1. Introduction
Lisianthus (
Eustoma grandiflorum (Raf.) Shinn.) has become one of the most popular cut flowers. With its multi-flower stems, single, semi-double, and double rose-like flowers in a variety of colors, it is attractive for consumers worldwide. Its postharvest longevity varies between cultivars, ranging from five days [
1], to eight or nine days [
2,
3] and up to 28 days [
4]. However, as each stem bears both fully open flowers and closed buds, it is not always clear how the reported vase life lengths were evaluated. As the structure of the lisianthus “cut flower” is complex, it is difficult to develop a postharvest treatment that would simultaneously delay senescence of the open flowers and promote bud opening. Studies on senescence and postharvest treatments of lisianthus have been carried out for over two decades to improve its keeping qualities: the vase life, bud opening, and petal coloration [
5,
6,
7].
The longevity of cut flowers is shortened by several factors: disruption of the water uptake and transport, due mainly to microbial vessel blockage; depletion of the respiratory substrate, which limits the energy available to sustain life processes; and harmful effects of the reactive oxygen species (ROS) emerging during the oxidative stress after the flowering stems are detached from mother plants as well as the senescence-accelerating ethylene action in the ethylene-sensitive species [
8]. All these factors have been found to play a role in lisianthus. Reports on biocides capable of prolonging the lisianthus vase life by assuring uninterrupted water transport in cut stems include 8-hydroxyquinolite citrate [
3,
4,
9,
10,
11], aluminum sulfate [
2], sodium hypochlorite [
7,
9], silver nitrate [
9], nanosilver [
12,
13], nanoparticles of silicon and silver [
14], and peracetic acid [
15]. All of these efficiently limit the microbial population in vase water and prolong the vase life, especially when applied with sucrose.
Exogenous sucrose is indispensable for most cut flowers as a respiratory substrate. Cut flowers are able to absorb and metabolize sugars from vase water to provide energy required to support life and to open flower buds [
8,
16]. Sucrose is mostly used as a flower food and for lisianthus, it is effective in concentration between 2% and 6% [
10,
14], but glucose and fructose can also be used [
10]. Sugar accumulation in the vacuoles reduces the osmotic potential, thus enhancing water influx into petals and promoting their expansion during bud opening [
10].
Ethylene is involved in the flower senescence of lisianthus [
17]. Treatments with inhibitors of the ethylene action such as silver thiosulphate (STS) [
17], 1-methylcyclopropene (1-MCP), or salicylic acid (SA) [
18] extended flower longevity. A positive effect of acetaldehyde on decreasing ethylene synthesis and extending lisianthus longevity was reported by Seighalani et al. [
19], and that of silver nanoparticles by Kiamohammadi [
14]. According to Asil and Karimi, [
20] cytokinin benzyladenine (BA) also improved lisianthus longevity by delaying ethylene production. The enhanced resistance to ethylene may also be related to the action of exogenous sucrose [
5].
Numerous results suggest that chemicals such as polyamines [
21] or SA and sucrose [
3,
22] can increase the vase life of lisianthus cut flowers by improving the antioxidant system and reducing the damage caused by the oxidative stress during senescence.
The aim of this work was to evaluate the effect of conditioning lisianthus flowering stems with nanosilver and to assess the efficiency of the NS-based preservative
versus the standard preservative containing 8-HQC in improving longevity, water balance, and senescence-related processes in petals of
Eustoma. Silver is a strong antibacterial agent, which nowadays is applied in a form of nanoparticles. According to Damunpola and Joyce [
23], nanosilver (NS) interacts with bacteria membranes and destroys them. As it has relatively little toxicity to the environment, it has become a common substance used for postharvest treatments of cut flowers [
24].
2. Material and Methods
Bifurcated flowering stems of
Eustoma grandiflorum (Raf.) Shinn. ‘Mariachi Blue’, each bearing three flowers/buds on each of the two side shoots (
Figure 1), were harvested from mother plants and trimmed to 60 cm in length. The stems were stripped off excessive leaves, leaving only the upper pairs of leaves. One half of the stems were placed directly into calibrated cylinders (volume 0.5 dm
3) with the following holding aqueous solutions: 200 mg dm
−3 8-hydroxyquinoline citrate with 2% sucrose (8-HQC + S), 1 mg dm
−3 nanosilver with 2% sucrose (NS + S), and distilled water serving as a control. NS was obtained as a commercial preparation (Altermedica Laboratories, Żywiec, Poland). The other half of the stems were conditioned for 24 h in 5 mg dm
−3 nanosilver solution and then placed into the same holding solutions as above. Each treatment contained 10 stems, individually tagged, in individual cylinders, and treated as single replicates. The preservatives and water were not exchanged during the experiment, but they were replenished when the holding solution level dropped to 5 cm.
The experiment was carried out in a room with controlled temperature of 20 ± 1 °C, a relative humidity of 60%, and quantum irradiance of 35 μmol m−2 s−1, under the 12 h day/12 h night regime; the 24 h conditioning with NS was performed under the same conditions.
The vase life was expressed as a number of days elapsed between placing flowers into solutions (conditioning or holding) and the loss of decorative value of each flower in an inflorescence such as petal withering or peduncle drying and bending. Longevity of the youngest flower should be regarded as a total vase life of the whole flowering stem.
The water balance parameters were determined in a separate experiment where flowering stems were placed individually into 250 mL cylinders, 10 stems per each treatment.
Water uptake (a drop in solution volume) was measured on each day of the experiment and expressed in mL. The fresh weight of flowers was taken daily after solutions had been replenished, and expressed in grams (g). The transpiration rate was expressed as the difference between water uptake and weight change, and expressed in gram per stem per day.
Samples for all biochemical analyses were collected from a separate batch of flowers immediately after harvest (Day 0), and Days 9 and 20 after harvest, separately from the lower (open at harvest) and upper (buds at harvest) flowers (
Figure 1). For each analysis, four flowers were sampled from each treatment. The petals were finely cut, mixed, and three samples of 0.5 g each were weighed. Three extracts were prepared and three aliquots were taken from each extract, giving a total of nine readings for each data point. Additionally, three samples were taken for the dry weight (DW) measurements: plant material was dried at 105 °C until constant weight, as in Strzelecka et al. [
25].
Total soluble sugars were measured as described by Dubois et al. [
26] and expressed in mg glucose per g of DW. The material was homogenized in 80% ethanol (Chempur, Piekary Śląskie, Poland). The extracts were incubated for 20 min in a boiling water bath with 5% phenol (Chempur, Piekary Śląskie, Poland) and 96% H
2SO
4 (Stanlab, Lublin, Poland), and the extinction was measured spectrophotometrically (Shimadzu UV-1800, Japan) at 490 nm. The total sugar content was calculated from a previously plotted standard curve, prepared for glucose.
The reducing sugars content was measured by the Somogyi method as modified by Nelson [
27] and expressed in mg glucose g
−1 DW. The material was homogenized in 80% ethanol (Chempur, Piekary Śląskie, Poland). The extracts were incubated for 20 min in a boiling water bath with the copper reagent; the molybdenum arsenic reagent (Merck, Darmstad, Germany) was added and the extinction was measured at 520 nm. The amounts of reducing sugars were calculated from a previously plotted standard curve, prepared for glucose.
The free proline content in petals was tested according to Bates et al. [
28] by measuring the quantity of a colored reaction product of proline with ninhydric acid (Sigma, Saint Louis, USA). The absorbance was read at 520 nm. The amount of proline was calculated from a previously plotted standard curve and expressed in µmol g
−1 of DW.
The petal hydrogen peroxide (H
2O
2) content was measured spectrophotometrically after the reaction with potassium iodide (KI) (Sigma, Saint Louis, USA) as described by Jędrzejuk et al. [
29] and expressed at 390 nm as µg of hydrogen peroxide per gram on a DW basis.
The catalase (CAT) activity (EC 1.11.1.6) was determined spectrophotometrically as the rate of H
2O
2 disappearance at 405 nm according to Goth [
30] and expressed as mkatals per gram DW.
Results were statistically evaluated by ANOVA 1 or ANOVA 2 using IBM SPSS Statistics program (SPSS, Poland). Duncan’s test at α = 0.05 was applied to assess the significant differences between the means.
4. Discussion
Multi-flowered lisianthus (
Eustoma grandiflorum) stems are very showy so there is a growing demand worldwide for cut flowers, with cultivars from the Mariachi group considered particularly desirable. As the structure of the lisianthus “cut flower” is complex, it is difficult to design a postharvest treatment that on one hand would delay senescence of the flowers already opened, while on the other, would promote bud opening. Moreover, as each cut lisianthus stem bears fully open flowers and closed buds, it is not always clear how to evaluate the vase life length with considerable confusion in the literature. Bahrami et al. [
3] did not list the number of open flowers at harvest and considered the vase life ended when 50% of all flowers on a stem had wilted. Islam et al. [
4] used stems with three open flowers and several buds (without providing exact numbers), and also adopted the criterion of 50% wilted flowers. In the “half open stage’, the flowering stems were cut for the experiment of Kazemi et al. [
31] terminated when 50% flowers had wilted. Similarly, Ichimura [
5], Huang and Chen [
16] “recorded the vase life as a number of days from the end of pulse treatment until the when the last flower senesced”. However, again, no number of flowers per stem has been specified. For this study, we harvested flowering stems with one flower
per shoot open, reduced the bud number to two on each of the two side shoots, and evaluated the longevity of individual flowers.
Despite some confusion as to the exact state of the experimental material, all cited authors showed that a sugar combined with biocides, 8-HQS or 8-HQC, was an important factor in prolonging the vase life of lisianthus while the biocides alone were ineffective. This suggests that the microbial presence in vase water is a minor problem for cut lisianthus, and vessel blockages do not heavily obstruct water uptake and reduce the vase life. Such a conclusion was indeed drawn by Islam et al. [
4]. However, there are numerous reports on the positive effects of different biocides on the lisianthus vase life. Recently, a positive effect of silver nanoparticles in a concentration of 40 mg dm
−3 was reported by Kamiab et al. [
1], especially when combined with silicon nanoparticles and 4% sucrose. That mixture eliminated the microbial population and prolonged the vase life from five to 17 days. However, we consider such a concentration of silver nanoparticles in the holding solution as unnecessarily high, even though Jowkar et al. [
32] used NS at a similar range of concentrations (10–50 mg dm
−3) as a biocide for ‘Cherry Brandy‘ roses. Very high NS concentrations—75 and 125 mg dm
−3, with 2% sucrose—were tested as optimal for roses ‘High and Magic’ [
33]. Lü et al. [
12] used even higher concentrations of NS—50–250 mg dm
−3—for 1 h pulsing of ‘Movie Star’ roses, but the high end concentration turned out to be phytotoxic. We set out to compare the effectiveness of nanosilver against 8-HQC, which is a component of the long-known standard preservative [
8] and to test its effectiveness as a conditioner. However, just like Liu et al. [
34], who used 5 mg dm
−3 NS for conditioning gerberas, the concentrations we applied were much lower than those high ones listed above: 5 mg dm
−3 nanosilver for conditioning and 1 mg dm
−3 as a component of a vase solution. We were inspired by the fact that even lower NS concentrations—0.5 mg dm
−3—extended the vase life of ‘Movie Star’ roses [
13]. This is in line with now common tendency to use chemicals in the lowest concentrations possible, both for economic and environmental reasons.
Depending on the treatment, the longevity of the oldest flowers on our test stems ranged from 12 to 16 days while that of the youngest flower from 18 to 35 days. The latter included time to open the bud. Conditioning as such did not affect the longevity of individual flowers, but longevity was significantly increased by both preservatives containing 2% sucrose with nanosilver being more effective than 8-HQC + S. The overall mean for the vase life in NS + S for all six flowers/buds was 54% and 44% higher than for water controls in non-conditioned and conditioned stems, respectively, while such values for 8-HQC + S were 23% and 13%.
Numerous reports showed that exogenous sugar is indispensable for sustaining lisianthus flower life, both as a respiration substrate [
16] and as an osmolyte that promotes water influx into expanding petals, thus enhancing bud opening [
35]. The number of open buds on stems held in sucrose solutions was always higher than those in solutions without sugar [
15,
36]. With sucrose present in a holding solution, the reported increase in vase life was from 15% [
15] to 340% [
1]. Even a short 18 h pulse of a solution with 5% sucrose resulted in a 4.5 day longer vase life [
3].
As sucrose is a transportable form of carbohydrates within lisianthus stems [
35], it has mainly been used to extend the vase life, and generally it is more effective than glucose or fructose [
10]. The reported sucrose concentrations in holding solutions for lisianthus range between 2% [
4,
10,
17] and 6% [
14], while 5% has been used for 18 h conditioning [
3].
When sugar is transported from a vase solution to flower, it accumulates in leaves, causing severe damage, so a general rule is to use sucrose concentrations lower than 2% in leafy cut flowers such as roses, lilies, or alstremerias [
8]. To avoid potential leaf toxicity, we used 2% sucrose in both preservatives under study and did not observe any foliage problems. No information on leaf damage was given by Kiamohammadi [
14], who found 6% sucrose—in combination with an unusually high silver concentration (120 mg dm
−3 of AgNO
3)—as the best for lisianthus even though 4% sucrose was already harmful for leaves in the trials carried out Shimizu-Yumoto and Ichimura [
6] and Shimizu-Yumoto [
36].
As ethylene is involved in the senescence of lisianthus flowers [
6,
17,
36], pulse treatments with chemicals inhibiting its activity and limiting its biosynthesis should be beneficial in prolonging the postharvest longevity of cut flowers. Conditioning with silver thiosulfate (STS) has been applied to the ethylene-sensitive flowers for four decades, and its positive effect on lisianthus has also been reported [
6,
17,
36]. However, conditioning was more effective when STS was combined with sucrose. The role of sucrose in improving the postharvest physiology of lisianthus by delaying the ethylene production or reducing the sensitivity to C
2H
4 cannot be underestimated. To protect lisianthus flowers against the ethylene action, treatments with BA and sucrose resulted in the lowest ethylene production [
16].
Conditioning flowering stems with 5 mg dm
−3 NS did not prolong the vase life in our experiment. Perhaps the concentration of silver transported from the vase solution to flowers was too low to affect the ethylene synthesis and to increase flower longevity. According to Lü et al. [
12], silver concentration in pulsed rose stems was the highest in their basal ends and much lower in leaves and flowers. The authors used nanosilver in concentrations 10–50 times higher than ours, and successfully improved keeping qualities of roses with concentrations of 50 and 100 mg dm
−3. Even higher NS concentrations were used by Kasir et al. [
33]: 75 and 125 mg dm
−3 for 2 h pulse of ‘High and Magic’ roses. The concentration ranging between our 5 and 125 mg dm
−3 of the above cited authors leaves room for further trials to find the lowest but optimal NS concentration for conditioning lisianthus. As for NS as a biocide, the concentration of 1 mg dm
−3 appeared to be very low yet its efficiency—when applied with sucrose—was higher than that of the standard preservative. In our experiments with cut peonies, 1 mg dm
−3 NS affected keeping qualities in a manner comparable to 8-HQC, and has been recommended as its alternative treatment for peonies [
37].
Water balance is a crucial factor in controlling the longevity of cut flowers. When the water balance becomes negative due to dominance of transpiration over water uptake, flowers begin to wilt. Water deficiency affects not only the visual appearance of cut flowers but also accelerates the processes of senescence. The rate of water uptake is known to decline as lisinathus flowers senesce, regardless of the holding solution [
9] and this tendency was observed here. Treatments improving water balance (i.e., minimizing transpirational losses and stimulating uptake by eliminating bacterial vessel blockages) are commonly used in the cut flower industry. 8-HQC has long been known as a potent biocide as well as an antitranspirant [
8], so it is no surprise that when used in the standard preservative, it positively affected water balance in our experiments. However, it is noteworthy that higher average stem weights from the standard preservative—compared to water controls—appeared to be due to a reduced transpiration rate and not to stimulation of the uptake. Contrary to Lü et al. [
12], where NS inhibited the stomatal conductance and reduced the leaf water loss in roses, in our experiment, the transpiration rate was the highest in the NS + S-treated flowers. However, as nanosilver also stimulated the water uptake, it is the combination of the two processes that produced the highest average weights of flowering stems. It was also unexpected that conditioning with NS negatively affected the fresh weight, expressed both by its average daily values and the course of changes during 27 days of the vase life. Perhaps less sugar was delivered to the stems, as during the first day of the vase life, with a high uptake intensity, only pure nanosilver was absorbed. Sugar is the “driving force” for the solution uptake.
The fresh weight of flowering lisianthus stems depended not only on the volume of absorbed holding solutions, but on sucrose present in both of them. A crucial role of sucrose in enhancing the vase solution uptake was shown by Bahrami et al. [
3]. Stems from our sucrose containing solutions had their fresh weight significantly higher than those from water controls. Similar observations were made by Huang and Chen [
16], who kept cut lisianthus flowers in 2% sucrose or glucose, and observed increases in their fresh weight while that in the water-held stems decreased during vase life. They showed that exogenous sugars were absorbed from holding solutions where their concentrations dropped after several days, to be translocated to buds and flowers and promoted their opening and maintained water balance. Glucose, fructose, and sucrose were identified as endogenous carbohydrates in lisianthus petals and they accumulated in petals of flowers standing in the sugar-containing solution while decreased in flowers kept in water. Such a reduction in total soluble sugars in lisianthus flowers—regardless of the postharvest treatments and temperatures—were reported by Cavasini et al. [
18]. Apart from the above listed sugars,
myo-inositol and D-bornesitol were found in petals by Norikoshi et al. [
35], but glucose and fructose were the major carbohydrates in petals. Their accumulation in the vacuoles of flowers supplemented with exogenous carbohydrates contributed to the reduction of the osmotic potential, which in turn promoted water influx associated with flower opening. We did not identify individual sugars in petals; however, differences between the amounts of the total soluble and reducing sugars suggest that the latter constituted an important proportion of soluble carbohydrates in lisianthus. At harvest, open flowers were well provided with carbohydrates by the mother plant so the concentration of total soluble sugars was higher than that in the buds. At the same time, the initial reducing sugar concentrations were comparable in both flower types. Generally, flowers held in water had less endogenous carbohydrates during the vase life than those placed into the preservatives, and the highest sugar content in flowers from NS + S may be due to more intensive uptake of the vase solution in this treatment. While conditioning lowered the water uptake rate and thus affected the amounts of sugars delivered to petals, the upper, younger buds on conditioned stems had more total soluble and reducing sugars than those on non-conditioned stems. Carbohydrates needed for bud opening might have come from leaves, which are an intermediate station in the exogenous sugar translocation from the vase solution to flowers. This is only a speculation as unfortunately, we did not analyze the sugar levels in leaves. This might have elucidated certain ambiguities in carbohydrate changes in lisianthus supplemented with sucrose.
The rise in free proline is taken as an indicator of the progress of senescence and such a rise was observed during the vase life of cut lisianthus by Kazemi et al. [
31] and Bahrami et al. [
3]. Treatments extending the vase life limited proline accumulation and both authors found SA as a chemical effective for both purposes. Additionally, in cut peonies, an accumulation of free proline occurred at the end of the vase life and sugar-containing preservatives—either with 8-HQC or NS—reduced this process several-fold relative to water controls. In our experiments, free proline increased in senescing lower and upper flowers standing in water, while the preservatives generally reduced the free proline contents relative to their controls. Unexpectedly, relative to non-conditioned flowers, conditioning enhanced the accumulation of free proline in upper flowers on stems held in water. In contrast, conditioning limited the rise in free proline in lower and upper flowers on stems held in NS + S. Here, an extra dose of nanosilver absorbed during the first 24 h of the vase life might have supplemented the amount of silver taken up by the stems from the vase solution and tipped the balance, making the NS concentration sufficient for the antisenescence action. However, as both sugar-containing solutions reduced free proline level in flowers, it might be sucrose that acted here as the antioxidant defense factor [
38] and this remains to be elucidated.
During senescence, cell damage appears, caused by oxidative stress. Reactive oxygen species (ROS) such as hydrogen peroxide are generated, damaging cells and hastening their death. Living organisms have developed antioxidant defense mechanisms against ROS to reduce the damage caused by the oxidative stress during senescence. Scavenging enzymes such as superoxide dismutase (SOD), CAT, or ascorbate peroxidase (APX) neutralize ROS to maintain the redox balance.
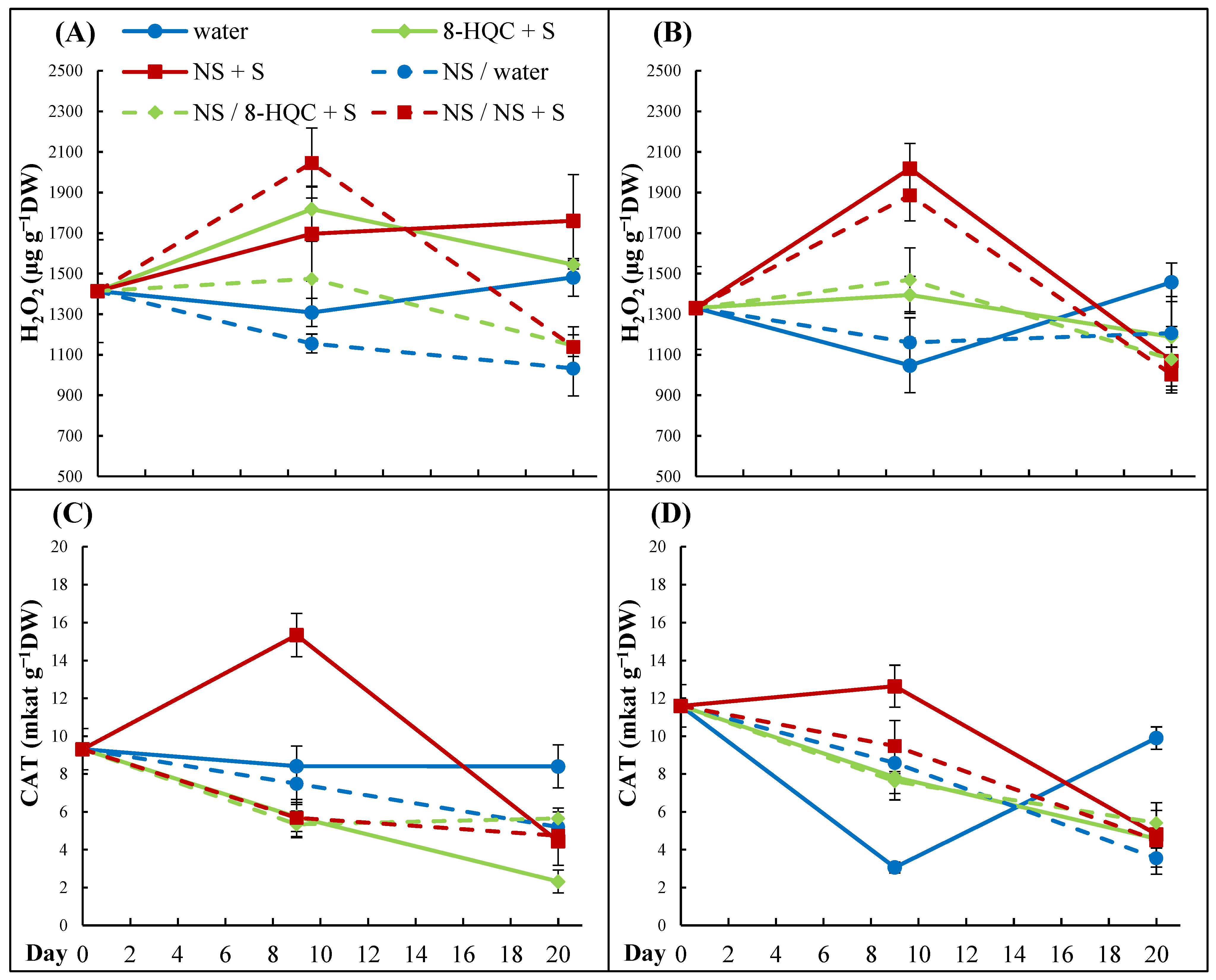
In our experiments, changes in the hydrogen peroxide contents were relatively small and the senescing lower flowers on conditioned stems contained less H
2O
2 than non-conditioned ones. In the upper flowers, striking differences in the H
2O
2 contents appeared on Day 9 between control flowers and those held in NS + S, the latter having the highest hydrogen peroxide concentration. In this case, the CAT activity peaked in synchrony with a rise in H
2O
2 which, however, did not reduce its content. Generally, the CAT activity was reduced with time in lower and upper flowers of all treatments, reaching the values at the end of the vase life than the values below the initial one. This does not confirm earlier reports that treatments limiting the rise in hydrogen peroxide and/or increasing the activity of antioxidant enzymes extends the vase life of cut lisianthus. Ataii et al. [
21] found such a positive effect of polyamine putrescine. Furthermore, SA extended longevity in lisianthus, reducing the H
2O
2 accumulation and enhancing the CAT activity in petals [
22]. A delay in senescence in lisianthus by endogenous hydrogen was also associated with increased activity of CAT [
39]. In cut peonies, the CAT activity increased during the vase life and was higher in flowers held in preservatives, especially in NS + S [
40].