Phosphorus and Carbohydrate Metabolism in Green Bean Plants Subjected to Increasing Phosphorus Concentration in the Nutrient Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Analysis of Carbohydrates Metabolism
2.3. Analysis of P Forms
2.4. Statistical Analysis
3. Results
3.1. Plant Parameters
3.2. Carbohydrates Metabolism
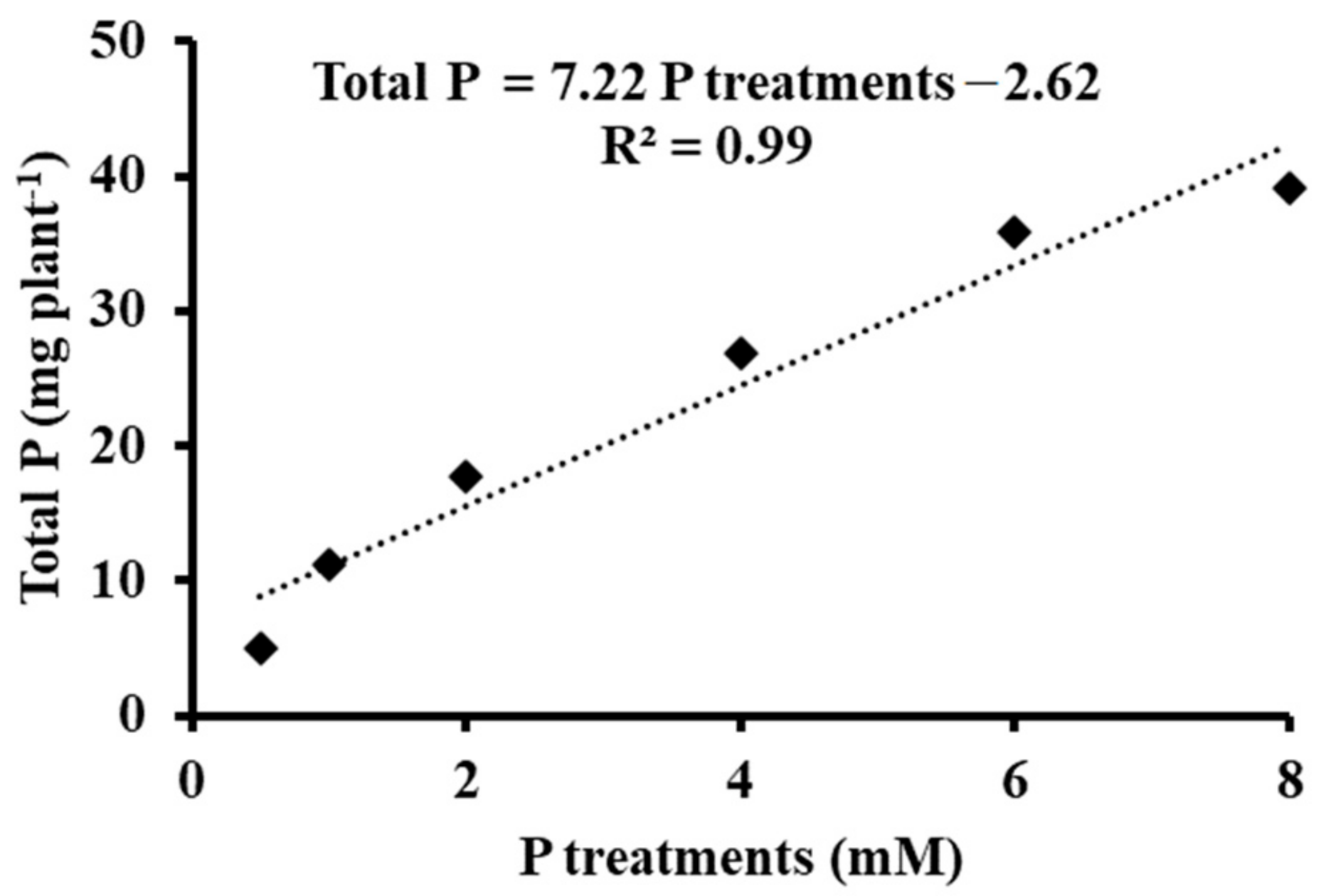
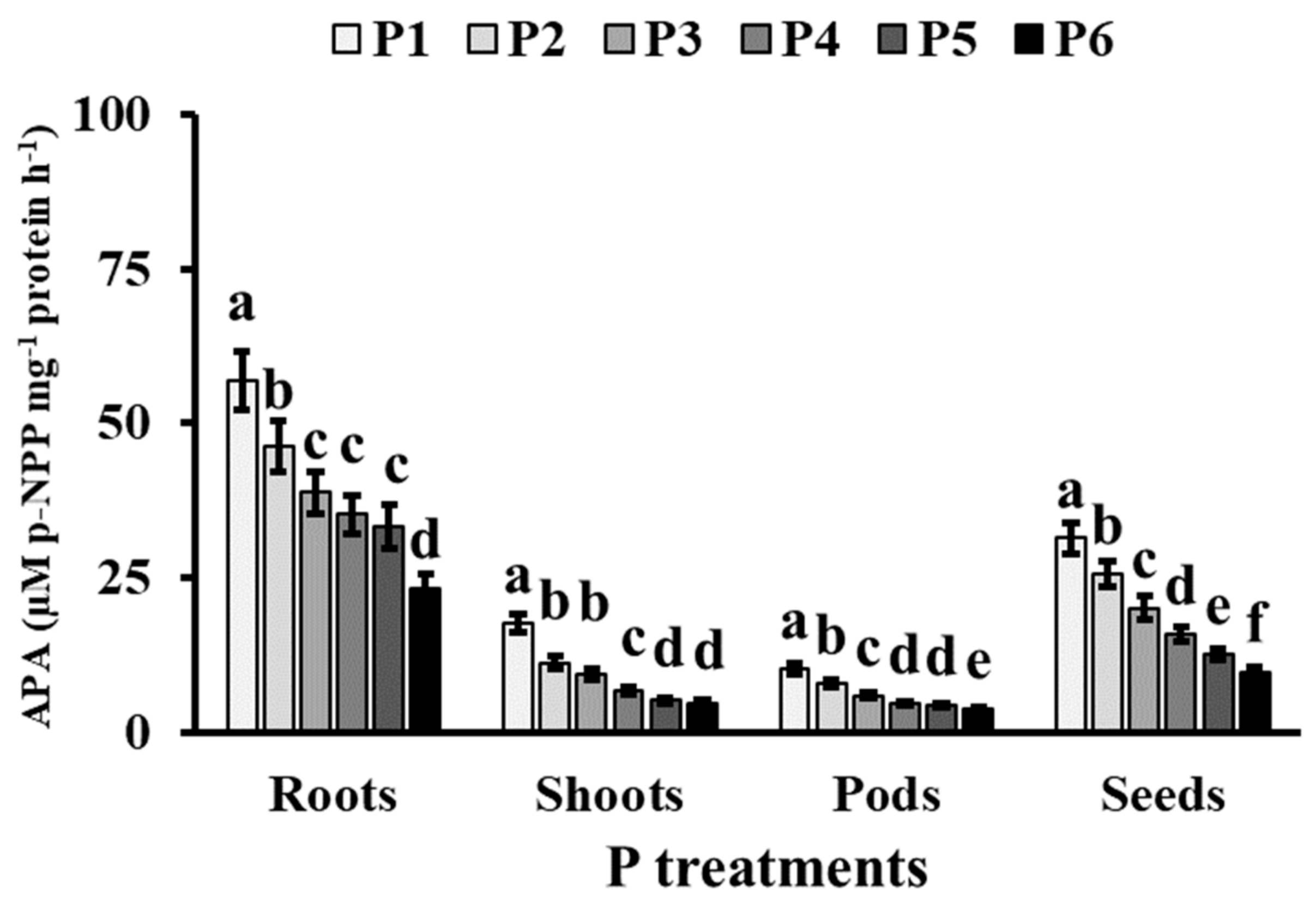
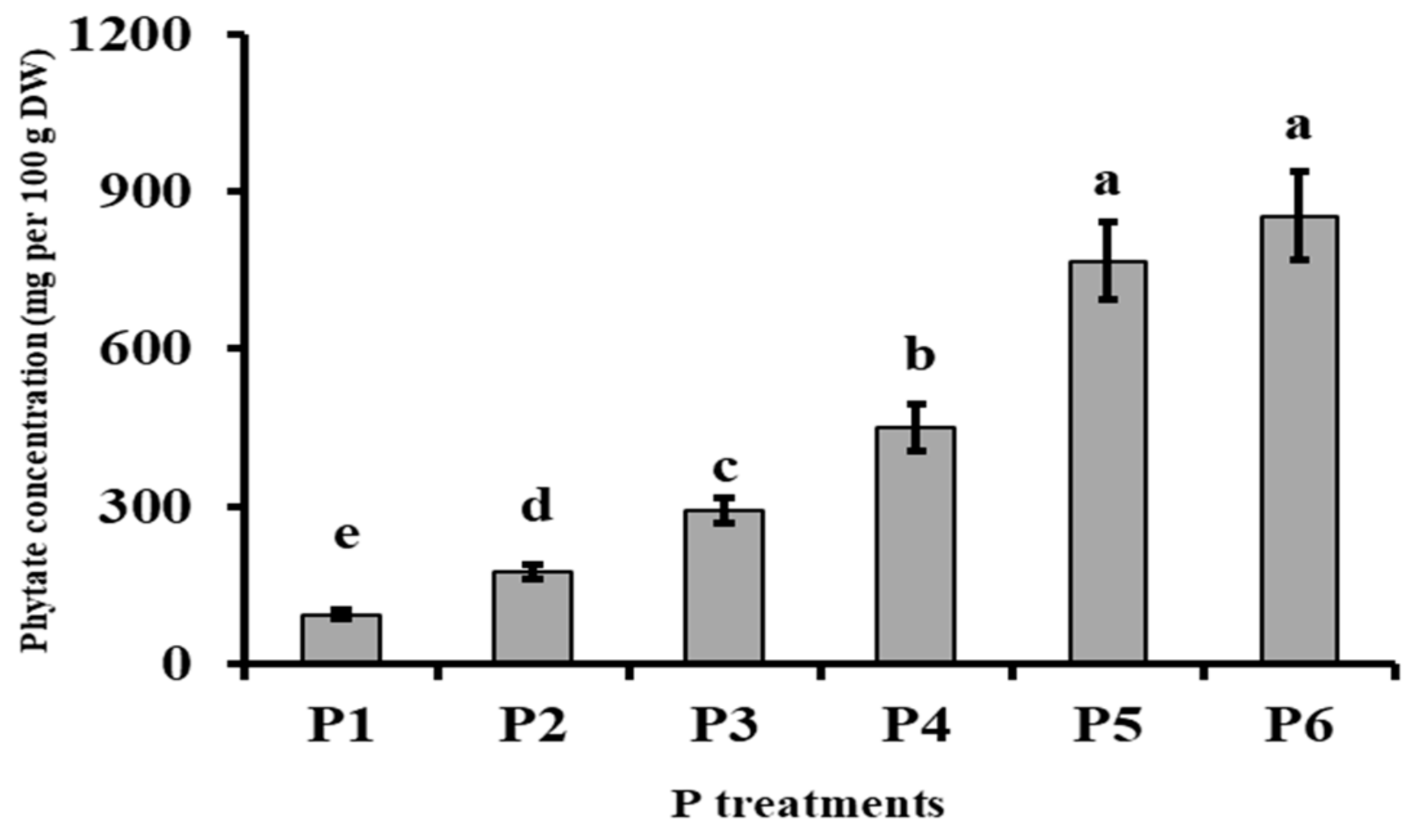
3.3. Phosphorus Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zafar, M.; Abbasi, M.K.; Rahim, N.; Khaliq, A.; Shaheen, A.; Jamil, M.; Shahid, M. Influence of integrated phosphorus supply and plant growth promoting rhizobacteria on growth, nodulation, yield and nutrient uptake in Phaseolus vulgaris. Afr. J. Biotechol. 2011, 10, 16781–16792. [Google Scholar]
- Myers, J.R.; Kmiecik, K. Common bean: Economic importance and relevance to biological science research. In The Common Bean Genome; Springer: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). FAO Statistics. 2018. Available online: http://www.fao.org/faostat/en/#home (accessed on 30 November 2020).
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Phosphorus forms and P-sorption properties in three alkaline soils after long-term mineral and manure applications in north-eastern Italy. Agric. Ecosyst. Environ. 2011, 141, 58–66. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Relationship between soil test phosphorus and phosphorus release to solution in three soils after long-term mineral and manure application. Agric. Ecosyst. Environ. 2016, 233, 214–223. [Google Scholar] [CrossRef]
- Saad, S.; Lam-Son, P.T. Legume Nitrogen Fixation in Soils with Low Phosphorus Availability; Springer: Berlin/Heidelberg, Germany, 2017; p. 286. [Google Scholar]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Yang, X.J.; Finnegan, P.M. Regulation of phosphate starvation responses in higher plants. Ann. Bot. 2010, 105, 513–526. [Google Scholar] [CrossRef]
- Hansen, J.C.; Cade-Menun, B.J.; Strawn, D.G. Phosphorus speciation in manure-amended alkaline soils. J. Environ. Qual. 2004, 33, 1521–1527. [Google Scholar] [CrossRef]
- Turner, B.L.; Richardson, A.E.; Mullaney, E.J. Inositol Phosphates: Linking Agriculture and the Environment; CAB International: Wallingford, UK, 2007; p. 304. [Google Scholar]
- Raghothama, K.G. Phosphate acquisition. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef]
- Bieleski, R.L. Phosphate pools, phosphate transport, and phosphate availability. Ann. Rev. Plant. Physiol. 1973, 24, 225–252. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef]
- Pang, J.; Ryan, M.H.; Lambers, H.; Siddique, K.H. Phosphorus acquisition and utilisation in crop legumes under global change. Curr. Opin. Plant Biol. 2018, 45, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Rees, T. Compartmentation of plant metabolism. In The Biochemistry of Plants; Davies, D.D., Ed.; Academic Press: San Diego, CA, USA, 1987; Volume 12: Physiology of, Metabolism, pp. 87–115. [Google Scholar]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and signaling molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef]
- MacRae, E.; Lunn, J. Control of sucrose biosynthesis. Ann. Plant. Rev. 2018, 22, 234–257. [Google Scholar]
- Tauzin, A.S.; Giardina, T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Liu, C.; Sun, Y.; Zhang, T.; Hou, M.; Huang, S.; Yuan, H. The evolutionary history of the sucrose synthase gene family in higher plants. BMC Plant Biol. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Granot, D.; Stein, O. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar]
- Wan, H.; Wu, L.; Yang, Y.; Zhou, G.; Ruan, Y.L. Evolution of sucrose metabolism: The dichotomy of invertases and beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef]
- Jin, J.; Tang, C.; Sale, P. The impact of elevated carbon dioxide on the phosphorus nutrition of plants: A review. Ann. Bot. 2015, 116, 987–999. [Google Scholar] [CrossRef]
- Raven, J.A.; Lambers, H.; Smith, S.E.; Westoby, M. Costs of acquiring phosphorus by vascular land plants: Patterns and implications for plant coexistence. New Phytol. 2018, 217, 1420–1427. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahmad, E. Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms. In Phosphate Solubilizing Microorganisms; Springer: Cham, Switzerland, 2014; pp. 31–62. [Google Scholar]
- Ciereszko, I.; Gniazdowska, A.; Mikulska, M.; Rychter, A.M. Assimilate translocation in bean plants (Phaseolus vulgaris L.) during phosphate deficiency. J. Plant Physiol. 1996, 149, 343–348. [Google Scholar] [CrossRef]
- Ciereszko, I.; Zambrzycka, A.; Rychter, A.M. Sucrose hydrolysis in bean roots (Phaseolus vulgaris L.) under phosphate deficiency. Plant Sci. 1998, 133, 139–144. [Google Scholar] [CrossRef]
- Ciereszko, I.; Miłosek, I.; Rychter, A.M. Assimilate distribution in bean plants (Phaseolus vulgaris L.) during phosphate limitation. Acta Soc. Bot. Pol. 1999, 68, 269–273. [Google Scholar] [CrossRef]
- Ciereszko, I.; Barbachowska, A. Sucrose metabolism in leaves and roots of bean (Phaseolus vulgaris L.) during phosphate deficiency. J. Plant Physiol. 2000, 156, 640–644. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in the concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant 1992, 8, 455–460. [Google Scholar] [CrossRef]
- Hubbard, N.L.; Huber, S.C.; Pharr, D.M. Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing muskmelon (Cucumis melo L.) fruits. Plant Physiol. 1989, 91, 1527–1534. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cheikh, N.; Brenner, M.L. Regulation of key enzymes of sucrose biosynthesis in soybean leaves. Plant Physiol. 1992, 100, 1230–1237. [Google Scholar] [CrossRef]
- Van Handel, E. Direct microdetermination of sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- Holaday, S.A.; Martindale, W.; Alred, R.; Brooks, A.L.; Leegood, R.C. Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiol. 1992, 98, 1105–1114. [Google Scholar] [CrossRef]
- Geladopoulus, T.P.; Sotiroudis, T.G.; Evangelopoulus, A.E. A malachite green colorimetric assay for protein phosphatase activity. Anal. Biochem. 1991, 192, 112–116. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Hogue, E.; Wilcow, G.E.; Cantlife, D.J. Effect of soil P on phosphate fraction in tomato leaves. J. Am. Soc. Hortic. Sci. 1970, 95, 174–176. [Google Scholar]
- López-Cantarero, I.; Ruiz, J.M.; Hernández, J.; Romero, L. Phosphorus metabolism and yield response to increases in nitrogen-phosphorus fertilization: Improvement in greenhouse cultivation of eggplant (Solanum melongena cv. Bonica). J. Agric. Food. Chem. 1998, 46, 1603–1608. [Google Scholar] [CrossRef]
- Besford, R.T. Phosphorus nutrition and acid phosphatase activity in leaves of seven plant species. J. Sci. Food Agric. 1979, 30, 282–285. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Belakbir, A.; Romero, L. Foliar level of phosphorus and its bioindicators in Cucumis melo grafted plants. A possible effect of rootstock. J. Plant Physiol. 1996, 149, 400–404. [Google Scholar] [CrossRef]
- Graf, E.; Dintzis, F.R. High-performance liquid chromatographic method for the determination of phytate. Anal. Biochem. 1982, 119, 413–417. [Google Scholar] [CrossRef]
- Rychter, A.M.; Randall, D.D. The effect of phosphate deficiency on carbohydrate metabolism in bean roots. Physiol. Plant 1994, 91, 383–388. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, C.; Lynch, J.P. The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Funct. Plant Biol. 2010, 37, 313–322. [Google Scholar] [CrossRef]
- Pieters, A.J.; Paul, M.J.; Lawlor, D.W. Low sink demand limits photosynthesis under Pi deficiency. J. Exp. Bot. 2001, 52, 1083–1091. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M.A. Phosphorus affects growth and partitioning of nitrogen to Rubisco in Pinus pinaster. Tree Physiol. 2002, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Passarinho, J.A.; Rodrigues, M.L.; Osorio, M.L.; Ricardo, C.P.; Chaves, M.M. Physiological responses of Lupinus mutabilis to phosphorus nutrition and season of growth. J. Plant. Nutr. 2000, 23, 487–505. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; 849p. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Acaemic Press: London, UK, 2011; 645p. [Google Scholar]
- Fredeen, A.L.; Rao, I.M.; Terry, N. Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol. 1989, 89, 225–230. [Google Scholar] [CrossRef]
- Rao, I.M.; Fredeen, A.L.; Terry, N. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet III. Diurnal changes in carbon partitioning and carbon export. Plant Physiol. 1990, 92, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.C.; Lecharny, A.; Willenbrink, J. Sucrose cleavage in relation to import and metabolism of sugars in sink organs. In Phloem Transport and Assimilate Compartmentation; Bonneman, J.L., Delrot, S., Lucas, W.J., Danty, J., Eds.; Intercept Ltd.: Andover, UK, 1991; pp. 178–186. [Google Scholar]
- Stitt, M.; Herzog, B.; Heldt, H.W. Control of photosynthetic sucrose synthesis by fructose 2,6-bisphosphate, I: Coordination of CO2 fixation and sucrose synthesis. Plant Physiol. 1981, 75, 548–553. [Google Scholar] [CrossRef]
- Black, C.C.; Mustardy, L.; Sung, S.S.; Kormanik, P.P.; Xu, D.P.; Paz, N. Regulation and roles for alternative pathways of hexose metabolism in plants. Physiol. Plant 1987, 69, 387–394. [Google Scholar] [CrossRef]
- Ross, H.A.; Davies, H.V. Purification and characterization of sucrose synthase from the cotyledons of Vicia faba L. Plant Physiol. 1992, 100, 1008–1013. [Google Scholar] [CrossRef]
- Schaffer, A.A. Invertases in young and mature leaves of Citrus sinensis. Phytochemistry 1986, 25, 2275–2277. [Google Scholar] [CrossRef]
- Thao, H.T.B.; Yamakawa, T.; Shibata, K. Effect of phosphite-phosphate interaction on growth and quality of hydroponic lettuce (Lactuca sativa). J. Plant. Nutr. Soil Sci. 2009, 172, 378–384. [Google Scholar] [CrossRef]
- Gidago, G.; Beyene, S.; Worku, W.; Sodo, E. The response of haricot bean (Phaseolus vulgaris L.) to phosphorus application on Ultisols at Areka, Southern Ethiopia. J. Biol. Agric. Healthc. 2011, 1, 38–49. [Google Scholar]
- Ávila, F.W.; Faquin, V.; da Silva Lobato, A.K.; Ávila, P.A.; Marques, D.J.; Silva Guedes, E.M.; Tan, D.K.Y. Effect of phosphite supply in nutrient solution on yield, phosphorus nutrition and enzymatic behavior in common bean (Phaseolus vulgaris L.) plants. Austr. J. Crop. Sci. 2013, 7, 713. [Google Scholar]
- Gulmezoglu, N.; Daghan, H. The interactive effects of phosphorus and salt on growth, water potential and phosphorus uptake in green beans. Appl. Ecol. Environ. Res. 2017, 15, 1831–1842. [Google Scholar] [CrossRef]
- Hussain, A.; Larsson, H.; Kuktaite, R.; Johansson, E. Mineral composition of organically grown wheat genotypes: Contribution to daily minerals intake. Inter. J. Environ. Res. Public Health 2010, 7, 3442–3456. [Google Scholar] [CrossRef] [PubMed]
- Constan-Aguilar, C.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Camacho, M.A.; Romero, L.; Ruiz, J.M.; Blasco, B. Physiological and nutritional evaluation of the application of phosphite as a phosphorus source in cucumber plants. Commun. Soil Sci. Plant. Anal. 2014, 45, 204–222. [Google Scholar] [CrossRef]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Nakanishi, T.M.; Thibaud, M.C. Phosphate import in plants: Focus on the PHT1 transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef]
- Gu, M.; Chen, A.; Sun, S.; Xu, G. Complex regulation of plant phosphate transporters and the gap between molecular mechanisms and practical application: What is missing? Mol. Plant 2016, 9, 396–416. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Ferguson, I.B. Physiology and metabolism of phosphate and its compounds. In Encyclopedia of Plant Physiology; Lauchli, A., Bieleski, R.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; Volume 15a, pp. 422–449. [Google Scholar]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to the roots. Ann. Plant Rev. 2015, 48, 3–22. [Google Scholar]
- Taliman, N.A.; Dong, Q.; Echigo, K.; Raboy, V.; Saneoka, H. Effect of phosphorus fertilization on the growth, photosynthesis, nitrogen fixation, mineral accumulation, seed yield, and seed quality of a soybean low-phytate line. Plants 2019, 8, 119. [Google Scholar] [CrossRef]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Tian, J.; Venkatachalam, P.; Liao, H.; Yan, X.; Raghothama, K. Molecular cloning and characterization of phosphorus starvation responsive genes in common bean (Phaseolus vulgaris L.). Planta 2007, 227, 151–165. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Raven, J.A. Protein turnover and plant RNA and phosphorus requirements in relation to nitrogen fixation. Plant Sci. 2012, 188, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Hurley, B.A.; Plaxton, W.C. Feeding hungry plants: The role of purple acid phosphatases in phosphate nutrition. Plant Sci. 2010, 179, 14–27. [Google Scholar] [CrossRef]
- Araujo, A.P.; Plassard, C.; Drevon, J.J. Phosphatase and phytase activities in nodules of common bean genotypes at different levels of phosphorus supply. Plant Soil 2008, 312, 129. [Google Scholar] [CrossRef]
- Wasaki, J.; Ando, M.; Ozawa, K.; Omura, M.; Osaki, M.; Ito, H.; Matsui, H. Properties of secretary acid phosphatase from lupin roots under phosphorus-deficient conditions. Soil Sci. Plant Nutr. 1997, 43, 981–986. [Google Scholar] [CrossRef]
- Hunter, D.A.; McManus, M.T. Comparison of acid phosphatases in two genotypes of white clover with different responses to applied phosphate. J. Plant Nutr. 1999, 22, 679–692. [Google Scholar] [CrossRef]
- George, T.S.; Gregory, P.J.; Hocking, P.; Richardson, A.E. Variation in root-associated phosphatase activities in wheat contributes to the utilization of organic P substrates in vitro, but does not explain differences in the P-nutrition of plants when grown in soils. Environ. Exp. Bot. 2008, 64, 239–249. [Google Scholar] [CrossRef]
- Plaxton, W.C. Plant Response to Stress: Biochemical Adaptations to Phosphate Deficiency; Encyclopedia of Plant and Crop Science; Marcel Dekker: New York, NY, USA, 2004; pp. 976–980. [Google Scholar]
- Lockhart, H.B.; Hurt, H.D. Nutrition of Oats; American Association of Cereal Chemists: St. Paul, MN, USA, 1986; pp. 297–308. [Google Scholar]
- White, P.J.; Veneklaas, E.J. Nature and nurture: The importance of seed phosphorus content. Plant Soil 2012, 357, 1–8. [Google Scholar] [CrossRef]
- Chen, R.; Song, S.; Li, X.; Liu, H.; Huang, D. Phosphorus deficiency restricts plant growth but induces pigment formation in the flower stalk of Chinese kale. Hortic. Environ. Biotechnol. 2013, 54, 243–248. [Google Scholar] [CrossRef]
- Gupta, K.; Barat, G.K.; Wagle, D.S.; Chawla, H.K.L. Nutrient contents and antinutritional factors in conventional and non-conventional leafy vegetables. Food Chem. 1989, 31, 105–116. [Google Scholar] [CrossRef]
- Kennedy, G.; Nantel, G. Basic Guidelines for Validation of a Simple Dietary Diversity Score as an Indicator of Dietary Nutrient Adequacy for Non-Breastfeeding Children 2–6 Years; FAO: Rome, Italy, 2006. [Google Scholar]

| Treatments | Glucose (mg g−1 FW) | Fructose (mg g−1 FW) | Sucrose (mg g−1 FW) | Starch (mg g−1 FW) |
|---|---|---|---|---|
| Roots | ||||
| P1 | 2.05 ± 0.18 d | 2.19 ± 0.19 d | 1.99 ± 0.17 d | 11.75 ± 1.02 a |
| P2 | 2.68 ± 0.23 c | 2.85 ± 0.25 c | 2.59 ± 0.23 c | 11.69 ± 1.02 a |
| P3 | 6.44 ± 0.56 a | 6.86 ± 0.60 a | 6.23 ± 0.54 a | 11.11 ± 0.97 a |
| P4 | 6.68 ± 0.58 a | 7.11 ± 0.62 a | 6.46 ± 0.56 a | 12.14 ± 1.06 a |
| P5 | 4.52 ± 0.39 b | 4.81 ± 0.42 b | 4.37 ± 0.38 b | 11.58 ± 1.27 a |
| P6 | 2.60 ± 0.23 c | 2.77 ± 0.24 c | 2.52 ± 0.22 c | 10.28 ± 0.90 a |
| Shoots | ||||
| P1 | 17.13 ± 1.49 a | 18.25 ± 1.59 a | 16.57 ± 1.44 a | 13.96 ± 1.65 a |
| P2 | 11.16 ± 0.97 b | 11.89 ± 1.04 b | 10.80 ± 0.94 b | 13.01 ± 1.40 a |
| P3 | 10.37 ± 0.90 b | 11.04 ± 0.96 b | 10.03 ± 0.87 b | 14.02 ± 1.36 a |
| P4 | 10.14 ± 0.88 b | 10.78 ± 0.88 b | 10.05 ± 0.88 b | 13.85 ± 1.29 a |
| P5 | 10.71 ± 1.11 b | 10.54 ± 1.18 b | 9.81 ± 0.86 b | 13.35 ± 1.16 a |
| P6 | 10.59 ± 1.05 b | 11.68 ± 1.02 b | 10.29 ± 1.07 b | 14.13 ± 1.23 a |
| Sucrose Biosynthesis | Sucrose Degradation | ||||
|---|---|---|---|---|---|
| Treatments | SPS (µmol Sucrose mg−1 prot. h−1) | F1,6BPase (µmol Pi mg−1 prot. min−1) | SS (µmol Sucrose mg−1 prot. h−1) | Acid Invertase (µmol Glucose mg−1 prot. h−1) | Alkaline Invertase (µmol Glucose mg−1 prot. h−1) |
| Roots | |||||
| P1 | 5.02 ± 0.44 c | 77.16 ± 6.73 d | 3.62 ± 0.32 c | 216.30 ± 18.86 b | 229.00 ± 19.96 d |
| P2 | 5.17 ± 0.45 c | 86.64 ± 7.55 d | 3.39 ± 0.30 c | 222.90 ± 19.92 b | 234.80 ± 20.47 d |
| P3 | 8.79 ± 0.77 a | 226.10 ± 19.71 a | 6.14 ± 0.54 a | 272.60 ± 23.76 a | 411.10 ± 35.84 a |
| P4 | 6.04 ± 0.53 b | 165.80 ± 14.45 b | 4.29 ± 0.37 b | 300.61 ± 26.21 a | 452.90 ± 39.48 a |
| P5 | 3.73 ± 0.40 d | 121.10 ± 10.56 c | 2.87 ± 0.24 c | 238.82 ± 20.82 b | 342.20 ± 29.83 b |
| P6 | 4.17 ± 0.36 d | 70.18 ± 6.12 d | 2.97 ± 0.26 c | 166.81 ± 14.54 c | 272.82 ± 23.78 c |
| Shoots | |||||
| P1 | 4.27 ± 0.37 c | 84.03 ± 7.33 d | 3.51 ± 0.31 c | 46.87 ± 4.09 b | 155.10 ± 13.52 a |
| P2 | 4.56 ± 0.40 c | 108.80 ± 10.36 c | 3.22 ± 0.28 c | 21.63 ± 1.89 c | 96.18 ± 8.38 b |
| P3 | 7.47 ± 0.65 a | 179.70 ± 15.67 a | 5.76 ± 0.50 a | 14.39 ± 1.25 e | 37.54 ± 3.27 e |
| P4 | 6.60 ± 0.40 b | 127.60 ± 11.12 b | 4.43 ± 0.39 b | 17.65 ± 1.54 d | 59.10 ± 5.15 d |
| P5 | 4.17 ± 0.36 c | 107.90 ± 9.41 c | 3.70 ± 0.32 c | 55.34 ± 4.82 a | 77.40 ± 6.88 c |
| P6 | 4.42 ± 0.30 c | 105.01 ± 9.15 c | 3.31 ± 0.29 c | 59.72 ± 5.21 a | ± 7.12 c |
| Treatments | Total P (mg 100 g−1 DW) | Inorganic P (mg 100 g−1 DW) | Organic P (mg 100 g1 DW) | Organic P/Inorganic P |
|---|---|---|---|---|
| Roots | ||||
| P1 | 247.64 ± 22.18 f | 86.67 ± 8.17 f | 164.38 ± 16.21 d | 0.53 ± 0.04 e |
| P2 | 531.85 ± 50.23 e | 228.61 ± 21.43 e | 303.59 ± 30.32 c | 0.75 ± 0.06 d |
| P3 | 686.31 ± 64.25 d | 329.42 ± 29.64 d | 357.98 ± 33.60 c | 0.92 ± 0.08 c |
| P4 | 953.82 ± 90.47 c | 476.90 ± 44.58 c | 477.76 ± 39.52 b | 1.00 ± 0.09 c |
| P5 | 1407.91 ± 141.22 b | 814.58 ± 79.28 b | 593.19 ± 45.44 a | 1.35 ± 0.06 b |
| P6 | 1655.56 ± 153.28 a | 991.82 ± 80.22 a | 664.58 ± 65.27 a | 1.49 ± 0.07 a |
| Shoots | ||||
| P1 | 167.08 ± 14.49 f | 50.12 ± 4.72 f | 117.11 ± 13.59 d | 0.43 ± 0.04 d |
| P2 | 354.12 ± 29.91 e | 141.64 ± 11.94 e | 213.06 ± 21.74 c | 0.66 ± 0.06 c |
| P3 | 449.02 ± 38.90 d | 188.59 ± 16.87 d | 261.76 ± 25.96 c | 0.72 ± 0.06 c |
| P4 | 815.52 ± 79.88 c | 375.14 ± 31.46 c | 440.10 ± 34.79 b | 0.85 ± 0.08 b |
| P5 | 1218.13 ± 114.11 b | 596.88 ± 57.66 b | 622.26 ± 59.01 a | 0.96 ± 0.08 b |
| P6 | 1539.16 ± 145.45 a | 769.58 ± 71.17 a | 570.87 ± 56.64 a | 1.35 ± 0.11 a |
| Pods | ||||
| P1 | 144.52 ± 11.78 f | 40.46 ± 3.94 f | 104.46 ± 9.19 e | 0.39 ± 0.04 d |
| P2 | 206.99 ± 19.57 e | 74.51 ± 6.94 e | 132.88 ± 14.04 d | 0.56 ± 0.06 c |
| P3 | 343.67 ± 25.97 d | 134.02 ± 10.87 d | 209.60 ± 19.76 c | 0.64 ± 0.05 c |
| P4 | 526.20 ± 49.78 c | 231.53 ± 19.88 c | 294.71 ± 29.03 b | 0.79 ± 0.06 b |
| P5 | 1021.42 ± 100.11 b | 439.84 ± 40.26 b | 582.63 ± 58.18 a | 0.75 ± 0.06 b |
| P6 | 1265.17 ± 111.25 a | 594.63 ± 47.07 a | 671.43 ± 69.42 a | 0.89 ± 0.07 a |
| Seeds | ||||
| P1 | 295.59 ± 21.49 f | 70.93 ± 6.44 e | 225.02 ± 25.59 e | 0.32 ± 0.02 a |
| P2 | 486.67 ± 40.46 e | 107.06 ± 8.90 d | 379.33 ± 39.01 d | 0.28 ± 0.02 b |
| P3 | 679.62 ± 62.93 d | 135.92 ± 10.43 c | 544.94 ± 59.96 c | 0.25 ± 0.02 b |
| P4 | 977.44 ± 89.79 c | 175.93 ± 13.88 b | 802.51 ± 79.98 b | 0.22 ± 0.02 c |
| P5 | 1394.81 ± 101.15 b | 195.27 ± 16.86 b | 1200.47 ± 115.18 a | 0.16 ± 0.01 d |
| P6 | 1623.41 ± 103.45 a | 241.04 ± 19.97 a | 1383.57 ± 126.72 a | 0.17 ± 0.01 d |
| Treatments | Lipidic P (mg 100 g−1 DW) | Proteic P (mg 100 g−1 DW) | RNA-P (mg 100 g−1 DW) | DNA-P (mg 100 g−1 DW) |
|---|---|---|---|---|
| Roots | ||||
| P1 | 34.63 ± 3.17 e | 17.33 ± 1.70 f | 24.76 ± 2.19 e | 19.81 ± 1.18 e |
| P2 | 69.11 ± 5.45 d | 21.26 ± 2.23 e | 47.84 ± 4.55 d | 37.21 ± 2.83 d |
| P3 | 82.35 ± 7.42 c | 27.45 ± 2.54 d | 54.90 ± 5.60 d | 41.17 ± 3.59 d |
| P4 | 95.37 ± 8.26 c | 47.68 ± 4.06 c | 85.94 ± 7.92 c | 57.22 ± 4.58 c |
| P5 | 135.70 ± 12.01 b | 60.31 ± 5.38 b | 135.70 ± 12.12 b | 75.39 ± 7.39 b |
| P6 | 170.79 ± 14.19 a | 83.77 ± 8.22 a | 170.79 ± 13.54 a | 93.77 ± 7.23 a |
| Shoots | ||||
| P1 | 25.06 ± 1.84 f | 13.36 ± 1.31 e | 18.37 ± 1.59 e | 15.03 ± 1.07 e |
| P2 | 42.49 ± 3.24 e | 14.16 ± 1.40 e | 35.41 ± 3.04 d | 28.32 ± 1.97 d |
| P3 | 58.37 ± 4.64 d | 22.45 ± 2.01 d | 40.41 ± 3.96 d | 31.43 ± 2.09 d |
| P4 | 89.70 ± 7.88 c | 48.93 ± 4.08 c | 81.55 ± 7.88 c | 57.08 ± 4.86 c |
| P5 | 121.81 ± 11.01 b | 73.08 ± 6.86 b | 121.81 ± 11.18 b | 85.26 ± 8.04 b |
| P6 | 169.30 ± 14.07 a | 107.73 ± 10.07 a | 153.91 ± 12.89 a | 107.73 ± 9.26 a |
| Pods | ||||
| P1 | 20.23 ± 1.44 d | 13.00 ± 1.04 e | 15.89 ± 1.35 f | 14.45 ± 1.49 f |
| P2 | 22.76 ± 1.24 d | 14.48 ± 1.11 e | 20.69 ± 1.86 e | 18.62 ± 1.71 e |
| P3 | 41.23 ± 3.87 c | 27.49 ± 2.57 d | 34.36 ± 2.96 d | 30.92 ± 2.80 d |
| P4 | 47.35 ± 4.18 c | 47.35 ± 3.88 c | 57.88 ± 5.08 c | 47.35 ± 4.02 c |
| P5 | 81.71 ± 6.86 b | 91.92 ± 8.81 b | 112.35 ± 10.16 b | 91.92 ± 9.01 b |
| P6 | 139.16 ± 12.14 a | 111.21 ± 9.99 a | 126.51 ± 11.41 a | 111.21 ± 9.55 a |
| Seeds | ||||
| P1 | 23.64 ± 1.51 f | 11.82 ± 0.94 e | 17.73 ± 1.04 f | 11.82 ± 1.12 e |
| P2 | 34.06 ± 2.14 e | 19.46 ± 1.52 d | 29.19 ± 2.04 e | 24.33 ± 2.01 d |
| P3 | 47.57 ± 3.95 d | 27.18 ± 2.17 c | 40.77 ± 3.47 d | 33.98 ± 2.90 c |
| P4 | 78.19 ± 6.18 c | 48.87 ± 4.47 b | 55.64 ± 5.13 c | 58.64 ± 4.63 b |
| P5 | 97.63 ± 8.24 b | 55.79 ± 5.16 b | 69.73 ± 6.18 b | 69.73 ± 6.11 b |
| P6 | 133.63 ± 11.17 a | 69.93 ± 5.57 a | 88.16 ± 7.54 a | 84.16 ± 7.45 a |
| Regression Equation | R2 |
|---|---|
| Organic P = 0.13 P + 1.21 | 0.94 |
| Lipidic P = 0.09 P + 0.31 | 0.97 |
| Proteic P = 0.07 P − 0.15 | 0.97 |
| RNA-P = 0.09 P − 0.04 | 0.99 |
| DNA-P = 0.07 P + 0.10 | 0.99 |
| Phytates = 0.03 P + 0.02 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Caparrós, P.; Lao, M.T.; Preciado-Rangel, P.; Sanchez, E. Phosphorus and Carbohydrate Metabolism in Green Bean Plants Subjected to Increasing Phosphorus Concentration in the Nutrient Solution. Agronomy 2021, 11, 245. https://doi.org/10.3390/agronomy11020245
García-Caparrós P, Lao MT, Preciado-Rangel P, Sanchez E. Phosphorus and Carbohydrate Metabolism in Green Bean Plants Subjected to Increasing Phosphorus Concentration in the Nutrient Solution. Agronomy. 2021; 11(2):245. https://doi.org/10.3390/agronomy11020245
Chicago/Turabian StyleGarcía-Caparrós, Pedro, María Teresa Lao, Pablo Preciado-Rangel, and Esteban Sanchez. 2021. "Phosphorus and Carbohydrate Metabolism in Green Bean Plants Subjected to Increasing Phosphorus Concentration in the Nutrient Solution" Agronomy 11, no. 2: 245. https://doi.org/10.3390/agronomy11020245
APA StyleGarcía-Caparrós, P., Lao, M. T., Preciado-Rangel, P., & Sanchez, E. (2021). Phosphorus and Carbohydrate Metabolism in Green Bean Plants Subjected to Increasing Phosphorus Concentration in the Nutrient Solution. Agronomy, 11(2), 245. https://doi.org/10.3390/agronomy11020245







