Aluminum-Specific Upregulation of GmALS3 in the Shoots of Soybeans: A Potential Biomarker for Managing Soybean Production in Acidic Soil Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA/Protein Sequence, Phylogenetic Analyses
2.2. Hydroponic Culture
2.3. Acidic Soil Experiment
2.4. RNA Extraction and cDNA Synthesis
2.5. Primer Design
2.6. Transcript Analysis
2.7. Growth and Biomass Assay in Acidic Soil
2.8. Quantification of Al Content
2.9. Statistical Analysis
3. Results
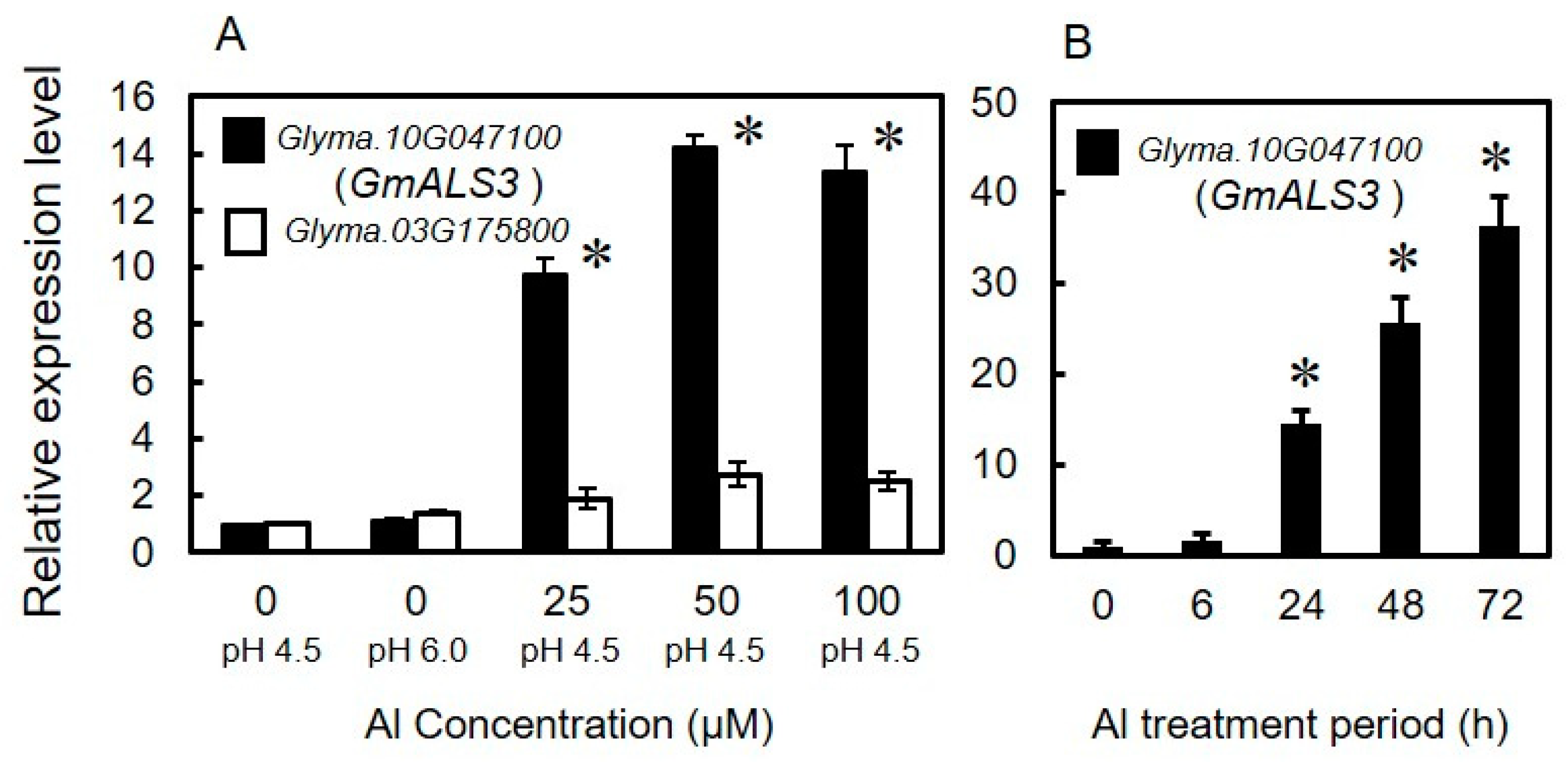
3.1. Identification of Orthologs of AtALS3 in Soybeans That Are Inducible by Aluminum
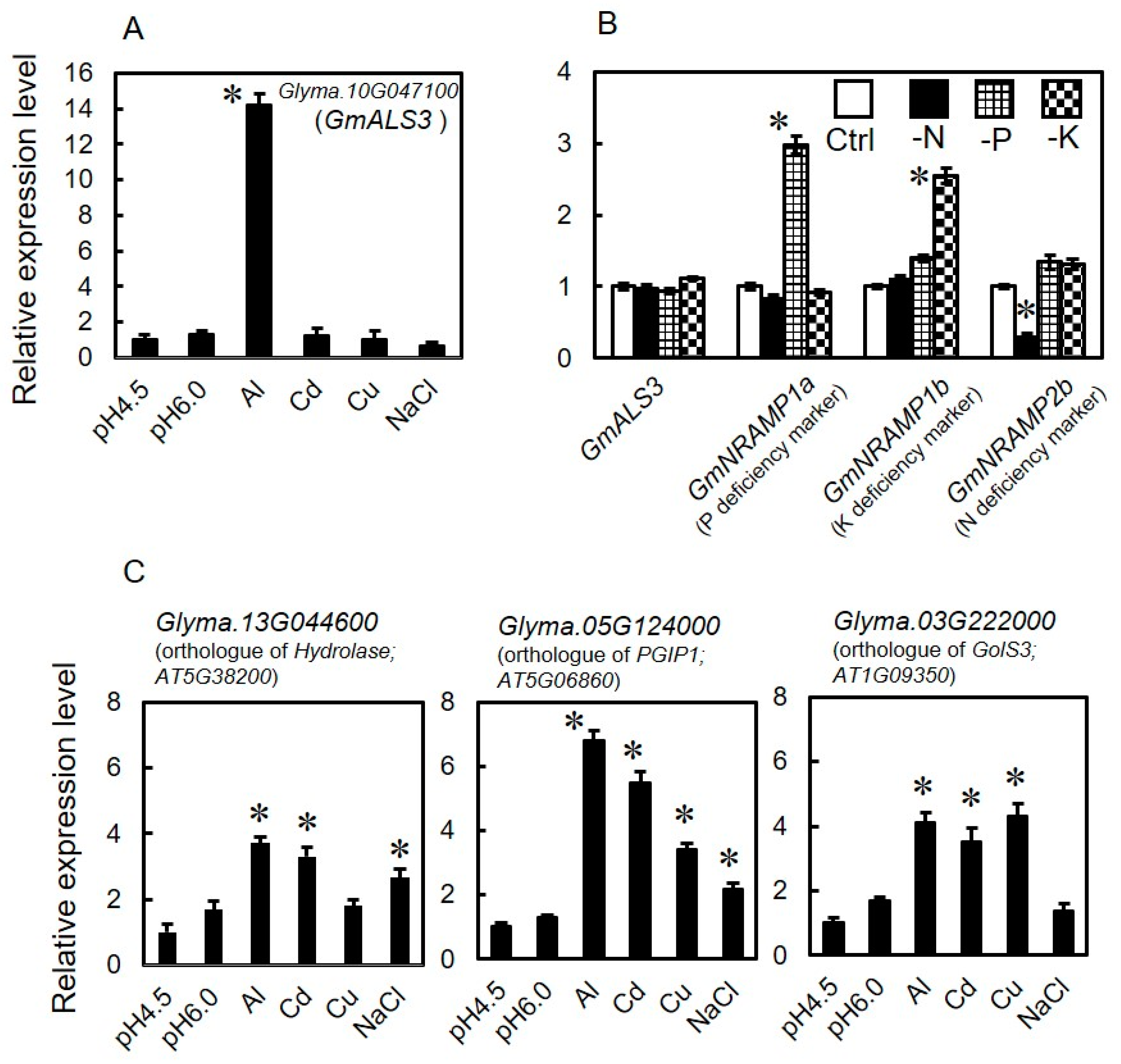
3.2. Expression Profile of GmALS3 in Response to Rhizotoxic Ion Stress and Nutrient-Deficiency Treatments under Hydroponic Culture Conditions
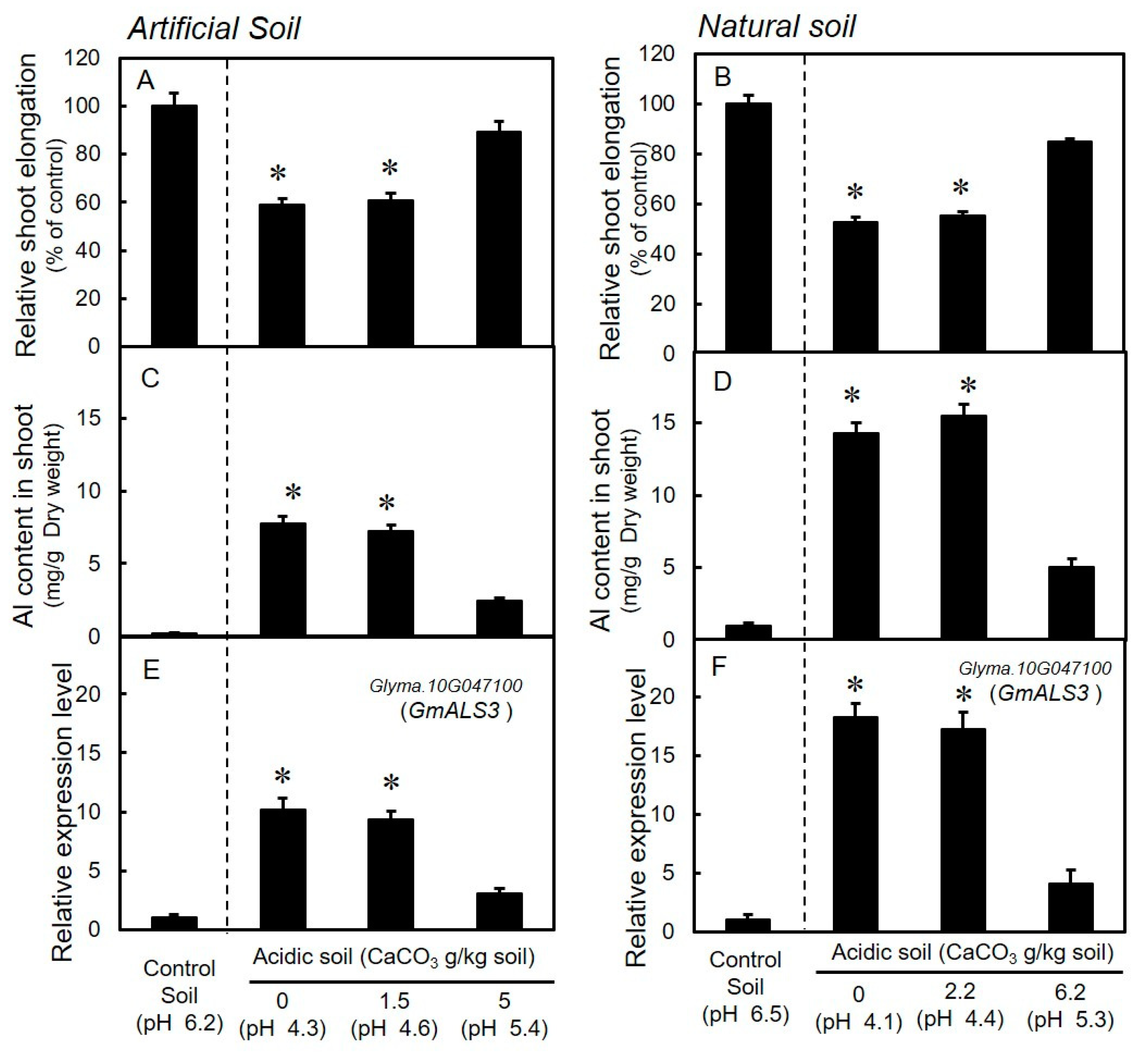
3.3. Growth and Expression Levels of GmALS3 in Soybeans Grown in Acidic Soils with Different Levels of CaCO3
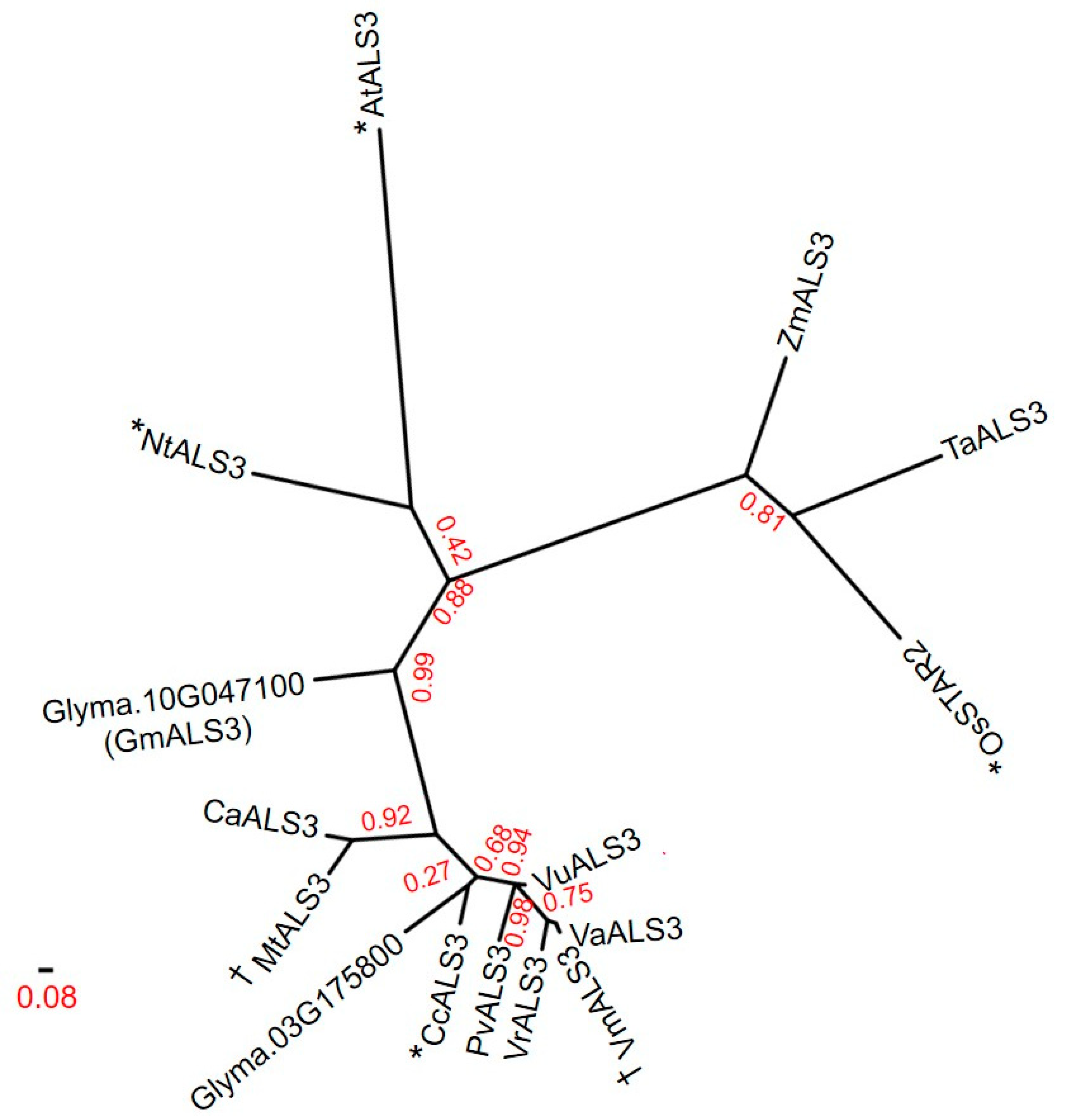
3.4. Protein Sequence and Phylogenetic Analyses for the Identification of GmALS3 Orthologs in Other Crops
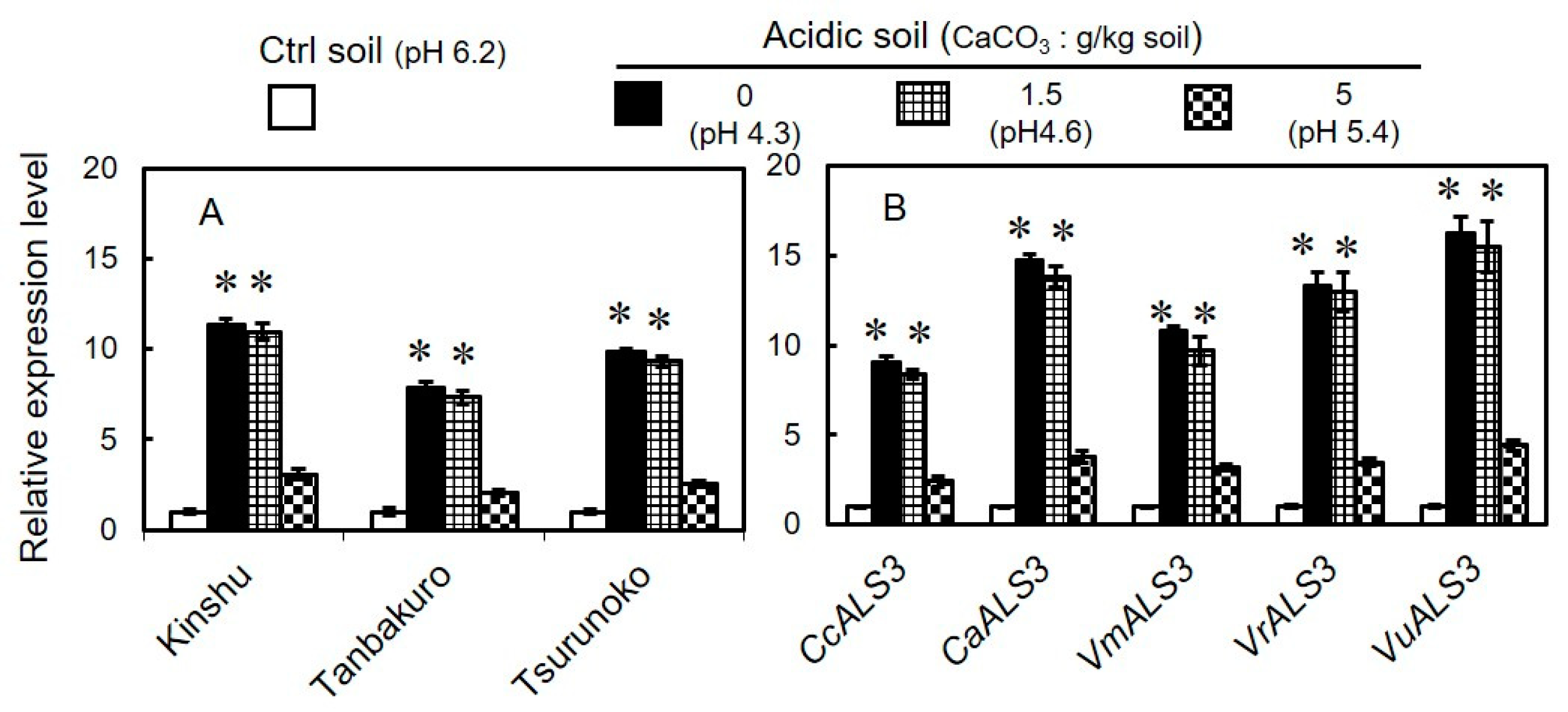
3.5. Expression of GmALS3 in Different Varieties of Soybeans and Its Orthologs in Other Legumes Grown in Acidic Soils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Komatsuzaki, M.; Ohta, H. Soil management practices for sustainable agro-ecosystems. Sustain. Sci. 2007, 2, 103–120. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Rengel, Z. Aluminum, manganese, and iron tolerance improves performance of wheat genotypes in waterlogged acidic soils. J. Plant Nutr. Soil Sci. 2010, 173, 461–468. [Google Scholar] [CrossRef]
- Porter, G.S.; Bajita-Locke, J.B.; Hue, N.V.; Strand, D. Manganese Solubility and Phytotoxicity Affected by Soil Moisture, Oxygen Levels, and Green Manure Additions. Commun. Soil Sci. Plant Anal. 2004, 35, 99–116. [Google Scholar] [CrossRef]
- Wood, M.; Cooper, J.E.; Holding, A.J. Aluminium toxicity and nodulation of Trifolium repens. Plant Soil 1984, 78, 367–379. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R. Aluminum Toxicity and Tolerance in Plants. Plant Physiol. 1995, 107, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, J.P.; Saha, B.; Regon, P.; Sahoo, S.; Chowra, U.; Pradhan, A.; Roy, A.; Panda, S.K. Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PLoS ONE 2017, 12, e0176357. [Google Scholar] [CrossRef]
- Carvalho, M.; Van Raij, B. Calcium sulphate, phosphogypsum and calcium carbonate in the amelioration of acid subsoils for root growth. Plant Soil 1997, 192, 37–48. [Google Scholar] [CrossRef]
- Mora, M.L.; Schnettler, B.; Demanet, R. Effect of liming and gypsum on soil chemistry, yield, and mineral composition of ryegrass grown in an acidic Andisol. Commun. Soil Sci. Plant Anal. 1999, 30, 1251–1266. [Google Scholar] [CrossRef]
- Fageria, N.; Baligar, V. Chapter 7 Ameliorating Soil Acidity of Tropical Oxisols by Liming For Sustainable Crop Production. Adv. Agron. 2008, 99, 345–399. [Google Scholar] [CrossRef]
- Von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Fageria, N.K.; Zimmermann, F.J.P.; Baligar, V. Lime and phosphorus interactions on growth and nutrient uptake by upland rice, wheat, common bean, and corn in an Oxisol. J. Plant Nutr. 1995, 18, 2519–2532. [Google Scholar] [CrossRef]
- Kamprath, E.J. Crop Response to Lime on Soils in the Tropics. In Soil Acidity and Liming, 2nd ed.; Adams, F., Ed.; Agronomy: Madison, WI, USA, 1984; Volume 12, pp. 349–368. [Google Scholar]
- Munns, D. Soil acidity and growth of a legume. III. Interaction of lime and phosphate on growth of Medicgo sativa L. in relation to aluminium toxicity and phosphate fixation. Aust. J. Agric. Res. 1965, 16, 757–766. [Google Scholar] [CrossRef]
- Grove, J.H.; Sumner, M.E.; Syers, J.K. Effect of Lime on Exchangeable Magnesium in Variable Surface Charge Soils. Soil Sci. Soc. Am. J. 1981, 45, 497–500. [Google Scholar] [CrossRef]
- Pavan, M.A.; Bingham, F.T.; Pratt, P.F. Redistribution of Exchangeable Calcium, Magnesium, and Aluminum Following Lime or Gypsum Applications to a Brazilian Oxisol. Soil Sci. Soc. Am. J. 1984, 48, 33–38. [Google Scholar] [CrossRef]
- Coventry, D.R.; Farhoodi, A.; Xu, R.K. Management Soil Acidification Through Crop Rotations in Southern Australia. In Handbook of Soil Acidity; Rengel, Z., Ed.; Marcel Dekker: New York, NY, USA, 2003; pp. 407–429. [Google Scholar]
- Yli-Halla, M. Comparison of a bioassay and three chemical methods for determination of plant-available P in cultivated soils of Finland. Agric. Food Sci. 1990, 62, 213–219. [Google Scholar] [CrossRef]
- Jinyang, L.; Meiqing, L.; Hanping, M.; Wenjing, Z. Diagnosis of potassium nutrition level in Solanum lycopersicum based on electrical impedance. Biosyst. Eng. 2016, 147, 130–138. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Ortiz-Melendez, A.D.J.; Guevara-Gonzalez, R.G.; Torres-Pacheco, I.; Herrera-Ruiz, G.; Contreras-Medina, L.M.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. An Analysis of Electrical Impedance Measurements Applied for Plant N Status Estimation in Lettuce (Lactuca sativa). Sensors 2014, 14, 11492–11503. [Google Scholar] [CrossRef]
- Yang, X.S.; Wu, J.; Ziegler, T.E.; Yang, X.; Zayed, A.; Rajani, M.S.; Zhou, D.; Basra, A.S.; Schachtman, D.P.; Peng, M.; et al. Gene Expression Biomarkers Provide Sensitive Indicators of in Planta Nitrogen Status in Maize. Plant Physiol. 2011, 157, 1841–1852. [Google Scholar] [CrossRef]
- Sadder, M.; Alsadon, A.; Wahb-Allah, M. Transcriptomic analysis of tomato lines reveals putative stress-specific biomarkers. Turk. J. Agric. For. 2014, 38, 700–715. [Google Scholar] [CrossRef]
- Iuchi, S.; Koyama, H.; Iuchi, A.; Kobayashi, Y.; Kitabayashi, S.; Ikka, T.; Hirayama, T.; Shinozaki, K.; Kobayashi, M.; Kobayashi, Y.; et al. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 9900–9905. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, Y.; Ito, H.; Kobayashi, Y.; Ikka, T.; Morita, A.; Kobayashi, M.; Imaizumi, R.; Aoki, T.; Komatsu, K.; Sakata, Y.; et al. Characterization of AtSTOP1 Orthologous Genes in Tobacco and Other Plant Species. Plant Physiol. 2013, 162, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, Y.; Iuchi, S.; Kobayashi, Y.; Kobayashi, Y.; Ikka, T.; Sakurai, N.; Fujita, M.; Shinozaki, K.; Shibata, D.; Kobayashi, M.; et al. STOP1 Regulates Multiple Genes That Protect Arabidopsis from Proton and Aluminum Toxicities. Plant Physiol. 2009, 150, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Huang, C.-F.; Nagao, S.; Yano, M.; Sato, Y.; Nagamura, Y.; Ma, J.F. A Zinc Finger Transcription Factor ART1 Regulates Multiple Genes Implicated in Aluminum Tolerance in Rice. Plant Cell 2009, 21, 3339–3349. [Google Scholar] [CrossRef]
- Daspute, A.A.; Kobayashi, Y.; Panda, S.K.; Fakrudin, B.; Kobayashi, Y.; Tokizawa, M.; Iuchi, S.; Choudhary, A.K.; Yamamoto, Y.Y.; Koyama, H. Characterization of CcSTOP1; a C2H2-type transcription factor regulates Al tolerance gene in pigeonpea. Planta 2018, 247, 201–214. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Gong, L.; Liu, R.; Sun, H.; You, J. Molecular characterization of GmSTOP1 homologs in soybean under Al and proton stress. Plant Soil 2018, 427, 213–230. [Google Scholar] [CrossRef]
- Fan, W.; Lou, H.Q.; Gong, Y.L.; Liu, M.Y.; Cao, M.J.; Liu, Y.; Yang, J.L.; Zheng, S.J. Characterization of an inducible C2H2-type zinc finger transcription factor VuSTOP1 in rice bean (Vigna umbellata) reveals differential regulation between low pH and aluminum tolerance mechanisms. New Phytol. 2015, 208, 456–468. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Q.; Li, P.; Yang, L.; Sun, X.; Benedito, V.A.; Wen, J.; Chen, B.; Mysore, K.S.; Zhao, J. Diverse functions of multidrug and toxin extrusion (MATE) transporters in citric acid efflux and metal homeostasis inMedicago truncatula. Plant J. 2017, 90, 79–95. [Google Scholar] [CrossRef]
- Larsen, P.B.; Geisler, M.; Jones, C.A.; Williams, K.M.; Cancel, J.D. ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 2005, 41, 353–363. [Google Scholar] [CrossRef]
- Sawaki, K.; Sawaki, Y.; Zhao, C.-R.; Kobayashi, Y.; Koyama, H. Specific transcriptomic response in the shoots of Arabidopsis thaliana after exposure to Al rhizotoxicity: Potential gene expression biomarkers for evaluating Al toxicity in soils. Plant Soil 2016, 409, 131–142. [Google Scholar] [CrossRef]
- Abdeljalil, S.; Borgi, I.; Carvalho, S.; Jmal-Hammami, L.; Gargouri, A. Molecular and bioinformatics analyses reveal two differentially expressed intracellular GH1 β-glucosidases from the rare alkalophilic fungus Stachybotrys microspora. Gene 2019, 703, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Hirai, M.Y.; Chino, M.; Komeda, Y.; Naito, S. Effects of Sulfur Nutrition on Expression of the Soybean Seed Storage Protein Genes in Transgenic Petunia. Plant Physiol. 1992, 99, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Watanabe, T.; Shaff, J.E.; Ohta, H.; Kochian, L.V.; Wagatsuma, T.; Kinraide, T.B.; Koyama, H.; Kobayashi, Y.; Kobayashi, Y. Molecular and Physiological Analysis of Al3+ and H+ Rhizotoxicities at Moderately Acidic Conditions. Plant Physiol. 2013, 163, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Kawamura, A.; Kihara, T.; Hara, T.; Takita, E.; Shibata, D. Overexpression of mitochondrial citrate synthase in Arabidopsis thaliana improved growth on a phosphorus-limited soil. Plant Cell Physiol. 2000, 41, 1030–1037. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.F.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hoekenga, O.A.; Itoh, H.; Nakashima, M.; Saito, S.; Shaff, J.E.; Maron, L.G.; Piñeros, M.A.; Kochian, L.V.; Koyama, H. Characterization of AtALMT1 Expression in Aluminum-Inducible Malate Release and Its Role for Rhizotoxic Stress Tolerance in Arabidopsis. Plant Physiol. 2007, 145, 843–852. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ohyama, Y.; Kobayashi, Y.; Ito, H.; Iuchi, S.; Fujita, M.; Zhao, C.-R.; Tanveer, T.; Ganesan, M.; Kobayashi, M.; et al. STOP2 Activates Transcription of Several Genes for Al- and Low pH-Tolerance that Are Regulated by STOP1 in Arabidopsis. Mol. Plant 2014, 7, 311–322. [Google Scholar] [CrossRef]
- Yim, A.K.-Y.; Wong, J.W.-H.; Ku, Y.-S.; Qin, H.; Chan, T.F.; Lam, H.-M. Using RNA-Seq Data to Evaluate Reference Genes Suitable for Gene Expression Studies in Soybean. PLoS ONE 2015, 10, e0136343. [Google Scholar] [CrossRef]
- Garg, R.; Sahoo, A.; Tyagi, A.K.; Jain, M. Validation of internal control genes for quantitative gene expression studies in chickpea (Cicer arietinum L.). Biochem. Biophys. Res. Commun. 2010, 396, 283–288. [Google Scholar] [CrossRef]
- Kundu, A.; Patel, A.; Pal, A. Defining reference genes for qPCR normalization to study biotic and abiotic stress responses in Vigna mungo. Plant Cell Rep. 2013, 32, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, B.; Cao, Z.; Su, Q.; Wang, Y.; Zhang, Z.; Wu, J.; Tian, J. A deep sequencing analysis of transcriptomes and the development of EST-SSR markers in mungbean (Vigna radiata). J. Genet. 2016, 95, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Amorim, L.L.B.; Ferreira-Neto, J.R.C.; Bezerra-Neto, J.P.; Pandolfi, V.; de Araújo, F.T.; da Silva Matos, M.K.; Santos, M.G.; Kido, E.A.; Benko-Iseppon, A.M. Cowpea and abiotic stresses: Identification of reference genes for transcriptional profiling by qPCR. Plant Methods 2018, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, A.; Enomoto, T.; Kobayashi, Y.; Watanabe, T.; Iuchi, S.; Kobayashi, M.; Sahoo, L.; Yamamoto, Y.Y.; Koyama, H. Sensitive to Proton Rhizotoxicity1 Regulates Salt and Drought Tolerance of Arabidopsis thaliana through Transcriptional Regulation of CIPK23. Plant Cell Physiol. 2019, 60, 2113–2126. [Google Scholar] [CrossRef]
- Watanabe, T.; Urayama, M.; Shinano, T.; Okada, R.; Osaki, M. Application of ionomics to plant and soil in fields under long-term fertilizer trials. SpringerPlus 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Qin, L.; Han, P.; Chen, L.; Walk, T.C.; Li, Y.; Hu, X.; Xie, L.; Liao, H.; Liao, X. Genome-Wide Identification and Expression Analysis of NRAMP Family Genes in Soybean (Glycine Max L.). Front. Plant Sci. 2017, 8, 1436. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, R.; Albrecht, U.; Padmanabhan, C.; Wang, A.; Coffey, M.D.; Girke, T.; Wang, Z.; Close, T.J.; Roose, M.; et al. Small RNA Profiling Reveals Phosphorus Deficiency as a Contributing Factor in Symptom Expression for Citrus Huanglongbing Disease. Mol. Plant 2013, 6, 301–310. [Google Scholar] [CrossRef]
- Ito, H.; Kobayashi, Y.; Yamamoto, Y.Y.; Koyama, H. Characterization of NtSTOP1-regulating genes in tobacco under aluminum stress. Soil Sci. Plant Nutr. 2019, 65, 251–258. [Google Scholar] [CrossRef]
- Sawaki, Y.; Kobayashi, Y.; Kihara-Doi, T.; Nishikubo, N.; Kawazu, T.; Kobayashi, M.; Kobayashi, Y.; Iuchi, S.; Koyama, H.; Sato, S. Identification of a STOP1-like protein in Eucalyptus that regulates transcription of Al tolerance genes. Plant Sci. 2014, 223, 8–15. [Google Scholar] [CrossRef]
- Liang, C.; Piñeros, M.A.; Tian, J.; Yao, Z.; Sun, L.; Liu, J.; Shaff, J.; Coluccio, A.; Kochian, L.V.; Liao, H. Low pH, Aluminum, and Phosphorus Coordinately Regulate Malate Exudation through GmALMT1 to Improve Soybean Adaptation to Acid Soils. Plant Physiol. 2013, 161, 1347–1361. [Google Scholar] [CrossRef]
- Tokizawa, M.; Kobayashi, Y.; Saito, T.; Kobayashi, M.; Iuchi, S.; Nomoto, M.; Tada, Y.; Yamamoto, Y.Y.; Koyama, H. Sensitive to proton rhizotoxicity1, calmodulin binding transcription activator2, and other transcription factors are involved in aluminum-activated malate transporter1 expression. Plant Physiol. 2015, 167, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Balzergue, C.; Dartevelle, T.; Godon, C.; Laugier, E.; Meisrimler, C.-N.; Teulon, J.-M.; Creff, A.; Bissler, M.; Brouchoud, C.; Hagège, A.; et al. Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nat. Commun. 2017, 8, 15300. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kobayashi, Y.; Sugimoto, M.; Lakshmanan, V.; Iuchi, S.; Kobayashi, M.; Bais, H.P.; Koyama, H. Characterization of the Complex Regulation of AtALMT1 Expression in Response to Phytohormones and Other Inducers. Plant Physiol. 2013, 162, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-F.; Yamaji, N.; Mitani, N.; Yano, M.; Nagamura, Y.; Ma, J.F. A Bacterial-Type ABC Transporter Is Involved in Aluminum Tolerance in Rice. Plant Cell 2009, 21, 655–667. [Google Scholar] [CrossRef] [PubMed]
| Peptide Sequence ID ‡ | Score § | E-Value ¶ | Sequence | Description † | |
|---|---|---|---|---|---|
| Similarity € | Identity ± | ||||
| Glyma.10G047100 | 428.7 | 6.2 × 10−151 | 90.1 | 77.94 | Putative ABC transport system permease protein |
| Glyma.03G175800 | 413.7 | 7.7 × 10−145 | 89.7 | 74.26 | Putative ABC transport system permease protein |
| Soil Type | Soil Condition | CaCO3 (g kg−1 soil) | Soil pH (H2O) | Exchangeable Al (meqkg−1 soil) |
|---|---|---|---|---|
| Neutral soil | 2 | 6.2 | 3 ± 0.5 | |
| Artificial | Acidic soil | 0 | 4.3 | 31.2 ± 0.3 |
| Acidic soil | 1.5 | 4.6 | 27.5 ± 0.1 | |
| Acidic soil | 5 | 5.4 | 8.4 ± 0.6 | |
| Neutral soil | 2 | 6.5 | 5.2 ± 0.2 | |
| Field | Acidic soil | 0 | 4.1 | 54.7 ± 0.4 |
| (natural) | Acidic soil | 2.2 | 4.4 | 48.9 ± 0.7 |
| Acidic soil | 6.2 | 5.3 | 13.5 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrahari, R.K.; Kobayashi, Y.; Borgohain, P.; Panda, S.K.; Koyama, H. Aluminum-Specific Upregulation of GmALS3 in the Shoots of Soybeans: A Potential Biomarker for Managing Soybean Production in Acidic Soil Regions. Agronomy 2020, 10, 1228. https://doi.org/10.3390/agronomy10091228
Agrahari RK, Kobayashi Y, Borgohain P, Panda SK, Koyama H. Aluminum-Specific Upregulation of GmALS3 in the Shoots of Soybeans: A Potential Biomarker for Managing Soybean Production in Acidic Soil Regions. Agronomy. 2020; 10(9):1228. https://doi.org/10.3390/agronomy10091228
Chicago/Turabian StyleAgrahari, Raj Kishan, Yuriko Kobayashi, Pankaj Borgohain, Sanjib Kumar Panda, and Hiroyuki Koyama. 2020. "Aluminum-Specific Upregulation of GmALS3 in the Shoots of Soybeans: A Potential Biomarker for Managing Soybean Production in Acidic Soil Regions" Agronomy 10, no. 9: 1228. https://doi.org/10.3390/agronomy10091228
APA StyleAgrahari, R. K., Kobayashi, Y., Borgohain, P., Panda, S. K., & Koyama, H. (2020). Aluminum-Specific Upregulation of GmALS3 in the Shoots of Soybeans: A Potential Biomarker for Managing Soybean Production in Acidic Soil Regions. Agronomy, 10(9), 1228. https://doi.org/10.3390/agronomy10091228







