Stomata and Xylem Vessels Traits Improved by Melatonin Application Contribute to Enhancing Salt Tolerance and Fatty Acid Composition of Brassica napus L. Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Experimental Treatments
2.2. Assessment of Growth Traits
2.3. Determination of Membrane Stability Index, Relative Water Content, Lipid Peroxidation, and Antioxidant Enzymes Activity
2.4. Determination of Ions, Proline, and Total Soluble Sugars Concentrations
2.5. Measurement of Chlorophyll Content, Photosynthetic Pigments and Gas Exchange Parameters
2.6. Assessment of Yield Traits
2.7. Assessment and Imaging of Green Siliques and Seeds Quality Traits
2.8. Quantified and Qualified Determinations of Rapeseed Oil
2.9. Anatomical Traits and Analyses
2.10. Data Analysis
3. Results
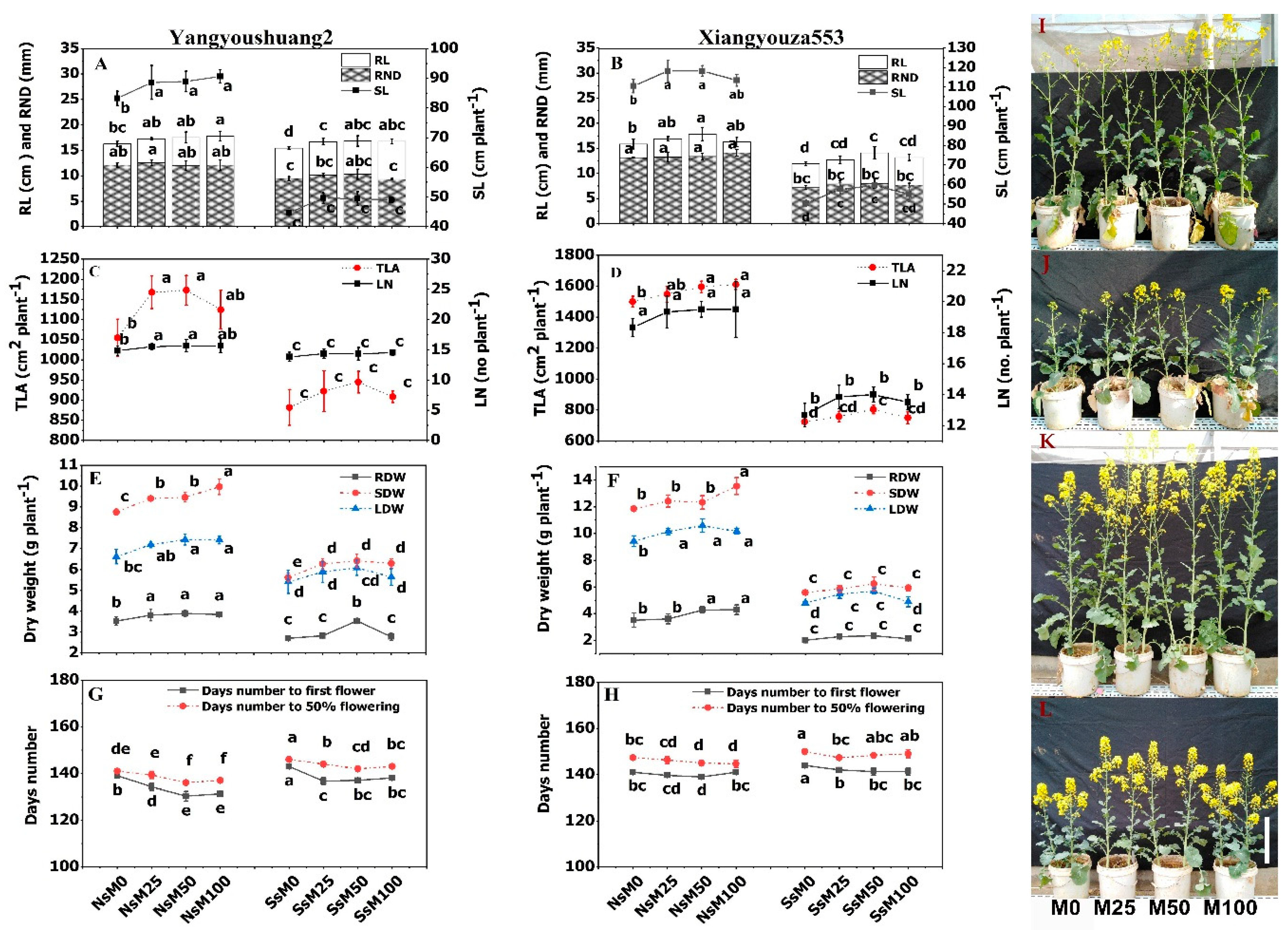
3.1. Melatonin Priming Improved Growth Traits of Rapeseed Cultivars
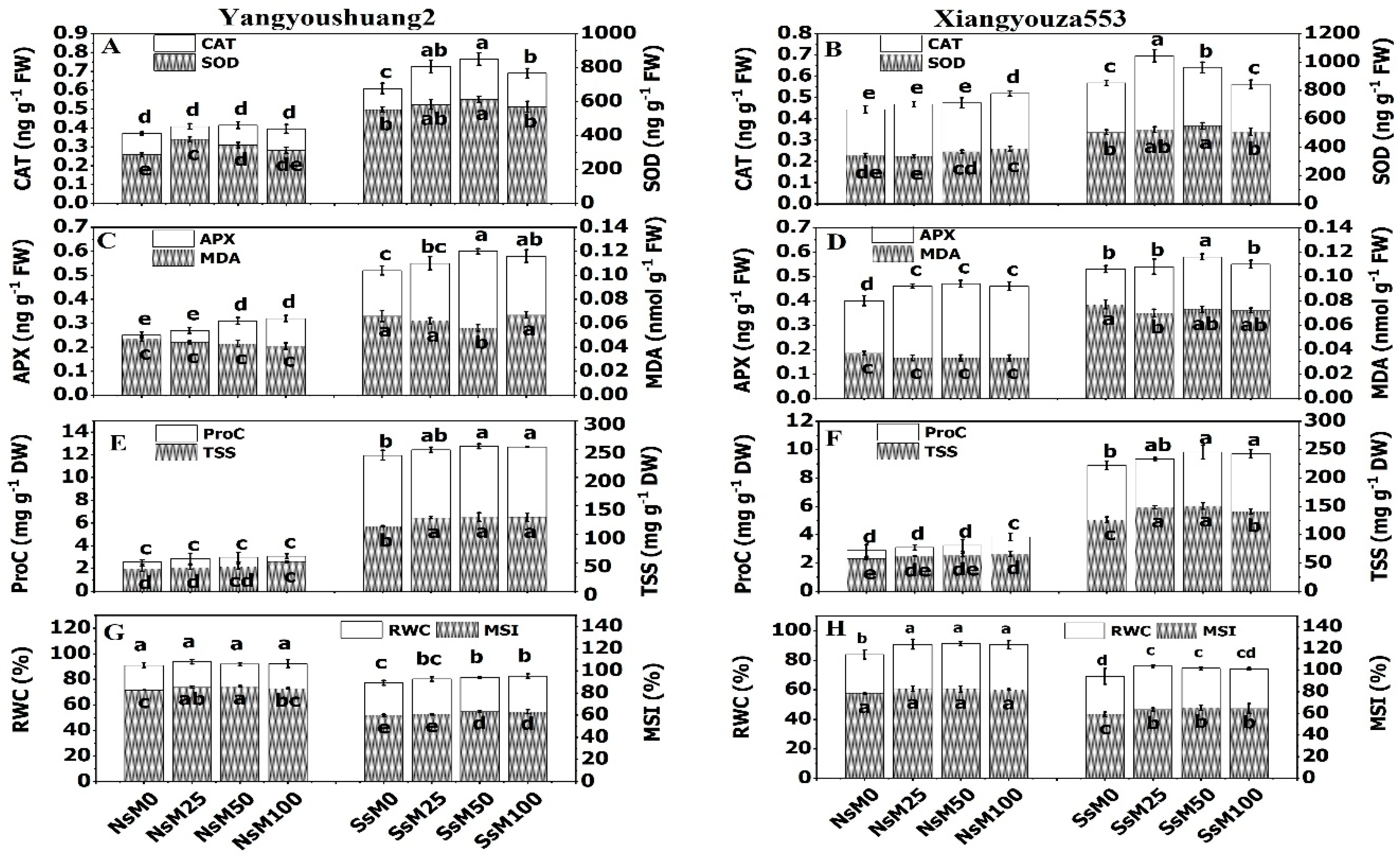
3.2. Melatonin Priming Improved Antioxidant Enzymes Activities and Decreased Malondialdehyde
3.3. Melatonin Priming and Accumulation of Osmolytes under NaCl Stress
3.4. Melatonin Priming Improved MSI and RWC
3.5. Melatonin Priming Improved K+/Na+ and Ca2+/Na+ Ratio
3.6. Melatonin Priming Improved Photosynthetic Pigments and Gas Exchange Parameters
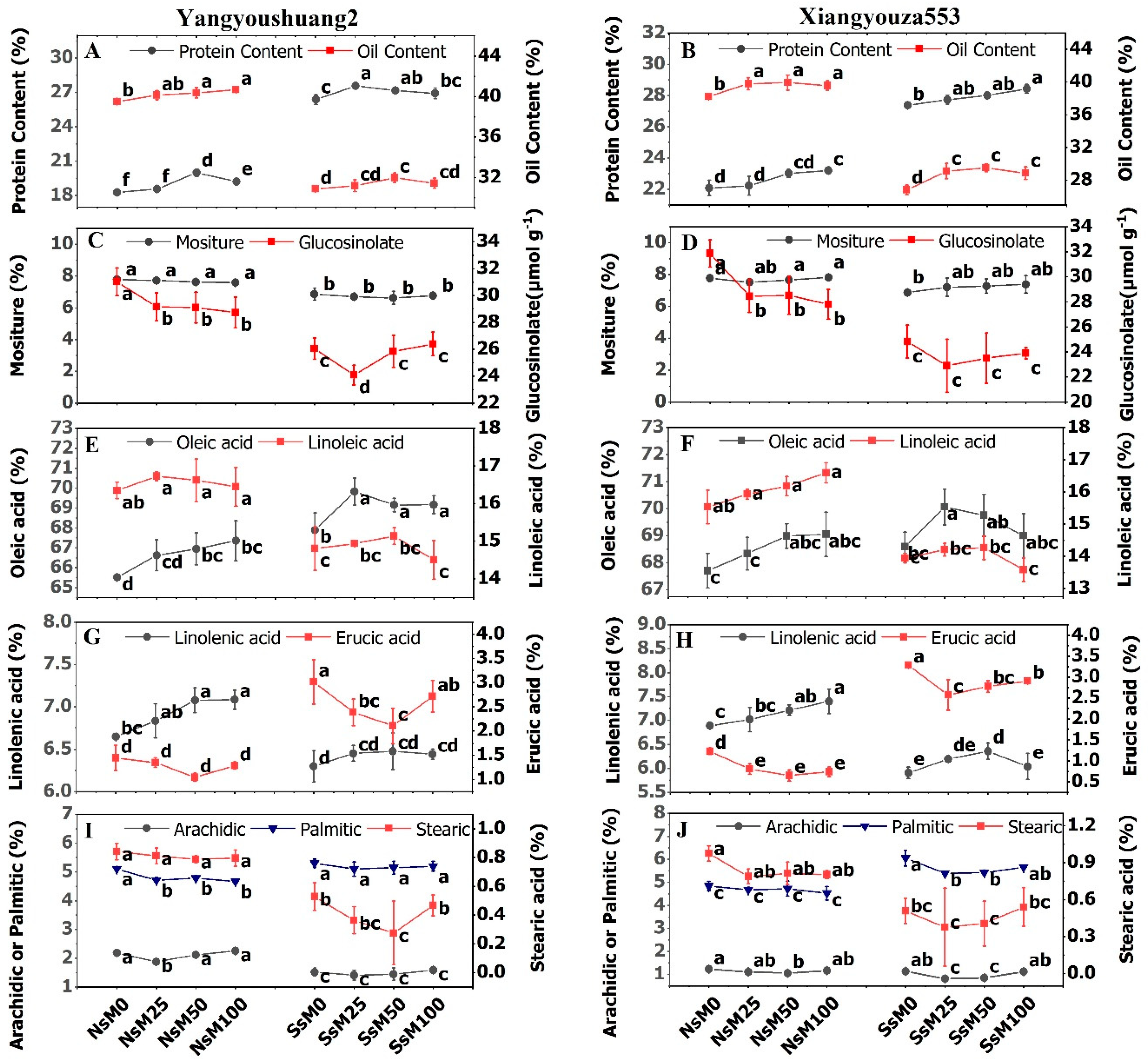
3.7. Melatonin Priming Improved Yield Traits and Fatty Acids Composition of Rapeseed Cultivars
3.8. Relationships
3.9. Melatonin Priming Improved Performance of Stomata and Transporting System by Xylem Vessels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Santangeli, M.; Capo, C.; Beninati, S.; Pietrini, F.; Forni, C. Gradual exposure to salinity improves tolerance to salt stress in rapeseed (Brassica napus L.). Water 2019, 11, 1667. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2017, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Penella, C.; Hernández, J.A.; Díaz-Vivancos, P.; Sánchez-Blanco, M.J.; Navarro, J.M.; Gómez-Bellot, M.J.; Barba-Espín, G. Towards a Sustainable Agriculture: Strategies Involving Phytoprotectants against Salt Stress. Agronomy 2020, 10, 194. [Google Scholar] [CrossRef]
- Chen, L.; Fan, J.; Hu, Z.; Huang, X.; Amombo, E.; Liu, A.; Bi, A.; Chen, K.; Xie, Y.; Fu, J. Melatonin is involved in regulation of Bermuda grass growth and development and response to low K+ stress. Front. Plant Sci. 2017, 8, 2038. [Google Scholar]
- Ketehouli, T.; Idrice Carther, K.F.; Noman, M.; Wang, F.-W.; Li, X.-W.; Li, H.-Y. Adaptation of Plants to Salt Stress: Characterization of Na+ and K+ Transporters and Role of CBL Gene Family in Regulating Salt Stress Response. Agronomy 2019, 9, 687. [Google Scholar] [CrossRef]
- Chen, T.-W.; Gomez Pineda, I.M.; Brand, A.M.; Stützel, H. Determining Ion Toxicity in Cucumber under Salinity Stress. Agronomy 2020, 10, 677. [Google Scholar]
- Agami, R.A. Applications of ascorbic acid or proline increase resistance to salt stress in barley seedlings. Biol. Plant. 2014, 58, 341–347. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Moez, H.; Chantal, E.; Mariama, N.; Laurent, L.; Khaled, M. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar]
- Anjum, S.A.; Farooq, M.; Xie, X.Y.; Liu, X.J.; Ijaz, M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- FAO Oil Crops, Oils and Meals. Available online: http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Oilcrops/Documents/Food_outlook_oilseeds/FO_Oilcrops.pdf (accessed on 12 August 2020).
- Miyamoto, S.; Oster, M.F.; Rostle, C.T.; Lenn, E.G. Salt tolerance of oilseed crops during establishment. J. Arid Land Stud. 2012, 22, 147–151. [Google Scholar]
- Purty, R.S.; Kumar, G.; Singla-Pareek, S.L.; Pareek, A. Towards salinity tolerance in Brassica: An overview. Physiol. Mol. Biol. Plants 2008, 14, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Banaei-Asl, F.; Farajzadeh, D.; Bandehagh, A.; Komatsu, S. Comprehensive proteomic analysis of canola leaf inoculated with a plant growth-promoting bacterium, Pseudomonas fluorescens, under salt stress. Biochim. Biophys. Acta (BBA)-Proteins Proteomics 2016, 1864, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.A.A.; Shalby, N.; Bai, C.; Qin, M.; Agami, R.A.; Jie, K.; Wang, B.; Zhou, G. Stomatal and photosynthetic traits are associated with investigating sodium chloride tolerance of Brassica napus l. Cultivars. Plants 2020, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator. Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Fan, J.; Yan, X.; Zaichao, Z.; Liang, C. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 2018, 8, 33. [Google Scholar] [CrossRef]
- Ye, J.; Yang, W.; Li, Y.; Wang, S.; Yin, L.; Deng, X. Seed Pre-Soaking with Melatonin Improves Wheat Yield by Delaying Leaf Senescence and Promoting Root Development. Agronomy 2020, 10, 84. [Google Scholar] [CrossRef]
- Zeng, L.; Cai, J.; Li, J.; Lu, G.; Li, C.; Fu, G.; Zhang, X.; Liu, Q.; Zou, X.; Cheng, Y. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J. Integr. Agric. 2018, 17, 328–335. [Google Scholar] [CrossRef]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous Melatonin Improves Salt Tolerance by Mitigating Osmotic, Ion, and Oxidative Stresses in Maize Seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P. Melatonin and its effects on plant systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Liu, B.; Zhang, S. Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci. Hortic. 2017, 219, 264–271. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. J. Pineal Res. 2017, 62, e12403. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Castaares, J.L.; Bouzo, C.A. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic. Plant J. 2019, 5, 37–45. [Google Scholar]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef]
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.J.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the Defense Mechanisms during Plant Oxidative Stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zheng, B. Melatonin mediated regulation of drought stress: Physiological and molecular aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.-J.; Sun, Q.-Q.; Cao, Y.-Y.; Li, X.; Zhao, B.; Wu, P.; Guo, Y.-D. Proteomic analysis reveals a role of melatonin in promoting cucumber seed germination under high salinity by regulating energy production. Sci. Rep. 2017, 7, 503. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.; Weeda, S.; Yang, C.; Yang, Z.; Ren, S.; Guo, Y. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Wang, H.; Li, D.; Bai, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Li, C. Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 2019, 14, e0216575. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.; Wang, C.; Li, K.; Zhang, X.; Yang, Z.; Li, M.; Wang, B. An effective screening method and a reliable screening trait for salt tolerance of Brassica napus at the germination stage. Front. Plant Sci. 2019, 10, 530. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Shu, K.; Qi, Y.; Chen, F.; Meng, Y.; Luo, X.; Shuai, H.; Zhou, W.; Ding, J.; Du, J.; Liu, J.; et al. Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front. Plant Sci. 2017, 8, 1372. [Google Scholar] [CrossRef]
- Benincasa, P.; Pace, R.; Quinet, M.; Lutts, S. Effect of salinity and priming on seedling growth in rapeseed (Brassica napus var oleifera Del.). Acta Sci. Agron. 2013, 35, 479–486. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Pramanik, K. Vegetable seed priming: A low cost, simple and powerful techniques for farmers’ livelihood. Int. J. Bio-Resour. Stress Manag. 2013, 4, 475–481. [Google Scholar]
- Zhao, G.; Zhao, Y.; Yu, X.; Kiprotich, F.; Han, H.; Guan, R.; Wang, R.; Wenbiao, S. Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 2018, 19, 1912. [Google Scholar] [CrossRef] [PubMed]
- Soil Survey Staff, Department of Agriculture. Keys to Soil Taxonomy; USDA–Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course, 2nd ed.; Parallel Press, University of Wisconsin-Madison: Madison, WI, USA, 2005; ISBN 1893311473. [Google Scholar]
- Farooq, M.; Irfan, M.; Aziz, T.; Ahmad, I.; Cheema, S.A. Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop Sci. 2013, 199, 12–22. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Springer: New York, NY, USA, 2001; pp. 207–212. [Google Scholar]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal. Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef]
- Yong, H.-Y.; Zou, Z.; Kok, E.-P.; Kwan, B.-H.; Chow, K.; Nasu, S.; Nanzyo, M.; Kitashiba, H.; Nishio, T. Comparative transcriptome analysis of leaves and roots in response to sudden increase in salinity in Brassica napus by RNA-seq. Biomed Res. Int. 2014, 467395. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Maness, N. Extraction and analysis of soluble carbohydrates. In Plant Stress Tolerance; Springer: New York, NY, USA, 2010; pp. 341–370. [Google Scholar]
- Wu, F.; Wu, L.; Xu, F. Chlorophyll meter to predict nitrogen sidedress requirements for short-season cotton (Gossypium hirsutum L.). Field Crop. Res. 1998, 56, 309–314. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Burns, M.J.; Barnes, S.R.; Bowman, J.G.; Clarke, M.H.E.; Werner, C.P.; Kearsey, M.J. QTL analysis of an intervarietal set of substitution lines in Brassica napus:(i) Seed oil content and fatty acid composition. Heredity 2003, 90, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chauhan, J.S.; Kumar, A. Screening for erucic acid and glucosinolate content in rapeseed-mustard seeds using near infrared reflectance spectroscopy. J. Food Sci. Technol. 2010, 47, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, S.E. Plant Microtechnique and Microscopy, 1st ed.; Oxford University Press: Oxford, UK, 1999; Volume 198. [Google Scholar]
- 3DHISTECH Ltd. CaseViewer 2.3 Software Program. Available online: https://www.3dhistech.com/solution/caseviewer/ (accessed on 12 August 2020).
- VSN International GENSTAT for Windows 17th Edition. Available online: https://www.vsni.co.uk/software/genstat (accessed on 12 August 2020).
- Rady, M.M.; Kuşvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kuşvuran, S. Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J. Plant Growth Regul. 2019, 38, 449–462. [Google Scholar] [CrossRef]
- Rady, M.M.; El-Azeem, M.M.A.; El-Mageed, T.A.A.; Abdelhamid, M.T. Integrative potassium humate and biochar application reduces salinity effects and contaminants, and improves growth and yield of eggplant grown under saline conditions. Int. J. Empir. Educ. Res. 2018, 1, 36–37. [Google Scholar] [CrossRef]
- Smoleń, S.; Lukasiewicz, A.; Klimek-Chodacka, M.; Baranski, R. Effect of Soil Salinity and Foliar Application of Jasmonic Acid on Mineral Balance of Carrot Plants Tolerant and Sensitive to Salt Stress. Agronomy 2020, 10, 659. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, X.; Yan, J.; Yuan, Z.; Gu, M. Effects of Salt Stress on Growth, Photosynthesis, and Mineral Nutrients of 18 Pomegranate (Punica granatum) Cultivars. Agronomy 2020, 10, 27. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 24. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2014, 66, 647–656. [Google Scholar] [CrossRef]
- Sami, A.; Shah, F.A.; Abdullah, M.; Zhou, X.; Yan, Y.; Zhu, Z.; Zhou, K. Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Wang, T.; Liu, H.; Tian, D.; Zhang, Y. Melatonin application improves salt tolerance of alfalfa (Medicago sativa L.) by enhancing antioxidant capacity. Plants 2020, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Sharma, A.; Tao, S.; Zheng, B.; Landi, M.; Yuan, H.; Yan, D. Melatonin Stimulates Activities and Expression Level of Antioxidant Enzymes and Preserves Functionality of Photosynthetic Apparatus in Hickory Plants (Carya cathayensis Sarg.) under PEG-Promoted Drought. Agronomy 2019, 9, 702. [Google Scholar] [CrossRef]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. Plant Stress Physiol. 2012, 1, 59–93. [Google Scholar]
- Chen, Z.; Newman, I.; Zhou, M.; Mendham, N.; Zhang, G.; Shabala, S. Screening plants for salt tolerance by measuring K+ flux: A case study for barley. Plant. Cell Environ. 2005, 28, 1230–1246. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- Sarropoulou, V.N.; Therios, I.N.; Dimassi-Theriou, K.N. Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus× P. canescens), and MxM 60 (P. avium× P. mahaleb). J. Pineal Res. 2012, 52, 38–46. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Awadi, M.E. Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biológica Colomb. 2015, 20, 223–235. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Li, X.; Brestic, M.; Tan, D.; Zivcak, M.; Zhu, X.; Liu, S.; Song, F.; Reiter, R.J.; Liu, F. Melatonin alleviates low PSI-limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef] [PubMed]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2019, 57, 108–115. [Google Scholar]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Naeem, M.S.; Jin, Z.L.; Wan, G.L.; Liu, D.; Liu, H.B.; Yoneyama, K.; Zhou, W.J. 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.). Plant Soil 2010, 332, 405–415. [Google Scholar] [CrossRef]
- Youssef, T.; Awad, M.A. Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-Aminolevulinic acid-based fertilizer. J. Plant Growth Regul. 2008, 27, 1–9. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, R.; Wang, L.; Sun, Q.; Li, D.; Cao, Y.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar]
- Tahjib-UI-Arif, M.; Sohag, A.A.M.; Afrin, S.; Bashar, K.K.; Afrin, T.; Mahamud, A.G.M.; Polash, M.A.S.; Hossain, M.; Sohel, M.; Taher, A. Differential response of sugar beet to long-term mild to severe salinity in a soil–pot culture. Agriculture 2019, 9, 223. [Google Scholar] [CrossRef]
- Sabagh, A.E.L.; Hossain, A.; Barutçular, C.; Islam, M.S.; Ratnasekera, D.; Kumar, N.; Meena, R.S.; Gharib, H.S.; Saneoka, H.; da Silva, J.A.T. Drought and salinity stress management for higher and sustainable canola (Brassica napus L.) production: A critical review. Aust. J. Crop Sci. 2019, 13, 88. [Google Scholar] [CrossRef]
- Elferjani, R.; Soolanayakanahally, R. Canola responses to drought, heat, and combined stress: Shared and specific effects on carbon assimilation, seed yield, and oil composition. Front. Plant Sci. 2018, 9, 1224. [Google Scholar] [CrossRef]
- Davoudi, A.; Mirshekari, B.; Shirani-Rad, A.; Farahvash, F.; Rashidi, V. Effect of selenium foliar application on oil yield, fatty acid composition and glucosinolate content of rapeseed cultivars under late-season thermal stress. OCL 2019, 26, 43. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Pascale, S. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2016, 29, 1–12. [Google Scholar]
- Fernández-García, N.; Olmos, E.; Bardisi, E.; García-De la Garma, J.; López-Berenguer, C.; Rubio-Asensio, J.S. Intrinsic water use efficiency controls the adaptation to high salinity in a semi-arid adapted plant, henna (Lawsonia inermis L.). J. Plant Physiol. 2014, 171, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A.; Medani, R.A.; Abd El-Mola, I.A.; Taha, R.S. Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress. Int. J. Environ. Agric. Res. 2016, 2, 78. [Google Scholar]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, I.A.A.; Shalby, N.; M. A. El-Badri, A.; Saleem, M.H.; Khan, M.N.; A. Nawaz, M.; Qin, M.; Agami, R.A.; Kuai, J.; Wang, B.; et al. Stomata and Xylem Vessels Traits Improved by Melatonin Application Contribute to Enhancing Salt Tolerance and Fatty Acid Composition of Brassica napus L. Plants. Agronomy 2020, 10, 1186. https://doi.org/10.3390/agronomy10081186
Mohamed IAA, Shalby N, M. A. El-Badri A, Saleem MH, Khan MN, A. Nawaz M, Qin M, Agami RA, Kuai J, Wang B, et al. Stomata and Xylem Vessels Traits Improved by Melatonin Application Contribute to Enhancing Salt Tolerance and Fatty Acid Composition of Brassica napus L. Plants. Agronomy. 2020; 10(8):1186. https://doi.org/10.3390/agronomy10081186
Chicago/Turabian StyleMohamed, Ibrahim A. A., Nesma Shalby, Ali M. A. El-Badri, Muhammad Hamzah Saleem, Mohammad Nauman Khan, Muhammad A. Nawaz, Meng Qin, Ramadan A. Agami, Jie Kuai, Bo Wang, and et al. 2020. "Stomata and Xylem Vessels Traits Improved by Melatonin Application Contribute to Enhancing Salt Tolerance and Fatty Acid Composition of Brassica napus L. Plants" Agronomy 10, no. 8: 1186. https://doi.org/10.3390/agronomy10081186
APA StyleMohamed, I. A. A., Shalby, N., M. A. El-Badri, A., Saleem, M. H., Khan, M. N., A. Nawaz, M., Qin, M., Agami, R. A., Kuai, J., Wang, B., & Zhou, G. (2020). Stomata and Xylem Vessels Traits Improved by Melatonin Application Contribute to Enhancing Salt Tolerance and Fatty Acid Composition of Brassica napus L. Plants. Agronomy, 10(8), 1186. https://doi.org/10.3390/agronomy10081186








