Improved assessments in the field. Appl. Soil Ecol. 2018, 132, 146–154. [Google Scholar] [CrossRef]
- Thompson, J.P.; Sheedy, J.G.; Robinson, N.A. Resistance of Wheat Genotypes to Root-Lesion Nematode (Pratylenchus thornei) Can be Used to Predict Final Nematode Population Densities, Crop Greenness, and Grain Yield in the Field. Phytopathology 2020, 110, 505–516. [Google Scholar] [CrossRef]
- Wallace, H. A Perception of Tolerance. Nematologica 1987, 33, 419–432. [Google Scholar] [CrossRef]
- Thompson, J.P.; Sheedy, J.G.; Robinson, N.A.; Clewett, T.G. Tolerance of wheat (Triticum aestivum) genotypes to root-lesion nematode (Pratylenchus thornei) in the subtropical grain region of eastern Australia. Euphytica 2021, 217, 1–30. [Google Scholar] [CrossRef]
- Cook, R.; Evans, K. Resistance and tolerance. In Principles and Practices of Nematode Control in Crops; Brown, R.H., Kerry, B.R., Eds.; Academic Press: Sydney, Australia, 1987; pp. 179–231. [Google Scholar]
- Roberts, P.A. Concepts and consequences of resistance. In Plant Resistance to Parasitic Nematodes; Starr, J.L., Cook, R., Bridge, J., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 23–41. [Google Scholar]
- Thompson, J.P.; Brennan, P.S.; Clewett, T.G.; Sheedy, J.G.; Seymour, N.P. Progress in breeding wheat for tolerance and resistance to root-lesion nematode (Pratylenchus thornei). Australas. Plant Pathol. 1999, 28, 45–52. [Google Scholar] [CrossRef]
- Matthews, P.; McCaffery, D. Winter Crop Variety Sowing Guide 2019; NSW Dep Primary Industries: Sydney, Australia, 2019; ISSN 1328-9535. [Google Scholar]
- Anon. 2020 Queensland Winter Crop Sowing Guide; GRDC Grains Research and Development Corporation: 2019; ISSN: 2652-3590.
- Thompson, J.P.; Seymour, N.P. And Inheritance of resistance to root-lesion nematode (Pratylenchus thornei) in wheat landraces and cultivars from the West Asia and North Africa (WANA) region. Crop. Pasture Sci. 2011, 62, 82–93. [Google Scholar] [CrossRef]
- Thompson, J.P.; Zwart, R.S.; Butler, D. Inheritance of resistance to root-lesion nematodes (Pratylenchus thornei and P. neglectus) in five doubled-haploid populations of wheat. Euphytica 2012, 188, 209–219. [Google Scholar] [CrossRef]
- Zwart, R.S.; Thompson, J.P.; Godwin, I.D. Genetic analysis of resistance to root-lesion nematode (Pratylenchus thornei) in wheat. Plant Breed. 2004, 123, 209–212. [Google Scholar] [CrossRef]
- Simpfendorfer, S.; McKay, A.; Ophel-Keller, K. New approaches to crop disease management in conservation agriculture. In Australian Agriculture in 2020: From Conservation to Automation; Pratley, J., Kirkegaard, J., Eds.; Agronomy Australia and Charles Sturt University: Wagga, Australia, 2019; pp. 173–188. [Google Scholar]
- Wildermuth, G.B.; Thomas, G.A.; Radford, B.J.; McNamara, R.B. Crown rot and common root rot in wheat grown under different tillage and stubble management treatments in southern Queensland. Aust. Soil Tillage Res. 1997, 44, 211–224. [Google Scholar] [CrossRef]
- Klein, T.; Burgess, L.; Ellison, F. The incidence and spatial patterns of wheat plants infected by Fusarium graminearum Group 1 and the effect of crown rot on yield. Aust. J. Agric. Res. 1991, 42, 399–407. [Google Scholar] [CrossRef]
- Backhouse, D. Modelling the behaviour of crown rot in wheat caused by Fusarium pseudograminearum. Australas. Plant Pathol. 2013, 43, 15–23. [Google Scholar] [CrossRef]
- Summerell, B.; Burgess, L.; Klein, T. The impact of stubble management on the incidence of crown rot of wheat. Aust. J. Exp. Agric. 1989, 29, 91–98. [Google Scholar] [CrossRef]
- Dodman, R.L.; Wildermuth, G.B. The effect of stubble retention and tillage practices in wheat and barley on crown rot caused by Fusarium graminearum Group 1. Plant Prot. Q. 1989, 3, 3–4. [Google Scholar]
- Burgess, L.; Backhouse, D.; Summerell, B.; Pattison, A.; Klein, T.; Esdaile, R.; Ticehurst, G. Long-term effects of stubble management on the incidence of infection of wheat by Fusarium graminearum Schw. Group 1. Aust. J. Exp. Agric. 1993, 33, 451–456. [Google Scholar] [CrossRef]
- Lush, D.J. (Ed.) Wheat Varieties for Queensland 2003, Crop Link Variety Update; Queensland Government Department of Primary Industries: Brisbane, Australia, 2003; ISSN 0727-6273. [Google Scholar]
- Rovira, A.D. Studies on soil fumigation I Effects on ammonium, nitrate and phosphate in soil and on the growth, nutrition and yield of wheat. Soil Biol. Biochem. 1976, 8, 241–247. [Google Scholar] [CrossRef]
- Thompson, J.P.; Mackenzie, J.; Sheedy, G.H. Root-lesion nematode (Pratylenchus thornei) reduces nutrient response, biomass and yield of wheat in sorghum–fallow–wheat cropping systems in a subtropical environment. Field Crops Res. 2012, 137, 126–140. [Google Scholar] [CrossRef]
- Reen, R.A.; Thompson, J.P.; Clewett, T.G.; Sheedy, J.G.; Bell, K.L. Yield response in chickpea cultivars and wheat following crop rotations affecting population densities of Pratylenchus thornei and arbuscular mycorrhizal fungi. Crop. Pasture Sci. 2014, 65, 428–441. [Google Scholar] [CrossRef]
- Thompson, J.; Clewett, T. Impacts of Root-Lesion Nematode (Pratylenchus thornei) on Plant Nutrition, Biomass, Grain Yield and Yield Components of Susceptible/Intolerant Wheat Cultivars Determined by Nematicide Applications. Agronomy 2021, 11, 296. [Google Scholar] [CrossRef]
- Isbell, R.F. The Australian Soil Classification, Revised ed.; CSIRO Publishing: Melbourne, Australia, 1996. [Google Scholar]
- Harris, P.; Biggs, A.J.W.; Stone, B.J.; Crane, L.N.; Douglas, N.J. Resource information book. In Central Darling Downs Land Management Manual. DNRQ990102; Queensland Department of Natural Resources: Brisbane, Australia, 1999. [Google Scholar]
- Cooper, M.; Powell, O.; Voss-Fels, K.P.; Messina, C.D.; Gho, C.; Podlich, D.W.; Technow, F.; Chapman, S.C.; A Beveridge, C.; Ortiz-Barrientos, D.; et al. Modelling selection response in plant-breeding programs using crop models as mechanistic gene-to-phenotype (CGM-G2P) multi-trait link functions. In Silico Plants 2021, 3, 1–21. [Google Scholar] [CrossRef]
- Williams, E.R. Row and column designs with contiguous replicates. Austral. J. Stat. 1986, 28, 154–163. [Google Scholar] [CrossRef]
- Klein, L. Methyl bromide as soil fumigant. In The Methyl Bromide Issue; Bell, C.H., Price, N., Chakrabarti, B., Eds.; John Wiley & Sons: New York, NY, USA, 1996; pp. 191–235. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Dalgliesh, N.; Foale, M. Soil Matters—Monitoring Soil Water and Nutrients in Dryland Farming, CSIRO/Agricultural Production Systems Research Unit: Technical Manual. 1998. Available online: https://www.apsim.info/wp-content/uploads/2019/10/Soil-matters.pdf (accessed on 11 March 2021).
- Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Peters, B.G. Toxicity tests with vinegar eelworm 1. Counting and culturing. J. Helminthol. 1952, 26, 97–110. [Google Scholar] [CrossRef]
- Fortuner, R. Pratylenchus thornei. C.I.H. Description of Plant-parasitic Nematodes Set 7; Commonwealth Institute of Helminthology: St Albans, UK, 1977; No. 93. [Google Scholar]
- Ophel-Keller, K.; McKay, A.; Hartley, D.; Herdina; Curran, J. Development of a routine DNA-based testing service for soilborne diseases in Australia. Australas. Plant Pathol. 2008, 37, 243–253. [Google Scholar] [CrossRef]
- Rayment, G.E.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press: Melbourne, Australia, 1992. [Google Scholar]
- Colwell, J. The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Aust. J. Exp. Agric. 1963, 3, 190–197. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iIron, mManganese, and cCopper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Best, E.K. An automated method for determining nitrate-nitrogen in soil extracts. Qld. J. Agric. Anim. Sci. 1976, 33, 161–166. [Google Scholar]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Johnson, O.M.; Ulrich, A. Analytical methods for use in plant analysis. Bull. Calif. Agric. Exp. Stn. 1959, 766, 32. [Google Scholar]
- VSN International. GenStat for Windows, 18th ed.; VSN International: Hemel Hempstead, UK, 2015. [Google Scholar]
- O’Brien, P.C. A Study on the Host Range of Pratylenchus thornei. Australas. Plant Pathol. 1982, 11, 3–5. [Google Scholar] [CrossRef]
- Thompson, J.P.; Reen, R.A.; Clewett, T.G.; Sheedy, J.G.; Kelly, A.M.; Gogel, B.J.; Knights, E.J. Hybridisation of Australian chickpea cultivars with wild Cicer spp. increases resistance to root-lesion nematodes (Pratylenchus thornei and P. neglectus). Australas. Plant Pathol. 2011, 40, 601–611. [Google Scholar] [CrossRef]
- Owen, K.J.; Sheedy, J.G.; Thompson, J.P.; Clewett, T.G.; O’Reilly, M.M. Resistance of Australian spring barley and wheat cultivars to root lesion nematode (P. thornei), 2007 Plant Disease Management Reports (Online); The American Phytopathological Society: St. Paul, MN, USA, 2008; Report 2:N034. [Google Scholar] [CrossRef]
- Summerell, B.A.; Burgess, L.W. Stubble Management Practices and the Survival of Fusarium graminearum Group 1 in Wheat Stubble Residues. Australas. Plant Pathol. 1988, 17, 88–93. [Google Scholar] [CrossRef]
- Oka, Y. From old-generation to next-generation nematicides. Agronomy 2020, 10, 1387. [Google Scholar] [CrossRef]
- Brown, G.; Jenkinson, D. Bromine in wheat grown on soil fumigated with methyl bromide. Commun. Soil Sci. Plant Anal. 1971, 2, 45–54. [Google Scholar] [CrossRef]
- Whish, J.; Thompson, J.; Clewett, T.; Wood, J.; Rostad, H. Predicting the slow decline of root lesion nematodes (Pratylenchus thornei) during host-free fallows to improve farm management decisions. Eur. J. Agron. 2017, 91, 44–53. [Google Scholar] [CrossRef]
- Martin, A.; Simpfendorfer, S.; Hare, R.A.; Sutherland, M.W. Introgression of hexaploid sources of crown rot resistance into durum wheat. Euphytica 2013, 192, 463–470. [Google Scholar] [CrossRef]

| Agronomic Practice | Traditional Systems | Conservation Farming Systems |
|---|---|---|
| Crop rotations |
|
|
|
| |
| Stubble management |
|
|
| Tillage practices |
|
|
|
| |
| Fertiliser practices |
|
|
| Soil water monitoring |
|
|
| Experiments 1 and 2 | Experiment 3 | Experiment 4 |
|---|---|---|
| 2001 Wheat cultivar experiments | 2002 Fumigation experiment | 2003 Fumigation Experiment |
| 2000–2001 Black gram (Vigna mungo cv. Regur) 1999 Wheat (Hybrid Mercury) | 2001 Chickpea (Cicer arietinum, mixed cultivars Jimbour, Kaniva, Howzat and Barwon) | 2002 Chickpea (Howzat) 2001 Wheat (Kennedy) 2000–2001 Black gram (Regur) |
| 1998 Barley (Skiff) | 2000 Barley (Tallon) | 1999 Wheat (Hybrid Mercury) |
| 1997–1998 Sorghum (Buster MR) | 1998–1999 Maize (Zea mays C76) | 1998 Barley (Skiff) |
| 1996–1997 Sorghum (Buster MR) | 1997–1998 Sorghum (Buster MR) | 1997–1998 Sorghum (Buster MR) |
| 1995–1996 Sorghum (Buster MR) | 1996–1997 Sorghum (Buster MR) | 1996–1997 Sorghum (Buster MR) |
| Growth Rating | Description of Plants |
|---|---|
| 1 | stunted with marked leaf chlorosis and necrosis |
| 2 | severe lower leaf chlorosis, reduced tillering, and poor canopy closure |
| 3 | lower leaf chlorosis, reduced biomass, and ~50% canopy closure of inter-row space |
| 4 | green with some lower leaf chlorosis, and ~60% canopy closure of the inter-row space |
| 5 | green with ~80–90% leaf closure of the inter-row space |
| 6 | dark green with full canopy closure of the inter-row space. |
| Rainfall (mm) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment No. | Year | J | F | M | A | M | J | J | A | S | O | N | D | Total |
| Exps 1 and 2 | 2001 | 32 | 135 | 41 | 54 | 14 | 0 | 21 | 8 | 18 | 83 | 119 | 44 | 568 |
| Exp 3 | 2002 | 58 | 60 | 88 | 1 | 5 | 48 | 0 | 61 | 7 | 30 | 22 | 92 | 472 |
| Exp 4 | 2003 | 30 | 108 | 150 | 50 | 14 | 51 | 13 | 18 | 8 | 89 | 22 | 154 | 707 |
| Macalister BOM | 83 | 75 | 57 | 33 | 42 | 31 | 31 | 28 | 32 | 65 | 69 | 92 | 636 | |
| Soil Depth (cm) | Fumigation | NH4–N (mg/kg) | NO3–N (mg/kg) | a P (mg/kg | b Zn (mg/kg) | c pH |
|---|---|---|---|---|---|---|
| Pre-incubation 0–15 | nil | 1.83 (0.65) | 16.50 (0.78) | 17.81 (1.55) | 0.67 (0.06) | 8.07 (0.05) |
| fumigated | 2.90 (0.41) 1 | 16.80 (0.67) | 17.86 (1.33) | 0.80 (0.09) | 8.19 (0.05) | |
| 15–30 | nil | 1.21 (0.92) | 15.48 (0.52) | 4.86 (0.40) | 0.27 (0.04) | 8.44 (0.04) |
| fumigated | 2.80 (1.16) | 12.80 (0.31) | 3.90 (0.33) | 0.23 (0.03) | 8.61 (0.02) | |
| Post-incubation 0–15 | nil | 8.24 (0.04) | 5.70 (0.17) | 14.91 (1.02) | 0.76 (0.10) | 8.11 (0.06) |
| fumigated | 2.01 (0.02) | 10.56 (0.17) | 13.77 (0.93) | 0.52 (0.06) | 8.14 (0.06) |
| Soil Depth (cm) | Fumigation | NH4–N (mg/kg) | NO3–N (mg/kg) | a P (mg/kg | b Zn (mg/kg) | c pH |
|---|---|---|---|---|---|---|
| Pre-incubation 0–15 | nil | 1.28 (0.18) | 6.11 (0.97) | 24.48 (1.55) | 1.26 (0.25) | 7.61 (0.07) |
| fumigated | 4.11 (0.83) 1 | 3.58 (0.91) | 24.30 (1.33) | 1.12 (0.14) | 7.65 (0.07) | |
| 15–30 | nil | 1.01 (0.00) | 8.13 (0.90) | 6.48 (0.22) | 0.25 (0.05) | 8.43 (0.05) |
| fumigated | 2.03 (0.33) | 5.02 (0.92) | 3.90 (0.33) | 0.23 (0.03) | 8.47 (0.04) | |
| Post-incubation 0–15 | nil | 0 (0) | 20.5 (1.37) | 24.62 (1.55) | 1.39 (0.25) | 7.58 (0.07) |
| fumigated | 1.3 (0.8) | 21.2 (1.54) | 24.26 (1.38) | 1.39 (0.22) | 7.60 (0.07) |
| Growth Rating | Biomass | Whiteheads | |
|---|---|---|---|
| Biomass | 0.937 *** | ||
| Whiteheads | −0.051 n.s. | −0.031 n.s. | |
| Grain yield | 0.580 *** | 0.561 *** | −0.739 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, J.P.; Clewett, T.G. Investigating the Impact of Root-Lesion Nematodes (Pratylenchus thornei) and Crown Rot (Fusarium pseudograminearum) on Diverse Cereal Cultivars in a Conservation Farming System. Agronomy 2021, 11, 867. https://doi.org/10.3390/agronomy11050867
Thompson JP, Clewett TG. Investigating the Impact of Root-Lesion Nematodes (Pratylenchus thornei) and Crown Rot (Fusarium pseudograminearum) on Diverse Cereal Cultivars in a Conservation Farming System. Agronomy. 2021; 11(5):867. https://doi.org/10.3390/agronomy11050867
Chicago/Turabian StyleThompson, John P., and Timothy G. Clewett. 2021. "Investigating the Impact of Root-Lesion Nematodes (Pratylenchus thornei) and Crown Rot (Fusarium pseudograminearum) on Diverse Cereal Cultivars in a Conservation Farming System" Agronomy 11, no. 5: 867. https://doi.org/10.3390/agronomy11050867
APA StyleThompson, J. P., & Clewett, T. G. (2021). Investigating the Impact of Root-Lesion Nematodes (Pratylenchus thornei) and Crown Rot (Fusarium pseudograminearum) on Diverse Cereal Cultivars in a Conservation Farming System. Agronomy, 11(5), 867. https://doi.org/10.3390/agronomy11050867






