Foliar Application of Zn Alleviates Salt Stress Symptoms of Pak Choi Plants by Activating Water Relations and Glucosinolate Synthesis
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Growth Conditions
- Salinity experiment.
- (i)
- Control: the plants were grown without NaCl (Hoagland solution).
- (ii)
- Salinity: NaCl concentrations of 40, 80, 120 or 160 mM were added to the nutrient solution.
The treatments were applied the same day and were maintained for 15 days. - Zn foliar application.
- (i)
- Control: the plants were grown without foliar Zn application.
- (ii)
- (Zn solutions: 3 mL per plant, containing zinc sulphate at concentrations of 50, 100, 500 or 1000 µM, with 0.1% non-ionic, organo-silicon surfactant, were sprayed onto the leaves three times a week for one week. As we had found previously that 25 µM zinc sulphate did not produce any negative effect on plant biomass [32], in this experiment, to check the levels of toxicity, the lowest concentration was 50 µM.
- Combination treatments.Based on the results of the previous experiments, main dosages of NaCl or Zn were used to carry out this one. The experiment was carried out for 15 days and included two levels of nutrient solution salinity (0 and 80 mM NaCl) for two weeks and three levels of Zn (0, 25 and 50 µM) applied as a foliar solution by spraying (3 mL per plant) three times in the last week of the experiment. One day after the last Zn application, the gas exchange parameters were determined, and tissue samples were collected for the rest of the measurements.All samples were collected 45 days after germination, washed thoroughly with tap water and 0.1% non-ionic detergent, and washed twice with distilled water before being frozen at −80 °C. Then, the samples were lyophilized and weighed to obtain the dry weight.
2.2. Gas Exchange Parameters
2.3. Ion Concentrations
2.4. Histochemical Staining and Malondialdehyde (MDA) Concentration
2.5. Zynpyr-1 Staining
2.6. GSLs Determination
2.7. Statistical Analysis
3. Results
3.1. Effect of Zn Foliar Application or Salinity on Plant Growth
3.2. Effects of Salinity and Zn Application on Pak Choi Plants
3.2.1. Plant Growth and Gas Exchange Parameters
3.2.2. Plasma Membrane Integrity and Lipid Peroxidation
3.2.3. Zn-Pyr Staining
3.2.4. Ion Concentrations
3.2.5. Glucosinolate Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, J.; Singh, V.P.; Prasad, S.M. NaCl-induced physiological and biochemical changes in two cyanobacteria Nostoc muscorum and Phormidium foveolarum acclimatized to different photosynthetically active radiation. J. Photochem. Photobiol. B Biol. 2015, 151, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Smith, S.E.; Smith, F.A. Plant growth and cation composition of two cultivars of spring wheat (Triticum aestivum L.) differing in P uptake efficiency. J. Exp. Bot. 2001, 52, 1277–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, M.; Martinez, V.; Alcaraz, C.F. Physiological function of water channels as affected by salinity in roots of paprika pepper. Physiol. Plant. 2002, 105, 95–101. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant. Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Bybordi, A.; Ebrahimian, E. Effect of salinity stress on activity of enzymes involved in nitrogen and phosphorous metabolism case study: Canola (Brassica napus L.). Asian J. Agric. Res. 2011, 5, 208–214. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant. Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Coleman, J.E. Zinc enzymes. Curr. Opin. Chem. Biol. 1998, 2, 222–234. [Google Scholar] [CrossRef]
- Berg, J.M.; Shi, Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef]
- Vallee, B.L.; Auld, D.S. Zinc Coordination, Function, and Structure of Zinc Enzymes and Other Proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef]
- Barak, P.; Helmke, P.A. The Chemistry of Zinc. In Zinc in Soils and Plants; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J. Plant. Nutr. 2016, 39, 172–180. [Google Scholar] [CrossRef]
- Iqbal, M.N.; Rasheed, R.; Ashraf, M.Y.; Ashraf, M.A.; Hussain, I. Exogenously applied zinc and copper mitigate salinity effect in maize (Zea mays L.) by improving key physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2018, 25, 23889–23896. [Google Scholar] [CrossRef] [PubMed]
- Alpaslan, M.; Inal, A.; Güneş, A.; Çikili, Y.; Özcan, H. Effect of zinc treatment on the alleviation of sodium and chloride injury in tomato (Lycopersicum esculentum (L.) Mill. cv. Lale) grown under salinity. Turk. J. Botany 1999, 23, 1–6. [Google Scholar]
- Redondo-Gómez, S.; Andrades-Moreno, L.; Mateos-Naranjo, E.; Parra, R.; Valera-Burgos, J.; Aroca, R. Synergic effect of salinity and zinc stress on growth and photosynthetic responses of the cordgrass, Spartina densiflora. J. Exp. Bot. 2011, 62, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Badakhshan, H. Effects of Zinc Application on Growth, Absorption and Distribution of Mineral Nutrients Under Salinity Stress in Soybean (Glycine Max L.). J. Plant. Nutr. 2014, 37, 2014. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.I.; Mottaleb, S.A. Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.Y.; Tariq, S.; Saleem, M.; Khan, M.A.; Hassan, S.W.U.; Sadef, Y. Calcium and zinc mediated growth and physio-biochemical changes in mungbean grown under saline conditions. J. Plant. Nutr. 2020, 43, 512–525. [Google Scholar] [CrossRef]
- Hanson, P.; Yang, R.Y.; Chang, L.C.; Ledesma, L.; Ledesma, D. Contents of carotenoids, ascorbic acid, minerals and total glucosinolates in leafy brassica pakchoi (Brassica rapa L. chinensis) as affected by season and variety. J. Sci. Food Agric. 2009, 89, 906–914. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 2013, 263, 398–403. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Olsson, K.; Engqvist, G.; Ekvall, J.; Olsson, M.; Nyman, M.; Åkesson, B. Variation in the content of glucosinolates, hydroxycinnamic acids, carotenoids, total antioxidant capacity and low-molecular-weight carbohydrates in Brassica vegetables. J. Sci. Food Agric. 2006, 86, 528–538. [Google Scholar] [CrossRef]
- Aires, A.; Fernandes, C.; Carvalho, R.; Bennett, R.N.; Saavedra, M.J.; Rosa, E.A.S. Seasonal effects on bioactive compounds and antioxidant capacity of six economically important brassica vegetables. Molecules 2011, 16, 6816–6832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila, F.W.; Faquin, V.; Yang, Y.; Ramos, S.J.; Guilherme, L.R.G.; Thannhauser, T.W.; Li, L. Assessment of the anticancer compounds Se -methylselenocysteine and glucosinolates in Se-biofortified broccoli (Brassica oleracea L. var. italica) sprouts and florets. J. Agric. Food Chem. 2013, 8, 532. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Moreno-Fernández, D.A.; Castejón, D.; Ochando, C.; Morandini, P.A.; Carvajal, M. The impact of the absence of aliphatic glucosinolates on water transport under salt stress in Arabidopsis thaliana. Front. Plant. Sci. 2015, 6, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, J.J.; Agudelo, A.; Moreno, D.A.; Carvajal, M. Growing broccoli under salinity: The influence of cultivar and season on glucosinolates content. Sci. Agric. 2020. [Google Scholar] [CrossRef] [Green Version]
- Keling, H.; Zhujun, Z. Effects of different concentrations of sodium chloride on plant growth and glucosinolate content and composition in pakchoi. Afr. J. Biotechnol. 2010, 28, 4428–4433. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235, 343–351. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Tan, Q.; Nie, Z.; Sun, X. Effects of molybdenum on water utilization, antioxidative defense system and osmotic-adjustment ability in winter wheat (Triticumaestivum) under drought stress. Plant. Physiol. Biochem. 2014, 83, 365–374. [Google Scholar] [CrossRef]
- Qin, L.; Guo, Y.; Chen, L.; Liang, R.; Gu, M.; Xu, G.; Zhao, J.; Walk, T.; Liao, H. Functional Characterization of 14 Pht1 Family Genes in Yeast and Their Expressions in Response to Nutrient Starvation in Soybean. PLoS ONE 2012, 7, e47726. [Google Scholar] [CrossRef]
- Fatemi, H.; Zaghdoud, C.; Nortes, P.A.; Carvajal, M.; del Carmen Martínez-Ballesta, M. Differential Aquaporin Response to Distinct Effects of Two Zn Concentrations after Foliar Application in Pak Choi (Brassica rapa L.) Plants. Agronomy 2020, 10, 450. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.S.; Yang, Z.M. Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant. Cell Physiol. 2005, 46, 1915–1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pompella, A.; Maellaro, E.; Casini, A.F.; Comporti, M. Histochemical detection of lipid peroxidation in the liver of bromobenzene-poisoned mice. Am. J. Pathol. 1987, 192, 295–301. [Google Scholar]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 1, 35–44. [Google Scholar] [CrossRef]
- Wu, H.; Shabala, L.; Zhou, M.; Stefano, G.; Pandolfi, C.; Mancuso, S.; Shabala, S. Developing and validating a high-throughput assay for salinity tissue tolerance in wheat and barley. Planta 2015, 242, 847–857. [Google Scholar] [CrossRef] [PubMed]
- López-Berenguer, C.; Martínez-Ballesta, M.C.; García-Viguera, C.; Carvajal, M. Leaf water balance mediated by aquaporins under salt stress and associated glucosinolate synthesis in broccoli. Plant. Sci. 2008, 174, 321–328. [Google Scholar] [CrossRef]
- Ashraf, M. Relationships between growth and gas exchange characteristics in some salt-tolerant amphidiploid Brassica species in relation to their diploid parents. Environ. Exp. Bot. 2001, 45, 155–163. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Craig, R.; Lynch, J.P. Salinity tolerance of Phaseolus species during germination and early seedling growth. Crop. Sci. 2002, 42, 1584. [Google Scholar] [CrossRef]
- Fässler, E.; Robinson, B.H.; Stauffer, W.; Gupta, S.K.; Papritz, A.; Schulin, R. Phytomanagement of metal-contaminated agricultural land using sunflower, maize and tobacco. Agric. Ecosyst. Environ. 2010, 136, 49–58. [Google Scholar] [CrossRef]
- Demim, S.; Drouiche, N.; Aouabed, A.; Semsari, S. CCD study on the ecophysiological effects of heavy metals on Lemna gibba. Ecol. Eng. 2013, 57, 302–313. [Google Scholar] [CrossRef]
- Sagardoy, R.; Morales, F.; López-Millán, A.F.; Abadía, A.; Abadía, J. Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant. Biol. 2009, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Sagardoy, R.; Vázquez, S.; Florez-Sarasa, I.D.; Albacete, A.; Ribas-Carbó, M.; Flexas, J.; Abadía, J.; Morales, F. Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytol. 2010, 187, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Sagardoy, R.; Morales, F.; Rellán-Álvarez, R.; Abadía, A.; Abadía, J.; López-Millán, A.F. Carboxylate metabolism in sugar beet plants grown with excess Zn. J. Plant. Physiol. 2011, 168, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S.; Abbas, F.; Bharwana, S.A.; Zhang, G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Sun, X.H.; Xu, H.L.; Mantri, N.; Lu, H.F. Optimal concentration of zinc sulfate in foliar spray to alleviate salinity stress in Glycine soja. J. Agric. Sci. Technol. 2014, 16, 445–460. [Google Scholar]
- Siddiqui, S.N.; Umar, S.; Iqbal, M. Zinc-induced modulation of some biochemical parameters in a high- and a low-zinc-accumulating genotype of Cicer arietinum L. grown under Zn-deficient condition. Protoplasma 2015, 252, 1335–1345. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley review no. 111: Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant. Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Maksimovic, I.; Ilin, Z. Effects of Salinity on Vegetable Growth and Nutrients Uptake. In Irrigation Systems and Practices in Challenging Environments; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Yermiyahu, U.; Nir, S.; Ben-Hayyim, G.; Kafkafi, U.; Kinraide, T.B. Root elongation in saline solution related to calcium binding to root cell plasma membranes. Plant. Soil 1997, 191, 67–76. [Google Scholar] [CrossRef]
- Yu, Q.; Rengel, Z. Drought and salinity differentially influence activities of superoxide dismutases in narrow-leafed lupins. Plant. Sci. 1999, 142, 1–11. [Google Scholar] [CrossRef]
- Sharma, P.N.; Tripathi, A.; Bisht, S.S. Zinc requirement for stomatal opening in cauliflower. Plant. Physiol. 1995, 107, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinton, R.; Cakmak, I.; Marschner, H. Effect of zinc deficiency on proton fluxes in plasma membrane-enriched vesicles isolated from bean roots. J. Exp. Bot. 1993, 44, 623–630. [Google Scholar] [CrossRef]
- López-Millán, A.F.; Duy, D.; Philippar, K. Chloroplast iron transport proteins—Function and impact on plant physiology. Front. Plant. Sci. 2016, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Neocleous, D.; Nikolaou, G.; Ntatsi, G.; Savvas, D. Impact of chelated or inorganic manganese and zinc applications in closed hydroponic bean crops on growth, yield, photosynthesis, and nutrient uptake. Agronomy 2020, 10, 881. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Qasim, M.; Ashraf, M.; Ashraf, M.Y.; Rehman, S.U.; Rha, E.S. Salt-induced changes in two canola cultivars differing in salt tolerance. Biol. Plant. 2003, 46, 629–632. [Google Scholar] [CrossRef]
- Grubb, C.D.; Abel, S. Glucosinolate metabolism and its control. Trends Plant. Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Chen, S.; Petersen, B.L.; Olsen, C.E.; Schulz, A.; Halkier, B.A. Long-distance phloem transport of glucosinolates in Arabidopsis. Plant. Physiol. 2001, 127, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Sarikamiş, G.; Çakir, A. Influence of salinity on aliphatic and indole glucosinolates in broccoli (Brassica oleracea var. Italica). Appl. Ecol. Environ. Res. 2017, 15, 1781–1788. [Google Scholar] [CrossRef]
- Tolrà, R.P.; Poschenrieder, C.; Alonso, R.; Barceló, D.; Barceló, J. Influence of zinc hyperaccumulation on glucosinolates in Thlaspi caerulescens. New Phytol. 2001, 151, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Noret, N.; Meerts, P.; Tolrà, R.; Poschenrieder, C.; Barceló, J.; Escarre, J. Palatability of Thlaspi caerulescens for snails: Influence of zinc and glucosinolates. New Phytol. 2005, 165, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kusznierewicz, B.; Baczek-Kwinta, R.; Bartoszek, A.; Piekarska, A.; Huk, A.; Manikowska, A.; Antonkiewicz, J.; Namieśnik, J.; Konieczka, P. The dose-dependent influence of zinc and cadmium contamination of soil on their uptake and glucosinolate content in white cabbage (Brassica oleracea var. capitata f. alba). Environ. Toxicol. Chem. 2012, 31, 2482–2489. [Google Scholar] [CrossRef] [PubMed]
- Barrameda-Medina, Y.; Blasco, B.; Lentini, M.; Esposito, S.; Baenas, N.; Moreno, D.A.; Ruiz, J.M. Zinc biofortification improves phytochemicals and amino-acidic profile in Brassica oleracea cv. Bronco. Plant. Sci. 2017, 258, 45–51. [Google Scholar] [CrossRef]

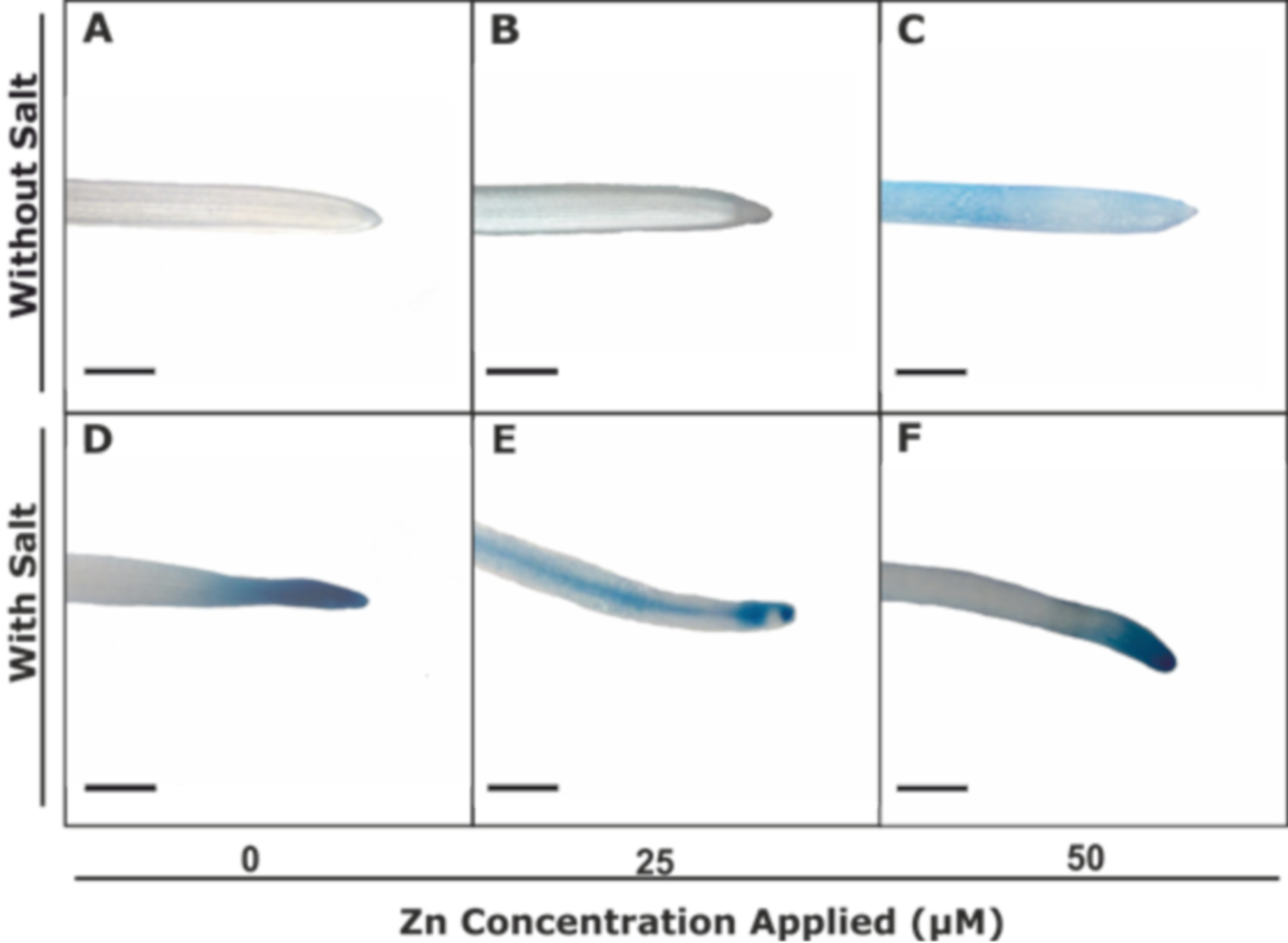
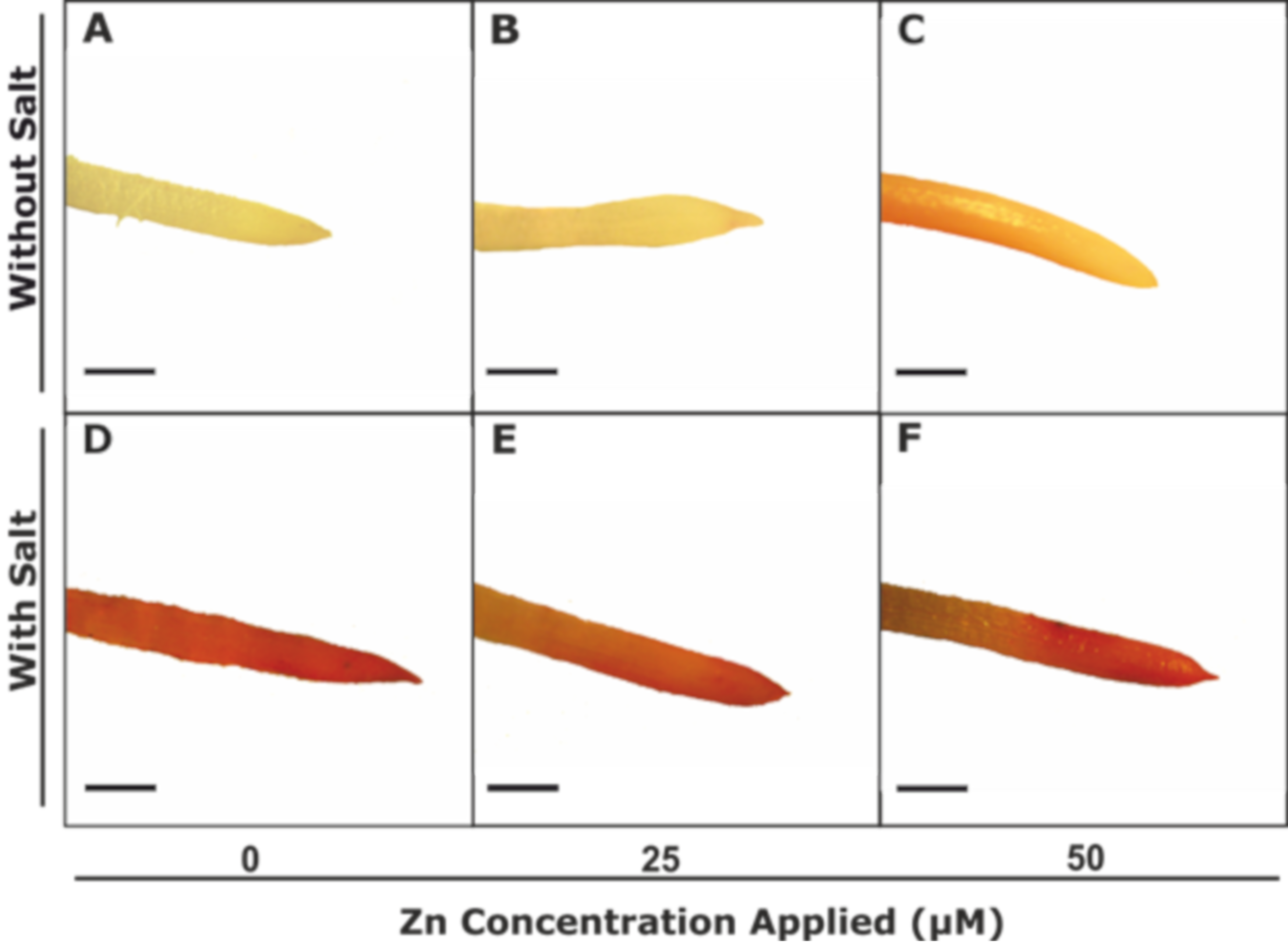

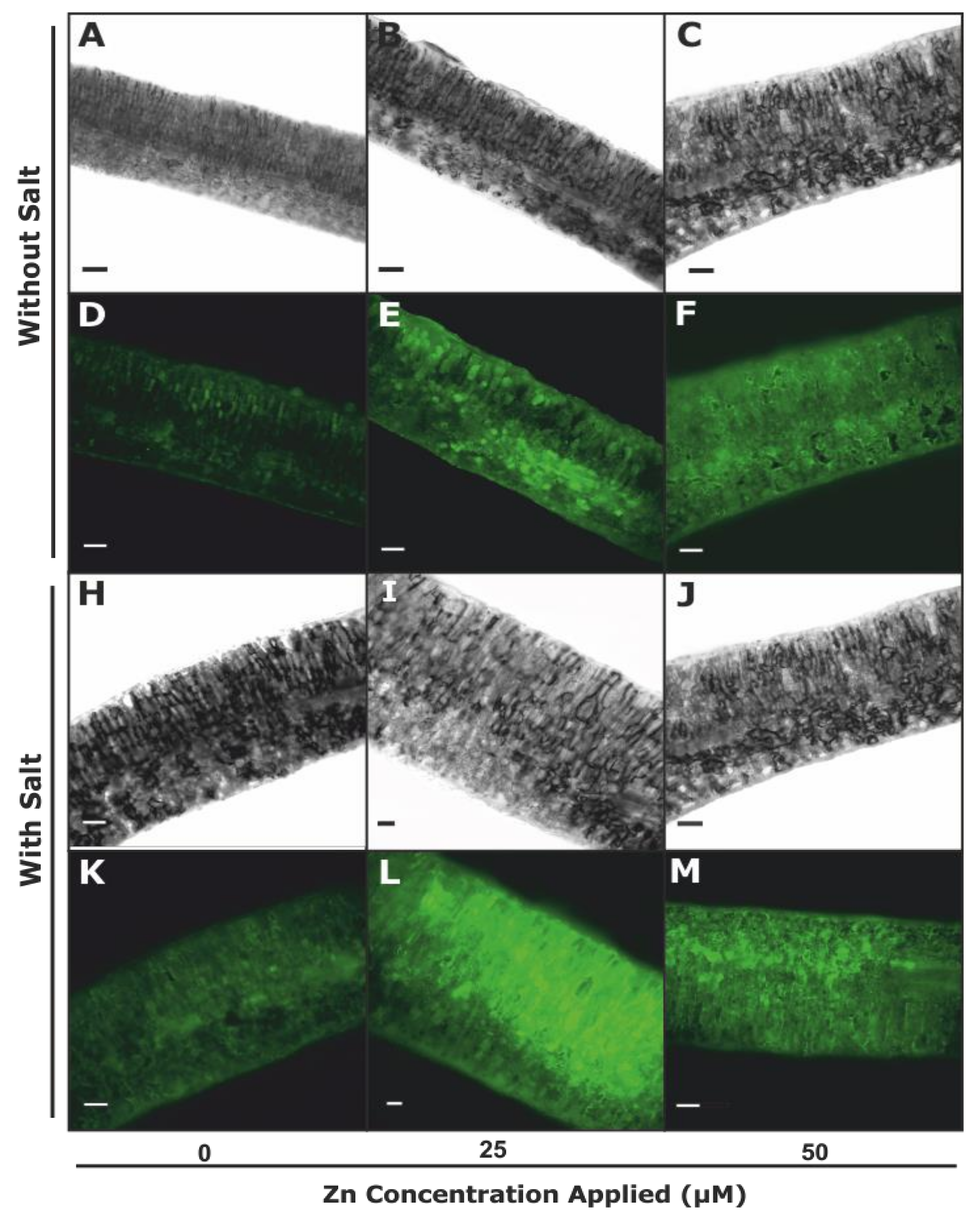

| Shoot Biomass (g DW) | Root Biomass (g DW) | Transpiration (mmol m−2 s−1) | Stomatal Conductance (mmol m−2 s−1)) | Assimilation (µmol m−2 s−1) | CI (mmol m−2 s−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Salinity (mM) | Salinity (mM) | Salinity (mM) | Salinity (mM) | Salinity (mM) | Salinity (mM) | ||||||
| Zn Applied (µM) | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 |
| 0 | 7.90 ± 0.47 | 4.66 ± 0.89 | 0.75 ± 0.07 | 0.81 ± 0.06 | 3.45 ± 0.21 | 3.74 ± 0.25 | 628.14 ± 50.71 | 524 ± 53.16 | 6.78 ± 0.79 | 9.90 ± 1.16 | 370.40 ± 26.73 | 206.50 ± 18.51 |
| 25 | 10.05 ± 0.93 | 7.84 ± 0.77 | 0.82 ± 0.07 | 1.01 ± 0.07 | 3.77 ± 0.20 | 6.12 ± 0.16 | 709.09 ± 44.87 | 618 ± 22.20 | 21.53 ± 4.34 | 16.8 ± 0.97 | 534.82 ± 66.92 | 333.01 ± 25.59 |
| 50 | 8.26 ± 1.06 | 7.52 ± 1.78 | 0.77 ± 0.05 | 1.12 ± 0.15 | 3.31 ± 0.22 | 5.72 ± 0.30 | 632.59 ± 41.19 | 630 ± 69.04 | 13.91 ± 1.82 | 15.64 ± 1.18 | 528.71 ± 58.37 | 294.3 ± 35.05 |
| p-value | ** | *** | n.s. | * | n.s | ** | n.s. | ** | *** | ** | ** | ** |
| LSD | 1.28 | 0.75 | 0.03 | 0.09 | 0.13 | 0.21 | 44.18 | 35.41 | 4.28 | 1.67 | 29.64 | 31.04 |
| Analysis of the variance | ||||||||||||
| Salinity | *** | n.s. | *** | n.s. | *** | *** | ||||||
| Zn Dosages | ** | n.s. | *** | n.s. | ** | *** | ||||||
| S x D | ** | n.s. | ** | * | ** | *** | ||||||
| LSD | 1.09 | 0.14 | 0.26 | 42.89 | 1.14 | 28.61 | ||||||
| Ca (mg g−1 DW) | Mg (mg g−1 DW) | K (mg g−1 DW) | Na (mg g−1 DW) | P (mg g−1 DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Salinity | Salinity | Salinity | Salinity | Salinity | ||||||
| Zn Applied (µM) | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | |
| 0 | 29.20 ± 1.4 | 23.11 ± 0.13 | 2.81 ± 0.08 | 2.2 ± 0.24 | 36.1 ± 1.82 | 24.15 ± 1.01 | 0.21 ± 0.08 | 27.21 ± 3.15 | 4.31 ± 0.39 | 5.9 ± 0.28 | |
| 25 | 30.91± 0.7 | 21.12 ± 4.40 | 3.03 ± 0.10 | 2.1 ± 0.16 | 39.7 ± 1.17 | 17.98 ± 0.37 | 0.18 ± 0.03 | 24.92 ± 3.54 | 4.01± 0.23 | 6.64 ± 0.75 | |
| 50 | 32.94± 0.6 | 20.4 ± 1.41 | 2.9 ± 0.39 | 2.0 ± 0.13 | 36.17 ± 3.34 | 18.95 ± 0.31 | 0.19 ± 0.01 | 24.21 ± 1.97 | 4.21 ± 0.45 | 5.14 ± 0.53 | |
| p-value | n.s. | n.s | n.s. | n.s. | n.s. | ** | n.s. | n.s. | n.s. | n.s. | |
| LSD | 0.84 | 0.59 | 0.12 | 0.17 | 1.22 | 0.86 | 0.06 | 1.93 | 0.33 | 0.6 | |
| Analysis of variance | |||||||||||
| Salinity | *** | n.s | ** | *** | * | ||||||
| Zn Dosages | n.s. | n.s | n.s. | n.s. | n.s | ||||||
| S x D | n.s. | n.s. | *** | *** | * | ||||||
| LSD | 0.15 | 0.11 | 0.97 | 1.71 | 0.48 | ||||||
| Fe (µg g−1 DW) | Mn (µg g−1 DW) | Cu (µg g−1 DW) | Zn (µg g−1 DW) | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Salinity | Salinity | Salinity | Salinity | ||||
| Zn Applied (µM) | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 |
| 0 | 48.31 ± 3.48 | 58.58 ± 3.14 | 60.67 ± 2.46 | 68.17 ± 2.32 | 1.97 ± 0.17 | 2.15 ± 0.23 | 45.36 ± 0.15 | 51.59 ± 1.21 |
| 25 | 50.41 ± 3.17 | 63.67 ± 5.13 | 56.91 ± 3.74 | 74.05 ± 5.44 | 2.24 ± 0.07 | 2.23 ± 0.07 | 58.62 ± 1.40 | 70.43 ± 1.25 |
| 50 | 48.43 ± 2.83 | 59.17 ± 5.04 | 5714 ± 5.42 | 73.52 ± 6.17 | 1.91 ± 0.32 | 2.21 ± 0.16 | 59.11 ± 2.42 | 66.43 ± 0.32 |
| p-value | n.s. | n.s. | n.s. | n.s. | n.s | n.s | *** | *** |
| LSD | 2.03 | 4.63 | 3.96 | 4.57 | 0.18 | 0.11 | 2.03 | 0.97 |
| Analysis of variance | ||||||||
| Salinity | n.s. | * | n.s | ** | ||||
| Zn Dosages | n.s. | * | n.s. | *** | ||||
| S x D | * | ** | n.s. | ** | ||||
| LSD | 4.11 | 2.57 | 0.24 | 1.16 | ||||
| Ca (mg g−1 DW) | Mg (mg g−1 DW) | K (mg g−1 DW) | Na (mg g−1 DW) | P (mg g−1 DW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Salinity | Salinity | Salinity | Salinity | Salinity | |||||
| Zn Applied (µM) | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 |
| 0 | 16.15 ± 1.03 | 6.69 ± 0.60 | 3.83 ± 0.07 | 2.74 ± 0.12 | 47.45 ± 1.73 | 26.35 ± 1.67 | 0.88 ± 0.07 | 29.90 ± 2.56 | 7.89 ± 0.30 | 7.39 ± 0.30 |
| 25 | 15.75 ± 1.4 | 7.81 ± 0.45 | 3.55 ± 0.22 | 2.81 ± 0.10 | 41.83 ± 2.45 | 29.86 ± 1.29 | 0.80 ± 0.12 | 31.54 ± 1.34 | 7.19 ± 0.45 | 7.72 ± 0.46 |
| 50 | 14.50 ± 0.43 | 8.00 ± 0.90 | 4.15 ± 0.12 | 3.83 ± 0.29 | 48.46 ± 1.78 | 28.39 ± 2.12 | 0.99 ± 0.10 | 28.39 ± 1.07 | 7.52 ± 0.19 | 7.72 ± 0.77 |
| p-value | n.s. | * | * | n.s. | * | ** | n.s. | n.s. | n.s. | n.s. |
| LSD | 1.23 | 0.59 | 0.19 | 0.23 | 1.55 | 2.03 | 0.09 | 1.28 | 0.38 | 0.27 |
| Analysis of variance | ||||||||||
| Salinity | *** | * | *** | *** | n.s. | |||||
| Zn Dosages | n.s. | n.s | ** | *** | n.s | |||||
| S x D | *** | *** | *** | *** | n.s. | |||||
| LSD | 0.23 | 0.17 | 1.58 | 0.98 | 0.11 | |||||
| Fe (µg g−1 DW) | Mn (µg g−1 DW) | Cu (µg g−1 DW) | Zn (µg g−1 DW) | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Salinity | Salinity | Salinity | Salinity | ||||
| Zn Applied (µM) | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 |
| 0 | 853.30 ± 50.96 | 573.97 ± 36.25 | 30.17 ± 1.08 | 24.02 ± 1.94 | 21.85 ± 1.24 | 19.22 ± 1.86 | 33.67 ± 1.52 | 27.01 ± 1.25 |
| 25 | 995.59 ± 94.03 | 684.41 ± 66.10 | 29.28 ± 1.68 | 30.19 ± 1.19 | 25.68 ± 2.14 | 22.47 ± 2.33 | 42.76 ± 2.01 | 45.01 ± 2.22 |
| 50 | 1184.37 ± 29.54 | 743.19 ± 67.02 | 31.62 ± 0.95 | 36.47 ± 1.91 | 24.38 ± 0.56 | 22.88 ± 1.80 | 43.04 ± 1.25 | 38.15 ± 2.19 |
| p-value | n.s. | * | n.s. | * | n.s | n.s | ** | *** |
| LSD | 62.55 | 34.51 | 1.12 | 0.92 | 1.25 | 1.94 | 2.08 | 2.34 |
| Analysis of variance | ||||||||
| Salinity | *** | n.s. | n.s | n.s. | ||||
| Zn Dosages | *** | * | n.s. | ** | ||||
| S x D | ** | n.s. | n.s. | ** | ||||
| LSD | 49.57 | 1.43 | 1.28 | 1.11 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatemi, H.; Carvajal, M.; Rios, J.J. Foliar Application of Zn Alleviates Salt Stress Symptoms of Pak Choi Plants by Activating Water Relations and Glucosinolate Synthesis. Agronomy 2020, 10, 1528. https://doi.org/10.3390/agronomy10101528
Fatemi H, Carvajal M, Rios JJ. Foliar Application of Zn Alleviates Salt Stress Symptoms of Pak Choi Plants by Activating Water Relations and Glucosinolate Synthesis. Agronomy. 2020; 10(10):1528. https://doi.org/10.3390/agronomy10101528
Chicago/Turabian StyleFatemi, Hamideh, Micaela Carvajal, and Juan José Rios. 2020. "Foliar Application of Zn Alleviates Salt Stress Symptoms of Pak Choi Plants by Activating Water Relations and Glucosinolate Synthesis" Agronomy 10, no. 10: 1528. https://doi.org/10.3390/agronomy10101528
APA StyleFatemi, H., Carvajal, M., & Rios, J. J. (2020). Foliar Application of Zn Alleviates Salt Stress Symptoms of Pak Choi Plants by Activating Water Relations and Glucosinolate Synthesis. Agronomy, 10(10), 1528. https://doi.org/10.3390/agronomy10101528







