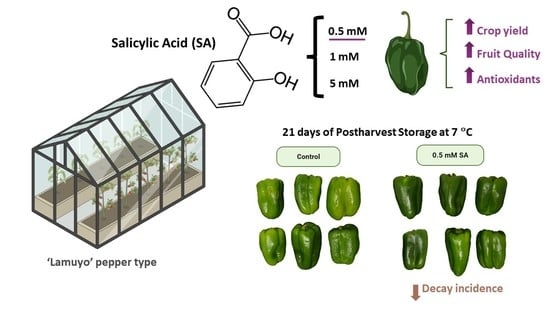
Salicylic Acid Foliar Application Increases Crop Yield and Quality Parameters of Green Pepper Fruit during Postharvest Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments and Growth Conditions
2.2. Crop Yield
2.3. Experimental Postharvest Storage Design
2.4. Quality Parameters of Green Pepper Fruit
2.5. Total Phenolics Content and Hydrophilic and Lipophilic Total Antioxidant Activity
2.6. Incidence Decay
2.7. Statistical Analysis
3. Results
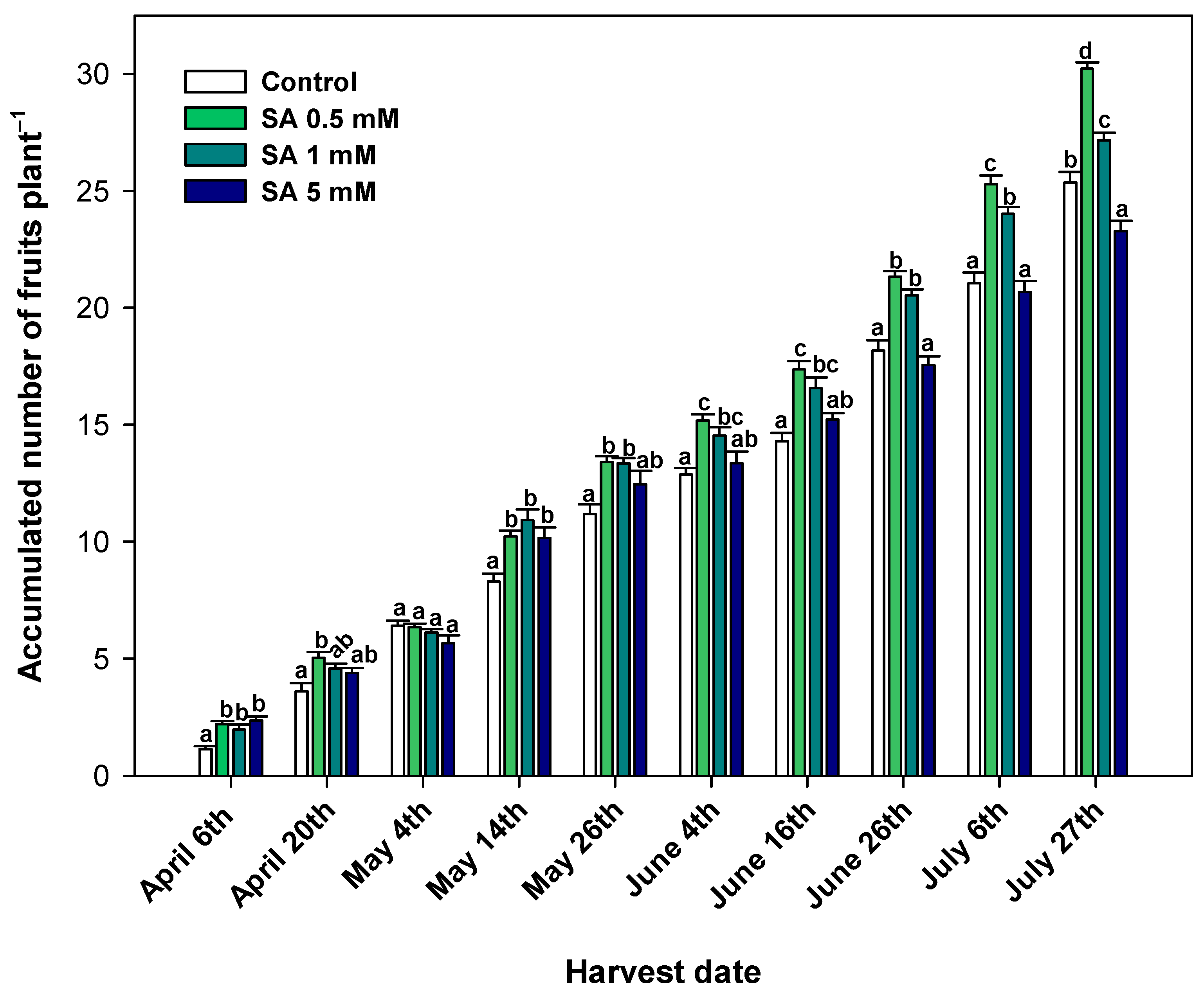
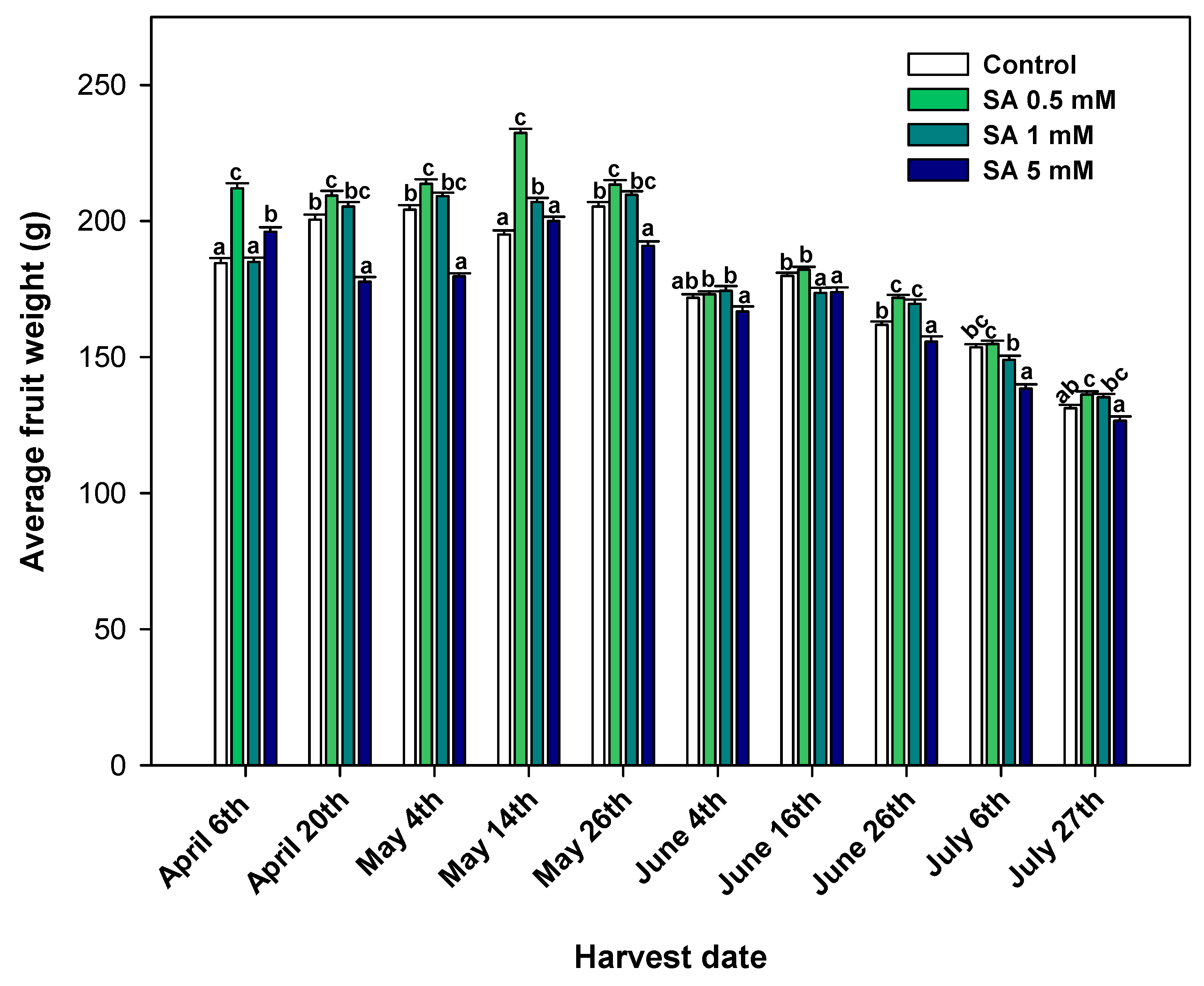
3.1. Effect of SA Preharvest Treatment on Crop Yield
3.2. Effect of SA Preharvest Treatment on Weight Loss, Respiration Rate and Physico-Quemical Parameters at Harvest and during Storage
3.3. Effect of SA Preharvest Treatment on Total Phenolics Content and Total Antioxidant Activity at Harvest and during Storage
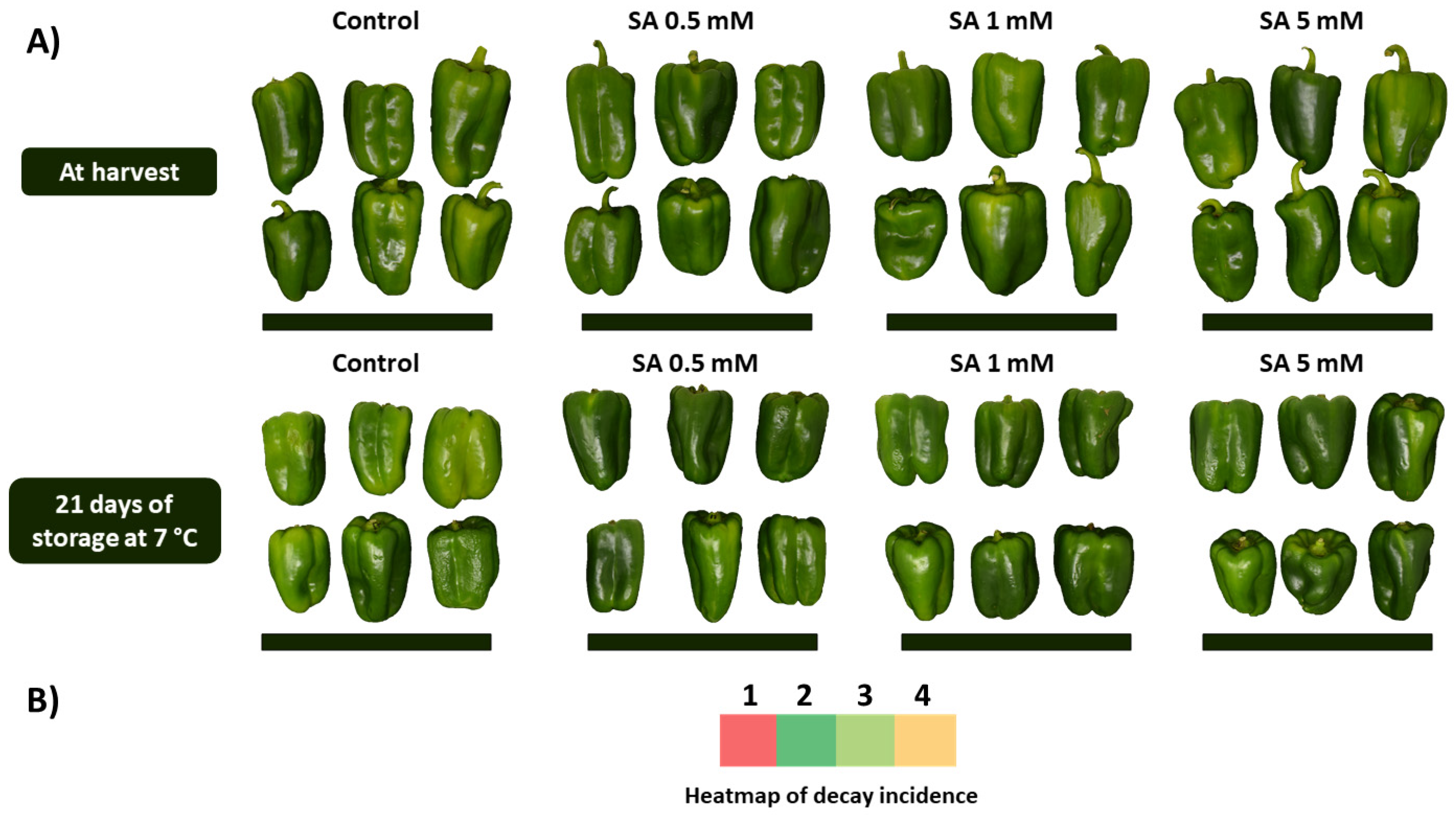
3.4. Effect of SA Preharvest Treatment on Visual Aspect and Decay Incidence of Green Pepper Fruit during Storage
4. Discussion
4.1. SA Preharvest Treatment Applied at Low Concentration Tested Increases Crop Yield
4.2. SA Preharvest Treatment Applied at Low Concentration Tested Improves Fruit Quality Parameters and Functional Quality at Harvest and during Storage
4.3. SA Preharvest Treatment Applied at Low Concentration Tested Induces Fruit Tolerance against Decay Incidence during Storage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majeed, A.; Muhammad, Z. Salinity: A Major Agricultural Problem—Causes, Impacts on Crop Productivity and Management Strategies. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 83–99. ISBN 978-3-030-06117-3. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; El-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; Martínez-Romero, D.; Díaz-Mula, H.M.; Serrano, M. Preharvest treatments with salicylates enhance nutrient and antioxidant compounds in plum at harvest and after storage. J. Sci. Food Agric. 2018, 98, 2742–2750. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, M.; Pazos-Navarrob, M.; López-Marina, A.; Galvez, A.; Varóc, P.; del Amora, F.M. Foliar application of plant growth regulators changes the nutrient composition of sweet pepper (Capsicum annuum L.). Sci. Hortic. 2015, 194, 188–193. [Google Scholar] [CrossRef]
- Souri, M.K.; Tohidloo, G. Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem. Biol. Technol. Agric. 2019, 6, 26. [Google Scholar] [CrossRef]
- Ge, W.; Zhao, Y.; Kong, X.; Sun, H.; Luo, M.; Yao, M.; Wei, B.; Ji, S. Combining salicylic acid and trisodium phosphate alleviates chilling injury in bell pepper (Capsicum annuum L.) through enhancing fatty-acid desaturation efficiency and water retention. Food Chem. 2020, 327, 127057. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma 2019, 256, 79–99. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid in plant salinity stress signaling and tolerance. Plant Growth Regul. 2015, 76, 25–40. [Google Scholar] [CrossRef]
- Sahu, G.K. Salicylic acid: Role in plant physiology and stress tolerance. G.R. Rout and A.B. Das eds. In Molecular Stress Physiology of Plants; Springer: Bhubaneswar, India, 2013; pp. 217–239. [Google Scholar] [CrossRef]
- Rao, T.V.R.; Gol, N.B.; Shah, K.K. Effect of postharvest treatments and storage temperatures on the quality and shelf life of sweet pepper (Capsicum annum L.). Sci. Hortic. 2011, 132, 18–26. [Google Scholar] [CrossRef]
- Blanco-Ríos, A.K.; Medina-Juárez, L.A.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of the phenolic and oily fractions of different sweet peppers. J. Mex. Chem. Soc. 2013, 57, 137–143. [Google Scholar] [CrossRef]
- Maalekuu, K.; Elkind, Y.; Tuvia-Alkalai, S.; Fallik, E.; Shalom, Y. The influence of harvest season and cultivar type on several quality traits and quality stability of three commercial sweet bell peppers during the harvest period. Adv. Hortic. Sci. (Riv. Dell’orthofloro Fruttic. Ital.) 2004, 18, 1000–1005. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Šunić, L.; Fallik, E. Quality evaluation and antioxidant activity of mini sweet pepper cultivars during storage in modified atmosphere packaging (MAP). Rom. Biotechnol. Lett. 2017, 22, 12214–12223. [Google Scholar]
- Raybaudi-Massilia, R.; Suárez, A.I.; Arvelo, F.; Zambrano, A.; Sojo, F.; Calderón-Gabaldón, M.I.; Mosqueda-Melgar, J. Cytotoxic, antioxidant and antimicrobial properties of red sweet pepper (Capsicum annuum L. Var. Llanerón) extracts: In vitro study. Int. J. Food Stud. 2017, 6, 222–231. [Google Scholar] [CrossRef]
- Dobón-Suárez, A.; Giménez, M.J.; Castillo, S.; García-Pastor, M.E.; Zapata, P.J. Influence of the Phenological Stage and Harvest Date on the Bioactive Compounds Content of Green Pepper Fruit. Molecules 2021, 26, 3099. [Google Scholar] [CrossRef] [PubMed]
- Nyanjage, M.O.; Nyalala, S.P.O.; Illa, A.O.; Mugo, B.W.; Limbe, A.E.; Vulimu, E.M. Extending post-harvest life of sweet pepper (Capsicum annum L. ‘California Wonder’) with modified atmosphere packaging and storage temperature. Agric. Trop. Subtrop. 2005, 38, 28–34. [Google Scholar]
- Fung, R.W.; Wang, C.Y.; Smith, D.L.; Gross, K.C.; Tian, M. MeSA and MeJA increase steady-state transcript levels of alternative oxidase and resistance against chilling injury in sweet peppers (Capsicum annuum L.). Plant Sci. 2004, 166, 711–719. [Google Scholar] [CrossRef]
- Konsue, W.; Dethoup, T.; Limtong, S. Biological control of fruit rot and anthracnose of postharvest mango by antagonistic yeasts from economic crops leaves. Microorganisms 2020, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, J.-C.; Jeon, W.B.; Chung, Y.-S.; Sung, S.; Choi, D.; Joung, Y.H.; Oh, B.-J. The salicylic acid-induced protection of non-climacteric unripe pepper fruit against Colletotrichum gloeosporioides is similar to the resistance of ripe fruit. Plant Cell Rep. 2009, 28, 1573–1580. [Google Scholar] [CrossRef]
- Kamara, A.; El-Argawy, E.; El Korany, A.; Amer, G. Potential of certain cultivars and resistance inducers to control gray mould (Botrytis cinerea) of pepper (Capsicum annuum L.). Afr. J. Microbiol. Res. 2016, 10, 1926–1937. [Google Scholar] [CrossRef][Green Version]
- Mekawi, E.M.; Khafagi, E.Y.; Abdel-Rahman, A. Effect of pre-harvest application with some organic acids and plant oils on antioxidant properties and resistance to Botrytis cinerea in pepper fruits. Sci. Hortic. 2019, 257, 108736. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.A.; Khafagi, E.Y.; Soliman, M.S.; Shoala, T.; Ahmed, Y. Preharvest application of salicylic acid induces some resistant genes of sweet pepper against black mold disease. Eur. J. Plant Pathol. 2021, 159, 755–768. [Google Scholar] [CrossRef]
- Elwan, M.W.M.; El-Hamahmy, M.A.M. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 2009, 122, 521–526. [Google Scholar] [CrossRef]
- Veloso, L.L.A.; Lima, G.S.; Silva, A.A.R.; Souza, L.P.; Lacerda, C.N.; Silva, I.J.; Chaves, L.H.G.; Fernandes, P.D. Attenuation of salt stress on the physiology and production of bell peppers by treatment with salicylic acid. Semin. Ciências Agrárias Londrina 2021, 42, 2751–2768. [Google Scholar] [CrossRef]
- Mahmood, N.; Abbasi, N.A.; Hafiz, I.; Ali, I.; Zakia, S. Effect of biostimulants on growth, yield and quality of bell pepper cv. Yolo Wonder. Pak. J. Agr. Sci. 2017, 54, 311–317. [Google Scholar] [CrossRef]
- Ibrahim, A.; Abdel-Razzak, H.; Wahb-Allah, M.; Alenazi, M.; Alsadon, A.; Dewir, Y.H. Improvement in Growth, Yield, and Fruit Quality of Three Red Sweet Pepper Cultivars by Foliar Application of Humic and Salicylic Acids. HortTechnology 2019, 29, 170–178. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2010. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Zapata, P.J. Enhancement of Antioxidant Systems and Storability of Two Plum Cultivars by Preharvest Treatments with Salicylates. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef] [PubMed]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Guillén, F.; Valero, D.; Serrano, M. The Effects of Salicylic Acid and Its Derivatives on Increasing Pomegranate Fruit Quality and Bioactive Compounds at Harvest and During Storage. Front. Plant Sci. 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Zapata, P.J.; Castillo, S.; Serrano, M. Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of ‘Early Lory’ sweet cherry. Postharvest Biol. Technol. 2016, 117, 102–109. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Giménez, M.J.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Preharvest application of methyl jasmonate increases crop yield, fruit quality and bioactive compounds in pomegranate ‘Mollar de Elche’ at harvest and during postharvest storage. J. Sci. Food Agric. 2020, 100, 145–153. [Google Scholar] [CrossRef]
- Tucuch-Hass, C.J.; Pérez-Balam, J.V.; Díaz-Magaña, K.B.; Castillo-Chuc, J.M.; Dzib-Ek, M.G.; Alcántar-González, G.; Vergara-Yoisura, S.; Larqué-Saavedra, A. Role of Salicylic Acid in the Control of General Plant Growth, Development, and Productivity. In Salicylic Acid: A Multifaceted Hormone, 1st ed.; Nazar, R., Iqbal, N., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 1–15. ISBN 978-981-10-6067-0. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Cicek, N.; Guneri, E.; Eraslan, F.; Guzelordu, T. Effects of exogenously applied salicylic acid on the induction of multiple stress tolerance and mineral nutrition in maize (Zea mays L.). Arch. Agron. Soil Sci. 2005, 51, 687–695. [Google Scholar] [CrossRef]
- Barkosky, R.R.; Einhellig, F.A. Effects of salicylic acid on plant water relationship. J. Chem. Ecol. 1993, 19, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef] [PubMed]
- El-Yazied, A.A. Effect of foliar application of salicylic acid and chelated zinc on growth and productivity of sweet pepper (Capsicum annuum L.) under autumn planting. Res. J. Agric. Biol. Sci. 2011, 7, 423–433. [Google Scholar]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M.; Guillén, F. Preharvest Salicylate Treatments Enhance Antioxidant Compounds, Color and Crop Yield in Low Pigmented-Table Grape Cultivars and Preserve Quality Traits during Storage. Antioxidants 2020, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Jacxsens, L.; Devlieghere, F.; Debevere, J. Temperature dependence of shelf-life as affected by microbial proliferation and sensory quality of equilibrium modified atmosphere packaged fresh produce. Postharvest Biol. Technol. 2002, 26, 59–73. [Google Scholar] [CrossRef]
- Rodríguez-Burruezo, A.N.; Kollmannsberger, H.; González-Mas, M.C.; Nitz, S.; Fernando, N. HS-SPME comparative analysis of genotypic diversity in the volatile fraction and aroma-contributing compounds of Capsicum fruits from the Annuum−Chinense−Frutescens complex. J. Agric. Food Chem. 2010, 58, 4388–4400. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Dwivedi, U.N. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef]
- Hanieh, A.; Mojtaba, D.; Zabihollah, Z.; Vahid, A. Effect of pre-sowing salicylic acid seed treatment on seed germination and growth of greenhouse sweet pepper plants. Indian J. Sci. Technol. 2013, 6, 3868–3871. [Google Scholar] [CrossRef]
- Koner, S.; Chatterjee, R.; Datta, S. Effect of planting dates and varieties on growth, fruit yield and quality of bell pepper (Capsicum annuum L.). J. Appl. Nat. Sci. 2015, 7, 734–738. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Buczkowska, H.; Thanoon, A.H. Effect of biological preparations on content of saccharides in sweet pepper fruits. Acta Sci. Pol. Hortorum Cultus 2016, 15, 65–75. [Google Scholar]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytologist. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Kim, S.H.; Chung, I.M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. Pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- Sanmartin, M.; Drogoudi, P.D.; Lyons, T.; Pateraki, I.; Barnes, J.; Kanellis, A.K. Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 2003, 216, 918–928. [Google Scholar] [CrossRef]
- Östbring, K.; Sjöholm, I.; Rayner, M.; Erlanson-Albertsson, C. Effects of Storage Conditions on Degradation of Chlorophyll and Emulsifying Capacity of Thylakoid Powders Produced by Different Drying Methods. Foods 2020, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Sun, X.; Wen, T.; Liu, M.; Yang, M.; Chen, X. Implications of terminal oxidase function in regulation of salicylic acid on soybean seedling photosynthetic performance under water stress. Plant Physiol. Biochem. 2017, 112, 19–28. [Google Scholar] [CrossRef]
- Cisternas-Jamet, J.; Salvatierra-Martínez, R.; Vega-Gálvez, A.; Stoll, A.; Uribe, E.; Goñi, M.G. Biochemical composition as a function of fruit maturity stage of bell pepper (Capsicum annum L.) inoculated with Bacillus amyloliquefaciens. Sci. Hortic. 2020, 263, 109107. [Google Scholar] [CrossRef]
- Li, L.; Zou, Y. Induction of disease resistance by salicylic acid and calcium ion against Botrytis cinerea in tomato (Lycopersicon esculentum). Emir. J. Food Agric. 2017, 29, 78–82. [Google Scholar] [CrossRef]

| Treatments | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|
| Dates | 24 February | 17 March | 6 April | 29 April | 19 May | 9 June | 12 July |
| Days | Control | SA 0.5 mM | SA 1 mM | SA 5 mM | |
|---|---|---|---|---|---|
| Weight loss (%) | 0 | - | - | - | - |
| 7 | 2.62 ± 0.16 aA | 2.29 ± 0.17 aA | 2.42 ± 0.19 aA | 2.54 ± 0.17 aA | |
| 14 | 5.24 ± 0.44 bB | 3.90 ± 0.24 aB | 4.51 ± 0.25 abB | 4.72 ± 0.41 abB | |
| 21 | 7.50 ± 0.52 bC | 5.81 ± 0.35 aC | 6.62 ± 0.38 abC | 6.70 ± 0.49 abC | |
| Respiration rate (mg CO2 kg−1 h−1) | 0 | 86.62 ± 2.91 cC | 65.02 ± 1.62 aC | 73.51 ± 2.63 bC | 74.65 ± 2.86 bC |
| 7 | 18.43 ± 0.44 cB | 12.73 ± 0.44 aB | 14.37 ± 0.43 bB | 14.67 ± 0.41 bB | |
| 14 | 17.34 ± 0.75 bB | 11.47 ± 0.72 aAB | 12.22 ± 0.72 aAB | 12.15 ± 0.67 aA | |
| 21 | 14.15 ± 0.35 bA | 10.63 ± 0.39 aA | 11.73 ± 0.59 aA | 11.43 ± 0.38 aA | |
| Firmness (N mm−1) | 0 | 4.77 ± 0.11 aC | 5.84 ± 0.11 cD | 5.51 ± 0.14 bcC | 5.35 ± 0.11 bC |
| 7 | 3.31 ± 0.17 aB | 4.65 ± 0.17 bC | 3.55 ± 0.19 aB | 3.69 ± 0.19 aB | |
| 14 | 2.94 ± 0.15 aB | 3.70 ± 0.13 bB | 3.20 ± 0.15 abB | 3.03 ± 0.18 aB | |
| 21 | 1.08 ± 0.18 aA | 2.20 ± 0.18 bA | 1.84 ± 0.11 bA | 1.73 ± 0.12 bA | |
| Color (b*) | 0 | 23.57 ± 0.63 aC | 21.75 ± 0.69 aC | 22.04 ± 0.68 aC | 23.43 ± 0.64 aC |
| 7 | 20.89 ± 0.51 bB | 16.67 ± 0.58 aB | 17.22 ± 0.61 aB | 18.70 ± 0.63 aB | |
| 14 | 17.05 ± 0.35 bA | 15.34 ± 0.54 aAB | 15.70 ± 0.55 abAB | 15.68 ± 0.59 abA | |
| 21 | 16.00 ± 0.38 bA | 14.50 ± 0.31 aA | 15.00 ± 0.51 abA | 15.00 ± 0.51 abA | |
| TSS (g kg−1) | 0 | 45.33 ± 1.02 aA | 47.16 ± 1.17 aA | 46.16 ± 1.42 aA | 46.00 ± 1.20 aA |
| 7 | 46.66 ± 1.16 aA | 49.00 ± 1.73 aA | 48.00 ± 1.31 aA | 47.66 ± 1.19 aA | |
| 14 | 47.90 ± 1.42 aA | 49.46 ± 1.35 aA | 48.66 ± 1.62 aA | 48.33 ± 1.85 aA | |
| 21 | 48.30 ± 1.50 aA | 50.83 ± 1.16 aA | 50.33 ± 1.23 aA | 49.90 ± 1.42 aA | |
| TA (g kg−1) | 0 | 1.91 ± 0.07 aB | 2.26 ± 0.05 cC | 2.17 ± 0.07 bcC | 1.96 ± 0.06 abB |
| 7 | 1.75 ± 0.04 aB | 1.92 ± 0.04 bB | 1.90 ± 0.06 abB | 1.89 ± 0.07 abB | |
| 14 | 1.54 ± 0.06 aA | 1.87 ± 0.07 bAB | 1.81 ± 0.05 bAB | 1.75 ± 0.08 abAB | |
| 21 | 1.35 ± 0.08 aA | 1.68 ± 0.06 bA | 1.61 ± 0.07 bA | 1.52 ± 0.06 abA |
| Days | Control | SA 0.5 mM | SA 1 mM | SA 5 mM | |
|---|---|---|---|---|---|
| Total phenolic content (g kg−1) | 0 | 0.707 ± 0.034 aA | 0.854 ± 0.027 bA | 0.850 ± 0.036 bA | 0.828 ± 0.036 abA |
| 7 | 0.800 ± 0.031 aAB | 0.928 ± 0.032 bAB | 0.900 ± 0.036 abAB | 0.914 ± 0.036 abAB | |
| 14 | 0.870 ± 0.029 aBC | 0.987 ± 0.028 bBC | 0.952 ± 0.037 abAB | 0.950 ± 0.025 abB | |
| 21 | 0.947 ± 0.026 aC | 1.049 ± 0.024 bC | 1.017 ± 0.040 abB | 0.993 ± 0.037 abB | |
| H-TAA (g kg−1) | 0 | 0.750 ± 0.059 aA | 1.012 ± 0.049 bA | 1.008 ± 0.043 bA | 1.004 ± 0.039 bA |
| 7 | 1.028 ± 0.053 aB | 1.401 ± 0.055 bB | 1.287 ± 0.048 bB | 1.282 ± 0.044 bB | |
| 14 | 1.212 ± 0.049 aBC | 1.614 ± 0.040 bC | 1.522 ± 0.060 bC | 1.498 ± 0.054 bC | |
| 21 | 1.407 ± 0.063 aC | 1.866 ± 0.058 cD | 1.620 ± 0.036 bC | 1.673 ± 0.035 bD | |
| L-TAA (g kg−1) | 0 | 0.357 ± 0.022 aA | 0.468 ± 0.033 bA | 0.381 ± 0.017 abA | 0.383 ± 0.020 abA |
| 7 | 0.386 ± 0.027 aAB | 0.521 ± 0.038 bA | 0.395 ± 0.024 aAB | 0.392 ± 0.035 abAB | |
| 14 | 0.430 ± 0.029 aAB | 0.647 ± 0.030 bB | 0.497 ± 0.041 aBC | 0.493 ± 0.043 aAB | |
| 21 | 0.467 ± 0.030 aB | 0.729 ± 0.033 bB | 0.539 ± 0.036 aC | 0.535 ± 0.050 aB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobón-Suárez, A.; Giménez, M.J.; García-Pastor, M.E.; Zapata, P.J. Salicylic Acid Foliar Application Increases Crop Yield and Quality Parameters of Green Pepper Fruit during Postharvest Storage. Agronomy 2021, 11, 2263. https://doi.org/10.3390/agronomy11112263
Dobón-Suárez A, Giménez MJ, García-Pastor ME, Zapata PJ. Salicylic Acid Foliar Application Increases Crop Yield and Quality Parameters of Green Pepper Fruit during Postharvest Storage. Agronomy. 2021; 11(11):2263. https://doi.org/10.3390/agronomy11112263
Chicago/Turabian StyleDobón-Suárez, Alicia, María J. Giménez, María E. García-Pastor, and Pedro J. Zapata. 2021. "Salicylic Acid Foliar Application Increases Crop Yield and Quality Parameters of Green Pepper Fruit during Postharvest Storage" Agronomy 11, no. 11: 2263. https://doi.org/10.3390/agronomy11112263
APA StyleDobón-Suárez, A., Giménez, M. J., García-Pastor, M. E., & Zapata, P. J. (2021). Salicylic Acid Foliar Application Increases Crop Yield and Quality Parameters of Green Pepper Fruit during Postharvest Storage. Agronomy, 11(11), 2263. https://doi.org/10.3390/agronomy11112263