Rootstock Genotypes Shape the Response of cv. Pinot gris to Water Deficit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Leaf Gas Exchange and Stomatal Characterization under Optimal Conditions
2.3. Treatment Application and Stress Conditions
2.4. Non-Destructive Estimation of Dynamic Leaf Area
2.5. Gas Exchange and Chlorophyll Fluorescence
2.6. Midday Leaf Water Potential
2.7. Shoot Destructive Analysis
2.8. Statistical Analysis
3. Results
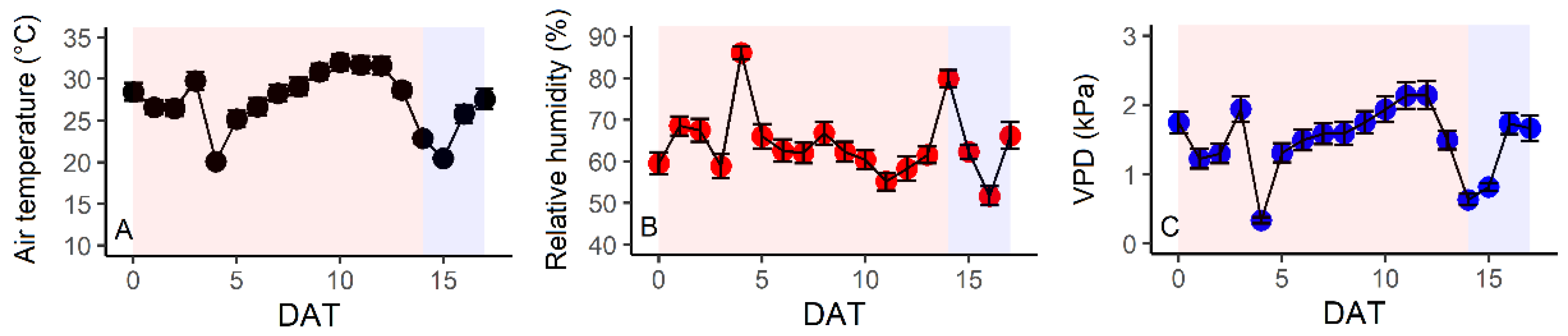
3.1. Environmental Conditions
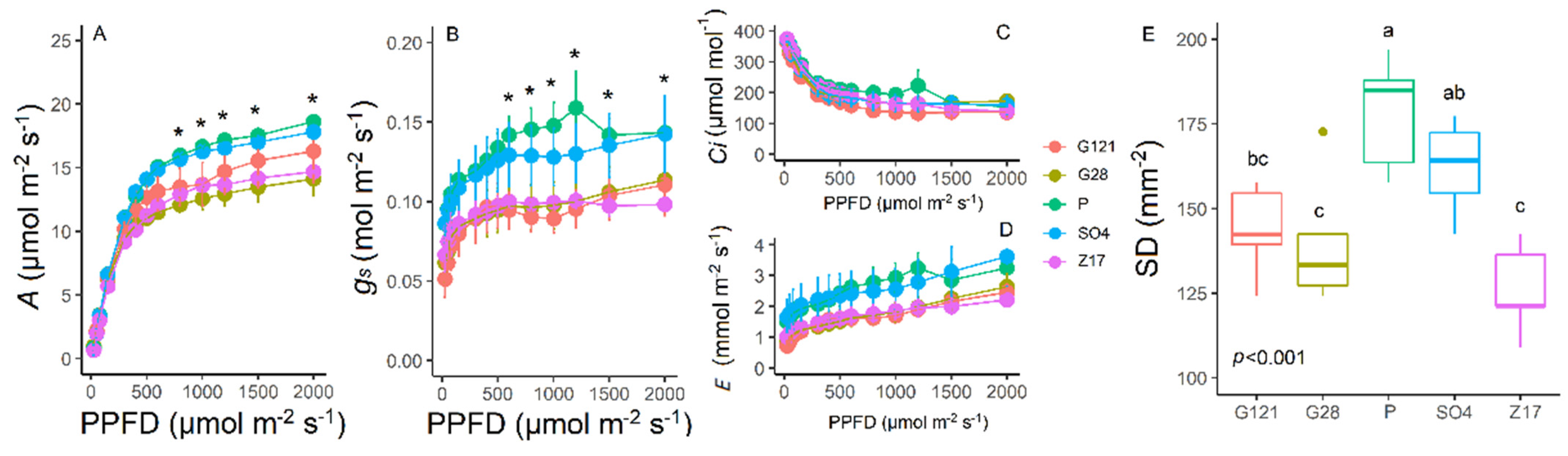
3.2. Light Response Curves and Stomatal Density
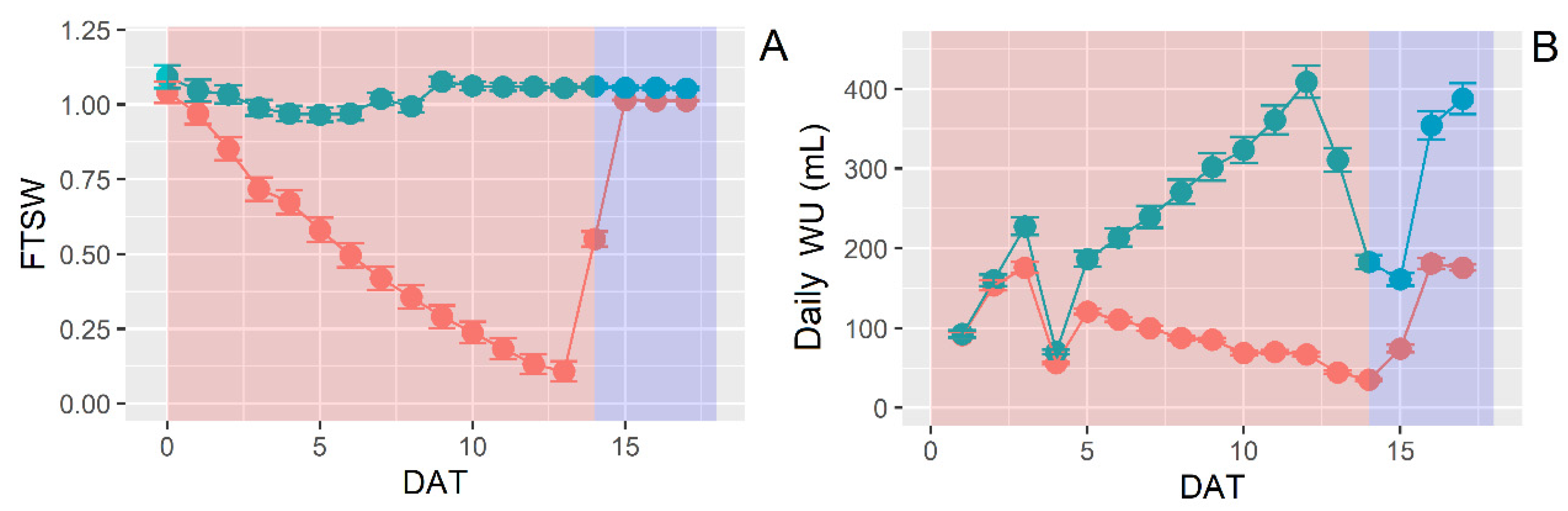
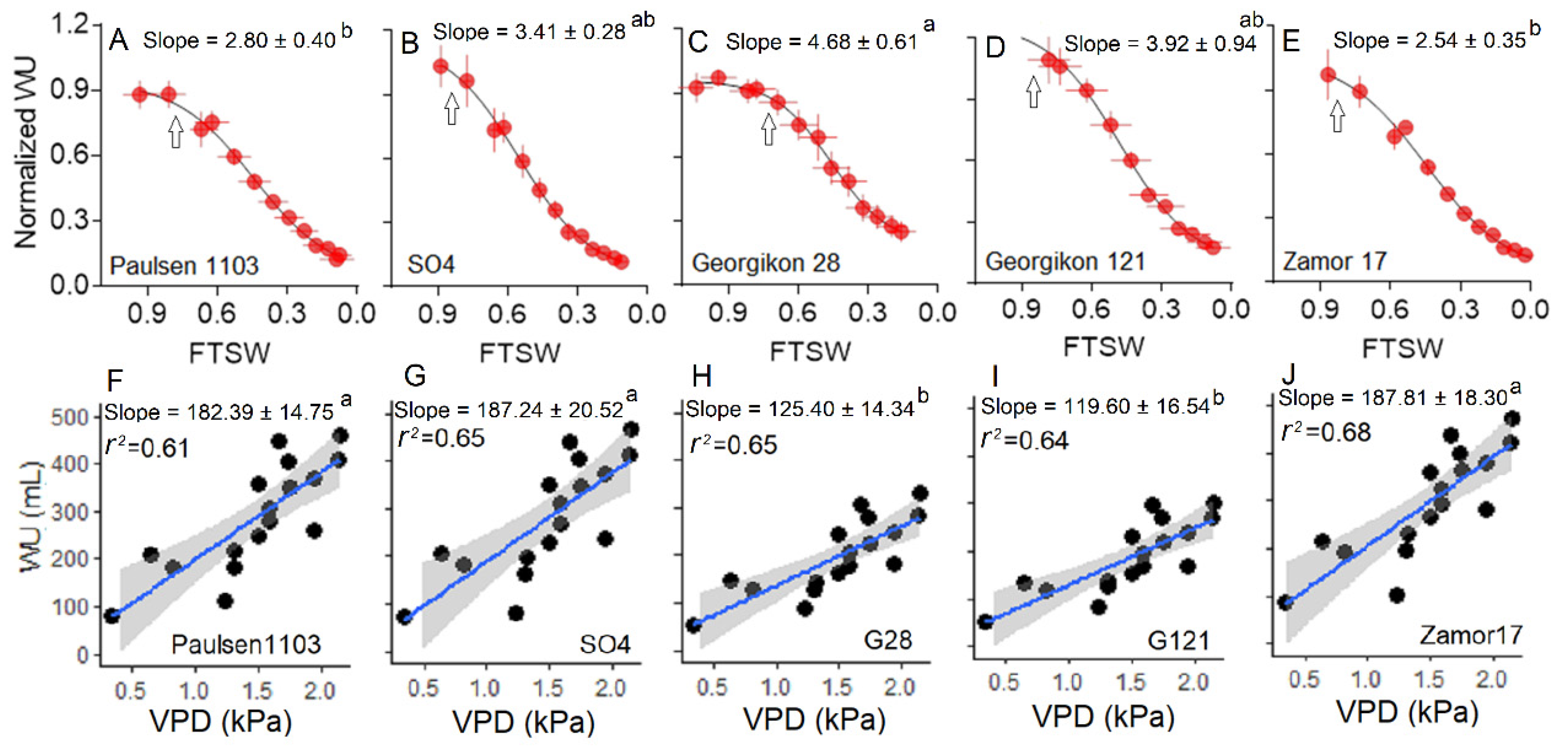
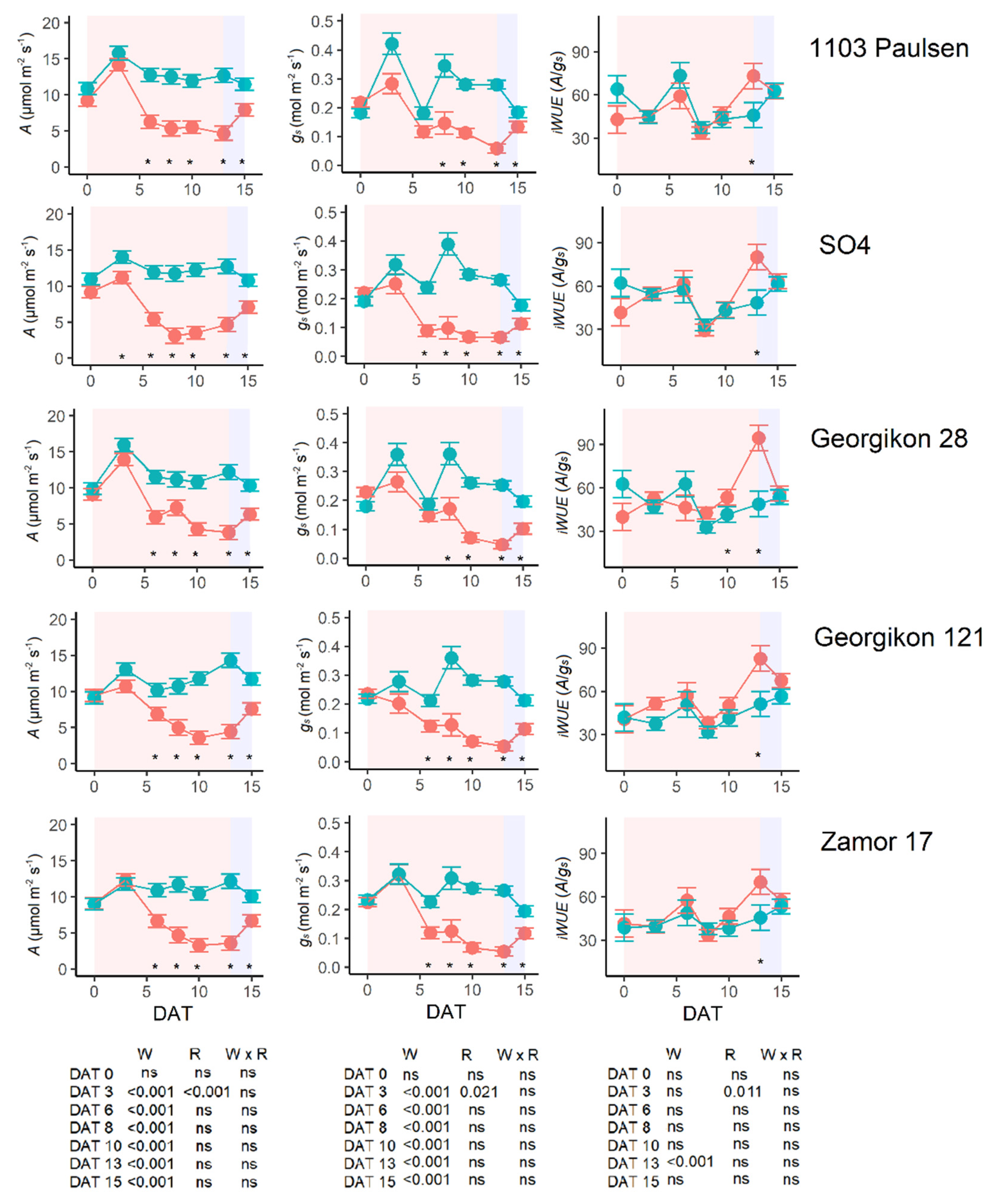
3.3. Water Use Behavior under Water Stress and Increasing VPD
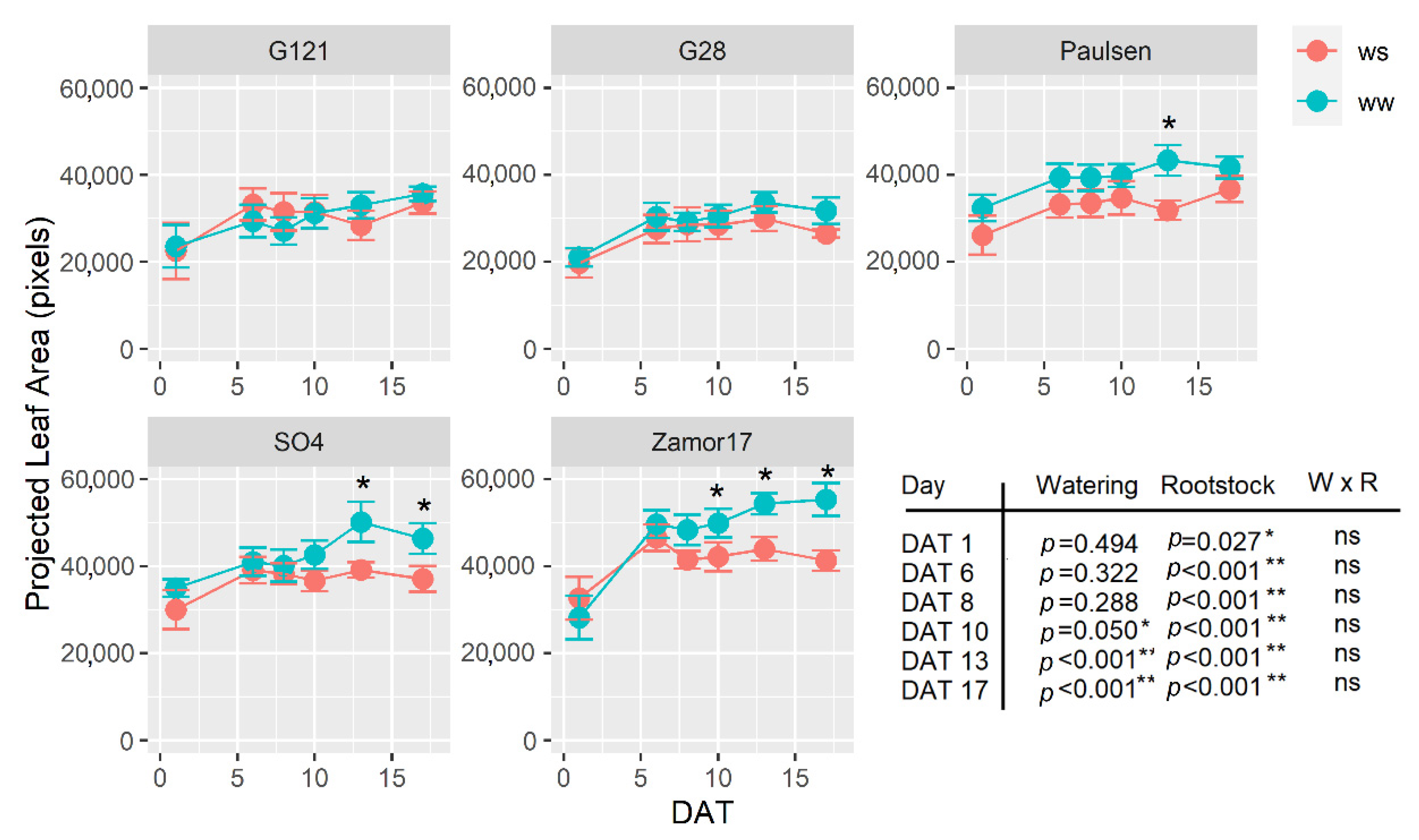
3.4. Dynamic Leaf Area
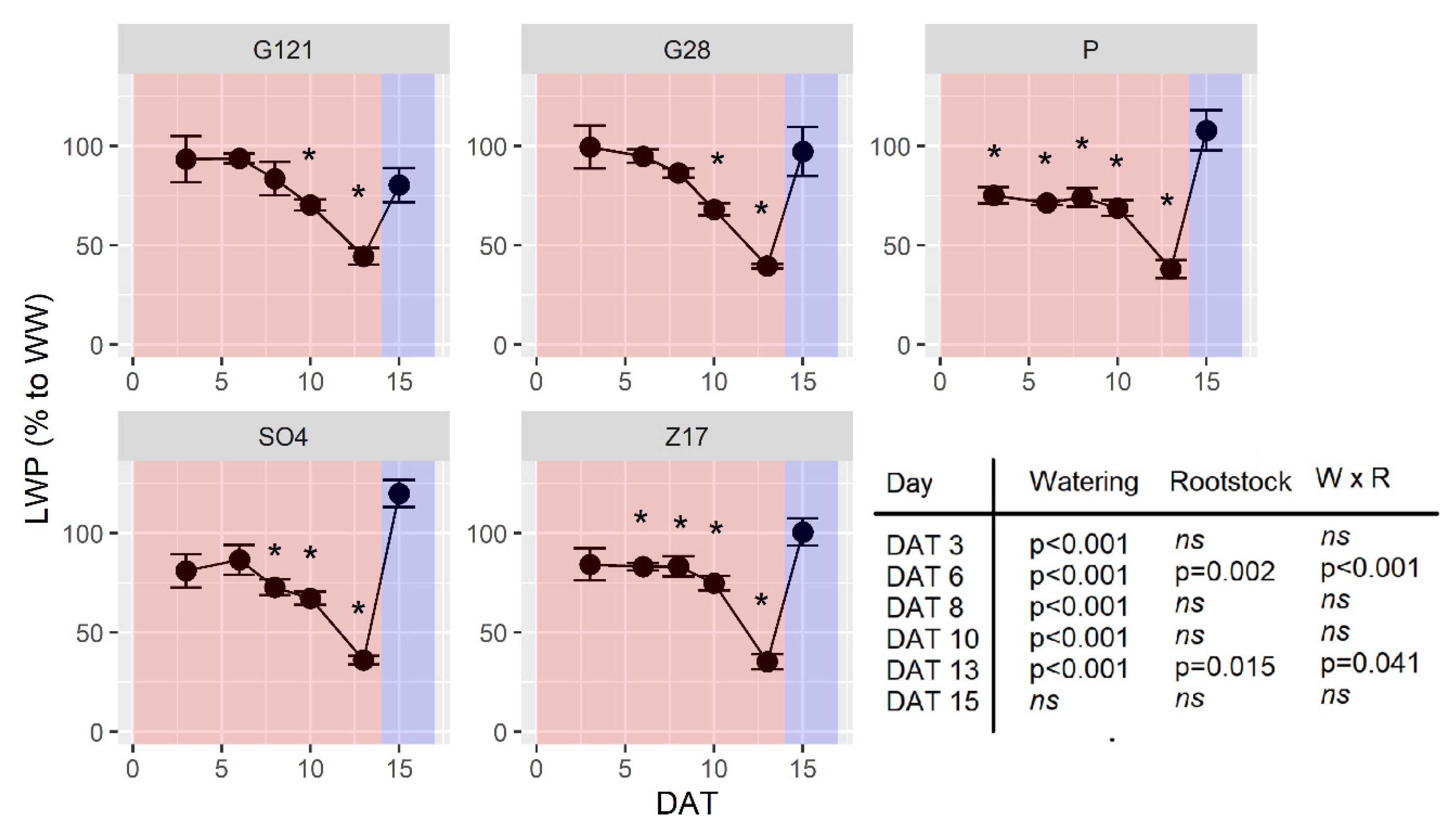
3.5. Leaf Water Potential
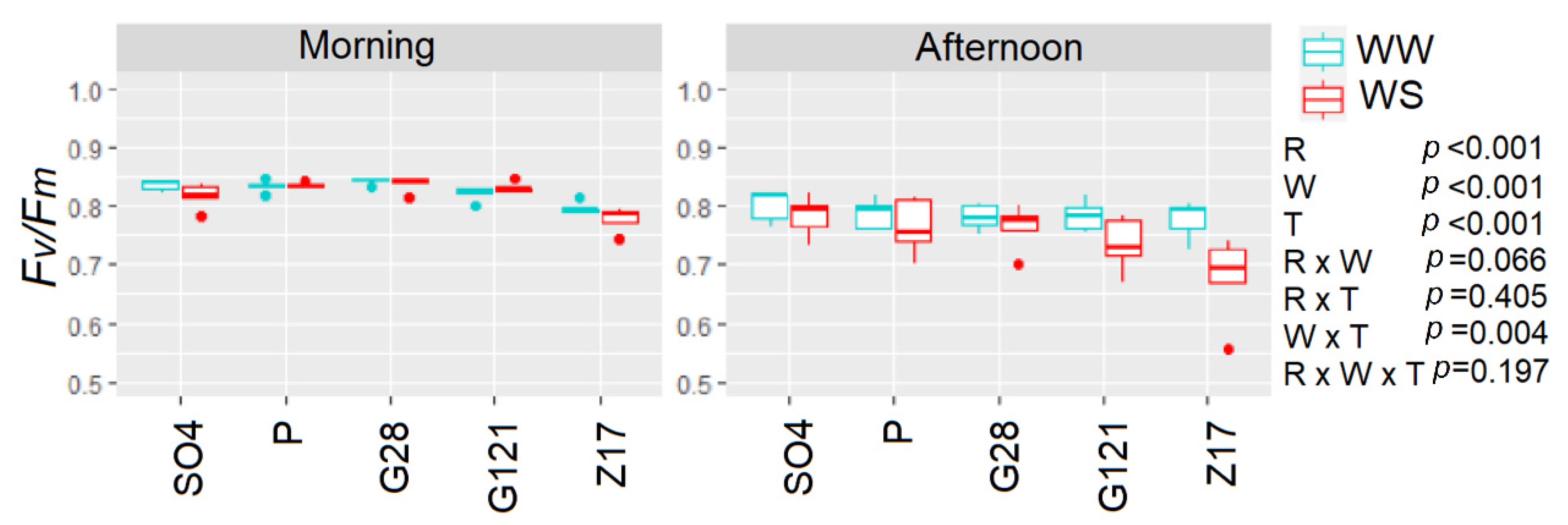
3.6. Gas Exchange and Chlorophyll Fluorescence
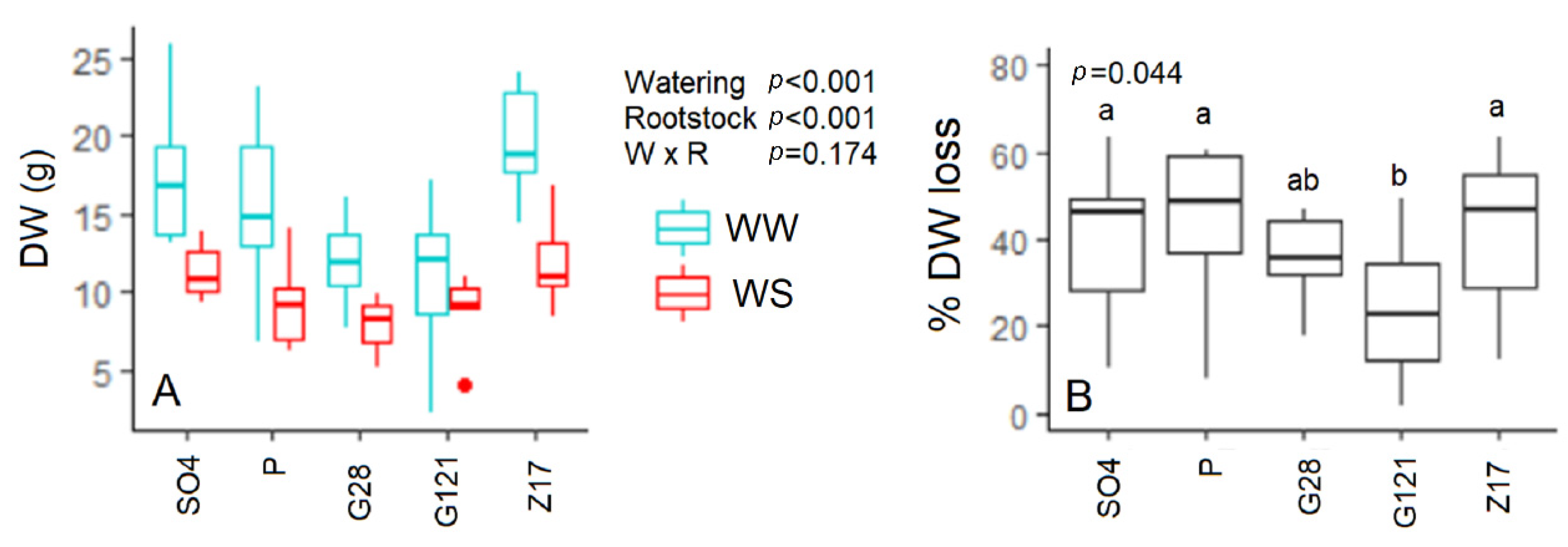
3.7. Biomass Dry Weight
4. Discussion
4.1. Pinot gris Vigor and Physiological Traits Are Modulated by The Rootstock under Optimal Conditions
4.2. Rootstocks Induced Different Water Stress Tolerance Response in Pinot gris
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medrano, H.; Tomás, M.; Martorell, S.; Escalona, J.-M.; Pou, A.; Fuentes, S.; Flexas, J.; Bota, J. Improving water use efficiency of vineyards in semi-arid regions. A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Serra, I.; Strever, A.; Myburgh, P.A.; Deloire, A. Review: The interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine Res. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; De Rességuier, L.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Medrano, H.; Escalona, J.M.; Cifre, J.; Bota, J.; Flexas, J. A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: Effects of water availability from leaf photosynthesis to grape yield and quality. Funct. Plant Biol. 2003, 30, 607–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilpart, N.; Metay, A.; Gary, C. Grapevine bud fertility and number of berries per bunch are determined by water and nitrogen stress around flowering in the previous year. Eur. J. Agron. 2014, 54, 9–20. [Google Scholar] [CrossRef]
- Marguerit, E.; Brendel, O.; Lebon, E.; Van Leeuwen, C.; Ollat, N. Rootstock control of scion transpiration and its acclimation to water deficit are controlled by different genes. New Phytol. 2012, 194, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Frioni, T.; Biagioni, A.; Squeri, C.; Tombesi, S.; Gatti, M.; Poni, S. Grafting cv. grechetto gentile vines to new m4 rootstock improves leaf gas exchange and water status as compared to commercial 1103p rootstock. Agronomy 2020, 10, 708. [Google Scholar] [CrossRef]
- Vivier, M.A.; Pretorius, I.S. Genetically tailored grapevines for the wine industry. Trends Biotechnol. 2002, 20, 472–478. [Google Scholar] [CrossRef]
- Carbonneau, A. The Early Selection of Grapevine Rootstocks for Resistance to Drought Conditions. Am. J. Enol. Vitic. 1985, 36, 195–198. [Google Scholar]
- Tramontini, S.; Vitali, M.; Centioni, L.; Schubert, A.; Lovisolo, C. Rootstock control of scion response to water stress in grapevine. Environ. Exp. Bot. 2013, 93, 20–26. [Google Scholar] [CrossRef]
- Prinsi, B.; Negri, A.S.; Failla, O.; Scienza, A.; Espen, L. Root proteomic and metabolic analyses reveal specific responses to drought stress in differently tolerant grapevine rootstocks. BMC Plant Biol. 2018, 18, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, F.; Distefano, G.; Gentile, A.; Allegra, M.; Lakso, A.N.; Nicolosi, E. Scion—Rootstock interactions influence the growth and behaviour of the grapevine root system in a heavy clay soil. Aust. J. Grape Wine Res. 2020, 26, 68–78. [Google Scholar] [CrossRef]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardieu, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [Green Version]
- Douthe, C.; Medrano, H.; Tortosa, I.; Escalona, J.M.; Hernández-Montes, E.; Pou, A. Whole-plant water use in field grown grapevine: Seasonal and environmental effects on water and carbon balance. Front. Plant Sci. 2018, 9, 1540. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P.; Navarro, J.M. Selecting rootstocks to improve vine performance and vineyard sustainability in deficit irrigated Monastrell grapevines under semiarid conditions. Agric. Water Manag. 2018, 209, 73–93. [Google Scholar] [CrossRef]
- Schultz, H.R. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Gerzon, E.; Biton, I.; Yaniv, Y.; Zemach, H.; Netzer, Y.; Schwartz, A.; Fait, A.; Ben-Ari, G. Grapevine anatomy as a possible determinant of isohydric or anisohydric behavior. Am. J. Enol. Vitic. 2015, 66, 340–347. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A Plant-Environment Interaction Rather Than a Simple Hydraulic Trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef]
- Levin, A.D.; Williams, L.E.; Matthews, M.A. A continuum of stomatal responses to water deficits among 17 wine grape cultivars (Vitis vinifera). Funct. Plant Biol. 2020, 47, 11–25. [Google Scholar] [CrossRef]
- Koundouras, S.; Tsialtas, I.T.; Zioziou, E.; Nikolaou, N. Rootstock effects on the adaptive strategies of grapevine (Vitis vinifera L. cv. Cabernet-Sauvignon) under contrasting water status: Leaf physiological and structural responses. Agric. Ecosyst. Environ. 2008, 128, 86–96. [Google Scholar] [CrossRef]
- Galbignani, M.; Merli, M.C.; Magnanini, E.; Bernizzoni, F.; Talaverano, I.; Gatti, M.; Tombesi, S.; Palliotti, A.; Poni, S. Gas exchange and water-use efficiency of cv. Sangiovese grafted to rootstocks of varying water-deficit tolerance. Irrig. Sci. 2016, 34, 105–116. [Google Scholar] [CrossRef]
- Alsina, M.M.; Smart, D.R.; Bauerle, T.; De Herralde, F.; Biel, C.; Stockert, C.; Negron, C.; Save, R. Seasonal changes of whole root system conductance by a drought-tolerant grape root system. J. Exp. Bot. 2011, 62, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandonnet, J.-P.; Cookson, S.J.; Vivin, P.; Ollat, N. Scion genotype controls biomass allocation and root development in grafted grapevine. Aust. J. Grape Wine Res. 2010, 16, 290–300. [Google Scholar] [CrossRef]
- Riaz, S.; Pap, D.; Uretsky, J.; Laucou, V.; Boursiquot, J.M.; Kocsis, L.; Walker, M.A. Genetic diversity and parentage analysis of grape rootstocks. Theor. Appl. Genet. 2019, 132, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Vršič, S.; Kocsis, L.; Pulko, B. Influence of substrate pH on root growth, biomass and leaf mineral contents of grapevine rootstocks grown in pots. J. Agric. Sci. Technol. 2016, 18, 483–490. [Google Scholar]
- Müller-Linow, M.; Wilhelm, J.; Briese, C.; Wojciechowski, T.; Schurr, U.; Fiorani, F. Plant Screen Mobile: An open-source mobile device app for plant trait analysis. Plant Methods 2019, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Faralli, M.; Williams, K.S.; Han, J.; Corke, F.M.K.; Doonan, J.H.; Kettlewell, P.S. Water-Saving Traits Can Protect Wheat Grain Number Under Progressive Soil Drying at the Meiotic Stage: A Phenotyping Approach. J. Plant Growth Regul. 2019, 38, 1562–1573. [Google Scholar] [CrossRef]
- Albacete, A.; Martínez-Andújar, C.; Martínez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; Pérez-Alfocea, F. Unravelling rootstock×scion interactions to improve food security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef] [Green Version]
- Migicovsky, Z.; Cousins, P.; Jordan, L.M.; Myles, S.; Striegler, R.K.; Verdegaal, P.; Chitwood, D.H. Grapevine rootstocks affect growth-related phenotypes. bioRxiv 2020, 864850. [Google Scholar] [CrossRef] [Green Version]
- Rives, M. Vigour, pruning, cropping in the grapevine (Vitis vinifera L.). I. A literature review. Agronomie 2000, 20, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, T.R. Limited-Transpiration Rate Under Elevated Atmospheric Vapor Pressure Deficit. In Water-Conservation Traits to Increase Crop Yields in Water-Deficit Environments; Sinclair, T.R., Ed.; Springer: Berlin, Germany, 2017; pp. 11–16. ISBN 9783319563213. [Google Scholar]
- Sivasakthi, K.; Tharanya, M.; Kholová, J.; Muriuki, R.W.; Thirunalasundari, T.; Vadez, V. Chickpea genotypes contrasting for vigor and canopy conductance also differ in their dependence on different water transport pathways. Front. Plant Sci. 2017, 8, 1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, T.; Blatt, M.R.; Yu, L.; Shi, D.; Li, J.; Kong, Y.; Yu, Y.; Chai, G.; Hu, R.; Wang, J.; et al. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faralli, M.; Matthews, J.; Lawson, T. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Curr. Opin. Plant Biol. 2019, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Manuck, C.M.; Drucker, S.T.; Shaghasi, T.; Fort, K.; Matthews, M.A.; Walker, M.A.; McElrone, A.J. The relationship between root hydraulics and scion vigour across Vitis rootstocks: What role do root aquaporins play? J. Exp. Bot. 2012, 63, 6445–6455. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Williams, L.E. Interaction of rootstock and applied water amounts at various fractions of estimated evapotranspiration (ETc) on productivity of Cabernet Sauvignon. Aust. J. Grape Wine Res. 2010, 16, 434–444. [Google Scholar] [CrossRef]
- Jones, H.G. How do rootstocks control shoot water relations? New Phytol. 2012, 194, 301–303. [Google Scholar] [CrossRef]
- Zhang, L.; Marguerit, E.; Rossdeutsch, L.; Ollat, N.; Gambetta, G.A. The influence of grapevine rootstocks on scion growth and drought resistance. Theor. Exp. Plant Physiol. 2016, 28, 143–157. [Google Scholar] [CrossRef]
- Hochberg, U.; Degu, A.; Fait, A.; Rachmilevitch, S. Near isohydric grapevine cultivar displays higher photosynthetic efficiency and photorespiration rates under drought stress as compared with near anisohydric grapevine cultivar. Physiol. Plant. 2013, 147, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Bühler, J.; Van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faralli, M.; Bianchedi, P.L.; Bertamini, M.; Varotto, C. Rootstock Genotypes Shape the Response of cv. Pinot gris to Water Deficit. Agronomy 2021, 11, 75. https://doi.org/10.3390/agronomy11010075
Faralli M, Bianchedi PL, Bertamini M, Varotto C. Rootstock Genotypes Shape the Response of cv. Pinot gris to Water Deficit. Agronomy. 2021; 11(1):75. https://doi.org/10.3390/agronomy11010075
Chicago/Turabian StyleFaralli, Michele, Pier Lugi Bianchedi, Massimo Bertamini, and Claudio Varotto. 2021. "Rootstock Genotypes Shape the Response of cv. Pinot gris to Water Deficit" Agronomy 11, no. 1: 75. https://doi.org/10.3390/agronomy11010075
APA StyleFaralli, M., Bianchedi, P. L., Bertamini, M., & Varotto, C. (2021). Rootstock Genotypes Shape the Response of cv. Pinot gris to Water Deficit. Agronomy, 11(1), 75. https://doi.org/10.3390/agronomy11010075







