Abstract
Plant bioactive compounds (PBC) are widespread in the plant kingdom, including in forage species, but their impact on silage fermentation and ruminant use of PBC-containing silage has been under-researched. The beneficial effects of PBC include plant-protein protection against excessive degradation by tannins or polyphenol oxidase leading to reduced soluble nitrogen (N) and better N use efficiency by animals, reduced emissions of pollutants such as enteric methane (CH4), improved animal health through antimicrobial, anthelmintic or antioxidant activities, and positive effects on animal product quality—especially greater increased polyunsaturated fatty acid (PUFA) content. However, there are still gaps in the research that require an interdisciplinary effort to ensure a balanced approach that co-addresses the economic, environmental and health pillars of sustainability. Here we review the potential offered by PBC to improve silage quality, nutrient use efficiency, performances and health of ruminants, and product quality. In addition, we use an example of cross-fertilization between disciplines to show that incorporating PBC-containing legume species in grass silage can provide multiple and additive effects from silage fermentation to product quality.
1. Introduction
Several approaches have been proposed in an effort to improve the sustainability of animal production systems by adapting agroecological principles [1]: reducing inputs needed for production, reducing pollution, integrating the management of animal health, exploiting system diversity and preserving biological diversity [2]. Applying these general principles and approaches to resource use for ruminant nutrition creates a number of challenges. There is a need to increase nutrient use efficiency by the animals while decreasing nitrogen (N) excretion and methane (CH4) emissions at the animal level [3], to reduce livestock exposure to pathogens, toxins and oxidative stress [4], and to improve animal product quality, for instance through better meat fatty acid (FA) profiles or oxidative stability [5].
All these challenges come into play for silages, which are an important source of forage outside the growing season in many countries. The demand for high-quality silage to provide forage for ruminants is also made more acute by heightening food–feed competition over limited arable land resources, especially for monogastric livestock. The issue of energy and protein losses (with concomitant pollutant emissions) is even more crucial, as they begin with enzymatic and microbial processes in the silos, long before actual intake of the silage. These losses come in the form of fermentation gases and juices that contain various amounts of energy and soluble N depending on harvest-plant and fermentative process factors [6]. Moreover, silage quality strongly affects silage use by the animals, especially as previously partly-degraded protein will be more degraded in the rumen and thus more N will get excreted as urea [7,8]. There are also potential health issues specific to silage due to the presence of pathogens or mycotoxins produced during the silage-making process [9,10] that can have impacts on ruminant health and performances [11,12] or lead to residues in animal products [13].
Plant bioactive compounds (PBC) have long been recognized as a fertile source of new drugs in the pharmaceutical industry. Mounting public concern over the use of chemicals and pharmaceuticals in ruminant production systems has driven interest in PBC as alternative rumen modifiers and performance enhancers [14]. The biological activity of PBC depends mainly on their chemical nature and concentration in animal diets, with contrasted effects on animal responses. The main desired effects are those promoting animal health (antimicrobial, anthelmintic, anti-bloating, antioxidant, immune stimulator) and performance (production, N use efficiency) and product quality [15], although some PBC (including the beneficial ones used at an undesirable concentration) can be anti-nutritional or even toxic to animals. There has been extensive investigation into the use of PBC in ruminant nutrition, but the value of PBC in silage remains under-researched.
Here we review the state of the art on PBC use in silage as part of practices to promote more sustainable ruminant production systems. Our scope includes opportunities opened up by PBC to improve: (i) silage lactic fermentation and plant protein protection to increase nutrient use efficiency and decrease pollutant losses in the form of greenhouse gases and ammonia (NH3), (ii) animal health (e.g., digestive diseases), and (iii) product quality, for example through FA profiles in meat and milk or increased oxidative stability. We also report results from the EU-funded Marie Curie project LegumePlus (FP7-PEOPLE-2011-ITN-289377, 2012–2015) in which these different aspects were covered together. One objective of the LegumePlus project was to check whether different pillars of sustainability (productivity, environment and product quality) can be co-achieved using two PBC-containing forage legumes, i.e., sainfoin (containing condensed tannins) and red clover (containing polyphenol oxidase; PPO) included in grass-based silage. Their potential benefits and associative effects on silage quality, voluntary intake, digestion, animal performances and meat quality were measured in sheep [16]. The results of this work are reported throughout the manuscript and summarized in Figure 1. The paper concludes by identifying gaps in the research needed to integrate PBC-enhanced silage into farming practices.
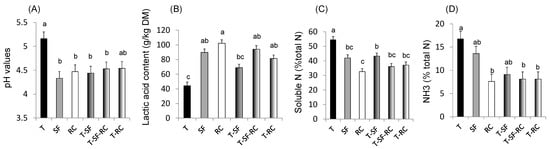
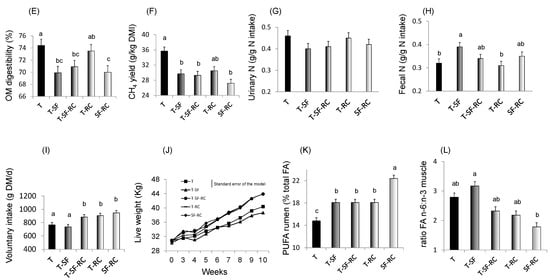
Figure 1.
(A) pH values, (B) lactic acid concentration, and proportions of (C) soluble nitrogen (N) and of (D) ammonia (NH3) in total N in microscale silos of pure timothy (T, black bars), pure sainfoin (SF, grey bars), pure red clover (RC, white bars), binary mixture T–SF (50:50%), ternary mixture T–SF–RC (50:25:25%), and binary mixture T–RC (50:50%) (n = 3 for each type of silage) [33]. (E) Organic matter (OM) digestibility, (F) methane (CH4) yield, (G) urinary and (H) faecal N in sheep fed silages of T, T–SF, T–SF–RC, T–RC and SF–RC (binary mixtures: 50:50%, ternary mixture: 50:25:25%) (n = 10 sheep allocated in a replicated Latin square design) [36]. (I) Voluntary intake, (J) kinetics of live weigh, (K) polyunsaturated fatty acids (PUFA) in rumen content and (L) ratio of n-6:n-3 fatty acids (FA) in Longissimus muscle of growing lambs (n = 8 lambs per group) fed the same silages as in (E–H) [37,38]. a,b,c For each sub-figure, values with different letters significantly differ (p < 0.05).
2. Classes of Plant Bioactive Compounds and Silages
The plant kingdom features a hugely diverse array of bioactive compounds, many of which are found in forage whole plants, feeds or plant extracts that can be incorporated into silage. PBC can be found in grassland plants, crops, agroindustry byproducts, and plant extracts or essential oils in which PBC have all been characterized to varying degrees.
Despite growing interest in the use of PBC in animal nutrition, their use in silages remains limited. Table 1 recaps the results of a literature search on specific classes of plant compounds. Plant molecules were searched based on chemical structure (lipid, aromatic, alkaloid, polyphenol, tannin, saponin, terpene) in the Web of Science, and each class was refined using the terms “bioactive or antimicrobial or anthelmintic or antibiotic”, “silage”, or both. The results highlight the contrast between the large number of articles reporting bioactivity for these compounds and the little literature focused on their effects in silage. Reasons that may explain this substantive lack of research are the low value of silage, the need for farmers to make silage as easily as possible, and other factors known to improve silage quality (ensiling techniques, inoculants). However, although difficult to assess, the economic impact of poor-quality silage, animal disease and reduced performances can be significant. Some bioactive compounds naturally present in a number of forage species or byproducts are potentially cheap yet could bring complementary benefits to existing solutions. The small amount of literature available on PBC bioactivity in silage tends to focus on polyphenols, and especially tannins.

Table 1.
Number of references associated to plant bioactive compounds and silage.
3. Improving Silage Quality
Silage quality is highly dependent on the capacity to maintain the integrity of plants at harvest. Plant degradation and decrease in nutritive value start with the post-harvest continuation of plant respiration leading to hydrolysis of carbohydrates into CO2 and consumption of sugars, which are essential substrates for lactic fermentation [17]. Consequently, the delay between harvest and ensiling should be reduced as much as possible. During silage fermentation, the proteins, which typically represent 70–80% of the total N of the plant, are partly degraded by plant proteases to soluble N and especially amino acids and NH3. Proteolysis raises the soluble N content to 40–60% of total N and continues until the pH drops below 4.0 [17]. The usual way to reach anaerobiosis and the desired pH as rapidly as possible is through an optimal management of silage: correct dry matter concentration at harvest, rapid silo filling, perfect mass sealing, high mass compaction [6]. Unappropriated management procedures and unloading technique, or the use of machinery not suitable to ensure sufficient compaction can lead to air penetration and consequent aerobic deterioration of the ensiled mass. The use of inoculants based on homo- and/or hetero-fermentative lactic acid bacteria can offer a significant help in some situations [18]. Chemical additives can provide a more consistent effect than inoculants because they are less dependent on biological processes, but their higher cost or toxicity may be a limit to adoption by farmers. For instance, benzoate or sorbate have been shown to be safe and of high effectiveness against molds and yeasts, while formaldehyde could be toxic due to irritation of the skin, eye and respiratory organs [19] (for a detailed review on the silage additives, see [18]).
PBCs offer a complementary option to these solutions as they can be used at negligible cost when incorporated in the form of bioactive forages or agroindustry byproducts. Research efforts have focused more on the ability of PBC to reduce protein degradation in the silo, and less on their ability to improve lactic fermentation. A recent meta-analysis unequivocally showed that tannins effectively and efficiently improve silage quality, especially by limiting the extensive proteolysis that may occur during ensiling [20]. Tannin-rich forage legumes are also packed with protein, which make them particularly good candidates to include in silage. Ensiling temperate tannin-rich legumes such as sainfoin or sulla can protect plant proteins during fermentation, reduce NH3 production, and improve nutritive value compared to tannin-free forages [21,22,23]. Tannins have also been shown to limit proteolysis in high-protein silage such as alfalfa, moringa, indigofera and other legume silages [20,24,25]. In a study comparing the effects of tannin levels in sorghum silages supplemented or not with tannin-inactivating polyethylene glycol (PEG), silages without PEG had higher protein levels and lower NH3 concentrations than silages with PEG [26]. Tannins can also be successfully supplemented in silage via byproducts (e.g., green tea waste, [27] and plant extracts containing hydrolysable tannins from chestnut and oak [28,29] or condensed tannins from quebracho [30].
PPO is another bioactive compound found in forages, especially red clover. PPO is an enzyme that can catalyze the oxidation of phenols to quinones, which are highly reactive protein-binding molecules, and thus provide effective protection against proteolysis and lipolysis [31]. PPO is particularly relevant for silages because PPO action requires (i) cellular damages in order to obtain contact between PPO located in chloroplasts and phenols located in vacuoles, and these damages are obtained during harvest, wilting and silage-making processes, and (ii) a time window of aerobiosis for oxidation, which occurs before the stabilization of the silo but not at grazing where the period of aerobiosis between chewing the forage and entry into the rumen is not enough long [32].
Mixing a grass (timothy) with sainfoin and/or red clover in micro-scale silos has been demonstrated to improve fermentation and protein protection against degradation through lower pH values, decreased soluble N and NH3 contents, and higher concentrations of lactic acid compared to pure ensiled grass (Figure 1A–D), thus confirming the benefits of condensed tannins and PPO for silage quality [33].
Other PBC including erythritol byproduct solution and lignosulphonates from wood molasses were studied as silage additives to reduce in-silo proteolysis but were shown to be less efficient compared to tannin solutions [29]. In contrast, spraying essential oils (eugenol, thymol, cinnamaldehyde and carvacrol) before ensiling ryegrass was shown to reduce protein degradation, but usually had to be sprayed at a concentration of 2 g/kg fresh forage to observe inhibition of amino acid deamination [34]. Finally, without really being considered as PBC, sugars added at ensiling or present in high-sugar grasses are well known to facilitate lactic fermentation, especially when the sugar content of forage is a limiting factor [35]. It should be noted that sugars can also be a substrate source for undesirable microorganisms if other inhibitory factors are missing or the silo management is inadequate. In particular, the saccharolytic Clostridia are able to ferment sugars to butyric acid, and excessive concentration of sugars can decrease aerobic stability, as residual sugars are rapidly used by spoilage yeasts and molds [18].
4. Increasing Nutrient Use Efficiency and Animal Performances in Ruminants
Beyond improving silage quality, PBC—and particularly tannins—may have the potential to enhance nutrient (N and energy) use efficiency in ruminants and, in turn, animal performances. However, it remains to be seen whether the PBC provide further biological effects once ingested by ruminants. The question is a relevant one, as the hydroxyl groups in the structure of tannins are already used to bind protein in the silage, which may leave insufficient hydroxyl groups to deliver any further effects in the rumen. However, PBC in silage can still improve N use efficiency only when specific bioactives also protect against excessive microbial protein degradation in the rumen, which means they can simultaneously reduce rumen-degradable protein and increase rumen-undegradable protein. Increased rumen-undegradable protein, which is often called rumen bypass protein, leads to an increase in metabolizable protein, i.e., protein that can be digested and absorbed in the small intestine. A key indicator of protein degradation in the rumen is NH3. Although rumen microbes require a certain amount of to synthesize microbial protein, NH3 overage in the rumen is a form of inefficiency of N use and can subsequently stimulate higher N excretion to the environment.
Various experimental results have shown that tannins in silage could modify rumen fermentation and proteolysis. For instance, increasing supplementation level of Flemingia macrophylla silage, a tropical shrub legume containing condensed tannins, reduced in vitro ruminal NH3 concentration in both high-roughage and low-roughage diets [39]. Apart from being natively present in the material ensiled, tannins can also be added to silage to modulate fermentation processes. Adding tannin extract from chestnut (on a 2% and 4% dry matter basis) to moringa and indigofera legume silages decreased NH3 concentration both in the respective silages and during in vitro rumen fermentation [20]. In agreement with in vitro results, an in vivo experiment in beef cattle showed that adding tannic acid (a hydrolysable tannin) to an alfalfa silage-based diet reduced ruminal NH3 concentration by 18.1% more than control [24].
A decrease of excessive proteolysis during rumen fermentation when PPO-containing red clover is ensiled together with other forage species has been evidenced in vitro both with temperate [40] and tropical grasses [41]. Note, however, that as PPO is inactive in the anaerobic conditions of the rumen, the protection of plant protein can only be the result of pre-ingestive events [42]. In vivo, a significant reduction of rumen NH3 release per unit of dietary N consumed is observed for red clover-containing silages compared with other forages [43,44], reflected by a higher flow of non-NH3-N to the small intestine corresponding to undegraded or partially degraded dietary protein. This results in an increase in N use efficiency when dietary N intake is balanced. However, when surplus dietary N is not balanced with more fermentable energy to maximize microbial protein synthesis in the rumen, urinary N losses increase [42].
The enhanced silage quality when sainfoin and red clover were included in grass silage was reflected in rumen digestion as shown in an in vitro rumen fermentation assay in which several parameters were positively affected [45]. In particular, ensiling red clover or red clover-sainfoin mixture with timothy has been shown to reduce the ratio of NH3 to insoluble N (as an indicator of rumen proteolysis) by 25.2% and 28.9%, respectively, compared to controls. A positive associative effect between timothy and red clover on the production of volatile fatty acids (VFA) was also observed. The effects of timothy silages with vs. without sainfoin, red clover or both on total tract digestion were then investigated on sheep in metabolic cages [36]. Results showed that N excretion was directed towards feces rather than urine with the timothy–sainfoin mixture (Figure 1G,H). This finding suggests that the tannin-protein complexes did not completely dissociate during their transit through the digestive tract, or may confirm the hypothesis that the complexes can re-form under the alkaline conditions of the intestine after dissociation in the acidic conditions of the abomasum [46]. This ‘shift’ in N excretion from urine to feces is environmentally beneficial, because urinary N is more volatile and therefore more quickly converted into greenhouse gases (nitrous oxide) than fecal N [47]. However, it did not yield any productive benefit, as the proportion of N retained in animal tissues was not significantly improved. N digestibility was greater for pure grass and the timothy–red mixture clover than for the timothy–sainfoin mixture, and the amount of N retained daily by the animals was greater for red clover-containing silages than for other silages [36].
Turning to energy use efficiency, PBC have been shown to mitigate enteric CH4 emissions from ruminants [48]. Apart from its contribution to global warming, enteric CH4 emission per se is a form of animal energy loss. Approximately 4–14% of ruminant gross energy intake is lost as CH4, so mitigating CH4 emissions also contributes to more efficient energy utilization by ruminants. Increasing the proportion of red clover silage to orchardgrass linearly reduced CH4 emissions per unit of dry matter intake in sheep [49]. Increasing the level of Flemingia macrophylla silage supplementation in rice straw-based diets decreased in vitro CH4 emissions and was accompanied by a decrease in protozoa population [39]. The use of this tannin-containing local fodder shrub in silage has also been shown to improve nutrient digestibility, rumen fermentation efficiency, and milk production in lactating dairy cows [50]. In vitro, adding chestnut tannin extract into moringa and indigofera legume silages reduced CH4 emissions [20]. In vivo CH4 emissions were lower in beef cattle consuming an alfalfa silage-based diet treated by gallic acid, a subunit of hydrolysable tannins [24]. The ability of tannins to mitigate enteric CH4 emissions is possible via a number of mechanisms. Tannins inhibit microbial degradation of plant cell wall such as cellulose and hemicellulose, and also reduce the formation of hydrogen, which is a major substrate for methanogenesis. PBC also inhibit methanogens [51], the microbial group responsible for methanogenesis in the rumen, and reduce populations of protozoa [52] that live in symbiosis with methanogens. Note that the tannin-induced reduction in CH4 emissions is often accompanied by a reduction of organic matter digestibility, as shown in metabolic cages with the inclusion of sainfoin in silage compared to pure timothy (Figure 1E,F; [36]).
PBC-containing silage can enhance the productive performance of ruminants via improved N and energy use efficiency, provided that the bioactive compounds are present in moderate concentrations, i.e., not too low and not too high. The effect of PBC in silage on nutrient use efficiency and animal performance is not, therefore, linear. For instance, increasing proportion of red clover silage to orchardgrass silage quadratically decreased urinary N loss and tended to quadratically increase N retention in sheep. Red clover silage at a 25% feed inclusion level had the highest N retention, whereas higher levels led to a decline in N retention [49]. Dietary tannins have to be at a moderate level to generate optimal N use efficiency and animal performance [46,53]. Negligible or very low dietary tannin levels would not affect the performance, while overly-high dietary tannins (typically > 5% dry matter) would reduce rumen fermentation, digestion parameters and animal performance. A precondition for successful use of dietary tannins is that the compounds bind and protect protein from microbial degradation in the rumen by forming tannin–protein complexes, but the complex needs to be released at low pH in the abomasum to make the released protein available for further digestion and absorption in the small intestine. At too high dietary tannin concentrations, tannin-to-protein binding is very strong and hard to release in the abomasum, making the protein unavailable in the small intestine. A study comparing animal performances in growing lambs fed different silage mixtures containing or not sainfoin and red clover (or both) found strong differences among PBC-containing forages [37]. Lambs fed mixtures containing red clover showed higher voluntary dry matter intake compared to lambs fed pure timothy or the timothy–sainfoin mixture (Figure 1I), and lambs fed silage containing 50% red clover had 29.8% average daily gain than lambs fed pure timothy (Figure 1J). Logically, this led to higher growth rates, reaching up to 235 g/day for the timothy–red clover mixture (against 145 g/day for the timothy–sainfoin mixture), and to carcass weights of up to 20.5 kg for the timothy–red clover mixture (against 17.2 kg for the timothy–sainfoin mixture).
5. Maintaining or Improving Ruminant Health
The ensiling process is managed on-farm by farmers who do not have the same kind of quality control resources as manufacturers of marketed feeds. The compliance of good practices in silo management remains the best way to limit the risk that silages contain undesirables affecting the animal. Silage-related hazards to ruminant health are mainly due to undesirable micro-organisms competing for nutrients with lactic acid bacteria or using lactate during the fermentation (Enterobacteria, Clostridia), aerobic spoilage due to yeasts and molds, mycotoxins, and metabolic disorders [54]. There are very few papers reporting the effects of PBC on ruminant health specifically in silage. However, PBC that have been shown to be active in non-silage diets can also be active when incorporated in silage, as long as they do not get removed or biotransformed by microorganisms and enzymes during ensiling.
Mycotoxins in silage pose a major risk for ruminant health [10]. The main toxic effects and risks are reduced feed intake and production, reproductive problems, immunosuppression, and there is also a non-negligible risk of certain metabolites (e.g., aflatoxin M1 or ochratoxin A) transferring into meat and milk and potentially causing problems for human health. To our knowledge, in contrast with other additives such as chemicals and inoculants [11], PBC have never been investigated or shown as a possible solution against the production of mycotoxins in silage. With the exclusion of Penicillium species, typical silage molds (Fusarium, Aspergillus, Monascus, Rhizopus, Geotrichum) are intolerant to low-oxygen. Thus, the adoption of correct ensiling procedures enabling to reduce the area exposed to risk of air penetration, such as appropriate dry matter content and particle sizes at ensiling or optimal mass compression, remains the best strategy to limit the presence of molds and mycotoxins in silage. It should be noted that, although high concentration of sugars in ensiled material helps stimulate fermentation, it tends to aerobic instability, as a lot of sugar may still be available for aerobic microorganisms [18].
Parasitism with gastrointestinal nematodes remains ubiquitous in grazing ruminants, and on-farm control largely depends on regular use of anthelmintic drugs, resulting in increased nematode resistance and reduced drug efficacy worldwide [55]. Thus, the identification of complementary or alternative solutions such as cures of PBC with anthelmintic activity is crucial to achieve sustainable parasite control in ruminants. Several forage plants have been shown to have anti-parasitic activity, including when used as silage. Chicory silage containing sesquiterpene lactones significantly reduced Ostertagia ostertagi adult burdens in cattle [56]. Sericea lespedeza and sainfoin silages containing condensed tannins demonstrated anthelmintic activity in vitro by inhibiting the exsheathment of Haemonchus contortus larvae, which is a crucial step in the life-cycle of parasites since it represents the transition from the free-living to the parasitic stages, and in vivo by decreasing adult worm burden and fecal egg counts in small ruminants [57,58].
There is evidence in farm animals of a detrimental effect of inflammation and oxidative stress characterized by imbalance between the formation of oxidants (radicals and non-radicals) and their detoxification by the antioxidant system, causing compromised immune responses and health disorders [4]. Although many of the antioxidant defense capacities of ruminants are derived from vitamins and minerals [59], supplying PBC in ruminant’s diet appears to be a promising approach. The main PBC reported to limit inflammation and oxidative processes are polyphenols, but the modes of action are still not fully elucidated [60]. It was shown that grape polyphenols may induce cytoprotective mechanisms after calving to cope with oxidative and inflammatory stress [61]. An additional study also indicated a potential reduction in endoplasmic reticulum stress and metabolic stress in the liver by the plant polyphenols [62]. Another effect of plant antioxidants is that they help prevent lipid oxidation, especially for polyunsaturated fatty acids (PUFA) that are particularly susceptible to oxidative damage, resulting in lipid peroxidation of membrane phospholipids that can affect normal cellular functions [63,64,65,66]. The PBC with an antioxidant potential can be supplied through silage feeding, and studies usually show far higher concentrations in herbaceous species than in corn [67], with variations according to the botanical families, seasons, and production systems [68].
6. Effects on Product Quality
An additional beneficial effect of PBC with antioxidant potential as described above is an increase of PUFA content and oxidative stability in ruminant products, allowing to produce animal-source foods with health-promoting properties for human. This attribute has been partly assigned to PUFA, particularly n-3 FA [69] and conjugated linoleic acid (CLA; [70]), but not assigned to saturated FA (SFA). Unfortunately, large amounts of PUFA undergo transformation processes in the rumen through microbial biohydrogenation to form FA with a higher degree of saturation [71]. This explains the high SFA content in ruminant products and the potential health risks tied to their consumption. The biohydrogenation process therefore needs to be controlled in order to obtain better FA profiles in farmed animal products, which makes it vital to develop nutritional strategies for optimizing the process while maintaining optimal rumen function and fermentation.
Increasing the PUFA content, especially omega-3 FA, in ruminant products is a desirable way forward, as unlike SFA, omega-3 FA are thought to bring health benefits such as reducing the risk of cardiovascular disease and lowering plasma cholesterol levels [69]. Promoting the level of c9,t11 C18:2, which is produced from partial biohydrogenation of C18:2 n-6 in the rumen, is of interest since it has been shown to prevent cancer proliferation, decrease atherosclerosis, and improve immune response [72]. In relation to the respective CLA isomer, t11 C18:1 is also desirable since it can be converted in ruminant tissues of to c9,t11 C18:2 via the action of the enzyme Δ-9 desaturase (stearoyl-CoA desaturase) by adding a cis9-double bond [71]. Any nutritional measure for manipulating the biohydrogenation of FA in the rumen is therefore directed towards these objectives. Feeding forages to ruminants is associated with improved FA profiles in the products, since lipids in forages tend to be naturally rich in PUFA [73]. For instance, it has been shown that feeding grass-based diets results in higher levels of C18:3 n-3 in muscle compared to concentrate-based diets [74]. Another study observed that feeding grass silage to fattening bulls led to higher C18:3 n-3 in the adipose tissue compared to bulls fed maize silage [75]. The positive effect of dietary grass supplementation on the fatty acid profile of milk and derived products (e.g., cheese) has also been demonstrated in different lactating species such as cows [76], buffalo [77] and ewes [78]. Forage species, cultivar, conservation method and level of inclusion are all sources of variation in the rates and extents of ruminal biohydrogenation of dietary FA from forages as well as their transfer to products [79].
Tannin has been reported to reduce PUFA biohydrogenation, accumulate t11 C18:1 and/or decrease C18:0 concentration [80,81,82,83,84], and to increase the rate of C18:3 n-3 transfer from feed to milk [85], and may thus alter FA composition in the animal products [74]. In vitro incubations of tropical [83] and alpine plants [84] with added linseed oil suggest the ability of plant phenolics to modulate FA biohydrogenation, i.e., by decelerating the process right from the first step. This was indicated by lower disappearance of C18:3 n-3 and C18:2 n-6 in the incubations of both tropical and alpine plants containing high phenolics. Such effects may be explained by the toxicity of phenolics, particularly tannin phenolics, to bacterial species involved in FA biohydrogenation [80,86]. It is also possible that phenolics inhibit the process of lipolysis, which is a prerequisite to further transformation of FA, i.e., biohydrogenation [87,88]. In line with this argument, research has found a negative relationship between tannins present in Vicia sativa and Trifolium incarnatum and the biohydrogenation of C18:3 n-3 [82]. Other phenolics are thought to drive the increase in C18:3 n-3 transfer from feed to milk in animals fed buckwheat [85], phenolics which might be rutin or fagopyrin [89].
Regarding the influence of plant phenolics on the occurrence of biohydrogenation intermediates, an in vitro study [80] found a considerable increase of t11 C18:1 to the expense of C18:0 when adding Acacia mearnsii extract (source of condensed tannins) at 79 g/kg dry matter to a mixed grass–clover hay diet supplemented with linseed oil. The authors suggested that the tannins inhibited the terminal step of FA biohydrogenation. The accumulation of t11 C18:1 in the presence of tannin phenolics was confirmed by another in vitro study [81]. This effect might be related to the toxicity of phenolics, especially tannin, to bacterial species involved in FA biohydrogenation, through selective inhibition of cell wall synthesis [90], interaction of phenols with microbial proteins [91], and direct phenol–lipid interactions [92]. Clostridium proteoclasticum, a bacterial species responsible for the terminal step of biohydrogenation, i.e., the conversion from t11 C18:1 to C18:0 [93], was proved to decrease by 31% in the rumen of lambs fed a tannin-supplemented diet compared to a control diet [86].
The different structures of tannins, i.e., hydrolysable and condensed tannins, may serve to modify the biohydrogenation pattern. Hydrolysable tannins were the main class of tannins that prevented C18:3 n-3 and C18:2 n-6 from biohydrogenation, whereas condensed tannins were more closely correlated with the appearance of c9,t11 C18:2 [83]. This illustrates that both types of tannins are involved in the inhibition of biohydrogenation, but in different steps, i.e., hydrolysable tannins in the first step, and condensed tannins in the second step. By contrast, it has been observed in vitro and in vivo that adding condensed tannin extracts to diet led no difference in concentration of conjugated C18:2 in the ruminal fluid but instead led to a considerable increase of t11 C18:1 to the expense of C18:0 [80]. This indicates that inhibition of the third step of biohydrogenation took place. Different tannins thus have a differentiated and not-always-coherent influence on the biohydrogenation pathway.
Note that the effect of tannins on FA biohydrogenation cannot be found for all the dietary resources [94], and it appears that phenolics have only minor effects when the concentration is below a certain threshold [83,95]. For instance, supplementation of commercially-available tannin extracts, i.e., a 1:1 mixture of Castanea sativa and Schinopsis lorentzii (quebracho, a source of condensed tannins) at a level of 10 mg/g dry matter to a diet containing sunflower oil did not alter the proportions of the major FA classes in milk, i.e., PUFA, mono-unsaturated FA (MUFA) and SFA, nor the proportions of c9,t11 C18:2 and t11 C18:1 [96]. The low dose of the tannin mixture in that study was presumed to have been the reason for the lack of change in FA found. It appears that both the levels of dietary polyphenols and the sources from which they were derived are among the factors affecting ruminal FA biohydrogenation and general rumen fermentation [83].
Tannins in silages continue to demonstrate effects on biohydrogenation. Adding oak tannin extract at 26 g/kg dry matter to grass silage increased unsaturated FA content and reduced SFA content in the milk of lactating dairy cows [97]. The effect of PPO in red clover included in silage has also been confirmed [31]. The exact mode of action of PPO protecting PUFA against biohydrogenation in the rumen is not fully elucidated, though several potential mechanisms have been evoked: quinone binding to polar lipids, reduction of microbial lipase activity following the formation of protein complexes around glycerol-based lipid, or changes in rumen microbial populations [42].
The partial or total substitution of timothy with sainfoin or red clover was associated with greater concentrations of PUFA and lower concentrations of MUFA in the rumen content (Figure 1K, [38]). In particular, the inclusion of these two bioactive legumes in silage favoured the accumulation of 18:3 n-3, with the greatest concentrations being observed for the sainfoin–red clover mixture. The dietary treatment also tended to affect the proportions of MUFA and PUFA in the intramuscular fat of lamb longissimus muscle. Sainfoin and red clover clearly had an additive effect on reducing omega-6/omega-3 FA ratio (Figure 1L), which is a desirable way to also reduce the risk of many chronic diseases. Therefore, this study indicated that the beneficial effects of bioactive PPO and tannins on rumen biohydrogenation and meat quality appear to be similar and additive. It was also shown that feeding lambs with silages containing sainfoin and red clover can improve the oxidative stability of the meat, as evidenced when meat was subjected to strong oxidative challenge, such as cooking and incubation with pro-oxidant catalysts [98]. This is particularly beneficial as meat with high PUFA and poor oxidative stability results in deterioration of its flavor, odor and color [64], and lipid oxidation end-products may facility carcinogenic processes in the colon [65].
7. Conclusions and Directions for Future Research
Silage quality can impact all the dimensions of ruminant nutrition, from nutritive value to palatability and voluntary intake and on to animal health and product quality. This greatly impacts the inputs needed for animal performances (energy and protein concentrates, drugs, and so on) as well as the pollutant emissions that need to minimized as far as possible to support a more sustainable model of agriculture and agroecology. The use of PBC emerges as a natural solution, relatively cheap especially if PBC are supplied by ensiled forages, and well accepted by consumers. In this review, we have highlighted the ability of PBS, especially tannins and PPO contained in some legumes or in plant extracts, to reduce degradation of dietary protein into soluble N and NH3 in the silo and in the rumen. This can lead, but not always, to increased nutrient use efficiency and animal performances, and decreased pollutions under the form of urinary N excretion and CH4 emissions. Polyphenolic compounds can have beneficial effects on ruminant health, especially through the anthelmintic activity of tannins and their antioxidant capacity to cope with inflammatory and oxidative stress. Finally, some PBS have been shown to reduce FA biohydrogenation resulting in increased PUFA content and oxidative stability in ruminant products. However, despite major strides forward in recent years on the effects of PBC in animal nutrition, questions remain as to how to translate research results into practices:
What agronomic, harvesting, or ensiling measures can ensure that PBC deliver consistent results, taking into account the diversity of PBC and expected effects?
How do the animals—and especially their rumen ecosystem—adapt to PBC? What are the consequences for long-term PBC efficiency?
How can we develop tools for improving PBC analysis in silage and digesta to better understand and predict PBC effects in the silo and in the animal?
How can we modulate the stability of the tannin–protein complexes throughout the digestive tract to allow a greater intestinal N absorption and improved N use efficiency?
When PBCs have multiple effects, is there a competition between these effects? For instance, are tannins already engaged in protein binding in the silo are still available to act on methane emissions or parasites?
How can we better integrate the researches on the antioxidant capacity of some PBC, encompassing effects on animal health and human health-promoting animal products?
Do PBCs hold equivalent potential in temperate and tropical areas, given that local PBC contents and profiles are substantially different? Which does this mean for use in practice?
Interdisciplinary research and cross-fertilization of disciplines are both key to co-addressing the different pillars of sustainability. Practical solutions need to be developed through collaboration between chemists, ruminant nutritionists, farmers, veterinarians, producers and consumers, to deliver sustainable innovations and produce high-quality products profitably while also maintaining the quality of the environment.
Author Contributions
Bibliographic review, V.N. and A.J.; writing—review and editing V.N. and A.J.; supervision, V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Altieri, M.A. Agroecological Principles and Strategies for Sustainable Agriculture. In Agroecological Innovations: Increasing Food Production with Participatory Development; Uphoff, N.T., Ed.; Earthscan Publication Ltd.: London, UK, 2002; pp. 40–46. [Google Scholar]
- Dumont, B.; Fortun-Lamothe, L.; Jouven, M.; Thomas, M.; Tichit, M. Prospects from Agroecology and Industrial Ecology for Animal Production in the 21st Century. Animal 2013, 7, 1028–1043. [Google Scholar] [CrossRef] [PubMed]
- Gislon, G.; Ferrero, F.; Bava, L.; Borreani, G.; Dal Prà, A.; Pacchioli, M.T.; Sandruccia, A.; Zucalia, M.; Tabacco, E. Forage Systems and Sustainability of Milk Production: Feed Efficiency, Environmental Impacts and Soil Carbon Stocks. J. Clean. Prod. 2020, 260, 121012. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and Antioxidants in Disease: Oxidative Stress in Farm Animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Bekhit, A.E.D.; Bruce, H.; Scollan, N.D.; Muchenje, V.; Silva, P.; Jacobs, J.L. Production Strategies and Processing Systems of Meat: Current Status and Future Outlook for Innovation—A Global Perspective. In Sustainable Meat Production and Processing; Galanakis, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 17–44. [Google Scholar]
- Muck, R.E. Factors Influencing Silage Quality and Their Implications for Management. J. Dairy Sci. 1998, 71, 2992–3002. [Google Scholar] [CrossRef]
- Peyraud, J.L.; Vérité, R.; Delaby, L. Nitrogen Excretion by Dairy Cows: Influence of the diet and of the Level of Production. Fourrages 1995, 142, 131–144. [Google Scholar]
- Charmley, E. Towards Improved Silage Quality—A Review. Can. J. Anim. Sci. 2001, 81, 157–168. [Google Scholar] [CrossRef]
- Queiroz, O.C.M.; Ogunade, I.M.; Weinberg, Z.; Adesogan, A.T. Silage Review: Foodborne Pathogens in Silage and Their Mitigation by Silage Additives. J. Dairy Sci. 2018, 101, 4132–4142. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vias, D.; Adesogan, A.T. Silage Review: Mycotoxins in Silage: Occurrence, Effects, Prevention, and Mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef]
- Driehuis, F.; Wilkinson, J.M.; Jiang, Y.; Ogunade, I.; Adesogan, A.T. Silage Review: Animal and Human Health Risks from Silage. J. Dairy Sci. 2018, 101, 4093–4110. [Google Scholar] [CrossRef]
- Flores-Flores, M.E.; Lizarraga, E.; de Cerain, A.L.; González-Peñas, E. Presence of Mycotoxins in Animal Milk: A Review. Food Control 2015, 53, 163–176. [Google Scholar] [CrossRef]
- Greathead, H. Plants and Plant Extracts for Improving Animal Productivity. Proc. Nutr. Soc. 2003, 62, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, S.; Parker, A.J.; Dunshea, F.R. Plant Bioactives for Ruminant Health and Productivity. Phytochemistry 2008, 69, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Copani, G. Benefit of Including Bioactive Legumes (Sainfoin, Red Clover) in Grass-Based Silages on Ruminant Production and Pollutant Emissions. Ph.D. Thesis, Université Clermont Auvergne, Clermont-Ferrand, France, 10 September 2015. Available online: https://hal.archives-ouvertes.fr/tel-01276667/ (accessed on 23 December 2020).
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Aberystwyth, UK, 1991. [Google Scholar]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung Jr, L. Silage Review: Recent Advances and Future Uses of Silage Additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Golden, R. Identifying an Indoor Air Exposure Limit for Formaldehyde Considering Both Irritation and Cancer Hazards. Crit. Rev. Toxicol. 2011, 41, 672–721. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Sujarnoko, T.U.; Ridla, M.; Kondo, M.; Kreuzer, M. Silage Quality as Influenced by Concentration and Type of tannins Present in the Material Ensiled: A Meta-Analysis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 456–465. [Google Scholar] [CrossRef]
- Albrecht, K.A.; Muck, R.E. Proteolysis in Ensiled Forage Legumes That Vary in Tannin Concentration. Crop Sci. 1991, 31, 464–469. [Google Scholar] [CrossRef]
- Niezen, J.H.; Waghorn, G.C.; Lyons, T.B.; Corson, D.C. The Potential Benefits of Ensiling the Forage Legume Sulla Compared with Pasture. Proc. New Zealand Grassl. Assoc. 1998, 60, 105–109. [Google Scholar] [CrossRef]
- Lorenz, M.M.; Eriksson, T.; Uden, P. Effect of Wilting, Silage Additive, PEG Treatment and Tannin Content on the Distribution of N between Different Fractions after Ensiling of Three Different Sainfoin (Onobrychis viciifolia) Varieties. Grass Forage Sci. 2010, 65, 175–184. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Koenig, K.M.; Zhao, G.Y.; Beauchemin, K.A. Use of Gallic Acid and Hydrolyzable Tannins to Reduce Methane Emission and Nitrogen Excretion in Beef Cattle Fed a Diet Containing Alfalfa Silage. J. Anim. Sci. 2019, 97, 2230–2244. [Google Scholar] [CrossRef]
- He, L.; Lv, H.; Chen, N.; Wang, C.; Zhou, W.; Chen, X.; Zhang, Q. Improving Fermentation, Protein Preservation and Antioxidant Activity of Moringa oleifera Leaves Silage with Gallic Acid and Tannin Acid. Bioresour. Technol. 2020, 297, 122390. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.G.; Berchielli, T.T.; Reis, R.; Vechetini, M.E.; dos Santos Pedreira, M. Fermentative Characteristics and Aerobic Stability of Sorghum Silages Containing Different Tannin Levels. Anim. Feed Sci. Technol. 2009, 154, 1–8. [Google Scholar] [CrossRef]
- Kondo, M.; Naoki, N.; Kazumi, K.; Yokota, H. Enhanced Lactic Acid Fermentation of Silage by the Addition of Green Tea Waste. J. Agric. Food Sci. 2004, 84, 728–734. [Google Scholar] [CrossRef]
- Tabacco, E.; Borreani, G.; Crovetto, G.M.; Galassi, G.; Colombo, D.; Cavallarin, L. Effect of Chestnut Tannin on Fermentation Quality, Proteolysis, and Protein Rumen Degradability of Alfalfa Silage. J. Dairy Sci. 2006, 89, 4736–4746. [Google Scholar] [CrossRef]
- Herremans, S.; Decruyenaere, V.; Beckers, Y.; Froidmont, E. Silage Additives to Reduce Protein Degradation during Ensiling and Evaluation of In Vitro Ruminal Nitrogen Degradability. Grass Forage Sci. 2019, 74, 86–96. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Salawu, M.B. The Effect of Different Additives on the Fermentation Quality, Aerobic Stability and In Vitro Digestibility of Pea/Wheat Bi-crop Silages Containing Contrasting Pea to Wheat Ratios. Grass Forage Sci. 2002, 57, 25–32. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Scott, M.B.; Tweed, J.K.S.; Minchin, F.R.; Davies, D.R. Effects of Polyphenol Oxidase on Lipolysis and Proteolysis of Red Clover Silage with and without a Silage Inoculant (Lactobacillus plantarum L54). Anim. Feed Sci. Technol. 2008, 144, 125–136. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Tweed, J.K.; Minchin, F.R.; Winters, A.L. Red Clover Polyphenol Oxidase: Activation, Activity and Efficacy under Grazing. Anim. Feed Sci. Technol. 2009, 149, 250–264. [Google Scholar] [CrossRef]
- Copani, G.; Ginane, C.; Le Morvan, A.; Niderkorn, V. Bioactive Forage Legumes as a Strategy to Improve Silage Quality and Minimise Nitrogenous Losses. Anim. Prod. Sci. 2014, 54, 1826–1829. [Google Scholar] [CrossRef]
- Foskolos, A.; Cavini, S.; Ferret, A.; Calsamiglia, S. Effects of Essential Oil Compounds Addition on Ryegrass Silage Protein Degradation. Can. J. Anim. Sci. 2016, 96, 100–103. [Google Scholar] [CrossRef]
- Ferris, C.P.; Mayne, C.S. The Effects of Incorporating Sugar-Beet Pulp with Herbage at Ensiling on Silage Fermentation, Effluent Output and In-Silo Losses. Grass Forage Sci. 1994, 49, 216–228. [Google Scholar] [CrossRef]
- Niderkorn, V.; Copani, G.; Martin, C.; Maxin, G.; Torrent, A.; Anglard, F.; Rochette, Y.; Ginane, C. Effects of Including Bioactive Legumes in Grass Silage on Digestion Parameters, Nitrogen Balance and Methane Emissions in Sheep. Grass Forage Sci. 2019, 74, 626–635. [Google Scholar] [CrossRef]
- Copani, G.; Niderkorn, V.; Anglard, F.; Quereuil, A.; Ginane, C. Silages Containing Bioactive Forage Legumes: A Promising Protein-Rich Feed Source for Growing Lambs. Grass Forage Sci. 2016, 71, 622–631. [Google Scholar] [CrossRef]
- Campidonico, L.; Toral, P.G.; Priolo, A.; Luciano, G.; Valenti, B.; Hervás, G.; Frutos, P.; Copani, G.; Ginane, C.; Niderkorn, V. Fatty Acid Composition of Ruminal Digesta and Longissimus Muscle from Lambs Fed Silage Mixtures Including Red Clover, Sainfoin, and Timothy. J. Anim. Sci. 2016, 94, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Viennasay, B.; Wanapat, M.; Totakul, P.; Phesatcha, B.; Ampapon, T.; Cherdthong, A. Effect of Flemingia macrophylla Silage on In Vitro Fermentation Characteristics and Reduced Methane Production. Anim. Prod. Sci. 2020, 60, 1918–1924. [Google Scholar] [CrossRef]
- Merry, R.J.; Lee, M.R.F.; Davies, D.R.; Dewhurst, R.J.; Moorby, J.M.; Scollan, N.D.; Theodorou, M.K. Effects of High-Sugar Ryegrass Silage and Mixtures with Red Clover Silage on Ruminant Digestion. 1. In Vitro and In Vivo Studies of Nitrogen Utilization. J. Anim. Sci. 2006, 84, 3049–3060. [Google Scholar] [CrossRef]
- Guzatti, G.C.; Duchini, P.G.; Kozloski, G.V.; Niderkorn, V.; Ribeiro-Filho, H.M.N. Associative Effects between Red Clover and Kikuyu Grass Silage: Proteolysis Reduction and Synergy during In Vitro Organic Matter Degradation. Anim. Feed Sci. Technol. 2017, 231, 107–110. [Google Scholar] [CrossRef]
- Lee, M.R.F. Forage Polyphenol Oxidase and Ruminant Livestock Nutrition. Front. Plant Sci. 2014, 5, 694. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Kuoppala, K.; Ahvenjärvi, S.; Rinne, M. Effects of Feeding Grass or Red Clover Silage Cut at Two Maturity Stages in Dairy Cows. 1. Nitrogen Metabolism and Supply of Amino Acids. J. Dairy Sci. 2009, 92, 5620–5633. [Google Scholar] [CrossRef]
- Guzatti, G.C.; Duchini, P.G.; Kozloski, G.V.; Niderkorn, V.; Ribeiro-Filho, H.M.N. Red Clover Silage: An Alternative for Mitigating the Impact of Nitrogen Excretion in Ovine Production Systems. Rev. Bras. Zootec. 2019, 48, e20190044. [Google Scholar] [CrossRef]
- Copani, G.; Ginane, C.; Le Morvan, A.; Niderkorn, V. Patterns of In Vitro Rumen Fermentation of Silage Mixtures Including Sainfoin and Red Clover as Bioactive Legumes. Anim. Feed Sci. Technol. 2015, 208, 220–224. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the Conundrum of Tannins in Animal Nutrition and Health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Varel, V.H.; Nienaber, J.A.; Freetly, H.C. Conservation of Nitrogen in Cattle Feedlot Waste with Urease Inhibitors. J. Anim. Sci. 1999, 77, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. Special topics—Mitigation of Methane and Nitrous Oxide Emissions from Animal Operations: I. A Review of Enteric Methane Mitigation Options. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef] [PubMed]
- Niderkorn, V.; Martin, C.; Rochette, Y.; Julien, S.; Baumont, R. Associative Effects between Orchardgrass and Red Clover Silages on Voluntary Intake and Digestion in Sheep: Evidence of a Synergy on Digestible Dry Matter Intake. J. Anim. Sci. 2015, 93, 4967–4976. [Google Scholar] [CrossRef]
- Viennasay, B.; Wanapat, M. Enhancing Lactating Dairy Cow Rumen Fermentation and Production with Flemingia Silage Containing Phytonutrients. Livest. Sci. 2020, 241, 104201. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.; Becker, K. Divergence between Purified Hydrolysable and Condensed Tannin Effects on Methane Emission, Rumen Fermentation and Microbial Population In Vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the Nature of Tannins on In Vitro Ruminal Methane and Volatile Fatty Acid Production and on Methanogenic Archaea and Protozoal Populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and Fate of Tannins in Ruminant Animals, Adaptation to Tannins, and Strategies to Overcome Detrimental Effects of Feeding Tannin-Rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Wilkinson, J.M. Silage and Animal Health. Nat. Toxins 1999, 7, 221–232. [Google Scholar] [CrossRef]
- Lanusse, C.; Canton, C.; Virkel, G.; Alvarez, L.; Costa-Junior, L.; Lifschitz, A. Strategies to Optimize the Efficacy of Anthelmintic Drugs in Ruminants. Trends Parasitol. 2018, 34, 664–682. [Google Scholar] [CrossRef] [PubMed]
- Pena-Espinoza, M.; Thamsborg, S.M.; Desrues, O.; Hansen, T.V.; Enemark, H.L. Anthelmintic Effects of Forage Chicory (Cichorium intybus) against Gastrointestinal Nematode Parasites in Experimentally Infected Cattle. Parasitology 2016, 143, 1279–1293. [Google Scholar] [CrossRef]
- Heckendorn, F.; Häring, D.A.; Maurer, V.; Zinsstag, J.; Langhans, W.; Hertzberg, H. Effect of Sainfoin (Onobrychis viciifolia) Silage and Hay on Established Populations of Haemonchus contortus and Cooperia curticei in Lambs. Vet. Parasitol. 2006, 142, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Terrill, T.H.; Griffin, E.; Kommuru, D.S.; Miller, J.E.; Mosjidis, J.A.; Kearney, M.T.; Burke, J.M. Effect of Ensiling on Anti-Parasitic Properties of Sericea lespedeza. In Proceedings of the AFGC Annual Meeting, Baton Rouge, LA, USA, 10–13 January 2016. [Google Scholar]
- Sordillo, L.M. Nutritional Strategies to Optimize Dairy Cattle Immunity. J. Dairy Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Animal Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Colitti, M.; Stefanon, B. Effect of Natural Antioxidants on Superoxide Dismutase and Glutathione Peroxidase mRNA Expression in Leukocytes from Periparturient Dairy Cows. Vet. Res. Commun. 2006, 30, 19–27. [Google Scholar] [CrossRef]
- Gessner, D.K.; Koch, C.; Romberg, F.J.; Winkler, A.; Dusel, G.; Herzog, E.; Most, E.; Eder, K. The Effect of Grape Seed and Grape Marc Meal Extract on Milk Performance and the Expression of Genes of Endoplasmic Reticulum Stress and Inflammation in the Liver of Dairy Cows in Early Lactation. J. Dairy Sci. 2015, 98, 8856–8868. [Google Scholar] [CrossRef]
- Gladine, C.; Rock, E.; Morand, C.; Bauchart, D.; Durand, D. Bioavailability and Antioxidant Capacity of Plant Extracts Rich in Polyphenols, Given as a Single Acute Dose, in Sheep Made Highly Susceptible to Lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701. [Google Scholar] [CrossRef]
- Durand, D.; Scislowski, V.; Gruffat, D.; Chilliard, Y.; Bauchart, D. High-fat Rations and Lipid Peroxidation in Ruminants: Consequences on the Health of Animals and Quality of Their Products. In Indicators of Milk and Beef Quality; Hocquette, J.F., Gigli, S., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005; pp. 151–162. [Google Scholar]
- Guéraud, F.; Taché, S.; Steghens, J.P.; Milkovic, L.; Borovic-Sunjic, S.; Zarkovic, N.; Gaultier, E.; Naud, N.; Héliès-Toussaint, C.; Pierre, F.; et al. Dietary Polyunsaturated Fatty Acids and Heme Iron Induce Oxidative Stress Biomarkers and a Cancer Promoting Environment in the Colon of Rats. Free Radic. Biol. Med. 2015, 83, 192–200. [Google Scholar] [CrossRef]
- Delosière, M.; Durand, D.; Bourguet, C.; Terlouw, E.C. Lipid Oxidation, Pre-Slaughter Animal Stress and Meat Packaging: Can Dietary Supplementation of Vitamin E and Plant Extracts Come to the Rescue? Food Chem. 2020, 309, 125668. [Google Scholar] [CrossRef]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A Review of Fatty Acid Profiles and Antioxidant Content in Grass-Fed and Grain-Fed Beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Kuhnen, S.; Moacyr, J.R.; Mayer, J.K.; Navarro, B.B.; Trevisan, R.; Honorato, L.A.; Maraschin, M.; Pinheiro Machado Filho, L.C. Phenolic Content and Ferric Reducing—Antioxidant Power of Cow’s Milk Produced in Different Pasture-Based Production Systems in Southern Brazil. J. Sci. Food Agric. 2014, 94, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-Linolenic Acid and Its Conversion to Longer Chain n-3 Fatty Acids: Benefits for Human Health and a Role in Maintaining Tissue n-3 Fatty Acid Levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Spener, F. Conjugated Linoleic Acids as Functional Food: An Insight into Their Health Benefits. Nutr. Metab. 2009, 6, 36. [Google Scholar] [CrossRef]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, Rumen Biohydrogenation and Nutritional Quality of Cow and Goat Milk Fat. Eur. J. Lipid Sci. Technol. 2007, 109, 828–855. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Lock, A.L.; Shingfield, K.J.; Bauman, D.E. Biosynthesis of Conjugated Linoleic Acid in Ruminants and Humans. Adv. Food Nutr. Res. 2005, 50, 179–217. [Google Scholar]
- Lourenço, M.; Van Ranst, G.; Vlaeminck, B.; De Smet, S.; Fievez, V. Influence of Different Dietary Forages on the Fatty Acid Composition of Rumen Digesta as Well as Ruminant Meat and Milk. Anim. Feed Sci. Technol. 2008, 145, 418–437. [Google Scholar] [CrossRef]
- Doreau, M.; Bauchart, D.; Chilliard, Y. Enhancing Fatty Acid Composition of Milk and Meat Through Animal Feeding. Anim. Prod. Sci. 2011, 51, 19–29. [Google Scholar] [CrossRef]
- Staerfl, S.M.; Soliva, C.R.; Leiber, F.; Kreuzer, M. Fatty Acid Profile and Oxidative Stability of the Perirenal Fat of Bulls Fattened on Grass Silage and Maize Silage Supplemented with Tannins, Garlic, Maca and Lupines. Meat Sci. 2011, 89, 98–104. [Google Scholar] [CrossRef]
- Corazzin, M.; Romanzin, A.; Sepulcri, A.; Pinosa, M.; Piasentier, E.; Bovolenta, S. Fatty Acid Profiles of Cow’s Milk and Cheese as Affected by Mountain Pasture Type and Concentrate Supplementation. Animals 2019, 9, 68. [Google Scholar] [CrossRef]
- Uzun, P.; Masucci, F.; Serrapica, F.; Napolitano, F.; Braghieri, A.; Romano, R.; Manzo, N.; Esposito, G.; Di Francia, A. The Inclusion of Fresh Forage in the Lactating Buffalo Diet Affects Fatty Acid and Sensory Profile of Mozzarella Cheese. J. Dairy Sci. 2018, 101, 6752–6761. [Google Scholar] [CrossRef] [PubMed]
- Serrapica, F.; Masucci, F.; Di Francia, A.; Napolitano, F.; Braghieri, A.; Esposito, G.; Romano, R. Seasonal Variation of Chemical Composition, Fatty Acid Profile, and Sensory Properties of a Mountain Pecorino Cheese. Foods 2020, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, R.J.; Shingfield, K.J.; Lee, M.R.F.; Scollan, N.D. Increasing the Concentrations of Beneficial Polyunsaturated Fatty Acids in Milk Produced by Dairy Cows in High-Forage Systems. Anim. Feed Sci. Technol. 2006, 131, 168–206. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Bryner, S.F.; Scheeder, M.R.L.; Wettstein, H.R.; Leiber, F.; Kreuzer, M.; Soliva, C.R. Evidence for the Inhibition of the Terminal Step of Ruminal α-Linolenic Acid Biohydrogenation by Condensed Tannins. J. Dairy Sci. 2009, 92, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Vasta, V.; Mele, M.; Serra, M.; Scerra, M.; Luciano, G.; Lanza, M.; Priolo, A. Metabolic Fate of Fatty Acids Involved in Ruminal Biohydrogenation in Sheep Fed Concentrate or Herbage with or without Tannins. J. Anim. Sci. 2009, 87, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Cabiddu, A.; Salis, L.; Tweed, J.K.; Molle, G.; Decandia, M.; Lee, M.R.F. The Influence of Plant Polyphenols on Lipolysis and Biohydrogenation in Dried Forages at Different Phenological Stages: In Vitro Study. J. Sci. Food Agric. 2010, 90, 829–835. [Google Scholar] [CrossRef]
- Jayanegara, A.; Kreuzer, M.; Wina, E.; Leiber, F. Significance of Phenolic Compounds in Tropical Forages for the Ruminal Bypass of Polyunsaturated Fatty Acids and the Appearance of Biohydrogenation Intermediates as Examined In Vitro. Anim. Prod. Sci. 2011, 51, 1127–1136. [Google Scholar] [CrossRef]
- Jayanegara, A.; Kreuzer, M.; Leiber, F. Ruminal Disappearance of Polyunsaturated Fatty Acids and Appearance of Biohydrogenation Products When Incubating Linseed Oil with Alpine Forage Plant Species In Vitro. Livest. Sci. 2012, 147, 104–112. [Google Scholar] [CrossRef]
- Kälber, T.; Meier, J.S.; Kreuzer, M.; Leiber, F. Flowering Catch Crops Used as Forage Plants for Dairy Cows: Influence on Fatty Acids and Tocopherols in Milk. J. Dairy Sci. 2011, 94, 1477–1489. [Google Scholar] [CrossRef]
- Vasta, V.; Yáñez-Ruiz, D.R.; Mele, M.; Serra, A.; Luciano, G.; Lanza, M.; Biodi, L.; Priolo, A. Bacterial and Protozoal Communities and Fatty Acid Profile in the Rumen of Sheep Fed a Diet Containing Added Tannins. Appl. Environ. Microbiol. 2010, 76, 2549–2555. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid Metabolism in the Rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Lourenço, M.R.A.; Ramos-Morales, E.; Wallace, R.J. The Role of Microbes in Rumen Lipolysis and Biohydrogenation and Their Manipulation. Animal 2010, 4, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Leiber, F.; Kunz, C.; Kreuzer, M. Influence of Different Morphological Parts of Buckwheat (Fagopyrum esculentum) and Its Major Secondary Metabolite Rutin on Rumen Fermentation In Vitro. Czech J. Anim. Sci. 2012, 57, 10–18. [Google Scholar] [CrossRef]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial Mechanisms to Overcome Inhibitory Effects of Dietary Tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef]
- Silanikove, N.; Perevolotsky, A.; Provenza, F.D. Use of Tannin-Binding Chemicals to Assay for Tannins and Their Negative Postingestive Effects in Ruminants. Anim. Feed Sci. Technol. 2001, 91, 69–81. [Google Scholar] [CrossRef]
- He, Q.; Shi, B.; Yao, K. Interactions of Gallotannins with Proteins, Amino Acids, Phospholipids and Sugars. Food Chem. 2006, 95, 250–254. [Google Scholar] [CrossRef]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.J.; Mosley, E.E. Board-Invited Review: Recent Advances in Biohydrogenation of Unsaturated Fatty Acids within the Rumen Microbial Ecosystem. J. Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef]
- Abbeddou, S.; Rischkowsky, B.; Richter, E.K.; Hess, H.D.; Kreuzer, M. Modification of Milk Fatty Acid Composition by Feeding Forages and Agro-Industrial Byproducts from Dry Areas to Awassi Sheep. J. Dairy Sci. 2011, 94, 4657–4668. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Soliva, C.R.; Kreuzer, M.; Leiber, F. Influence of Alpine Forage Either Employed as Donor Cow’s Feed or as Incubation Substrate on In Vitro Ruminal Fatty Acid Biohydrogenation. Livest. Sci. 2011, 140, 80–87. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Bichi, E.; Belenguer, Á.; Frutos, P. Tannins as Feed Additives to Modulate Ruminal Biohydrogenation: Effects on Animal Performance, Milk Fatty Acid Composition and Ruminal Fermentation in Dairy Ewes Fed a Diet Containing Sunflower Oil. Anim. Feed Sci. Technol. 2011, 164, 199–206. [Google Scholar] [CrossRef]
- Herremans, S.; Decruyenaere, V.; Cantalapiedra-Hijar, G.; Beckers, Y.; Froidmont, E. Effects of Hydrolysable Tannin-Treated Grass Silage on Milk Yield and Composition, Nitrogen Partitioning and Nitrogen Isotopic Discrimination in Lactating Dairy Cows. Animal 2020, 14, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Luciano, G.; Natalello, A.; Mattioli, S.; Pauselli, M.; Sebastiani, B.; Niderkorn, V.; Copani, G.; Benhissi, H.; Amanpour, A.; Valenti, B. Feeding Lambs with Silage Mixtures of Grass, Sainfoin and Red Clover Improves Meat Oxidative Stability under High Oxidative Challenge. Meat Sci. 2019, 156, 59–67. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).