An Optimized Extraction Procedure for Determining Acaricide Residues in Foundation Sheets of Beeswax by Using Gas Chromatography-Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. Standards
2.3. Sample Procurement and Treatment
2.3.1. Samples
2.3.2. Sample Preparation
2.4. GC-MS Conditions
2.5. Method Validation
3. Results and Discussion
3.1. Sample Treatment
3.2. Method Validation
3.3. Application of the Method
3.3.1. Analysis of Foundation Wax
3.3.2. Field Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Der Zee, R.; Pisa, L.; Andonov, S.; Brodschneider, R.; Charriére, J.D.; Chlebo, R.; Coffey, M.F.; Crailsheim, K.; Dahle, B.; Gajda, A.; et al. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008–2009 and 2009–2010. J. Apic. Res. 2012, 51, 100–114. [Google Scholar] [CrossRef]
- Evison, S.E.F.; Roberts, K.E.; Laurenson, L.; Pietravalle, S.; Hui, J.; Biesmeijer, J.C.; Smith, J.E.; Budge, G.; Hughes, W.O.H. Pervasiveness of parasites in pollinators. PLoS ONE 2012, 7, 30641. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Martín-Hernández, R.; Días, J.; García-Palencia, P.; Matabuena, M.; Juarranz, A.; Barrios, L.; Meana, A.; Nanetti, A.; Higes, M. The effect of induced queen replacement on Nosema spp. infection in honey bee (Apis mellifera iberiensis) colonies. Environ. Microbiol. 2012, 14, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Agriculture and Rural Development-European Commission. Available online: http://ec.europa.eu/agriculture/honey.en (accessed on 16 April 2021).
- Ministerio de Agricultura, Pesca y Alimentación (Spain). Available online: https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/indicadoreseconomicossectordelamiel2019_tcm30-419675.pdf (accessed on 16 April 2021).
- Chauzat, M.P.; Cauquil, L.; Roy, L.; Franco, S.; Hendrikx, P.; Ribière, M. Demographics of the European apicultural industry. PLoS ONE 2013, 8, 79018. [Google Scholar] [CrossRef]
- Cepero, A.; Ravoet, J.; Gómez-Moracho, T.; Bernal, J.L.; Nozal, M.J.; Bartolomé, C.; Maside, X.; Meana, A.; González-Porto, A.V.; De Graaf, D.C.; et al. Holistic screening of collapsing honey bee colonies in Spain: A case study. BMC Res. Notes 2014, 7, 649–659. [Google Scholar] [CrossRef]
- Martin, S.J. Acaricide (pyrethroid) resistance in Varroa destructor. Bee World 2004, 85, 67–69. [Google Scholar] [CrossRef]
- Gracia, M.J.; Moreno, C.; Ferrer, M.; Sanz, A.; Peribáñez, M.A.; Estrada, R. Field efficacy of acaricides against Varroa destructor. PLoS ONE 2017, 12, 0171633. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; Van Engelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, 9754. [Google Scholar] [CrossRef]
- Berry, J. Pesticides, bees and wax: An unhealthy, untidy mix. Bee Cult. 2009, 137, 33–35. [Google Scholar]
- Fulton, C.A.; Huff, K.E.; Reeve, J.A.; Lydy, J. An examination of exposure routes of fluvalinate to larval and adult honey bees (Apis mellifera). Environ. Toxicol. Chem. 2019, 38, 1356–1363. [Google Scholar] [CrossRef]
- Agrebi, N.; Willmart, O.; Urbain, B.; Danneels, E.L.; Graaf, D.C.; Saegerman, C. Belgian case study on flumethrin residues in beeswax: Possible impact on honeybee and prediction of the maximum daily intake for consumers. Sci. Total Environ. 2019, 687, 712–719. [Google Scholar] [CrossRef]
- Chauzat, M.P.; Faucon, J.P. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest Manag. Sci. 2007, 63, 1100–1106. [Google Scholar] [CrossRef]
- Ravoet, J.; Reybroeck, W.; de Graaf, D.C. Pesticides for apicultural and/or agricultural application found in Belgian honey bee wax combs. Bull. Environ. Contam. Toxicol. 2015, 94, 543–548. [Google Scholar] [CrossRef]
- Wilmart, O.; Legrève, A.; Scippo, M.-L.; Reybroeck, W.; Urbain, B.; de Graaf, D.C.; Steurbaut, W.; Delahaut, P.; Gustin, P.; Nguyen, B.K.; et al. Residues in beeswax: A health risk for the consumer of honey and beeswax? J. Agric. Food Chem. 2016, 64, 8425–8434. [Google Scholar] [CrossRef]
- Boi, M.; Serra, G.; Colombo, R.; Lodesani, M.; Massi, S.; Costa, C. A 10 year survey of acaricide residues in beeswax analysed in Italy. Pest Manag. Sci. 2016, 72, 1366–1372. [Google Scholar] [CrossRef]
- Bogdanov, S.; Kilchenmann, V.; Imdorf, A. Acaricide residues in beeswax and honey. In Bee Products, Properties, Applications, Apitherapy; Mizrahi, A., Lensky, Y., Eds.; Plenum Press: New York, NY, USA, 1997; pp. 239–246. [Google Scholar]
- Roszko, M.L.; Kaminska, M.; Szymczyk, K.; Jedrzejczak, R. Levels of selected persistent organic pollutants (PCB, PBDE) and pesticides in honey bee pollen sampled in Poland. PLoS ONE 2016, 11, 0167487. [Google Scholar] [CrossRef]
- Bommuraj, V.; Chen, Y.; Klein, H.; Sperling, R.; Barel, S.; Shimshoni, J.A. Pesticide and trace elements residues in honey and beeswax combs from Israel in association with human risk assessment and honey adulteration. Food Chem. 2019, 299, 125123. [Google Scholar] [CrossRef]
- Perugini, M.; Tulini, S.M.R.; Zezza, D.; Fenucci, S.; Conte, A.; Amorena, M. Occurrence of agrochemical residues in beeswax samples collected in Italy during 2013–2015. Sci. Total Environ. 2018, 625, 470–476. [Google Scholar] [CrossRef]
- Bajuk, B.P.; Babnik, K.; Snoj, T.; Milčinski, L.; Ocepek, M.P.; Škof, M.; Jenčič, V.; Filazi, A.; Štajnbaher, S. Coumaphos residues in honey, bee brood, and beeswax after Varroa treatment. Apidologie 2017, 48, 588–598. [Google Scholar] [CrossRef]
- Tsigouri, A.; Menkissoglu, U.; Thrasyvoulou, A.; Diamantidis, G. Fluvalinate residues in Greek honey and beeswax. Apiacta 2003, 38, 50–53. [Google Scholar]
- Jimenez, J.J.; Bernal, J.L.; Nozal, M.J.; Martín, M.T. Residues of organic contaminants in beeswax. Eur. J. Lipid Sci. Technol. 2005, 107, 896–902. [Google Scholar] [CrossRef]
- Adamczyk, S.; Lazaro, R.; Perez, C.; Bayarri, S.; Herrera, A. Impact of the use of fluvalinate on different types of beeswax from Spanish hives. Arch. Environ. Contam. Toxicol. 2010, 58, 733–739. [Google Scholar] [CrossRef]
- Serra, J.; Orantes, J. Acaricides and their residues in Spanish commercial beeswax. Pest. Manag. Sci. 2010, 66, 1230–1235. [Google Scholar] [CrossRef]
- Calatayud, P.; Calatayud, F.; Simó, E.; Pico, Y. Occurrence of pesticide residues in Spanish beeswax. Sci. Total. Environ. 2017, 605–606, 745–754. [Google Scholar] [CrossRef]
- Lozano, A.; Hernando, M.D.; Uclés, S.; Hakme, E.; Fernandez, A.R. Identification and measurement of veterinary drug residues in beehive products. Food Chem. 2019, 274, 61–70. [Google Scholar] [CrossRef]
- Morales, M.M.; Gomez, M.J.; Parrilla, P.; Diaz, F.J.; García, M.; Gámiz, V.; Flores, J.M.; Fernández, A.R. Distribution of chemical residues in the beehive compartments and their transfer to the honeybee brood. Sci. Total Environ. 2020, 710, 136288. [Google Scholar] [CrossRef]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 2014, 9, 77547. [Google Scholar] [CrossRef]
- Svečnjak, L.; Chesson, A.C.; Gallina, A.; Maia, M.; Martinello, M.; Mutinelli, F.; Muz, M.N.; Nunes, F.; Saucy, F.; Tipple, B.J.; et al. Standard methods for Apis mellifera beeswax research. J. Apic. Res. 2019, 58, 1–108. [Google Scholar] [CrossRef]
- Bonzini, S.; Tremolada, P.; Bernardinelli, I.; Colombo, M.; Vighi, M. Predicting pesticide fate in the hive (Part 1) experimentally determined tau-fluvalinate residues in bees, honey and wax. Apidologie 2011, 41, 378–390. [Google Scholar] [CrossRef]
- Bogdanov, S.; Kilchenmann, V.; Butikofer, U. Determination of acaricide residues in beeswax: Collaborative study. Apiacta 2003, 38, 235–245. [Google Scholar]
- Chauzat, M.P.; Faucon, J.P.; Martel, A.C.; Lachaize, J.; Gougoule, N.; Aubert, M. A survey of pesticide residues in pollen loads collected by honey bees in France. J. Econ. Entomol. 2006, 99, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kelley, R.A.; Anderson, T.D.; Lydy, M.J. Development and comparison of two multi-residue methods for the analysis of selected pesticides in honey bees, pollen and wax by gas chromatography quadrupole mass spectrometry. Talanta 2015, 40, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Alkassab, A.T.; Thorbain, D.; Frommberger, M.; Bischoff, G.; Pistorius, J. Effect of contamination and adulteration of wax foundations on the brood development of honeybees. Apidologie 2020, 51, 642–651. [Google Scholar] [CrossRef]
- Frison, S.; Breitkreitz, W.; Currie, R.; Nelson, D.; Sporns, P. The analysis of fluvalinate in beeswax using GC/MS. Food Res. Int. 1999, 32, 35–41. [Google Scholar] [CrossRef]
- Gil, M.D.; Uclés, S.; Lozano, A.B.; Sosa, A.; Fernández, A.R. Muliresidue method for trace pesticide analysis in honeybee wax comb by GC-QqQ-MS. Talanta 2017, 163, 54–64. [Google Scholar]
- Shimshoni, J.A.; Sperling, R.; Massarwa, M.; Chen, Y.; Bommuraj, V.; Borisover, M.; Barel, S. Pesticide distribution and depletion kinetic determination in honey and beeswax: Model for pesticide occurrence and distribution in beehive products. PLoS ONE 2019, 14, 0212631. [Google Scholar] [CrossRef]
- Ghini, S.; Fernández, M.; Pico, Y.; Marín, R.; Fini, F.; Mañes, J.; Girotti, S. Occurrence and distribution of pesticides in the province of Bologna, Italy, using honeybees as bioindicators. Arch. Environ. Contam. Toxicol. 2004, 47, 479–488. [Google Scholar] [CrossRef]
- Rossi, S.; Dalpero, A.; Ghini, S.; Colombo, R.; Sabatini, A.; Girotti, S. Multiresidual method for the gas chromatographic analysis of pesticides in honeybees cleaned by gel permeation chromatography. J. Chromatogr. A 2001, 905, 223–232. [Google Scholar] [CrossRef]
- Łozowicka, B. The development, validation and application of a GC-dual detector (NPD-ECD) multi-pesticide residue method for monitoring bee poisoning incidents. Ecotoxicol. Environ. Safety 2013, 97, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Niell, S.; Jesus, F.; Perez, C.; Mendoza, Y.; Diaz, R.; Franco, J.; Cesio, V.; Heinzen, H. QuEChERS adaptability for the analysis of pesticide residues in beehive products seeking the development of an agroecosystem sustainability monitor. J. Agric. Food Chem. 2015, 63, 4484–4492. [Google Scholar] [CrossRef]
- Lambert, O.; Piroux, M.; Puyo, S.; Thorin, C.; L’Hostis, M.; Wiest, L.; Buleté, A.; Delbac, F.; Pouliquen, H. Widespread occurrence of chemical residues in beehive matrices from apiaries located in different landscapes of western France. PLoS ONE 2013, 8, 67007. [Google Scholar] [CrossRef]
- Serra, J.; Orantes, F.J. Discoloration and adsorption of acaricides from beeswax. J. Food Process Eng. 2017, 40, 1–10. [Google Scholar]
- Navarro, M.D.; Orantes, F.J.; Sánchez, C.; Varela, A.; Giampieri, F.; Torres, C.; Serra, J.; Forbes, T.Y.; Reboredo, P.; Llopis, J.; et al. Industrial-scale decontamination procedure effects on the content of acaricides, heavy metals and antioxidant capacity of beeswax. Molecules 2019, 24, 1518. [Google Scholar]
- European Commission Directorate-General for Health and Food Safety, Document SANTE/12682/2019. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 16 April 2021).
- Lehotay, S.J. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef]
- Valverde, S.; Bernal, J.L.; Martín, M.T.; Nozal, M.J.; Bernal, J. Fast determination of neonicotinoid insecticides in bee pollen using QuEChERS and ultra-high performance liquid chromatography coupled to quadrupole mass spectrometry. Electrophoresis 2016, 37, 2470–2477. [Google Scholar] [CrossRef]
- Li, H.; Yin, J.; Liu, Y.; Shang, J. Effect of protein on the detection of fluoroquinolone residues in fish meat. J. Agric. Food Chem. 2012, 60, 1722–1727. [Google Scholar] [CrossRef]
- Valverde, S.; Ares, A.M.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Fast determination of neonicotinoid insecticides in beeswax by ultra-high performance liquid chromatography-tandem mass spectrometry using an enhanced matrix removal-lipid sorbent for clean-up. Microchem. J. 2018, 142, 70–77. [Google Scholar] [CrossRef]
| Compound | Analytical Range (µg/kg) | Slope | R2 | LOD (µg/kg) | LOQ (µg/kg) |
|---|---|---|---|---|---|
| Atrazine | 5–5000 | 38.54 | 0.9990 | 2 | 5 |
| Chlorpyrifos | 5–5000 | 17.95 | 0.9983 | 2 | 5 |
| Chlorfenvinphos | 5–5000 | 28.90 | 0.9991 | 2 | 5 |
| Alpha-Endosulfan | 10–5000 | 4.42 | 0.9981 | 3 | 10 |
| Bromopropylate | 10–5000 | 3.05 | 0.9984 | 3 | 10 |
| Coumaphos | 5–5000 | 22.16 | 0.9982 | 2 | 5 |
| Tau-Fluvalinate | 5–5000 | 52.66 | 0.9982 | 2 | 5 |
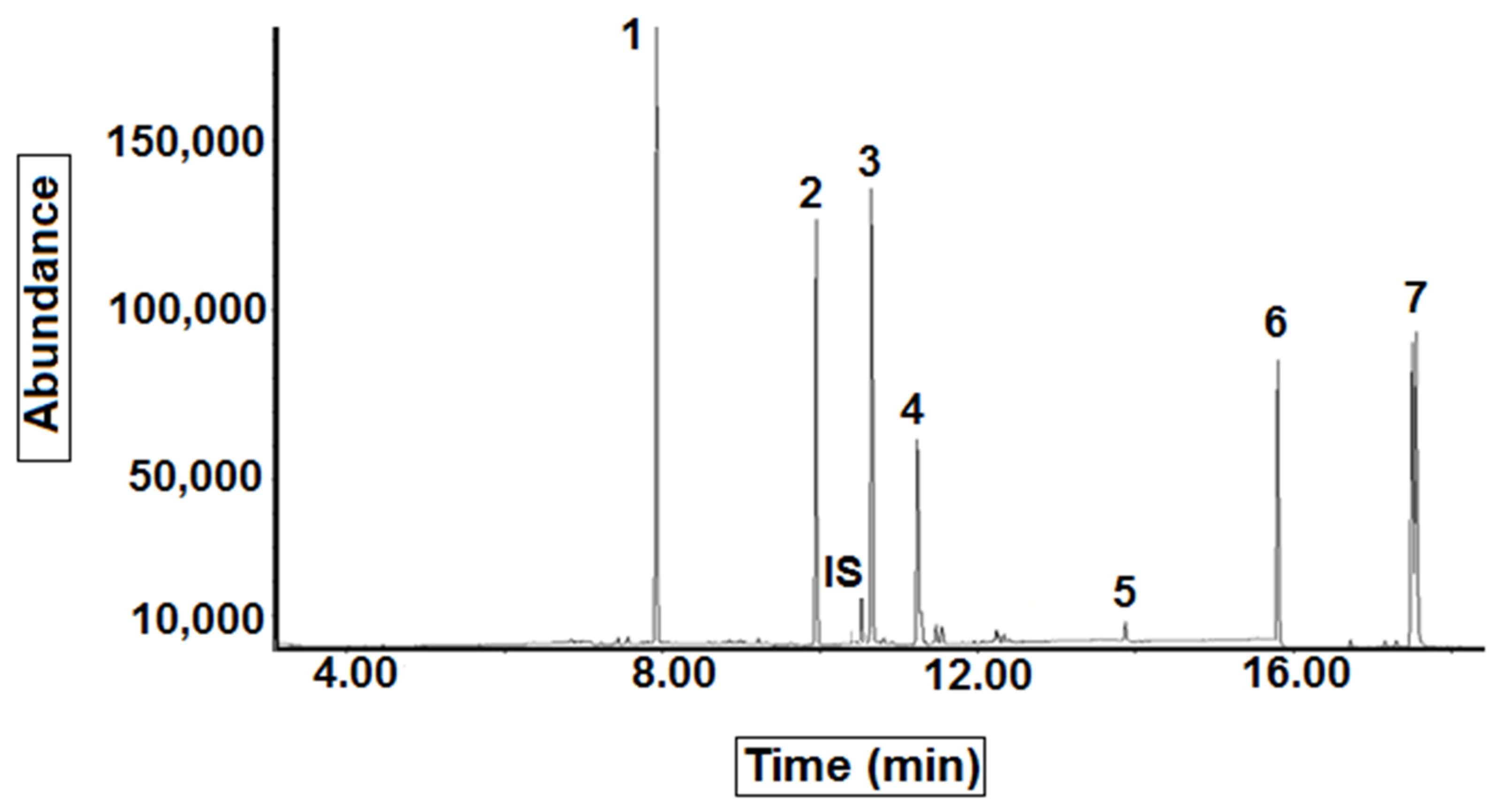
| Compound | Retention Time (min) | Target Ions (m/z) | Qualifier Ions (m/z) |
|---|---|---|---|
| Atrazine | 7.81 | 200 | 215, 173 |
| Chlorpyrifos | 9.72 | 197 | 315, 258 |
| Chlorfenvinphos | 10.48 | 267 | 329, 270 |
| Alpha-Endosulfan | 11.20 | 241 | 195, 207 |
| Bromopropylate | 13.82 | 345 | 185, 183 |
| Coumaphos | 15.62 | 362 | 226, 109 |
| Tau-Fluvalinate | 17.39 | 250 | 207, 181 |
| Chlorfenvinphos-d10 | 10.46 | 333 | - |
| Compound | Acetonitrile:Water (80:20, v/v) | Acetonitrile:Water (70:30, v/v) | Acetonitrile | 1% Acetic Acid in Acetonitrile |
|---|---|---|---|---|
| Atrazine | 79 ± 7 | 73 ± 7 | 86 ± 8 | 93 ± 7 |
| Chlorpyrifos | 78 ± 8 | 74 ± 8 | 85 ± 8 | 92 ± 8 |
| Chlorfenvinphos | 77 ± 7 | 73 ± 6 | 84 ± 7 | 94 ± 6 |
| Alpha-Endosulfan | 83 ± 5 | 76 ± 6 | 90 ± 4 | 95 ± 4 |
| Bromopropylate | 76 ± 7 | 66 ± 8 | 81 ± 8 | 91 ± 8 |
| Coumaphos | 82 ± 5 | 75 ± 4 | 91 ± 4 | 97 ± 3 |
| Tau-Fluvalinate | 80 ± 4 | 72 ± 5 | 82 ± 4 | 90 ± 4 |
| Compound | Evaluation of the Sample Treatment | Evaluation of the Matrix Effect | ||||
|---|---|---|---|---|---|---|
| Mean (%) ± RSD (%) | Mean (%) ± RSD (%) | |||||
| Low QC | Medium QC | High QC | Low QC | Medium QC | High QC | |
| Atrazine | 102 ± 3 | 106 ± 3 | 94 ± 3 | 100 ± 4 | 104 ± 2 | 99 ± 4 |
| Chlorpyrifos | 100 ± 3 | 103 ± 3 | 92 ± 4 | 94 ± 4 | 97 ± 4 | 99 ± 4 |
| Chlorfenvinphos | 104 ± 3 | 107 ± 4 | 105 ± 3 | 102 ± 4 | 105 ± 5 | 100 ± 3 |
| Alpha-Endosulfan | 93 ± 2 | 102 ± 3 | 90 ± 3 | 93 ± 4 | 102 ± 3 | 99 ± 4 |
| Bromopropylate | 101 ± 3 | 106 ± 2 | 93 ± 3 | 103 ± 3 | 106 ± 4 | 96 ± 4 |
| Coumaphos | 108 ± 2 | 105 ± 5 | 107 ± 5 | 131 ± 5 | 140 ± 5 | 128 ± 5 |
| Tau-Fluvalinate | 101 ± 4 | 103 ± 4 | 107 ± 4 | 122 ± 5 | 132 ± 4 | 127 ± 5 |
| Beeswax Origin | Chlorpyrifos | Chlorfenvinphos | Coumaphos | Tau-Fluvalinate |
|---|---|---|---|---|
| Organic white beeswax pellets (USA) | <LOD | <LOD | <LOD | <LOD |
| Raw beeswax (Álava, Spain) | <LOD | <LOD | 2350 | 207 |
| PBS (Guadalajara, Spain) | 101 | 162 | 433 | 43 |
| PBS from Perfection hive (Palencia, Spain) | <LOD | 75 | 983 | 173 |
| PBS from Dadant hive (Salamanca, Spain) | <LOD | 83 | 2217 | 413 |
| PBS from Layens hive (Salamanca, Spain) | <LOD | 105 | 1743 | 569 |
| DBS from Layens hive (León, Spain) | <LOD | 30 | 279 | 258 |
| LBS (Zamora, Spain) | <LOD | 113 | 1339 | 407 |
| LBS (Álava, Spain) | 37 | <LOD | 527 | 182 |
| LBS (Asturias, Spain) | 85 | 23 | <LOD | 179 |
| LBS (Salamanca, Spain) | 274 | 93 | 1513 | 284 |
| LBS (France) | <LOD | <LOD | <LOD | 451 |
| LBS (Córdoba, Spain) | <LOD | <LOD | <LOD | 485 |
| LBS (Guadalajara, Spain) | <LOD | 135 | 1764 | <LOD |
| DBS (Germany) | 52 | <LOD | <LOD | <LOD |
| DBS (France) | <LOD | <LOD | <LOD | 351 |
| DBS (Portugal) | <LOD | <LOD | <LOD | 210 |
| DBS (Spain) | <LOD | <LOD | <LOD | 326 |
| Apiary | Sample | Chlorpyrifos | Chlorfenvinphos | Coumaphos | Tau-Fluvalinate |
|---|---|---|---|---|---|
| VA1 | Foundation | 140 | 119 | 967 | 191 |
| Collected Beeswax | 94 | 96 | 148 | 153 | |
| VA2 | Foundation | 169 | 118 | 1399 | 386 |
| Collected Beeswax | 110 | 103 | 263 | 218 | |
| VA3 | Foundation | 94 | 95 | 139 | 180 |
| Collected Beeswax | 93 | 95 | 120 | 139 | |
| PA1 | Foundation | 114 | 111 | 910 | 765 |
| Collected Beeswax | 93 | 95 | 123 | 142 | |
| PA2 | Foundation | <LOD | 199 | 1701 | 265 |
| Collected Beeswax | <LOD | 87 | 332 | 134 | |
| GU1 | Foundation | <LOD | <LOD | <LOD | 326 |
| Collected Beeswax | <LOD | <LOD | <LOD | 147 | |
| GU2 | Foundation | 68 | 162 | 2183 | 221 |
| Collected Beeswax | 52 | 97 | 402 | 121 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozal, M.J.; Imaz, E.; Bernal, J.L.; Nieto, J.L.; Higes, M.; Bernal, J. An Optimized Extraction Procedure for Determining Acaricide Residues in Foundation Sheets of Beeswax by Using Gas Chromatography-Mass Spectrometry. Agronomy 2021, 11, 804. https://doi.org/10.3390/agronomy11040804
Nozal MJ, Imaz E, Bernal JL, Nieto JL, Higes M, Bernal J. An Optimized Extraction Procedure for Determining Acaricide Residues in Foundation Sheets of Beeswax by Using Gas Chromatography-Mass Spectrometry. Agronomy. 2021; 11(4):804. https://doi.org/10.3390/agronomy11040804
Chicago/Turabian StyleNozal, María Jesús, Edgar Imaz, José Luis Bernal, José Luis Nieto, Mariano Higes, and José Bernal. 2021. "An Optimized Extraction Procedure for Determining Acaricide Residues in Foundation Sheets of Beeswax by Using Gas Chromatography-Mass Spectrometry" Agronomy 11, no. 4: 804. https://doi.org/10.3390/agronomy11040804
APA StyleNozal, M. J., Imaz, E., Bernal, J. L., Nieto, J. L., Higes, M., & Bernal, J. (2021). An Optimized Extraction Procedure for Determining Acaricide Residues in Foundation Sheets of Beeswax by Using Gas Chromatography-Mass Spectrometry. Agronomy, 11(4), 804. https://doi.org/10.3390/agronomy11040804








