Adaptation to Water and Salt Stresses of Solanum pimpinellifolium and Solanum lycopersicum var. cerasiforme
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design and Greenhouse Conditions
2.3. Gas Exchange Measurements
2.4. Leaf Chlorophyll Concentration Measurement by Soil Plant Analysis Development (SPAD) Chlorophyll Meter
2.5. Agronomic and Phenotypic Traits
2.6. Statistical Analyses
3. Results
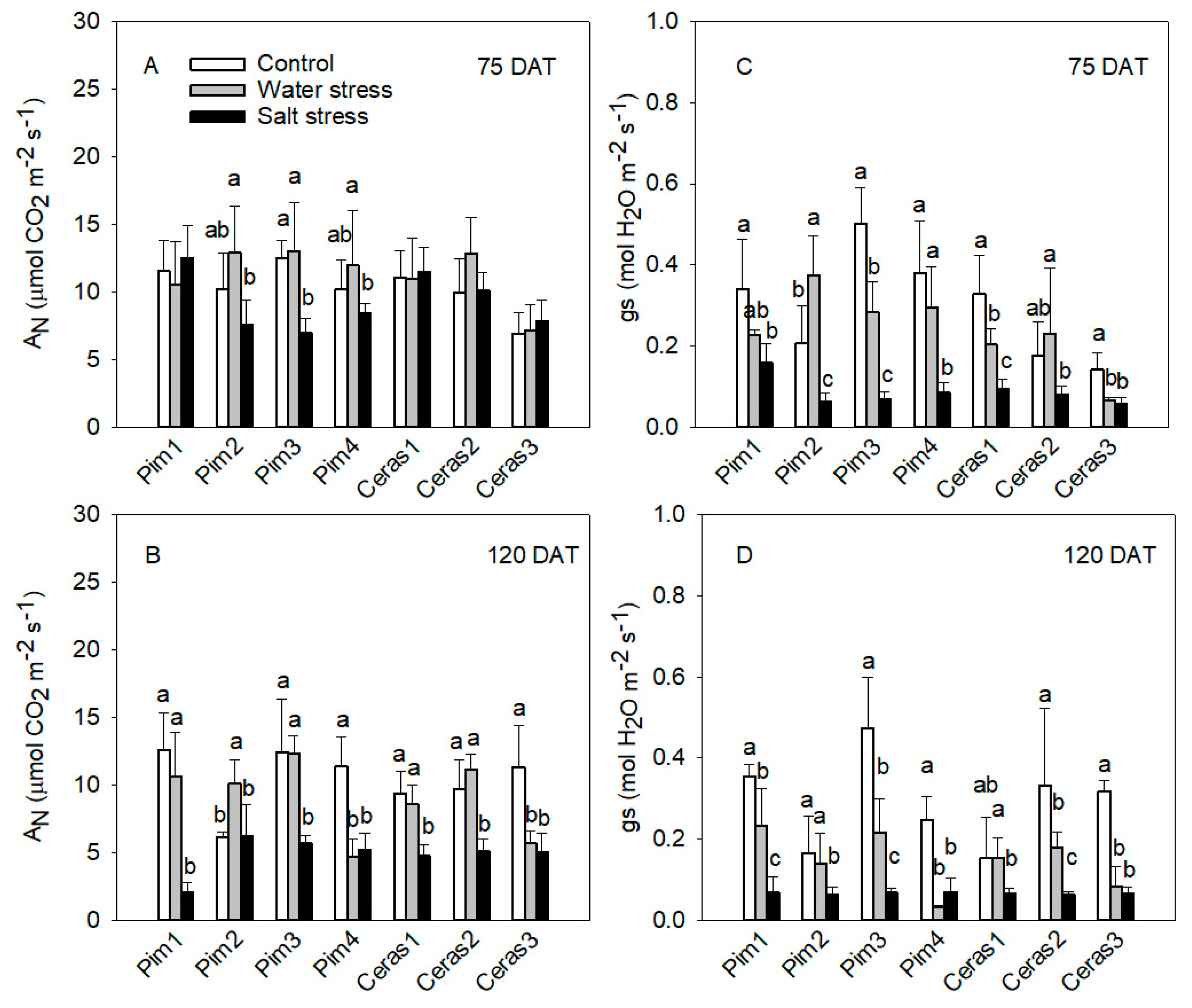
3.1. Gas Exchange Measurements
3.1.1. Photosynthetic Rate and Stomatal Conductance
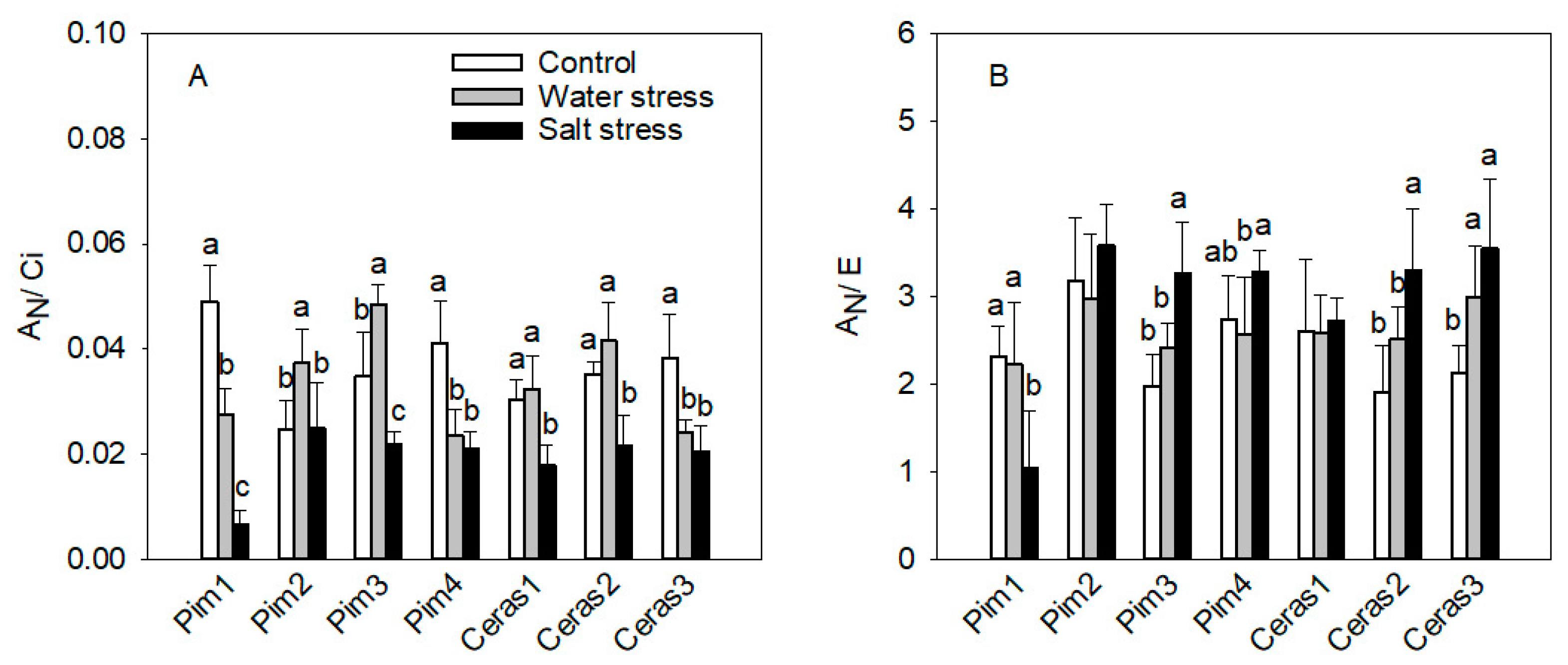
3.1.2. Instantaneous Carboxylation Efficiency
3.1.3. Instantaneous Water Use Efficiency
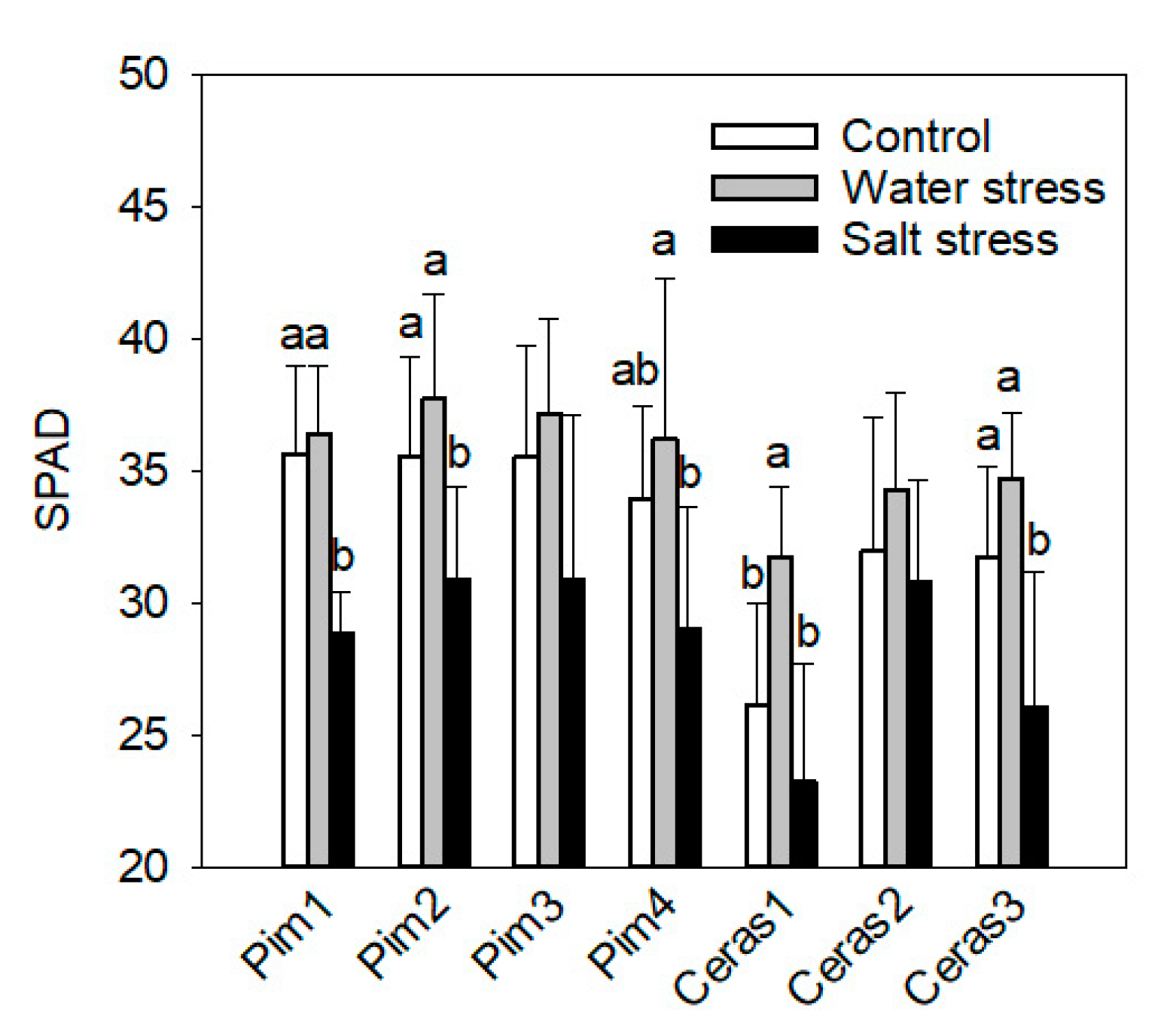
3.2. Leaf Chlorophyll Concentration Measurement by SPAD
3.3. Phenotypic Traits
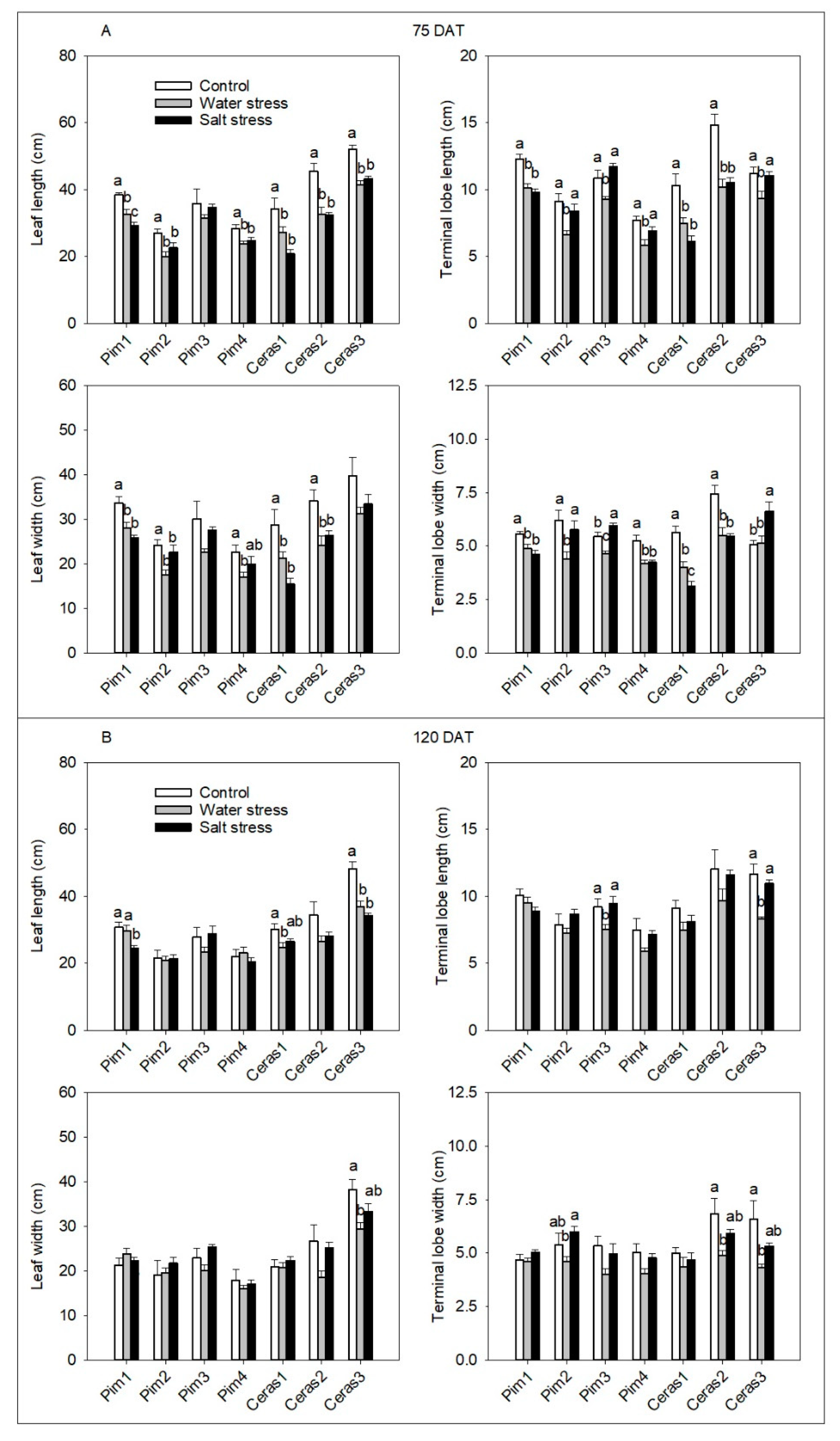
3.3.1. Leaf Morphological Parameters
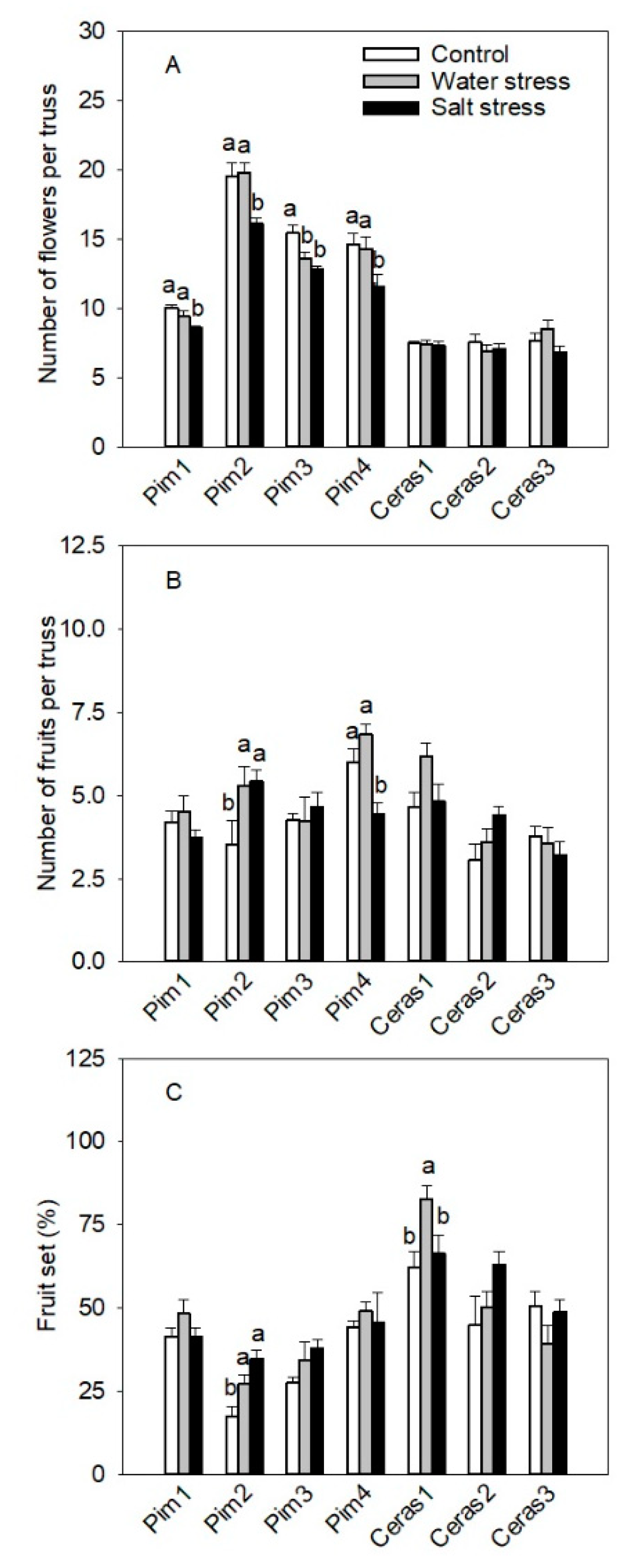
3.3.2. Flowers and Fruits per Truss Parameters
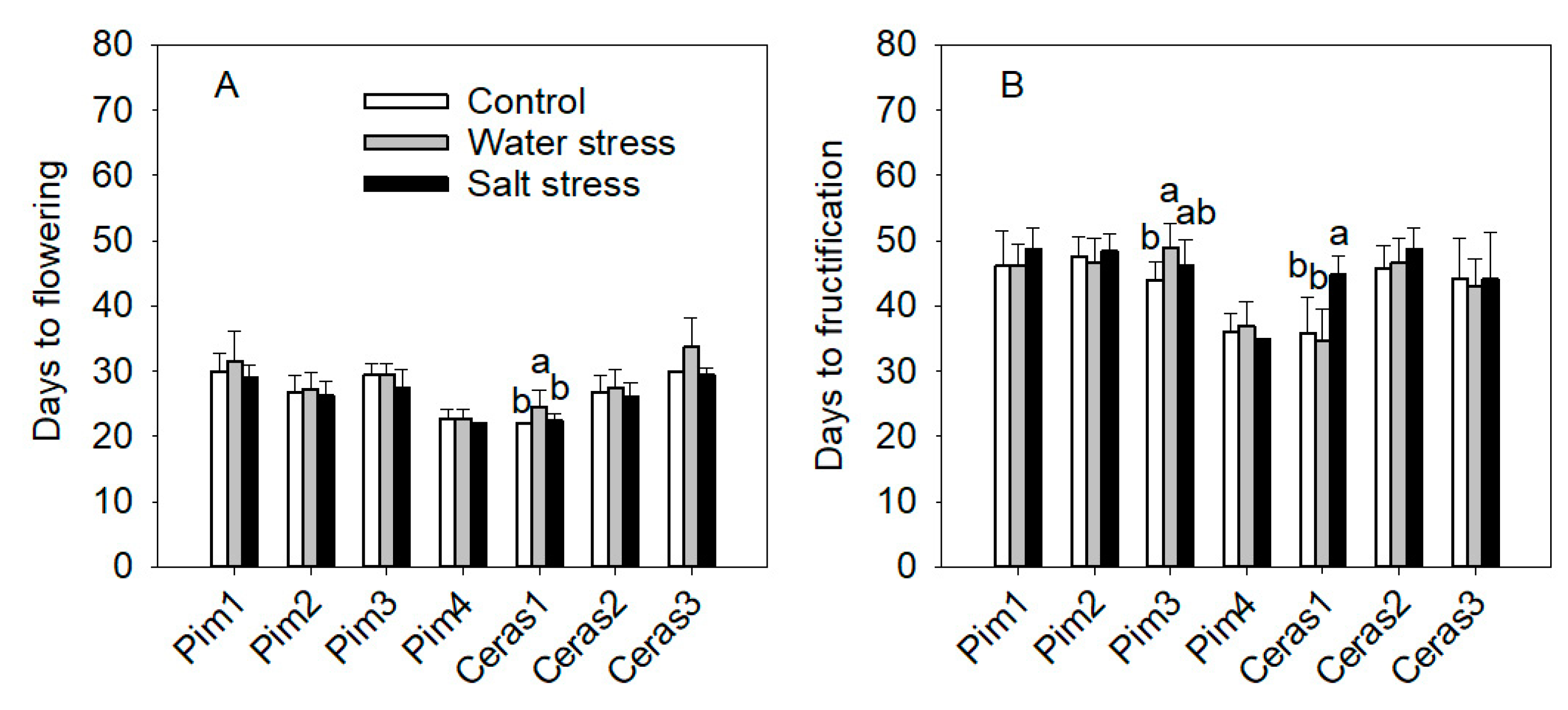
3.3.3. Flowering and Maturity Earliness
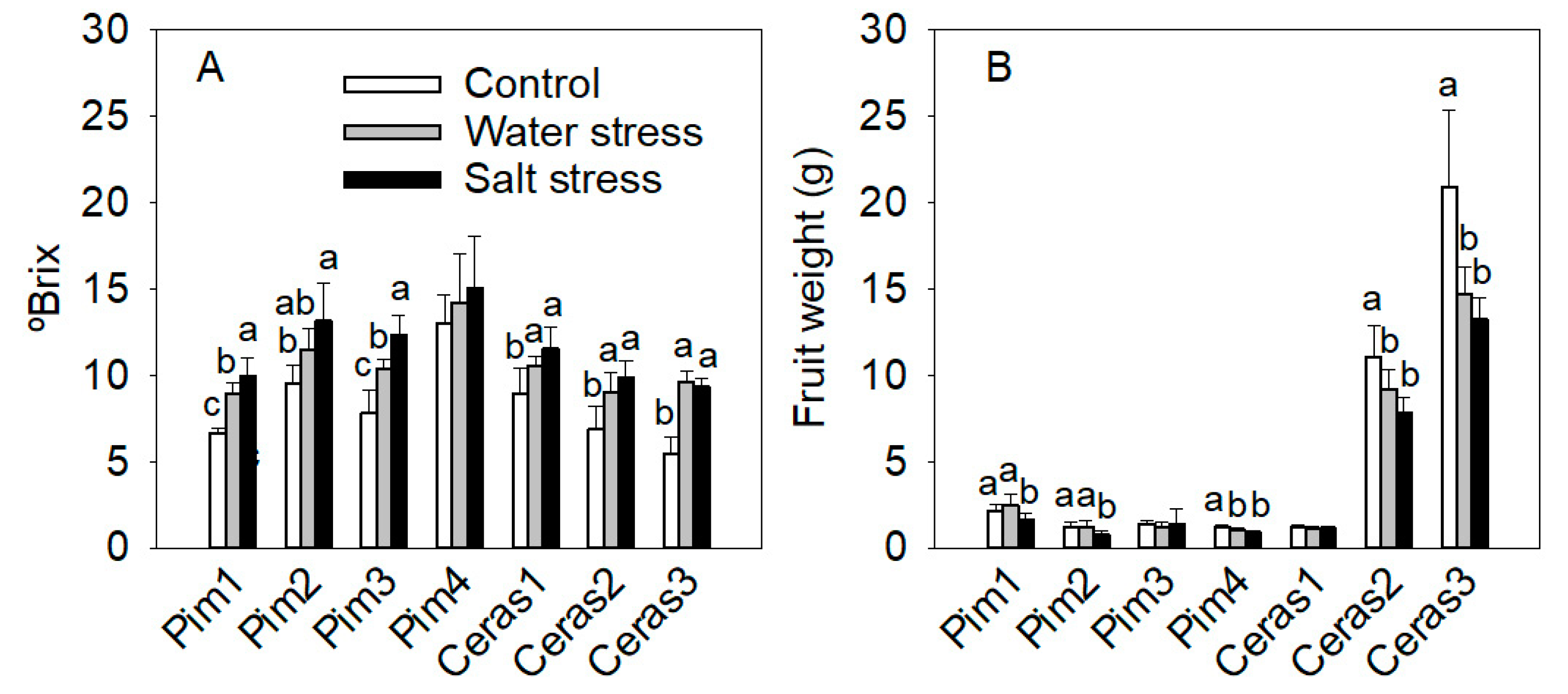
3.3.4. Fruit Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 1–14. [Google Scholar] [CrossRef]
- de Oliveira, A.B.; Mendes Alencar, N.L.; Gomes-Filho, E. Comparison between the water and salt stress effects on plant growth and development. In Responses of Organisms to Water Stress; InTech: London, UK, 2013; pp. 68–94. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; pp. 1–33. ISBN 9783642326. [Google Scholar]
- Niu, G.; Rodriguez, D.S.; Crosby, K.; Leskovar, D.; Jifon, J. Rapid screening for relative salt tolerance among chile pepper genotypes. HortScience 2010, 45, 1192–1195. [Google Scholar] [CrossRef]
- De Pascale, S.; Ruggiero, C.; Barbieri, G.; Maggio, A. Physiological responses of pepper to salinity and drought. J. Am. Soc. Hortic. Sci. 2003, 128, 48–54. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 25–87. ISBN 9781461447. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.N.K.; Bhatt, R.M.; Sadashiva, A.T. Tolerance to water stress in tomato cultivars. Photosynthetica 2000, 38, 465–467. [Google Scholar] [CrossRef]
- Scholberg, J.M.S.; Locascio, S.J. Growth response of snap bean and tomato as affected by salinity and irrigation method. HortScience 1999, 34, 259–264. [Google Scholar] [CrossRef]
- Singh, J.; Sastry, E.V.D.; Singh, V. Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol. Mol. Biol. Plants 2012, 18, 45–50. [Google Scholar] [CrossRef]
- Rick, C.M. Potential improvement of tomatoes by controlled introgression of genes from wild species. In Proceedings of the Broadening the Genetic Base of Crops, Wageningen, The Netherlands, 3–7 July 1978; Zeven, A.C., van Harten, A.M., Eds.; Pudoc: Wageningen, The Netherlands, 1979; pp. 167–173. [Google Scholar]
- Foolad, M.R. Recent advances in genetics of salt tolerance in tomato. Plant Cell. Tissue Organ Cult. 2004, 76, 101–119. [Google Scholar] [CrossRef]
- Albaladejo, I.; Meco, V.; Plasencia, F.; Flores, F.B.; Bolarin, M.C.; Egea, I. Unravelling the strategies used by the wild tomato species Solanum pennellii to confront salt stress: From leaf anatomical adaptations to molecular responses. Environ. Exp. Bot. 2017, 135, 1–12. [Google Scholar] [CrossRef]
- Egea, I.; Albaladejo, I.; Meco, V.; Morales, B.; Sevilla, A.; Bolarin, M.C.; Flores, F.B. The drought-tolerant Solanum pennellii regulates leaf water loss and induces genes involved in amino acid and ethylene/jasmonate metabolism under dehydration. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Zuriaga, E.; Blanca, J.M.; Cordero, L.; Sifres, A.; Blas-Cerdán, W.G.; Morales, R.; Nuez, F. Genetic and bioclimatic variation in Solanum pimpinellifolium. Genet. Resour. Crop Evol. 2009, 56, 39–51. [Google Scholar] [CrossRef]
- Zuriaga, E.; Blanca, J.; Nuez, F. Classification and phylogenetic relationships in Solanum section Lycopersicon based on AFLP and two nuclear gene sequences. Genet. Resour. Crop Evol. 2009, 56, 663–678. [Google Scholar] [CrossRef]
- Rick, C.M.; Fobes, J.F.; Holle, M. Genetic variation in Lycopersicon pimpinellifolium: Evidence of evolutionary change in mating systems. Plant Syst. Evol. 1977, 127, 139–170. [Google Scholar] [CrossRef]
- Villalta, I.; Reina-Sánchez, A.; Bolarín, M.C.; Cuartero, J.; Belver, A.; Venema, K.; Carbonell, E.A.; Asins, M.J. Genetic analysis of Na+ and K+ concentrations in leaf and stem as physiological components of salt tolerance in tomato. Theor. Appl. Genet. 2008, 116, 869–880. [Google Scholar] [CrossRef]
- Lin, K.H.; Yeh, W.L.; Chen, H.M.; Lo, H.F. Quantitative trait loci influencing fruit-related characteristics of tomato grown in high-temperature conditions. Euphytica 2010, 174, 119–135. [Google Scholar] [CrossRef]
- Bolarín, M.C.; Fernández, F.G.; Cruz, V.; Cuartero, J. Salinity tolerance in four wild tomato species using vegetative yield-salinity response curves. J. Am. Soc. Hortic. Sci. 1991, 116, 286–290. [Google Scholar] [CrossRef]
- Cuartero, J.; Yeo, A.R.; Flowers, T.J. Selection of donors for salt-tolerance in tomato using physiological traits. New Phytol. 1992, 121, 63–69. [Google Scholar] [CrossRef]
- Foolad, M.R.; Chen, F.Q. RFLP mapping of QTLs conferring salt tolerance during the vegetative stage in tomato. Theor. Appl. Genet. 1998, 97, 1133–1144. [Google Scholar] [CrossRef]
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hortic. (Amsterdam). 1999, 78, 83–125. [Google Scholar] [CrossRef]
- Foolad, M.R. Comparison of salt tolerance during seed germination and vegetative growth in tomato by QTL mapping. Genome 1999, 42, 727–734. [Google Scholar] [CrossRef]
- Foolad, M.R.; Zhang, L.P.; Lin, G.Y. Identification and validation of QTLs for salt tolerance during vegetative growth in tomato by selective genotyping. Genome 2001, 44, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Bolarín, M.C.; Estañ, M.T.; Caro, M.; Romero-Aranda, R.; Cuartero, J. Relationship between tomato fruit growth and fruit osmotic potential under salinity. Plant Sci. 2001, 160, 1153–1159. [Google Scholar] [CrossRef]
- Zhang, L.P.; Lin, G.Y.; Foolad, M.R. QTL comparison of salt tolerance during seed germination and vegetative stage in a Lycopersicon esculentum × L. pimpinellifolium RIL population. In Proceedings of the XXVI International Horticultural Congress: Environmental Stress and Horticulture Crops, Toronto, ON, Canada, 11 August 2002; pp. 59–67. [Google Scholar]
- Estañ, M.T.; Villalta, I.; Bolarín, M.C.; Carbonell, E.A.; Asins, M.J. Identification of fruit yield loci controlling the salt tolerance conferred by solanum rootstocks. Theor. Appl. Genet. 2009, 118, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Rao, E.S.; Kadirvel, P.; Symonds, R.C.; Ebert, A.W. Relationship between survival and yield related traits in Solanum pimpinellifolium under salt stress. Euphytica 2013, 190, 215–228. [Google Scholar] [CrossRef]
- Rao, E.S.; Kadirvel, P.; Symonds, R.C.; Geethanjali, S.; Thontadarya, R.N.; Ebert, A.W. Variations in DREB1A and VP1.1 genes show association with salt tolerance traits in wild tomato (Solanum pimpinellifolium). PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Razali, R.; Bougouffa, S.; Morton, M.J.L.; Lightfoot, D.J.; Alam, I.; Essack, M.; Arold, S.T.; Kamau, A.A.; Schmöckel, S.M.; Pailles, Y.; et al. The genome sequence of the wild tomato Solanum pimpinellifolium provides insights into salinity tolerance. Front. Plant Sci. 2018, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, T.; Goodwin, S.B.; Sutter, T.R. Comparative Transcriptome Analysis of Responses to Water Deficit in Solanum lycopersicum and S. pimpinellifolium Roots. Available online: https://omictools.com/88516daa3ab811e2cd922bd46ae0ade3-dataset (accessed on 26 February 2020).
- Rick, C.M. Tomato, Lycopersicon esculentum (Solanaceae). In Evolution of Crop Plants; Longman Group: London, UK, 1976; pp. 268–273. [Google Scholar]
- Warnock, S.J. Natural habitats of Lycopersicon species. HortScience 1991, 26, 466–471. [Google Scholar] [CrossRef]
- Diouf, I.A.; Derivot, L.; Bitton, F.; Pascual, L.; Causse, M. Water deficit and salinity stress reveal many specific QTL for plant growth and fruit quality traits in tomato. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Blanca, J.; Montero-Pau, J.; Sauvage, C.; Bauchet, G.; Illa, E.; Díez, M.J.; Francis, D.M.; Causse, M.; van der Knaap, E.; Cañizares, J. Genomic variation in tomato, from wild ancestors to contemporary breeding accessions. BMC Genomics 2015, 16, 257. [Google Scholar] [CrossRef]
- Blanca, J.; Cañizares, J.; Cordero, L.; Pascual, L.; Díez, M.J.; Nuez, F. Variation revealed by SNP genotyping and morphology provides insight into the origin of the tomato. PLoS ONE 2012, 7, e48198. [Google Scholar] [CrossRef]
- Climate-Data.org. Datos climáticos mundiales. Available online: https://es.climate-data.org/ (accessed on 5 March 2020).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future köppen-geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.N.; Hochmuth, G.J. Knott’s Handbook for Vegetable Growers; John Willey & Sons: New York, NY, USA, 2007; ISBN 978-0-471-73828-2. [Google Scholar]
- Allen, R.G. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Lang, S.P.; Tibbitts, T.W. Factors controlling intumescences development in tomato plants. J. Am. Soc. Hortic. Sci. 1983, 108, 93–98. [Google Scholar]
- Bolarín, M.C.; Cano, E.A.; Estañ, M.T.; Caro, M. Growth, fruit yield, and ion concentration in tomato genotypes after pre- and post-emergence salt treatments. J. Am. Soc. Hortic. Sci. 1993, 118, 655–660. [Google Scholar] [CrossRef]
- Jones, R.A.; Hashim, M.; El-Beltagy, A.S. Developmental responsiveness of salt-tolerant and salt-sensitive genotypes of Lycopersicon. In Arid Lands: Today and Tomorrow; Whitehead, E., Hutchison, F., Timmeman, B., Varazy, R., Eds.; Westview Press: Boulder, CO, USA, 1988; pp. 765–772. [Google Scholar]
- Maas, E.V. Salt tolerance of plants. Appl. Agric. Reasearch 1986, 1, 12–26. [Google Scholar]
- Cantore, V.; Lechkar, O.; Karabulut, E.; Sellami, M.H.; Albrizio, R.; Boari, F.; Stellacci, A.M.; Todorovic, M. Combined effect of deficit irrigation and strobilurin application on yield, fruit quality and water use efficiency of “cherry” tomato (Solanum lycopersicum L.). Agric. Water Manag. 2016, 167, 53–61. [Google Scholar] [CrossRef]
- López-Serrano, L.; Penella, C.; Bautista, A.S.; López-Galarza, S.; Calatayud, Á. Physiological changes of pepper accessions in response to salinity and water stress. Spanish J. Agric. Res. 2017, 15, e0804. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Lawlor, D.W. Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef]
- Nuruddin, M.M.; Madramootoo, C.A.; Dodds, G.T. Effects of water stress at different growth stages on greenhouse tomato yield and quality. HortScience 2003, 38, 1389–1393. [Google Scholar] [CrossRef]
- Yin, Y.G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ’Micro-Tom’) fruits in an ABA-and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Segura, V.; Gricourt, J.; Bonnefoi, J.; Derivot, L.; Causse, M. Association mapping reveals the genetic architecture of tomato response to water deficit: focus on major fruit quality traits. J. Exp. Bot. 2016, 67, 6413–6430. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.; Urban, L.; Brunel, B.; Bertin, N. Water deficit effects on tomato quality depend on fruit developmental stage and genotype. J. Plant Physiol. 2016, 190, 26–35. [Google Scholar] [CrossRef]
- Nakazato, T.; Bogonovich, M.; Moyle, L.C. Environmental factors predict adaptive phenotypic differentiation within and between two wild Andean tomatoes. Evolution (N. Y.) 2008, 62, 774–792. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Massaretto, I.L.; Albaladejo, I.; Purgatto, E.; Flores, F.B.; Plasencia, F.; Egea-Fernández, J.M.; Bolarín, M.C.; Egea, I. Recovering tomato landraces to simultaneously improve fruit yield and nutritional quality against salt stress. Front. Plant Sci. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Penella, C.; Nebauer, S.G.; Quiñones, A.; San Bautista, A.; López-Galarza, S.; Calatayud, Á. Some rootstocks improve pepper tolerance to mild salinity through ionic regulation. Plant Sci. 2015, 230, 12–22. [Google Scholar] [CrossRef]
- Praxedes, S.C.; de Lacerda, C.; DaMatta, F.M.; Prisco, J.T.; Gomes-Filho, E. Salt tolerance is associated with differences in ion accumulation, biomass allocation and photosynthesis in cowpea cultivars. J. Agron. Crop Sci. 2010, 196, 193–204. [Google Scholar] [CrossRef]
- Saleem, A.; Ashraf, M.; Akram, N.A. Salt (NaCl)-induced modulation in some key physio-biochemical attributes in Okra (Abelmoschus esculentus L.). J. Agron. Crop Sci. 2011, 197, 202–213. [Google Scholar] [CrossRef]
- Vargas, D.; Rodríguez, E.; Sánchez, J.J.; Montes, S.; Ruiz, A.; Lépiz, R.; Puente, P.; Martínez, J.L. Adaptación climática de Lycopersicum en el occidente de México. (Climatic adaptation of Lycopersicon in west Mexico). In Avances en la Investiación Científica en el CUCBA; Universidad de Guadalajara: Guadalajara, Mexico, 2005; pp. 207–210. ISBN 970-27-0770-6. [Google Scholar]
- Sánchez-Peña, P.; Oyama, K.; Núñez-Farfán, J.; Fornoni, J.; Hernández-Verdugo, S.; Márquez-Guzmán, J.; Garzón-Tiznado, J.A. Sources of resistance to whitefly (Bemisia spp.) in wild populations of Solanum lycopersicum var. cerasiforme (Dunal) spooner G.J. Anderson et R.K. Jansen in Northwestern Mexico. Genet. Resour. Crop Evol. 2006, 53, 711–719. [Google Scholar]
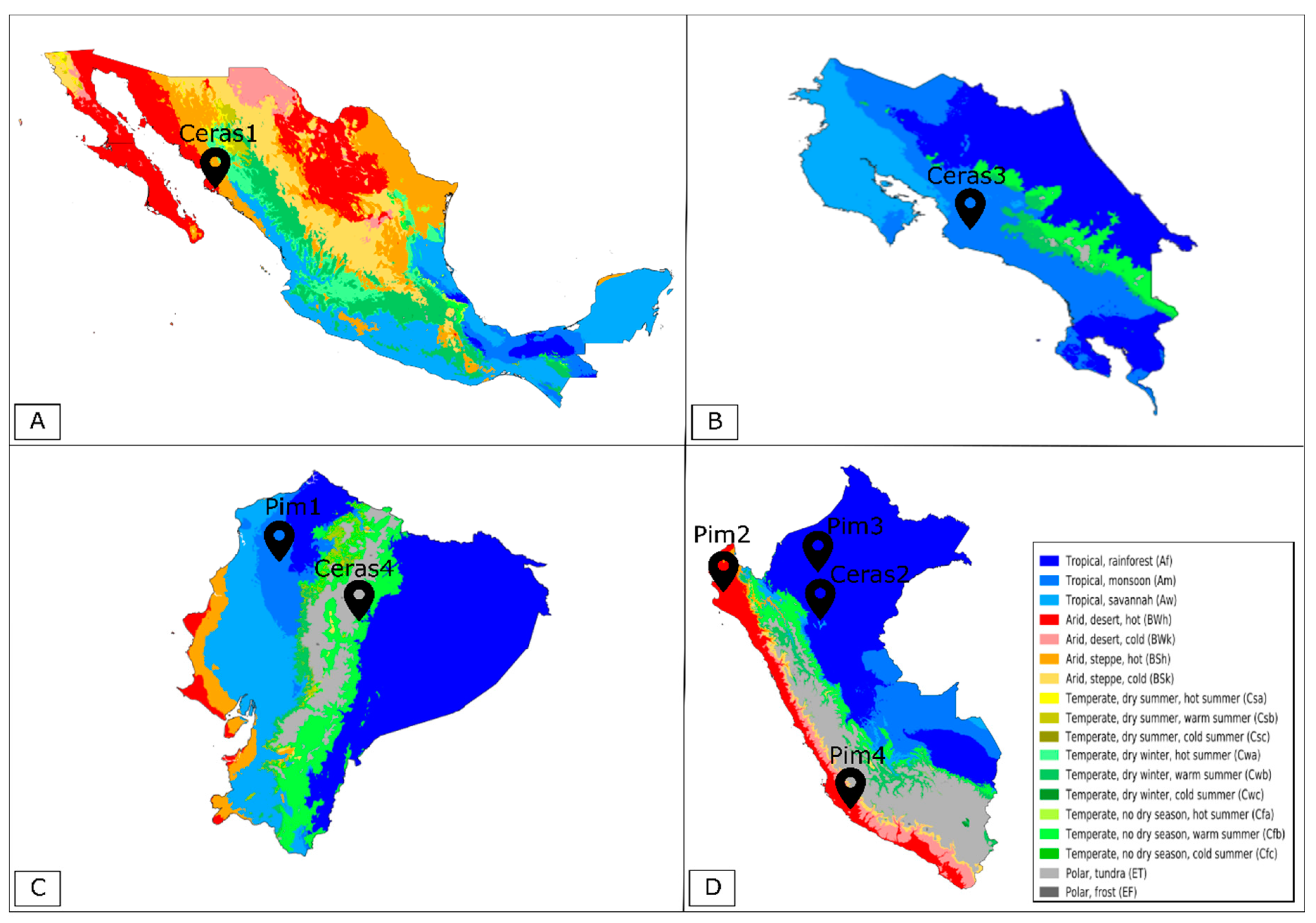
| S. pimpinellifolium Accessions | Pim1 | Pim2 | Pim3 | Pim4 |
| Genebank Code | BGV007145 | BGV006454 | BGV015382 | BGV013720 |
| Species | S. pimpinellifolium | S. pimpinellifolium | S. pimpinellifolium | S. pimpinellifolium |
| Country | Ecuador | Peru | Peru | Peru |
| State | Manabí | Piura | Amazonas | Ica |
| GPS Coordinates | 0°13′19.0″ S 79°29′20.0″ W | 5°08′51.0″ S 80°16′13.0″ W | 5°53′08.0″ S 78°10′34.0″ W | 14°34′49.0" S 74°53′14.0″ W |
| Altitude (m) | 238 | 36 | 637 | 1020 |
| Mean Annual Temperature (°C) | 25 | 24 | 22 | 13 |
| Temperature Seasonality (%) | 3 | 9 | 2 | 11 |
| Diurnal Temperature Range (°C) | 8 | 12 | 9 | 9 |
| Mean Annual Precipitation (mm) | 2806 | 49 | 732 | 258 |
| Precipitation Seasonality (%) | 83 | 134 | 35 | 140 |
| S. l. var. cerasiforme Accessions | Ceras1 | Ceras2 | Ceras3 | Ceras4 |
| Genebank Code | BGV007931 | LA2251 | PI487625 | BGV006769 |
| Species | S. l. var. cerasiforme | S. l. var. cerasiforme | S. l. var. cerasiforme | S. l. var. cerasiforme |
| Country | Mexico | Peru | Costa Rica | Ecuador |
| State | Sinaloa | San Martín | Los Diamantes | Napo |
| GPS Coordinates | 26°03′30.0″ N 109°22′30.0″ W | 6°08′30.0″ S 77°05′30.0″ W | 9°36′22.0″ N 84°08′27.0″ W | 1°01′52.0″ S 77°47′10.0″ W |
| Altitude (m) | 10 | 883 | 1465 | 512 |
| Mean Annual Temperature (°C) | 24 | 23 | 18 | 23 |
| Temperature Seasonality (%) | 20 | 3 | 3 | 2 |
| Diurnal Temperature Range (°C) | 13 | 6 | 10 | 8 |
| Mean Annual Precipitation (mm) | 341 | 1394 | 2274 | 4330 |
| Precipitation Seasonality (%) | 121 | 27 | 76 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cuenca, M.-R.; Pereira-Dias, L.; Soler, S.; López-Serrano, L.; Alonso, D.; Calatayud, Á.; Díez, M.J. Adaptation to Water and Salt Stresses of Solanum pimpinellifolium and Solanum lycopersicum var. cerasiforme. Agronomy 2020, 10, 1169. https://doi.org/10.3390/agronomy10081169
Martínez-Cuenca M-R, Pereira-Dias L, Soler S, López-Serrano L, Alonso D, Calatayud Á, Díez MJ. Adaptation to Water and Salt Stresses of Solanum pimpinellifolium and Solanum lycopersicum var. cerasiforme. Agronomy. 2020; 10(8):1169. https://doi.org/10.3390/agronomy10081169
Chicago/Turabian StyleMartínez-Cuenca, Mary-Rus, Leandro Pereira-Dias, Salvador Soler, Lidia López-Serrano, David Alonso, Ángeles Calatayud, and María José Díez. 2020. "Adaptation to Water and Salt Stresses of Solanum pimpinellifolium and Solanum lycopersicum var. cerasiforme" Agronomy 10, no. 8: 1169. https://doi.org/10.3390/agronomy10081169
APA StyleMartínez-Cuenca, M.-R., Pereira-Dias, L., Soler, S., López-Serrano, L., Alonso, D., Calatayud, Á., & Díez, M. J. (2020). Adaptation to Water and Salt Stresses of Solanum pimpinellifolium and Solanum lycopersicum var. cerasiforme. Agronomy, 10(8), 1169. https://doi.org/10.3390/agronomy10081169







