1. Introduction
Dracaena marginata Lam. var. Tricolor, commonly known as “red-edged dracaena” is well-valued as ornamental foliage plant [
1]. It is an evergreen shrub native to Madagascar belonging to the
Agavaceae family. The common name of this species is ascribed to the presence of multiple stems topped by a rosette of narrow ribbon-like green leaves edged in purplish-red [
2].
Nowadays, the production of containerized ornamental plants is facing increasing water and fertilizer costs, with higher efficiency and retention levels of water and nutrient use under constant scrutinization [
3]. Nevertheless, the water and nutrient management practices for containerized plants are rather difficult, mainly due to the characteristics of the substrate including low water and nutrient holding capacity, prompting the leaching of nutrients and the pollution of the environment [
4].
The reuse of drainage water for fertigation can reduce the water requirements and the overall problems of water pollution. Recycling of leaching in the same crop is the most common process to reuse runoff. This system allows the easy management of leachate, however the reuse of drainage on the same crop leads to an increase in the salinity level of the substrate solution, reducing the yield. To avoid this problem, it is necessary to make a periodic discharge which results in environmental pollution [
5]. One strategy for the sequential reuse of the drainage is the implementation of a serial biological concentration (SBC) or cascade cropping system. This system is used to grow increasingly salt-tolerant crops, based on the collection of drainage water from a first crop which is then used for the fertigation of another more salt-tolerant crop in the series, with the main aim of reducing almost entirely the drainage volume from the cascade cropping system [
6].
The implementation of this cropping system increases pathogen dispersion through the irrigation system [
7]. Under these conditions, the application of disinfectants in the leachate, such as hydrogen peroxide (H
2O
2), may result in a drastic reduction of pathogen dispersion [
8]. Besides this disinfectant power, the addition of H
2O
2 to the irrigation water can improve the level of oxygenation in the root zone and the consequent enhancement of the growth of the crop [
9,
10].
In reviewing the previous literature, we found several references regarding the enhancement of growth through the addition of H
2O
2 in the irrigation water in crops such as wheat [
11], zucchini, soybean, and cotton [
12], as well as in ornamental plants such as
Calibrachoa ×
hybrida and
Lobelia erinus [
13]. Nevertheless, there is scarce information about the application of H
2O
2 in an ornamental cascade cropping system and the consequent effects on crops. Therefore, in the present work, the leachates from an ornamental cascade cropping system under greenhouse conditions, including
Chrysalidocarpus lutescens and
Dracaena deremensis plants, were used for the fertigation of
Dracaena marginata potted plants. In this experiment, we aimed to test both the effect of the addition of H
2O
2 and the effect without this addition in the leachates reused, on biomass, pigment concentration, biochemical parameters, mineral nutrition, and water and nutrient use efficiencies in
D. marginata plants.
4. Discussion
The chemical composition of the leachates with or without H
2O
2 was higher in pH and EC with respect to the control treatment. The pH increase can be associated with the release of OH
−, as has been reported in similar systems [
23]. On the other hand, the fertigation with leachates with or without H
2O
2 resulted in an increase in EC mainly due to the accumulation of toxic ions such as Na
+ and Cl
− [
24,
25]. The reuse of the leachate from another crop and the nutrients released by this crop could be the reason for the high concentration of several nutrients in the leachate, as reported by Massa et al. [
26].
Our results reported that the
Dracaena marginata plants fertigated with leachates with H
2O
2 had an increased plant height. Similar results were reported by Almeida-Veloso et al. [
27] in soursop (
Annona muricata) plants fertigated with additional H
2O
2. Better oxygenation, associated with the addition of H
2O
2 to the leachate, may have enhanced root respiration and consequently increased the plant growth as reported Pendergast et al. [
28].
No variations in the root length between fertigation treatments can be associated to the availability of water and nutrients, which in some cases was excessive during the experimental period, suggesting that the plants did not need to vary their root length in search of water and nutrients. Also, the short duration of the experimental period can be considered relevant for the data obtained in our work.
The fertigation with leachates with or without H2O2 only increased the red index color. Although, the information about the effects of fertigation with leachates with or without H2O2 on the aesthetic values of ornamental plants are null, it is necessary to point out that the increase in red index color was highly valued in this species by local nursery growers resulting in more profitable sales.
The results of this experiment reported that the fertigation with raw leachates in
D. marginata resulted in a reduction in root and total dry weight compared to the fertigation with a standard nutrient solution, but the addition of H
2O
2 to the raw leachate enhanced root, shoot, and total dry weight. The reduction in root and total dry weight in our experiment is in agreement with the results obtained by several researchers studying ornamental plants grown under saline conditions [
29,
30,
31], and this decline in biomass can be associated with metabolic disorders in plants as a consequence of the osmotic and toxic effects caused by saline conditions [
32,
33]. On the other hand, the enhancement of the dry weight as a consequence of the addition of H
2O
2 to the leachate was in line with the findings reported in similar experiments. For instance, Hameed et al. [
11] noted an increase in the root dry weight of wheat plants with the addition of H
2O
2. Analogously, Bhattarai et al. [
12] reported an increase in the plant dry weight of soybean and cotton plants subjected to the application of H
2O
2.
Fertigation with raw leachates and additional H
2O
2 resulted in an increase in chlorophyll a and a decrease in chlorophyll b concentration, without changes in total chlorophyll concentration. Different results have been reported by other researchers who found an enhancement of pigment concentrations associated with the addition of H
2O
2 in several crops such as corn [
34], cotton, and soybean [
12] agree with our chlorophyll a results. However, there seems to be a compensatory effect occurring between both chlorophylls, without modification of the total amount of both.
Dracaena marginata plants fertigated with raw leachates had increased root proline concentration compared to the control treatment, with this concentration being even higher if the raw leachate had additional H
2O
2. Different results have been reported by other researchers who noted that the exogenous application of H
2O
2 resulted in an increase in proline content in cucumber plants [
35]. Nevertheless, leaf proline showed the highest value in plants fertigated with raw leachates without additional H
2O
2. The increase in proline concentration in both organs may be associated with its role as an osmoprotectant under stressed conditions, such as the ones occurring when higher salinity levels were present in the leachate [
36,
37,
38]. Regarding the concentration of total soluble sugars, the addition of H
2O
2 to the raw leachates decreased the root concentration. In the case of the concentration of leaf total soluble sugars, the fertigation with raw leachates showed the highest value. The decrease in the total soluble sugars in the root concentration can be associated to the consequent increase of proline since both may act as an osmoprotectant in plants, in addition to the fact that under better oxygenation conditions respiration rates may be higher consequently increasing the consumption of sugars at the root level.
As far as nutrient concentrations were concerned, it is necessary to highlight that the results obtained in our experiment revealed that the addition of H
2O
2 to the leachate resulted in an enhancement in N concentration, whereas in the case of P and K, there were no clear trends among the fertigation treatments. Ben-Noah and Friedman [
8] also noted a rise in N concentration in pepper plants fertigated with additional H
2O
2. No clear trends in P and K concentration in the different fertigation treatments assessed may be due to the antagonisms between Cl
− and H
2PO
4− and K
+/Na
+ that occur under saline conditions [
39]. The leaf N concentration obtained in our experiment in the plants fertigated with the standard nutrient solution and leachates without H
2O
2 were in the range (23 to 50 mg g
−1 DW) reported by Mills and Jones [
40], whereas plants fertigated with leachates with H
2O
2 had a higher value (58.59 mg g
−1 DW). Regarding leaf P concentration, only plants fertigated with leachates with H
2O
2 (3.43 mg g
−1 DW) were in the range reported by Mills and Jones [
40] (1.8 to 6 mg g
−1 DW), whereas the other fertigation treatments showed higher values. With respect to K, all the fertigation treatments were in the range proposed by Mills and Jones [
40] (25 to 45 mg g
−1 DW) except for the plants fertigated with leachates with H
2O
2 which showed higher values (51.89 mg g
−1 DW).
The decrease in water and nutrient use efficiencies in the plants fertigated with leachates compared to the fertigation with the standard nutrient solution may be related to the fact that under higher saline concentrations, as occurs in the leachate, the ability of crops to uptake water and nutrient is reduced mainly due to the osmotic and toxic effects associated with the reduction of growth under saline conditions [
41,
42]. Nevertheless, the addition of H
2O
2 to the leachate resulted in an enhancement of water and nutrient use efficiencies, agreeing with the results reported by Du et al. [
43] who noted that under better oxygenation conditions due to the addition of H
2O
2, there was a marked increase in crop water and fertilizer uptake.
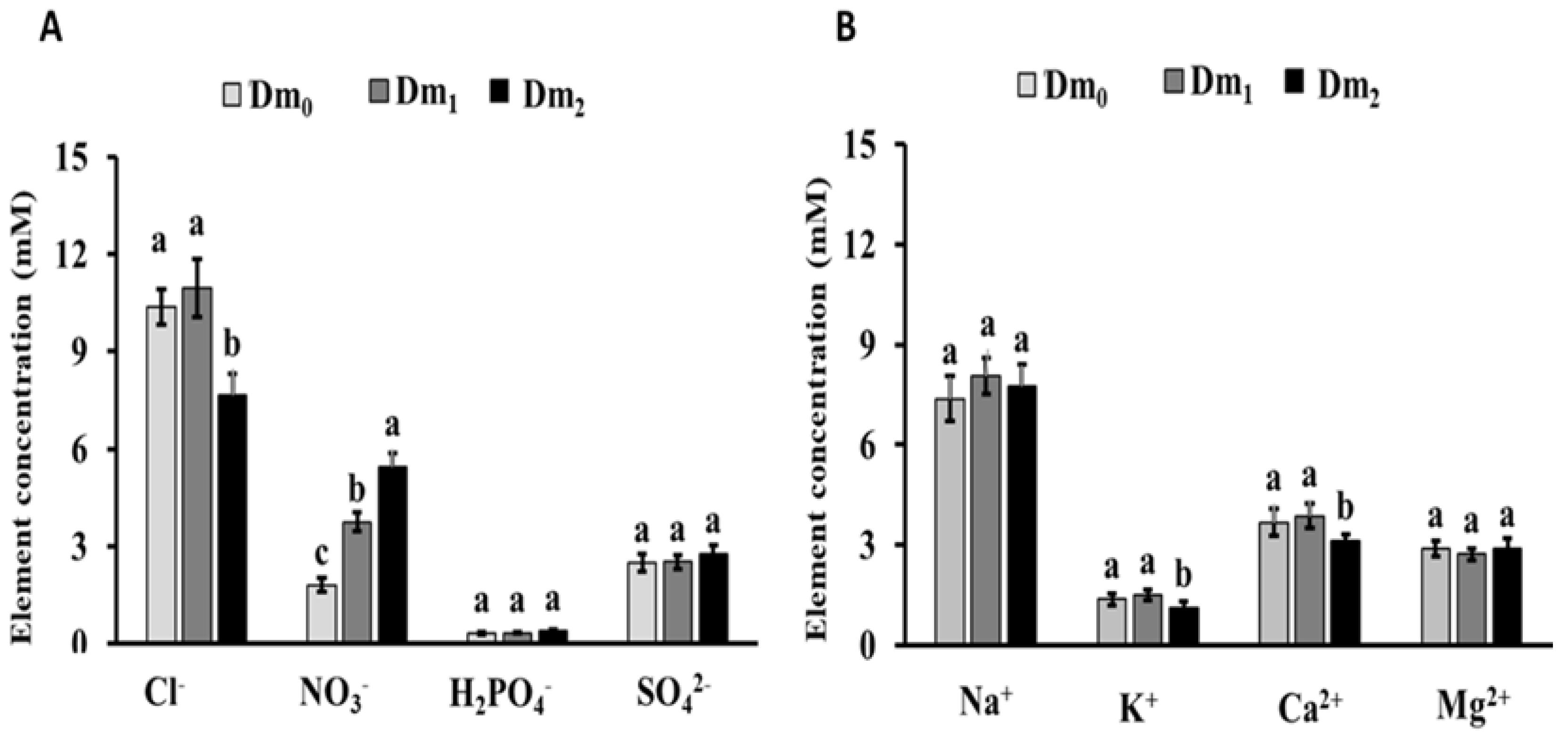
The chemical analysis of the substrate revealed that the fertigation with leachates with H
2O
2 resulted in a significant decline in Cl
−, K
+, and Ca
2+. On the other hand, the fertigation with raw leachates increased the concentration of NO
3− in the substrate. The decline of these nutrients in the substrate may be due to the possible modifications in the cation exchange capacity of the substrate as a consequence of the interaction between organic matter, H
2O
2, and reactive intermediates, as reported by Ben-Noah and Friedman [
8]. The decline in Cl
− and the increase in NO
3− in the substrate can be ascribed to the fact that under saline conditions some species tends to uptake Cl
− for the maintenance of the osmotic adjustment and consequently reduce their uptake of NO
3− due to antagonisms among these nutrients [
44].