Qualitative and Nutraceutical Characteristics after Storage of New Pear Selections in Emilia-Romagna Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Qualitative Parameters
2.3. HPLC Analysis for Sugars and Organic Acids Content
2.4. Total Phenolic Content
2.5. Ascorbic Acid Content and Total Antioxidant Activity
2.6. Chemicals and Reagents
2.7. Statistical Analysis
3. Results
3.1. Fruit Qualitative Traits
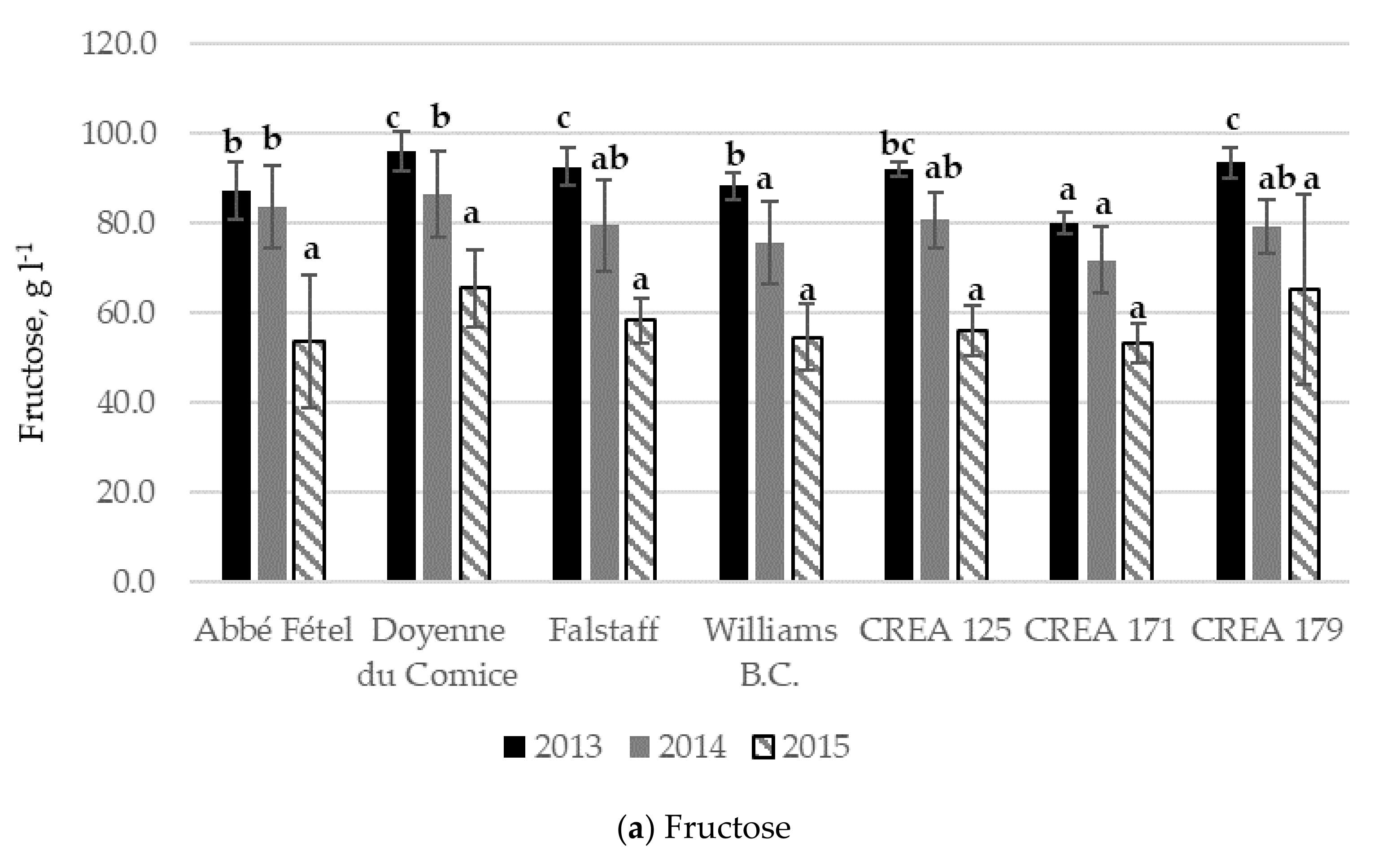
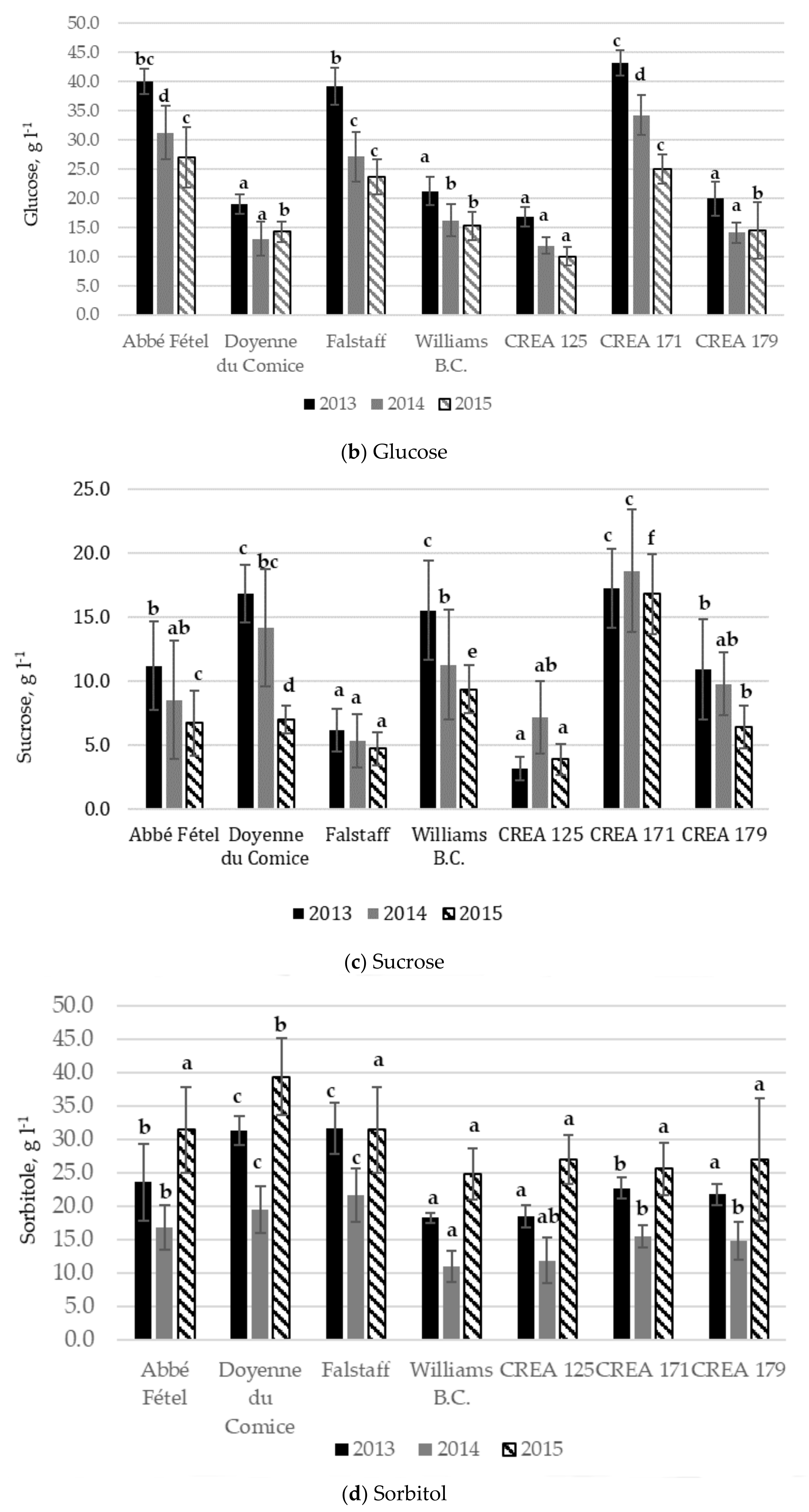
3.2. Sugar and Organic Acid Content
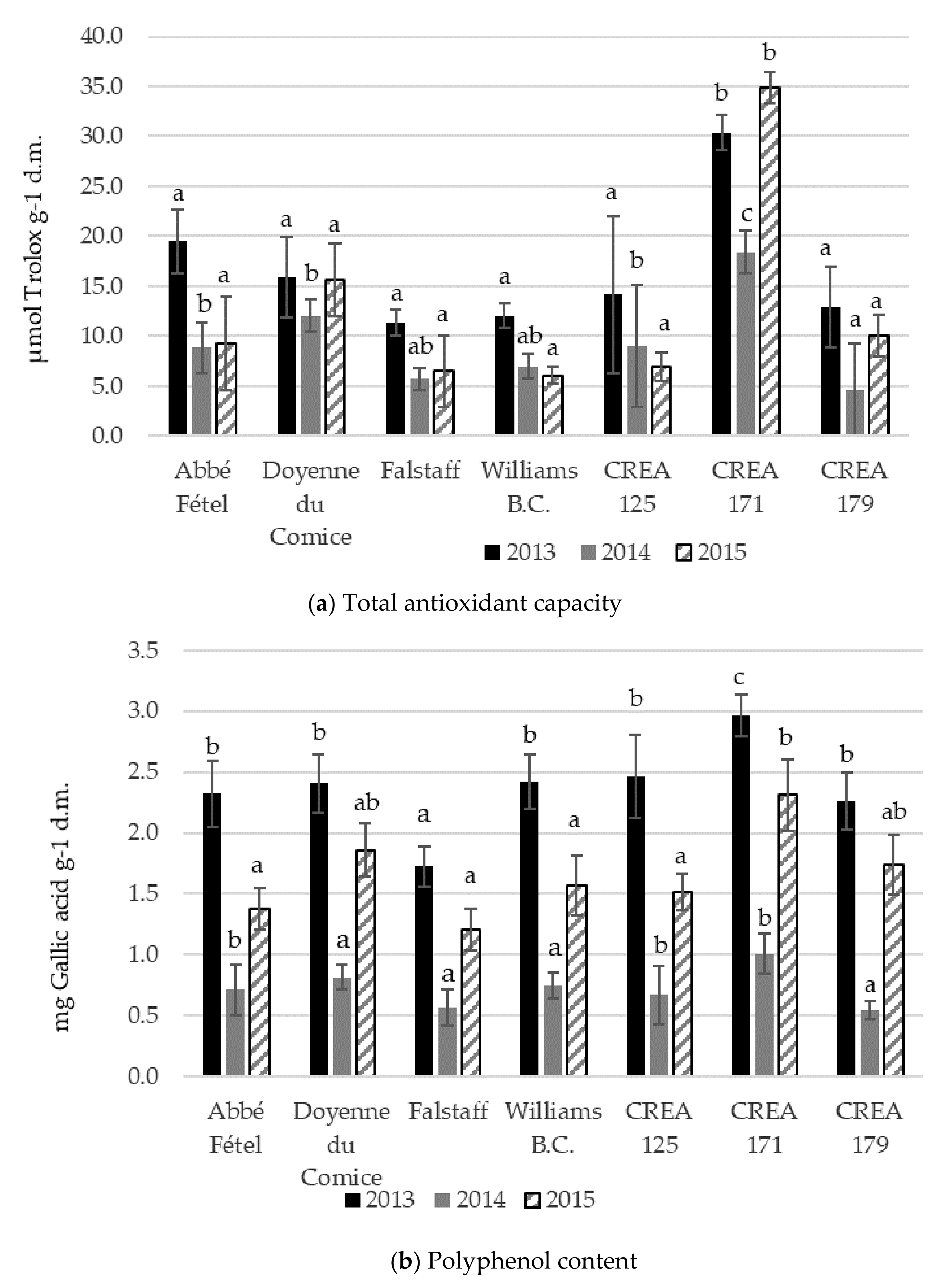
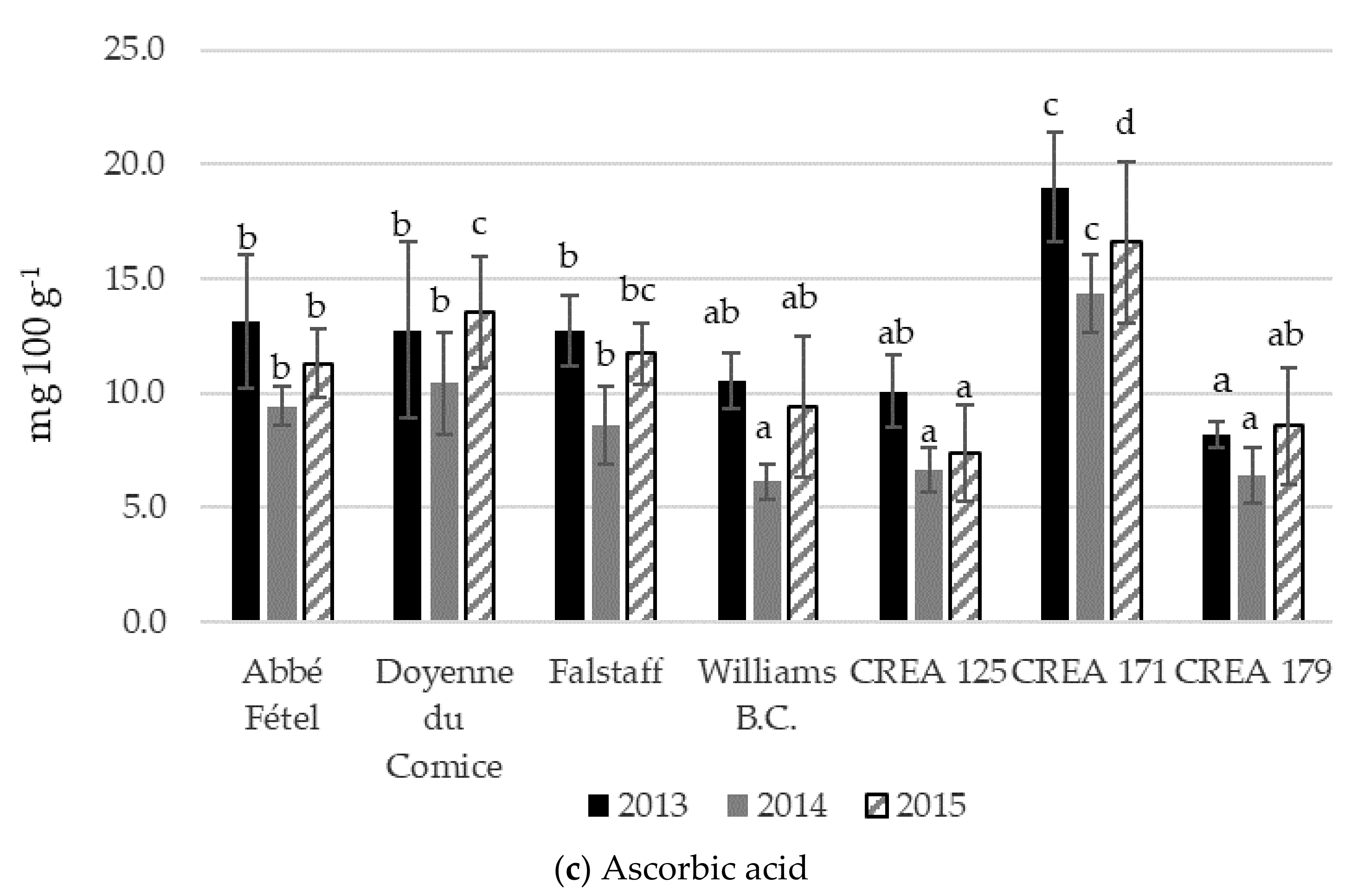
3.3. Total Antioxidant Capacity, Total Polyphenols, and Ascorbic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database; FAO: Rome, Italy, 2020. [Google Scholar]
- Centro Servizi Ortofrutticoli. Italia Consuntivo 2018 Pere; CSO Italy: Ferrara, Italy, 2019. [Google Scholar]
- Faedi, W.; Castagnoli, M.; Baruzzi, G.; Sirri, S. La creazione di nuove varietà presso il CRA-Frf di Forlì. Frutticoltura 2012, 10, 18–24. [Google Scholar]
- Brewer, L.; Volz, R. Genetics and Breeding of Pear. In The Pear Genome. Compendium of Plant Genomes; Korban, S., Ed.; Springer: Cham, Switzerland, 2019; Volume 4, pp. 63–101. [Google Scholar] [CrossRef]
- EccherZerbini, P. The quality of pear fruit. Acta Hortic. 2002, 596, 805–810. [Google Scholar] [CrossRef]
- Proteggente, A.; Pannala, A.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Van De Put, F.; Dacombe, C.; Rice-Evans, C. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. Critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chemistry 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Jaramillo, S.; Lopez, S.; Varela, L.M.; Rodriguez-Arcos, R.; Jimenez, A.; Abia, R.; Guillen, R.; Muriana, F.J.G. The flavonol isorhamnetin exhibits cytotoxic effects on human colon cancer cells. J. Agr. Food Chem. 2010, 58, 10869–10875. [Google Scholar] [CrossRef]
- Galvis Sànchez, A.C.; Gil-Izquierdo, A.; Gil, M.I. Comparative study of six pear cultivars in terms of their phenolic and vitamin C contents and antioxidant capacity. J. Sci. Food Agric. 2003, 83, 995–1003. [Google Scholar] [CrossRef]
- Caracciolo, G.; Sirri, S.; Baruzzi, G. Update on CREA Centro di Ricerca Olivicoltura, Frutticoltura e Agrumicoltura pear breeding program. Acta Hortic. 2021, 1303, 29–36. [Google Scholar] [CrossRef]
- Elgar, H.J.; Watkins, C.B.; Murray, S.H.; Gunson, F.A. “Quality of Buerre Bosc” and “Doyenne du Comice” pears in relation to harvest date and storage period. Postharvast Biol. Technol. 1997, 10, 29–37. [Google Scholar] [CrossRef]
- Sugar, D.; Richardson, D.G.; Chen, P.M.; Spotts, R.A.; Roberts, R.G.; Chand-Goya, T. Advances in improving the postharvest quality of pears. Acta Hortic. 1998, 475, 513–526. [Google Scholar] [CrossRef]
- Streif, J. Ripening management and postharvest fruit quality. Acta Hortic. 2010, 858, 121–129. [Google Scholar] [CrossRef]
- Saquet, A.A. Storage of pears. Sci. Hortic 2019, 246, 1009–1016. [Google Scholar] [CrossRef]
- Kupferman, E. Observations on harvest maturity and storage of apples and pears. Postharvest Inf. Netw. 2002, 1–7. [Google Scholar]
- Kupferman, E.; Spotts, R.; Sugar, D. Practices to reduce postharvest pear diseases. Postharvest Pomol. Newsl. 1985, 3, 11. [Google Scholar]
- Willett, M.; Kupferman, G.; Roberts, R.; Spotts, R.; Sugar, D.; Apel, G.; Ewart, H.W.; Bryant, B. Integrated management of postharvest diseases and disorders of apples, pears and cherries. Postharvest Pomol. Newsl. 1989, 7, 5–7. [Google Scholar]
- De Belie, N.; Hallett, I.C.; Harker, F.R.; De Baerdemaeker, J. Influence of Ripening and Turgor on the Tensile Properties of Pears: A Microscopic Study of Cellular and Tissue Changes. J. Am. Soc. Hortic. Sci. 2000, 125, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Pallotti, G.; Baruzzi, G.; Caracciolo, G.; Sirri, S. Nuovo interesse per le pere rosse, prime osservazioni sui cloni del Crea. Frutticoltura 2016, 10, 12–17. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual method. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Arzani, K.; Khoshghalb, H.; Malakouti, M.J.; Barzegar, M. Postaharvest Fruit Physicochemical changes and properties of Asia (Pyrus serotina Rehd.) and European (Pyrus communis L.) pear cultivars. Hort. Environ. Biotechnol. 2008, 49, 244–252. [Google Scholar]
- Chen, J.; Wang, Z.; Wu, J.; Wang, Q.; Hu, X. Chemical compositional characterization of eight pear cultivars grown in China. Food Chem. 2007, 104, 268–275. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Analysis of Botanicals and Dietary Supplements for Antioxidant Capacity: A Review. J. AOAC Int. 2000, 83, 950–956. [Google Scholar] [CrossRef] [Green Version]
- Kolniak-Ostek, J. Chemical composition, and antioxidant capacity of different anatomical parts of pear (Pyrus communis). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Hudina, M.; Stampa, F. Effect of Fruit Bagging on Quality of ‘Conference’ Pear (Pyrus communis L.). Europ. J. Hort. Sci. 2011, 76, 176–181. [Google Scholar]
- Kolniak-Ostek, J.; Kłopotowska, D.; Rutkowski, K.P.; Skorupinska, A. Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.). Molecules 2020, 25, 4444. [Google Scholar] [CrossRef]
- Premi, M.; Khan, K.A. Nutritional Values of Fruits and Vegetables: Macronutrients, Micronutrients, and Composition. In Technological Interventions in the Processing of Fruits and Vegetables; Sehrawat, R., Khan, K.A., Goyal, M.R., Paul, P.K., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Silva, F.J.P.; Gomes, M.H.; Fidalgo, F.; Rodrigue, J.A.; Almeida, D.P.F. Antioxidant properties and fruit quality during long-term storage of ‘Rocha’ pear: Effects of maturity and storage conditions. J. Food Qual. 2010, 33, 1–20. [Google Scholar] [CrossRef]
- Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Hafiz, A.R.; Suleria, A. Comparative Investigation on Phenolic Composition, Characterization and Antioxidant Potentials of Five Different Australian Grown Pear Varieties. Antioxidant 2021, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Veltman, R.H.; Lentheric, I.; Van Der Plas, L.H.W.W.; Peppelenboss, H.W. Internal browning in pear fruit (Pyrus communis L. cv Conference) may be a result of a limited availability of energy and antioxidants. Postharvest Biol. Technol. 2003, 28, 295–302. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Content of bioactive compounds and antioxidant capacity in skin tissues of pear. J. Funct. Foods 2016, 23, 40–51. [Google Scholar] [CrossRef]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Saltaa, J.; Martinsa, A.; Santosa, R.G.; Nenga, R.N.; Nogueiraa, M.F.; Justinob, J.; Rautera, A.P. Phenolic composition and antioxidant activity of Rocha pear and other pear cultivars—A comparative study. J. Funct. Foods 2010, 2, 153–157. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peel and flesh from 10 different pear varieties (Pyrus spp.). Food. Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
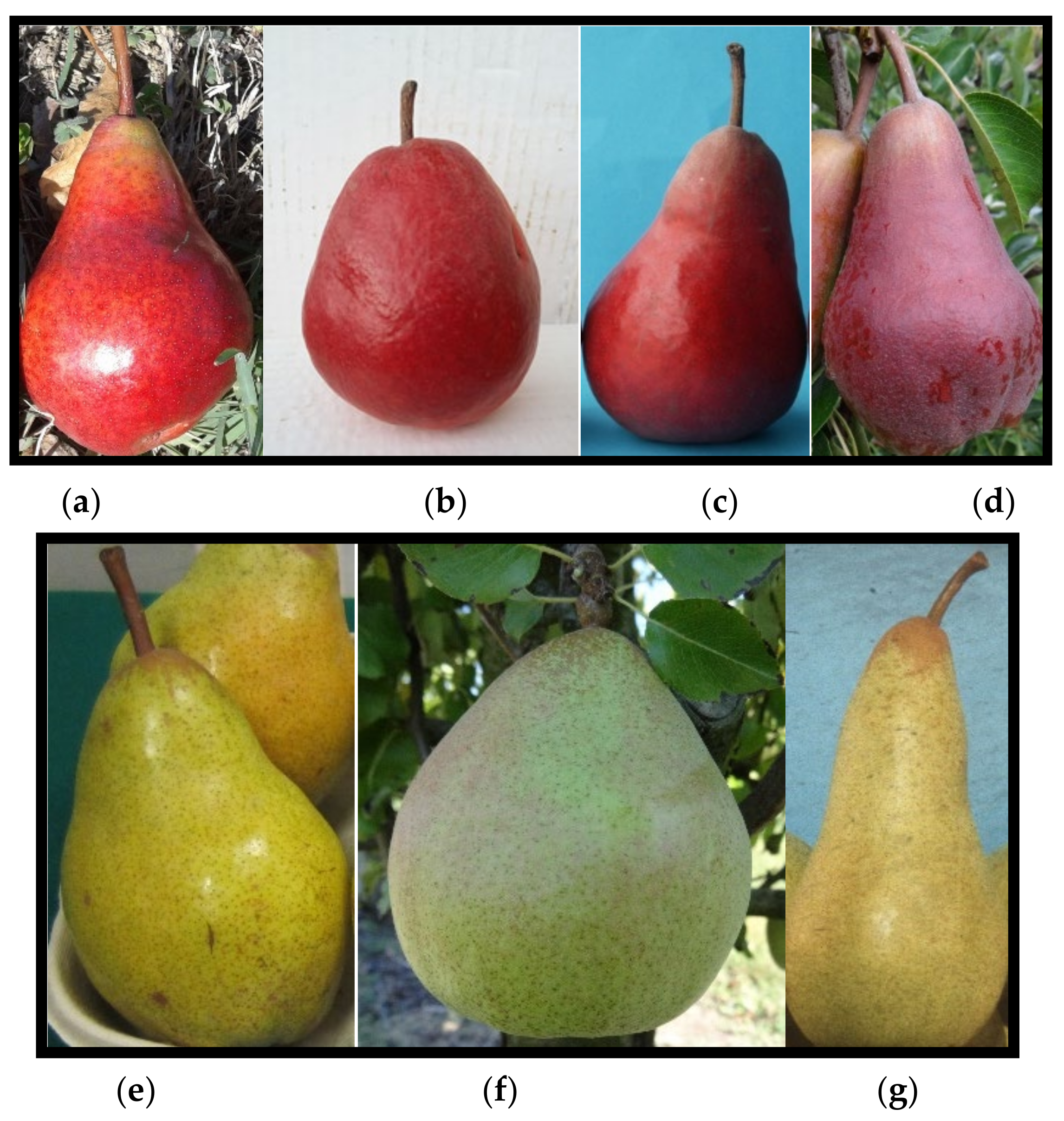

| Harvest Date | Firmness at Harvest, kg cm−2 | Average Weight, g | Size, mm | Colour | Red Over Colour, % | Rustiness, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbé Fétel | 28-Aug | 5.0 | ± | 0.5 | 310 | ± | 46 | 70–75 | green/yellow | 19 | 20 |
| Doyenne du Comice | 01-Sep | 4.5 | ± | 0.3 | 260 | ± | 18 | 75–80 | green/yellow | 0 | 5 |
| Falstaff | 23-Aug | 5.2 | ± | 0.3 | 290 | ± | 52 | 70–75 | yellow | 85 | 5 |
| William | 10-Aug | 6.5 | ± | 0.5 | 220 | ± | 29 | 70–75 | green/yellow | 10 | 0 |
| CREA 125 | 12-Aug | 5.6 | ± | 0.8 | 180 | ± | 26 | 65–70 | yellow | 90 | 0 |
| CREA 171 | 17-Sep | 6.6 | ± | 0.6 | 240 | ± | 49 | 70–75 | Red | 0 | 40 |
| CREA 179 | 27-Aug | 5.2 | ± | 0.4 | 220 | ± | 31 | 70–75 | yellow | 75 | 0 |
| Variety/Selection | Average Fruit Weight, g | TSS, °brix | TA, g Malic acid L-1 | pH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | ||||||||||||||||
| Abbé Fétel | 278.9 | ± | 22.8 | c | 15.8 | ± | 1.0 | c | 1.2 | ± | 0.27 | a | 4.9 | ± | 0.1 | bc |
| Doyenne du Comice | 279.7 | ± | 19.8 | c | 16.1 | ± | 0.5 | c | 1.8 | ± | 0.43 | b | 4.7 | ± | 0.1 | b |
| Falstaff | 315.5 | ± | 53.4 | d | 17.3 | ± | 0.8 | d | 1.2 | ± | 0.11 | a | 5.1 | ± | 0.1 | c |
| Williams B.C. | 233.2 | ± | 13.7 | b | 14.8 | ± | 0.2 | b | 3.0 | ± | 0.27 | d | 4.3 | ± | 0.1 | a |
| CREA 125 | 177.4 | ± | 23.9 | a | 13.9 | ± | 0.5 | a | 1.7 | ± | 0.18 | b | 4.6 | ± | 0.3 | b |
| CREA 171 | 213.2 | ± | 37.8 | b | 16.7 | ± | 0.5 | d | 2.2 | ± | 0.34 | c | 4.5 | ± | 0.2 | ab |
| CREA 179 | 224.1 | ± | 19.8 | b | 15.1 | ± | 0.5 | b | 2.3 | ± | 0.1 | c | 4.4 | ± | 0.2 | ab |
| 2014 | ||||||||||||||||
| Abbé Fétel | 343.5 | ± | 71.8 | c | 14.5 | ± | 0.6 | bc | 1.3 | ± | 0.2 | ab | 4.6 | ± | 0.3 | ab |
| Doyenne du Comice | 270.9 | ± | 22.1 | b | 14.1 | ± | 1.5 | b | 1.6 | ± | 0.2 | bc | 4.5 | ± | 0.4 | a |
| Falstaff | 286.7 | ± | 56.8 | b | 14.9 | ± | 0.7 | bc | 1.0 | ± | 0.3 | a | 5.0 | ± | 0.4 | b |
| Williams B.C. | 225.3 | ± | 35.1 | a | 12.4 | ± | 0.7 | a | 2.6 | ± | 0.6 | d | 4.1 | ± | 0.4 | a |
| CREA 125 | 194.7 | ± | 36.9 | a | 13.4 | ± | 0.8 | ab | 1.8 | ± | 0.3 | c | 4.2 | ± | 0.2 | a |
| CREA 171 | 294.1 | ± | 68.2 | b | 15.3 | ± | 0.5 | c | 2.0 | ± | 0.3 | c | 4.4 | ± | 0.2 | a |
| CREA 179 | 206.2 | ± | 31.9 | a | 13.5 | ± | 1.0 | b | 1.8 | ± | 0.2 | c | 4.3 | ± | 0.2 | a |
| 2015 | ||||||||||||||||
| Abbé Fétel | 316.8 | ± | 48.6 | d | 16.3 | ± | 1.3 | c | 1.9 | ± | 0.2 | c | 4.7 | ± | 0.1 | cd |
| Doyenne du Comice | 228.1 | ± | 12.4 | c | 14.9 | ± | 0.7 | b | 2.2 | ± | 0.3 | c | 4.6 | ± | 0.1 | c |
| Falstaff | 250.4 | ± | 39.8 | c | 15.9 | ± | 1.3 | bc | 2.2 | ± | 0.2 | c | 4.8 | ± | 0.1 | d |
| Williams B.C. | 184.6 | ± | 36.8 | b | 14.3 | ± | 0.5 | ab | 0.9 | ± | 0.2 | a | 4.2 | ± | 0.1 | b |
| CREA 125 | 153.3 | ± | 19.0 | a | 13.5 | ± | 0.5 | a | 1.3 | ± | 0.2 | b | 4.5 | ± | 0.1 | c |
| CREA 171 | 218.5 | ± | 45.9 | c | 16.5 | ± | 1.0 | c | 1.4 | ± | 0.3 | b | 4.1 | ± | 0.2 | a |
| CREA 179 | 228.7 | ± | 39.0 | c | 14.5 | ± | 0.6 | ab | 1.1 | ± | 0.2 | ab | 4.3 | ± | 0.1 | b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caracciolo, G.; Maltoni, M.L.; Faedi, G.; Sirri, S.; Baruzzi, G. Qualitative and Nutraceutical Characteristics after Storage of New Pear Selections in Emilia-Romagna Region. Agronomy 2021, 11, 2515. https://doi.org/10.3390/agronomy11122515
Caracciolo G, Maltoni ML, Faedi G, Sirri S, Baruzzi G. Qualitative and Nutraceutical Characteristics after Storage of New Pear Selections in Emilia-Romagna Region. Agronomy. 2021; 11(12):2515. https://doi.org/10.3390/agronomy11122515
Chicago/Turabian StyleCaracciolo, Giuseppina, Maria Luigia Maltoni, Giulia Faedi, Sandro Sirri, and Gianluca Baruzzi. 2021. "Qualitative and Nutraceutical Characteristics after Storage of New Pear Selections in Emilia-Romagna Region" Agronomy 11, no. 12: 2515. https://doi.org/10.3390/agronomy11122515
APA StyleCaracciolo, G., Maltoni, M. L., Faedi, G., Sirri, S., & Baruzzi, G. (2021). Qualitative and Nutraceutical Characteristics after Storage of New Pear Selections in Emilia-Romagna Region. Agronomy, 11(12), 2515. https://doi.org/10.3390/agronomy11122515






