Oxyfertigation and Transplanting Conditions of Strawberries
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Cropping System
2.2. Experimental Design
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abad, M.; Noguera, P.; Carrión, C. Los Sustratos en los Cultivos Sin Suelo. In Tratado de Cultivo Sin Suelo, 3rd ed.; Urrestarazu, M., Ed.; Mundi Prensa: Almería, Spain, 2004; pp. 113–158. [Google Scholar]
- Zhi, K.; Hu, W.H.; Dong, D.K.; Zhou, Y.H.; Yu, J.Q. Low O2 supply is involved in the poor growth in root-restricted plants of tomato (Solanum lycopersicum). Environ. Exp. Bot. 2007, 61, 181–189. [Google Scholar]
- Bonachela, S.; Vargas, J.; Acuña, R. Effect of increasing the dissolved oxygen in the nutrient solution to above-saturation levels in a greenhouse watermelon crop grown in perlite bags in a Mediterranean area. Acta Hortic. 2005, 697, 25–32. [Google Scholar] [CrossRef]
- Pivot, T.; Reist, A.; Gilliost, J.; Ryser, J. Water quality, climat environment and mineral of tomato (Solanum lycopersicum L.) in closed soilless cropping system. Acta Hortic. 1998, 458, 207–214. [Google Scholar] [CrossRef]
- Bonachela, S.; Acuña, R.; Casas, J. Environmental factors and management practices controlling oxygen dynamics in agricultural irrigation ponds in a semiarid Mediterranean region: Implications for pond agricultural functions. Water Res. 2007, 41, 1225–1234. [Google Scholar] [CrossRef]
- Barret-Lennard, E. The interaction between water logging and salinity in higher plants: Causes, consequences and implications. Plant Soil 2003, 253, 35–54. [Google Scholar] [CrossRef]
- Urrestarazu, M. Bases y Sistemas de los Cultivos Sin Suelo. In Tratado Internacional de Cultivo Sin Suelo; Urrestarazu, M., Ed.; Mundi Prensa: Almeria, Spain, 2004; pp. 3–48. [Google Scholar]
- Drew, M. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef]
- Irving, L.; Sheng, Y.; Woolley, D.; Matthew, C. Physiological effects of waterlogging on two lucerne varieties grown under glasshouse conditions. J. Agron. Crop Sci. 2007, 193, 345–356. [Google Scholar] [CrossRef]
- Horchani, F.; Gallusci, P.; Baldet, P.; Cabasson, C.; Maucourt, M.; Rolin, D.; Aschi-Smiti, S.; Raymond, P. Prolonged root hypoxia induces ammonium accumulation and decreases the nutritional quality of tomato fruits. J. Plant Physiol. 2008, 165, 1352–1359. [Google Scholar] [CrossRef]
- Horchani, F.; Khayati, H.; Raymond, P.; Brouquisse, R.; Aschi-Smiti, S. Contrasted effects of prolonged root hypoxia on tomato root and fruit (Solanum lycopersicum) metabolism. J. Agron. Crop Sci. 2009, 195, 313–318. [Google Scholar] [CrossRef]
- Devaux, C.; Baldet, P.; Joubès, J.; Dieuaide-Noubhani, M.; Just, D.; Chevalier, C. Physiological, biochemical and molecular analysis of sugar-starvation responses in tomato roots. J. Exp. Bot. 2003, 54, 1143–1151. [Google Scholar] [CrossRef]
- Marfá, O.; Cáceres, R.; Gurí, S. Oxyfertigation: A new technique for soilless culture under Mediterranean conditions. Acta Hortic. 2005, 697, 65–72. [Google Scholar] [CrossRef]
- Asplund, P.T.; Curtis, W.R. Intrinsic oxygen use kinetics of transformed plant root culture. Biotechnol. Prog. 2001, 17, 481–489. [Google Scholar] [CrossRef]
- Ityel, E.; Ben-Gal, A.; Silberbush, M.; Lazarovitch, N. Increased root zone oxygen by a capillary barrier is beneficial to bell pepper irrigated with brackish water in an arid region. Agric. Water Manag. 2014, 31, 108–114. [Google Scholar] [CrossRef]
- Bhattarai, S.; Su, N.; Midmore, D. Oxygation unlocks yield potentials of crops in oxygen-limited soil environments. Adv. Agron. 2005, 88, 313–377. [Google Scholar]
- Urrestarazu, M.; Mazuela, P. Effect of slow-release oxygen supply by fertigation on horticultural crops under soilless culture. Sci. Hortic. 2005, 106, 484–490. [Google Scholar] [CrossRef]
- Halliwell, B.; Clemente, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef]
- Long, L.H.; Evans, P.J.; Halliwell, B. Hydrogen peroxide in human urine: Implications for antioxidant defense and redox regulation. Biochem. Biophys. Res. Commun. 1999, 262, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Yorek, M.A. The role of oxidative stressin diabetic vascular and neural disease. Free Radic. Res. 2003, 37, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M. Hydrogen peroxide: Watery fuel for change in vascular biology. Arter. Thromb. Vas. Biol. 2006, 26, 1931–1933. [Google Scholar] [CrossRef]
- Marks, N.E.; Grandison, A.S.; Lewis, M.J. Use of hydrogen peroxide detection strips to determine the extent of pasteurization in whole milk. Int. J. Dairy Technol. 2001, 54, 20–22. [Google Scholar] [CrossRef]
- Notsu, H.; Tatsuma, T.; Fujishima, A. Tyrosinase-modified boron-doped diamond electrodes for the determination of phenol derivatives. J. Electroanal. Chem. 2002, 523, 86–92. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Krull, U.; Wang, J.; Mascini, M. Biosensors for direct monitoring of environmental pollutants in field. Kluwer Acad. Publ. 1998, 38, 220–226. [Google Scholar]
- Moller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant. Physiol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta -Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Neill, S.; Deskman, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enιas-Filho, J.; Medeiros, J.V.R.; Gomes-Filho, E. Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J. Plant Physiol. 2005, 162, 1114–1122. [Google Scholar] [CrossRef]
- Wahid, A.; Perveen, M.; Gelani, S.; Basra, S.M.A. Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J. Plant Physiol. 2007, 164, 283–294. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Yamaguchi, H.; Yuasa, T.; Iwaya-Inoue, M.; Arima, S.; Zheng, S.H. Hydrogen peroxide spraying alleviates drought stress in soybean plants. J. Plant Physiol. 2011, 168, 1562–1567. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, Y.K.; Lin, S.H.; Fang, Y.Y.; Bai, J.G. Hydrogen peroxide pretreatment alters the activity of antioxidant enzymes and protects chloroplast ultrastructure in heat-stressed cucumber leaves. Sci. Horti. 2010, 126, 20–26. [Google Scholar] [CrossRef]
- Yuan, L.; Wenquan, N.; Jingwei, W.; Jian, X.; Mingzhi, Z.; Kangyong, L. Review on advances of airjection irrigation. Int. J Agric. Biol. Eng. 2016, 9, 1–10. [Google Scholar]
- Horchani, F.; Aloui, A.; Brouquisse, R.; Aschi-Smiti, S. Physiological responses of tomato plants (Solanum lycopersicum) as affected by root hypoxia. J. Agron. Crop Sci. 2008, 194, 297–303. [Google Scholar] [CrossRef]
- Gislerod, H.; Kempton, R. The oxygen content of flowing nutrient solutions used for cucumber and tomato culture. Sci. Hortic. 1983, 20, 23–33. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Dixon, M. An upper limit for elevated root zone dissolved oxygen concentration for tomato. Sci. Hortic. 2007, 113, 162–165. [Google Scholar] [CrossRef]
- Gislerod, H.; Adams, P. Diurnal variations in the oxygen content and acid requirement of recirculating nutrient solutions and in the uptake of water and potassium by cucumber and tomato plants. Sci. Hortic. 1983, 21, 311–321. [Google Scholar] [CrossRef]
- Ehret, D.; Edwards, D.; Helmer, T.; Lin, W.; Jones, G.; Dorais, M. Effects of oxygen-enriched nutrient solution on greenhouse cucumber and pepper production. Sci. Hortic. 2010, 125, 602–607. [Google Scholar] [CrossRef]
- Bhattarai, S.; Huber, S.; Midmore, D. Aerated subsurface irrigation water gives growth and yield benefits to zucchini, vegetable soybean and cotton in heavy clay soils. Ann. Appl. Biol. 2004, 144, 285–298. [Google Scholar] [CrossRef]
- Larkindale, J.; Huang, B. Thermotolerance and antioxidant systems in Agrostis stolonifera: Involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J. Plant Physiol. 2004, 161, 405–413. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Khan, F.; Hassan, S.; Nasim, W.; Arif, M.; Wang, F.; Huang, J. Exogenously applied plant growth regulators affect heat stressed rice pollens. J. Agron. Crop Sci. 2016, 202, 139–150. [Google Scholar] [CrossRef]
- Orihuela, D.L.; Hernández, J.C.; Flores, F.; Tornos, P. La oxifertirrigación y su efecto sobre algunos parámetros productivos en fresa (Fragaria x ananassa D.). Comunicaciones SECH 2012, 20, 161–165. [Google Scholar]
- MAPA. Ministry of Agriculture, Fisheries and Food (Spain). 2017. Available online: https://www.mapa.gob.es/estadistica/pags/anuario/2018/CAPITULOSPDF/CAPITULO07/pdfc07_6.30.2.pdf (accessed on 30 November 2021).
- Palencia, P.; Martínez, F.; Medina, J.J.; Medina, J.L. Strawberry yield efficiency and its correlation with temperature and solar radiation. Hortic. Bras. 2013, 31, 93–99. [Google Scholar] [CrossRef][Green Version]
- Marfà, O.; Guri, S. Efectos de la aplicación de oxígeno en el agua de riego en cultivos sin suelo. Agrícola Vergel 2001, 239, 593–596. [Google Scholar]
- Ledesma, N.A.; Nakata, M.; Sugiyama, N. Effect of high temperature stress on the reproductive growth of strawberry cvs. “Nyoho” and “Toyonoka”. Sci. Hortic. 2008, 116, 186–193. [Google Scholar] [CrossRef]
- De Miranda, F.R.; da Silva, V.B.; dos Santos, F.S.R.; Guimaraes, A.; da Silva, C.F.B. Production of strawberry cultivars in closed hydroponic systems and coconut fibre substrate. Ciênc. Agronômica 2014, 45, 833–841. [Google Scholar] [CrossRef]
- Ben-Haj-Salah, H.; Tardieu, F. Temperature affects expansion rate of maize leaves without change in distribution of cell length. Plant Physiol. 1995, 109, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Basra, M.S.A.; Hussain, S.; Hussain, S.A.; Rehman, H.U.; Rehman, A.; Ali, A. Priming with ascorbic acid, salicylic acid and hydrogen peroxide improves seedling growth of spring maize at suboptimal temperature. J. Environ. Agric. Sci. 2015, 3, 14–22. [Google Scholar]
- Soto-Bravo, F. Oxifertirrigación química mediante riego en tomate hidropónico cultivado en invernadero. Agron. Mesoam. 2015, 26, 227–289. [Google Scholar] [CrossRef]
- Martínez, F.; Castillo, S.; Borrero, C.; Pérez, S.; Palencia, P.; Avilés, M. Effect of different soilless growing systems on the biological properties of growth media in strawberry. Sci. Hortic. 2013, 150, 59–64. [Google Scholar] [CrossRef]
- Caruso, G.; Villari, G.; Melchionnac, G.; Contic, S. Effects of cultural cycles and nutrient solutions on plant growth, yield and fruit quality of alpine strawberry (Fragaria vesca L.) grown in hydroponics. Sci. Hortic. 2011, 129, 479–485. [Google Scholar] [CrossRef]
- Adams, P. Nutritional Control in Hydroponics. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H.C., Eds.; Embryo Publications: Athens, Greece, 2002; pp. 211–261. [Google Scholar]
- Bonachela, S.; Acuña, R.A.; Magan, J.J.; Malfa, O. Oxygen Enrichment of Nutrient Solution of Substrate-Grown Vegetable Crops under Mediterranean Greenhouse Conditions: Oxygen Content Dynamics and Crop Response. Span. J. Agric. Res. 2010, 8, 1231–1241. [Google Scholar] [CrossRef]
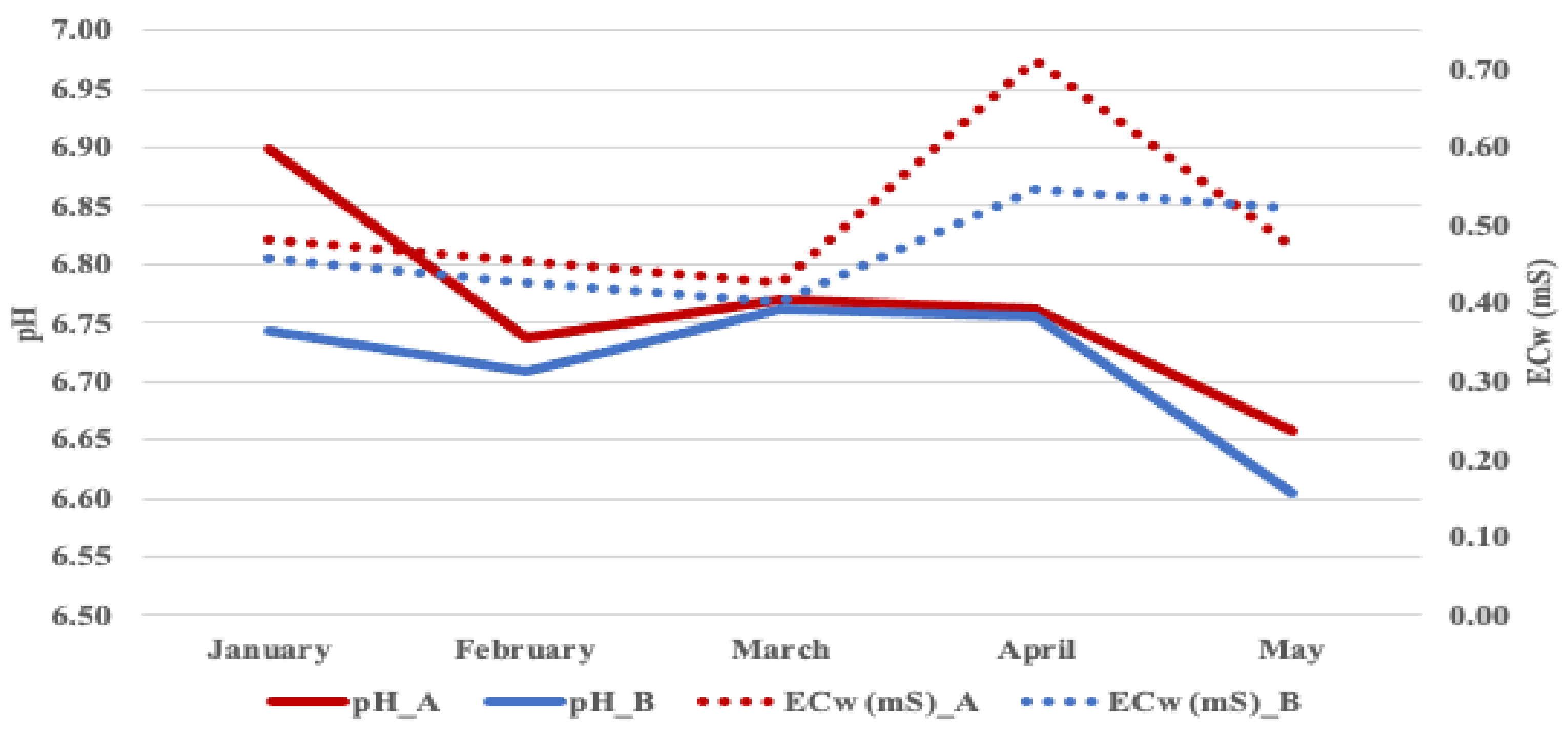
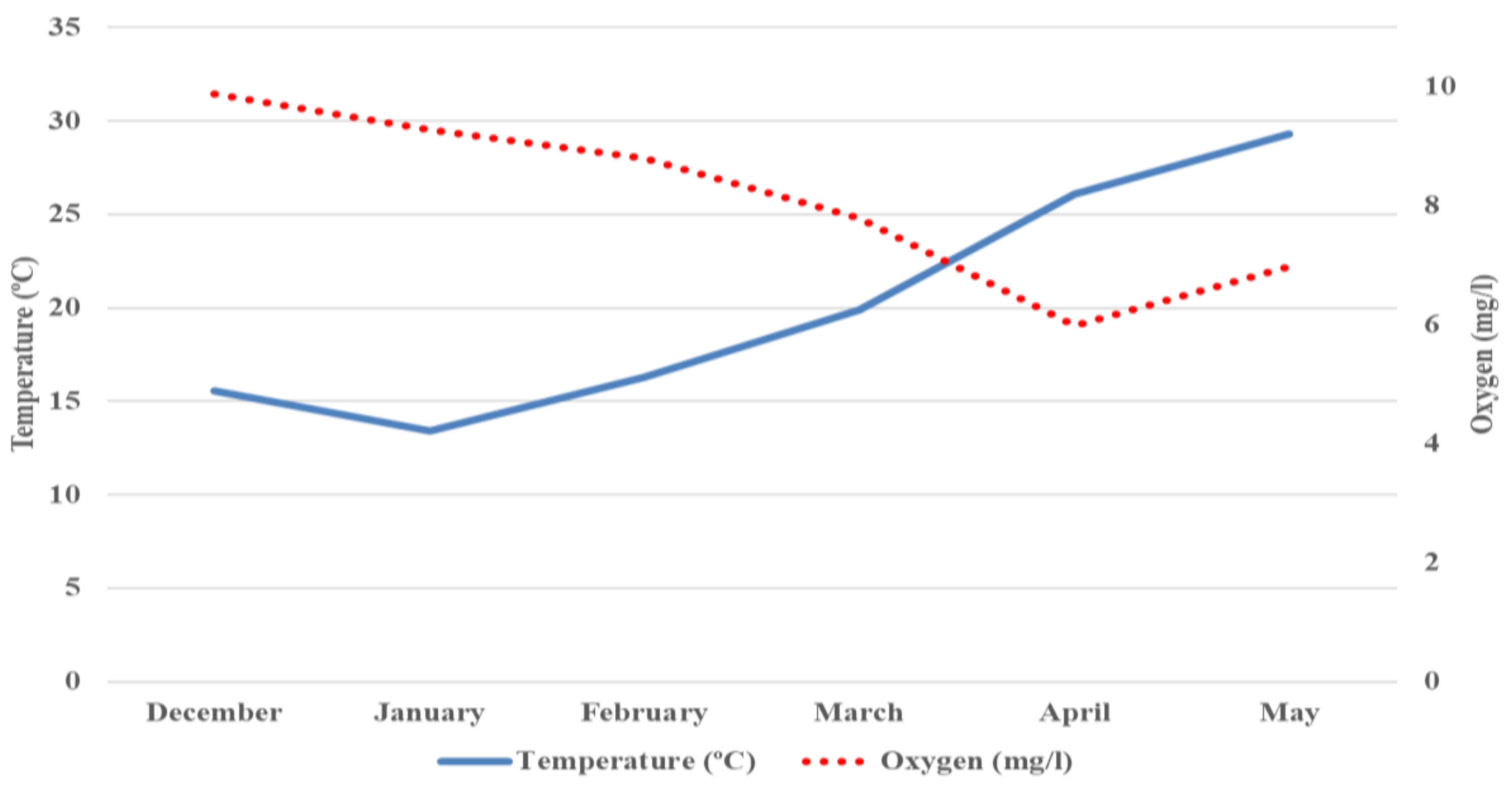
| Experimental Factor | Fruit per Plant | Yield per Plant | Fruit Weight | Fruit Size | Old SPAD | New SPAD | Crown | Diameter | Flowers |
|---|---|---|---|---|---|---|---|---|---|
| (Numbers) | (g plant−1) | (g fruit−1) | (mm) | (Numbers) | (mm) | (Numbers) | |||
| Transplanting conditions (T) 1 | |||||||||
| T1 (control) | 2.77 ± 1.85 | 59.46 ± 37.39 | 22.49 ± 7.36 b | 33.14 ± 4.62 b | 52.85 ± 4.27 a | 38.48 ± 5.00 | 1.85 ± 0.90 b | 34.50 ± 15.06 b | 5.13 ± 4.68 |
| T2 | 2.58 ± 1.28 | 58.18 ± 29.74 | 23.28 ± 8.24 b | 33.30 ± 4.68 b | 52.13 ± 3.75 ab | 38.83 ± 5.64 | 1.84 ± 0.83 b | 34.59 ± 13.64 b | 3.95 ± 3.64 |
| T3 | 2.55 ± 1.97 | 65.54 ± 40.45 | 28.08 ± 10.48 a | 36.51 ± 4.75 a | 50.41 ± 6.56 b | 37.32 ± 4.91 | 2.45 ± 1.01 a | 41.04 ± 15.87 a | 5.54 ± 5.19 |
| Significance | ns | ns | ** | ** | ** | ns | ** | ** | ns |
| Hydrogen peroxide (H2O2) | |||||||||
| H0 (without H2O2) | 2.40 ± 1.48 | 57.40 ± 33.94 | 25.23 ± 9.96 | 34.28 ± 5.21 | 51.95 ± 6.14 | 38.30 ± 5.40 | 1.90 ± 0.91 b | 35.78 ± 14.56 | 4.24 ± 4.09 |
| H1 (with H2O2) | 2.88 ± 1.93 | 65.52 ± 38.22 | 24.34 ± 8.33 | 34.59 ± 4.60 | 51.64 ± 3.79 | 38.13 ± 5.06 | 2.18 ± 0.98 a | 37.59 ± 15.68 | 5.50 ± 4.94 |
| Significance | ns | ns | ns | ns | ns | ns | * | ns | ns |
| Interaction T × H | ns | ns | ns | ns | ns | * | * | ns | ns |
| Experimental Factor | Fruit per Plant | Yield per Plant | Fruit Weight | Fruit Size | Old SPAD | New SPAD | Crown | Diameter | Flowers |
|---|---|---|---|---|---|---|---|---|---|
| (Numbers) | (g plant−1) | (g fruit−1) | (mm) | (Numbers) | (mm) | (Numbers) | |||
| Transplanting conditions (T) 1 | |||||||||
| T1 (control) | 3.33 ± 1.97 | 47.70 ± 30.49 ab | 15.19 ± 3.81 | 33.02 ± 3.97 | 49.93 ± 4.48 | 36.06 ± 2.51 | 2.77 ± 1.16 | 49.55 ± 9.25 b | 5.00 ± 3.36 |
| T2 | 3.00 ± 2.22 | 42.24 ± 31.50 b | 14.33 ± 4.15 | 31.52 ± 4.20 | 48.93 ± 4.48 | 37.13 ± 4.40 | 2.79 ± 1.26 | 53.45 ± 8.22 b | 3.22 ± 3.07 |
| T3 | 4.03 ± 2.11 | 62.06 ± 36.38 a | 15.70 ± 4.91 | 32.80 ± 4.05 | 49.08 ± 4.08 | 36.66 ± 3.46 | 3.44 ± 1.12 | 60.50 ± 5.95 a | 5.60 ± 5.48 |
| Significance | ns | * | ns | ns | ns | ns | ns | ** | ns |
| Hydrogen peroxide (H2O2) | |||||||||
| H0 (without H2O2) | 2.97 ± 1.55 b | 42.48 ± 26.84 b | 15.66 ± 4.33 | 32.31 ± 3.84 | 49.43 ± 5.20 | 36.60 ± 3.14 | 2.79 ± 1.26 | 51.29 ± 10.68 b | 3.47 ± 2.38 |
| H1 (with H2O2) | 3.95 ± 2.53 a | 58.98 ± 37.99 a | 15.08 ± 4.27 | 32.62 ± 4.35 | 49.20 ± 3.74 | 36.62 ± 3.91 | 3.18 ± 1.18 | 57.10 ± 6.18 a | 5.93 ± 5.21 |
| Significance | * | * | ns | ns | ns | ns | ns | ** | ns |
| Interaction T × H | ns | ns | ns | ns | ns | ns | ns | * | ns |
| Experimental Factor | Firmness | pH | Total Soluble Solids | Titratable Acidity | Total Soluble Solids TA−1 |
|---|---|---|---|---|---|
| (g cm−2) | (TSS) (mg kg−1) | (TA) (%) | |||
| Transplanting conditions (T) 1 | |||||
| T1 (control) | 282.13 ± 33.21 | 3.44 ± 0.28 | 6.11 ± 1.15 | 0.54 ± 0.08 b | 11.18 ± 2.78 |
| T2 | 290.69 ± 35.98 | 3.34 ± 0.25 | 6.07 ± 1.20 | 0.59 ± 0.10 b | 10.38 ± 2.81 |
| T3 | 294.74 ± 30.78 | 3.34 ± 0.44 | 6.58 ± 1.48 | 0.73 ± 0.09 a | 11.69 ± 2.76 |
| Significance | ns | ns | ns | * | ns |
| Hydrogen peroxide (H2O2) | |||||
| H0 (without H2O2) | 289.66 ± 34.85 | 3.36 ± 0.39 | 6.24 ± 1.43 b | 0.69 ± 0.08 | 10.79 ± 2.86 |
| H1 (with H2O2) | 289.61 ± 32.22 | 3.38 ± 0.27 | 6.31 ± 1.17 a | 0.56 ± 0.09 | 11.38 ± 2.76 |
| Significance | ns | ns | * | ns | ns |
| Interaction T × H | ns | ns | ns | ns | ns |
| Experimental Factor | Firmness | pH | Total Soluble Solids | Titratable Acidity | Total Soluble Solids TA−1 |
|---|---|---|---|---|---|
| (g cm−2) | (TSS) (mg kg−1) | (TA) (%) | |||
| Transplanting conditions (T) 1 | |||||
| T1 (control) | 304.11 ± 47.30 a | 3.19 ± 0.15 | 6.33 ± 1.51 ab | 0.57 ± 0.07 b | 12.63 ± 1.93 ab |
| T2 | 297.86 ± 49.14 ab | 3.16 ± 0.31 | 6.78 ± 1.70 a | 0.62 ± 0.06 a | 10.94 ± 3.51 b |
| T3 | 272.63 ± 55.58 b | 3.18 ± 0.12 | 5.79 ± 1.43 b | 0.49 ± 0.07 c | 13.82 ± 2.26 a |
| Significance | * | ns | * | ** | * |
| Hydrogen peroxide (H2O2) | |||||
| H0 (without H2O2) | 294.26 ± 49.65 | 3.22 ± 0.26 | 6.33 ± 1.65 | 0.59 ± 0.09 a | 12.60 ± 2.65 |
| H1 (with H2O2) | 291.19 ± 54.26 | 3.14 ± 0.12 | 6.23 ± 1.36 | 0.55 ± 0.06 b | 12.03 ± 3.06 |
| Significance | ns | ns | ns | * | ns |
| Interaction T × H | ns | ns | ns | ** | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palencia, P.; Martínez, F.; Vázquez, M.A. Oxyfertigation and Transplanting Conditions of Strawberries. Agronomy 2021, 11, 2513. https://doi.org/10.3390/agronomy11122513
Palencia P, Martínez F, Vázquez MA. Oxyfertigation and Transplanting Conditions of Strawberries. Agronomy. 2021; 11(12):2513. https://doi.org/10.3390/agronomy11122513
Chicago/Turabian StylePalencia, Pedro, Fátima Martínez, and Miguel A. Vázquez. 2021. "Oxyfertigation and Transplanting Conditions of Strawberries" Agronomy 11, no. 12: 2513. https://doi.org/10.3390/agronomy11122513
APA StylePalencia, P., Martínez, F., & Vázquez, M. A. (2021). Oxyfertigation and Transplanting Conditions of Strawberries. Agronomy, 11(12), 2513. https://doi.org/10.3390/agronomy11122513






