Nutritional Quality of Plant Species in Pyrenean Hay Meadows of High Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Vegetation Sampling
2.3. Chemical Analysis
2.4. Data Analysis
3. Results
3.1. Vegetation Cover
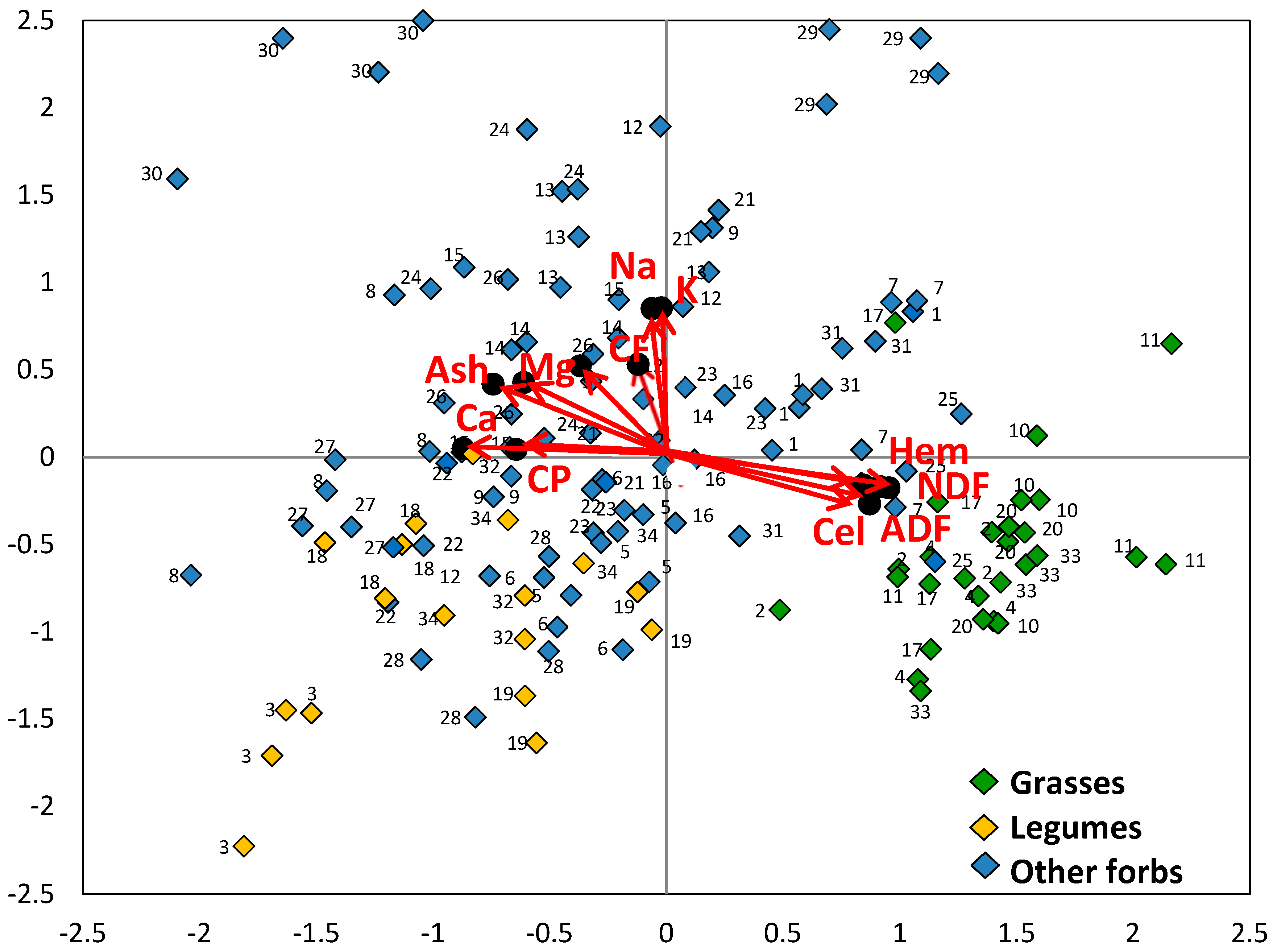
3.2. Chemical Composition of Species
3.3. Chemical Composition of Botanical Groups
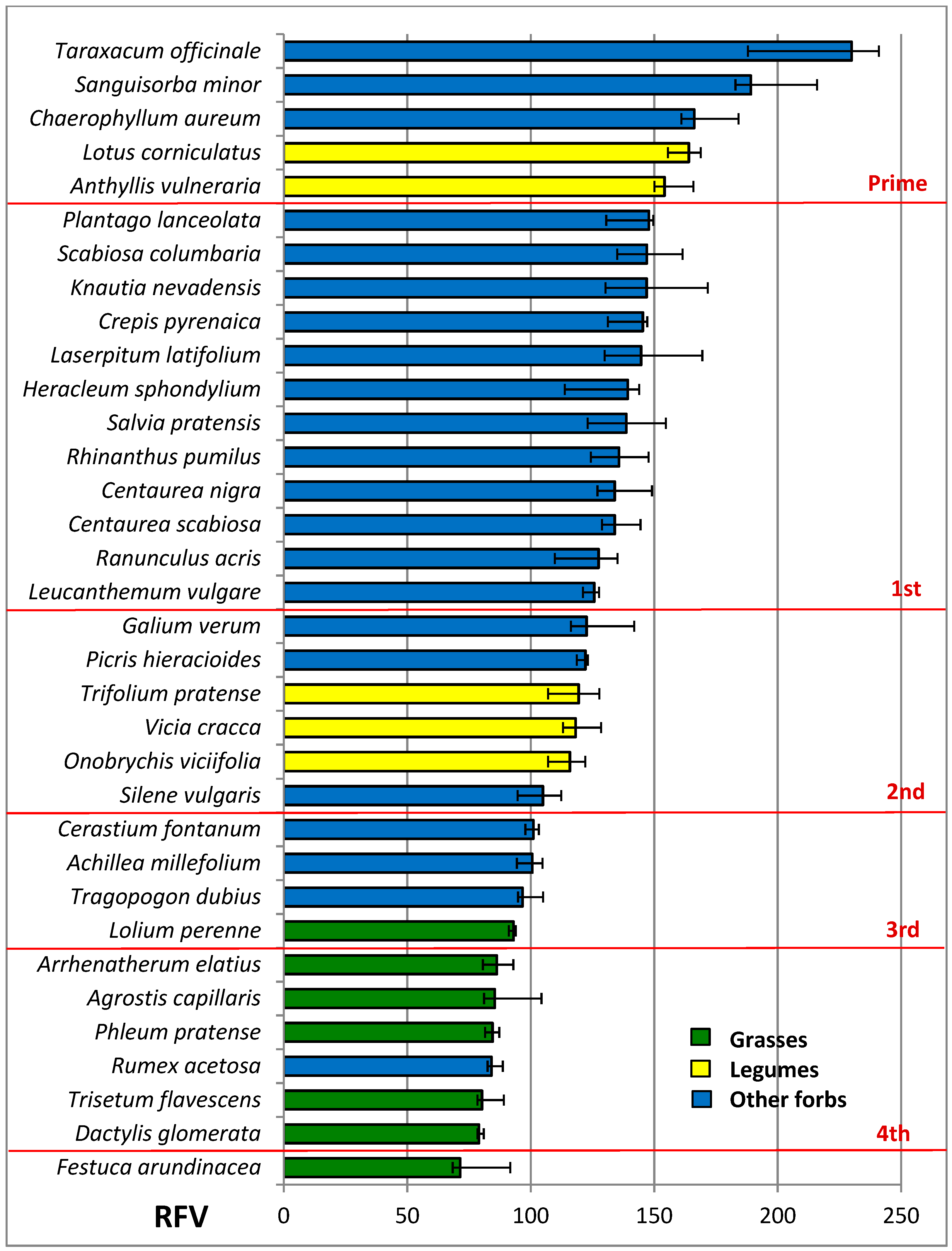
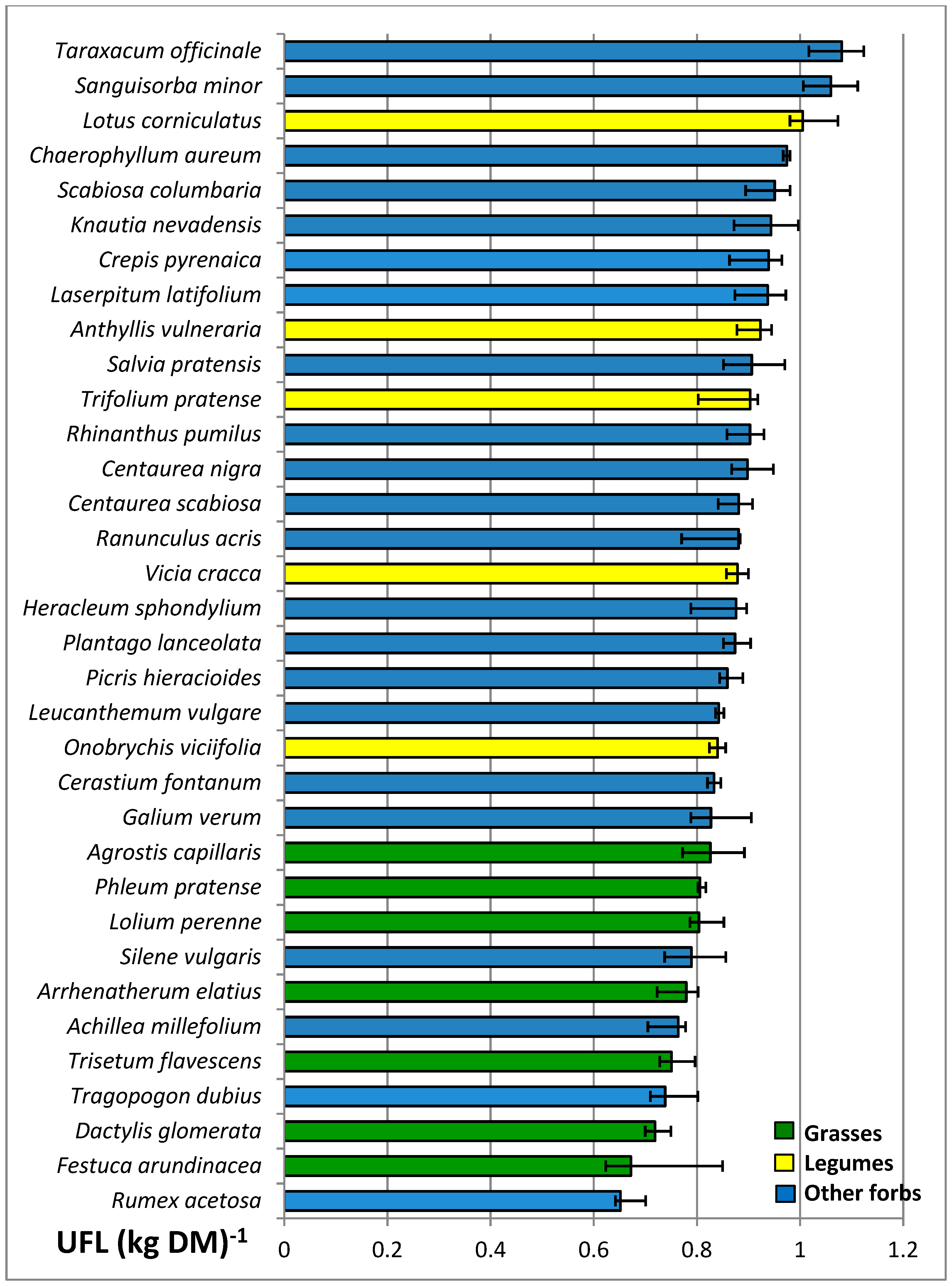
3.4. Nutritive Value of Species
3.5. Nutritive Value of Botanical Groups
4. Discussion
4.1. Species Diversity and Quality
4.2. Feeding Evaluation
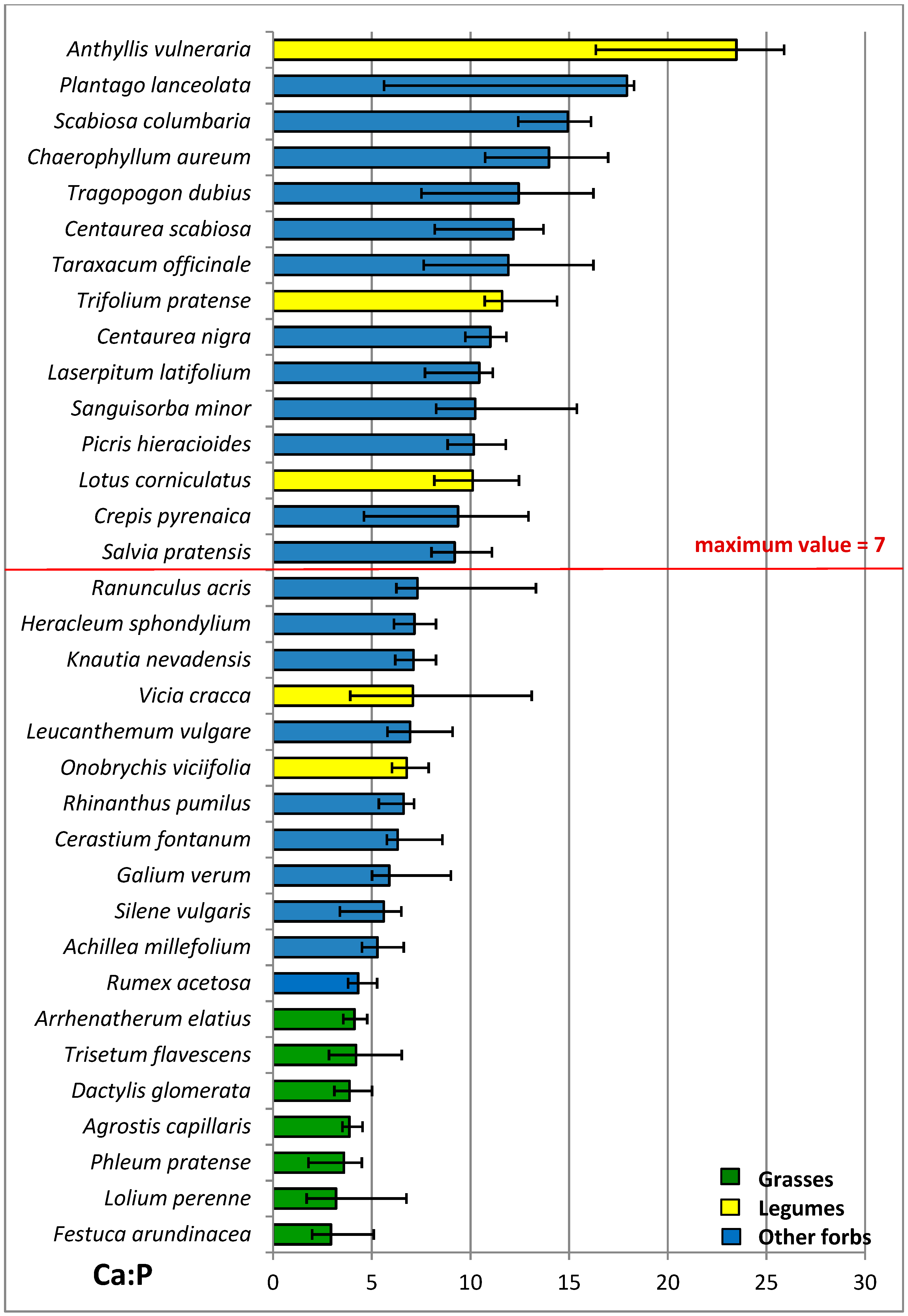
4.3. Mineral Contents
4.4. N:P Ratio
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buxton, D.R. Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim. Feed. Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
- Hopkins, A.; Holz, B. Grassland for agriculture and nature conservation: production, quality and multi-functionality. Agron. Res. 2006, 4, 3–20. [Google Scholar]
- Baumont, R.; Aufrère, J.; Niderkorn, V.; Andueza, D.; Surault, F.; Peccatte, J.R.; Delaby, L.; Pelletier, P. La diversité spécifique dans le fourrage: conséquences sur la valeur alimentaire. Fourrages 2008, 194, 189–206. Available online: https://www.researchgate.net/publication/273770331_La_diversite_specifique_dans_le_fourrage_consequences_sur_la_valeur_alimentaire (accessed on 9 May 2020).
- Baumont, R.; Aufrere, J.; Meschy, F. La valeur alimentaire des fourrages: rôle des pratiques de culture, de récolte et de conservation. Fourrages 2009, 198, 153–173. Available online: https://www.researchgate.net/publication/281798330_La_valeur_alimentaire_des_fourrages_role_des_pratiques_de_culture_de_recolte_et_de_conservation (accessed on 9 May 2020).
- Macheboeuf, D.; Coudert, L.; Bergeault, R.; Lalière, G.; Niderkorn, V. Screening of plants from diversified natural grasslands for their potential to combine high digestibility, and low methane and ammonia production. Animal 2014, 8, 1797–1806. [Google Scholar] [CrossRef]
- Reiné, R.; Barrantes, O.; Chocarro, C.; Juárez, A.; Broca, A.; Maestro, M.; Carlos, F.; Juárez-Escario, A. Pyrenean meadows in Natura 2000 network: grass production and plant biodiversity conservation. Span. J. Agric. Res. 2014, 12, 61. [Google Scholar] [CrossRef]
- Andueza, D.; Rodrigues, A.M.; Picard, F.; Rossignol, N.; Baumont, R.; Cecato, U.; Farruggia, A. Relationships between botanical composition, yield and forage quality of permanent grasslands over the first growth cycle. Grass Forage Sci. 2015, 71, 366–378. [Google Scholar] [CrossRef]
- Schlegel, P.; Wyss, U.; Arrigo, Y.; Hess, H. Mineral concentrations of fresh herbage from mixed grassland as influenced by botanical composition, harvest time and growth stage. Anim. Feed. Sci. Technol. 2016, 219, 226–233. [Google Scholar] [CrossRef]
- Pierik, M.E.; Gusmeroli, F.; Della Marianna, G.; Tamburini, A.; Bocchi, S. Meadows species composition, biodiversity and forage value in an Alpine district: Relationships with environmental and dairy farm management variables. Agric. Ecosyst. Environ. 2017, 244, 14–21. [Google Scholar] [CrossRef]
- Stevens, C.J.; Dise, N.B.; Mountford, J.O.; Gowing, D.J.G. Impact of Nitrogen Deposition on the Species Richness of Grasslands. Science 2004, 303, 1876–1879. [Google Scholar] [CrossRef]
- Horswill, P.; O’Sullivan, O.; Phoenix, G.K.; Lee, J.A.; Leake, J.R. Base cation depletion, eutrophication and acidification of species-rich grasslands in response to long-term simulated nitrogen deposition. Environ. Pollut. 2008, 155, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Hejcman, M.; Sochorová, L.; Pavlů, V.; Štrobach, J.; Diepolder, M.; Schellberg, J. The Steinach Grassland Experiment: Soil chemical properties, sward height and plant species composition in three cut alluvial meadow after decades-long fertilizer application. Agric. Ecosyst. Environ. 2014, 184, 76–87. [Google Scholar] [CrossRef]
- Schwab, A.; Dubois, D.; Fried, P.M.; Edwards, P.J. Estimating the biodiversity of hay meadows in north-eastern Switzerland on the basis of vegetation structure. Agric. Ecosyst. Environ. 2002, 93, 197–209. [Google Scholar] [CrossRef]
- Keenleyside, C.; Beaufoy, G.; Tucker, G.; Jones, G. High Nature Value Farming throughout EU-27 and Its Financial Support under the CAP. Report Prepared for DG Environment, Contract No ENV B.1/ETU/2012/0035; Institute for European Environmental Policy: London, UK, 2014; Available online: https://ieep.eu/uploads/articles/attachments/2e7adcbd-ba75--44db-89e6--0e1669076607/HNV_and_CAP_Full_Report.pdf?v=63664509849 (accessed on 9 May 2020).
- Wilman, D.; Riley, J.A. Potential nutritive value of a wide range of grassland species. J. Agric. Sci. 1993, 120, 43–50. [Google Scholar] [CrossRef]
- Scehovic, J. Effets de quelques plantes de prairies permanentes sur la qualité des associations végétales. Rev. Suisse Agric. 2000, 32, 195–200. Available online: https://abiodoc.docressources.fr/index.php?lvl=bulletin_display&id=24663 (accessed on 9 May 2020).
- Julier, B.; Huyghe, C. Quelles légumineuses fourragères (espèces et variétés) et quelles conduits pour améliorer l’autonomie protéique des élevages herbivores? Innov. Agron. 2010, 11, 101–114. Available online: https://prodinra.inra.fr/?locale=en#!ConsultNotice:173318 (accessed on 9 May 2020).
- Walzl, K.P.; Søegaard, K.; Jensen, H.H.; Eriksen, J.; Sanderson, M.A.; Rasmussen, J. Forage herbs improve mineral composition of grassland herbage. Grass Forage Sci. 2011, 66, 415–423. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, C.; Barry, T. Alternative temperate forages containing secondary compounds for improving sustainable productivity in grazing ruminants. Anim. Feed. Sci. Technol. 2005, 120, 179–201. [Google Scholar] [CrossRef]
- Hammond, K.; Humphries, D.; Westbury, D.; Thompson, A.; Crompton, L.; Kirton, P.; Green, C.; Reynolds, C.K. The inclusion of forage mixtures in the diet of growing dairy heifers: Impacts on digestion, energy utilisation, and methane emissions. Agric. Ecosyst. Environ. 2014, 197, 88–95. [Google Scholar] [CrossRef]
- Lukac, B.; Kramberger, B.; Meclic, V.; Verbic, J. Importance of non-leguminous forbs in animal nutrition and their ensiling properties: a review. Žemdirbyst Agric. 2012, 99, 3–8. Available online: http://www.lzi.lt/tomai/99(1)tomas/99_1_tomas_str1.pdf (accessed on 9 May 2020).
- Farruggia, A.; Martin, B.; Baumont, R.; Prache, S.; Doreau, M.; Hoste, H.; Durand, D. Quels intérêts de la diversité floristique des prairies permanentes pour les ruminants et les produits animaux? INRA Prod. Anim. 2008, 21, 181–200. [Google Scholar] [CrossRef]
- Tallowin, J.R.B.; Jefferson, R.G. Hay production from lowland semi-natural grasslands: A review of implications for ruminant livestock systems. Grass Forage Sci. 1999, 54, 99–115. [Google Scholar] [CrossRef]
- Sturludóttir, E.; Brophy, C.; Belanger, G.; Gustavsson, A.-M.; Jørgensen, M.; Lunnan, T.; Helgadottir, A. Benefits of mixing grasses and legumes for herbage yield and nutritive value in Northern Europe and Canada. Grass Forage Sci. 2013, 69, 229–240. [Google Scholar] [CrossRef]
- Dumont, B.; Garel, J.P.; Ginane, C.; Decuq, F.; Farruggia, A.; Pradel, P.; Rigolot, C.; Petit, M. Effect of cattle grazing a species-rich mountain pasture under different stocking rates on the dynamics of diet selection and sward structure. Animal 2007, 1, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Elgersma, A.; Søegaard, K. Changes in nutritive value and herbage yield during extended growth intervals in grass-legume mixtures: effects of species, maturity at harvest, and relationships between productivity and components of feed quality. Grass Forage Sci. 2017, 73, 78–93. [Google Scholar] [CrossRef]
- Lourenço, A.; Dias-Da-Silva, A.; Santos, A.S.; Rodrigues, M.; Cone, J.W.; Ferreira, L.M.M. Comparative digestibility of low-quality grass hay by two breeds of cattle differing in mature live weight. J. Anim. Physiol. Anim. Nutr. 2013, 98, 453–457. [Google Scholar] [CrossRef]
- Ascaso, J.; Reiné, R.; Barrantes, O. Evolution of Hay Meadows between 1956, 1986, and 2016 and Its Relation to the Characteristics and Location of the Parcels in the Valley of the River Esera (Pyrenees, Spain). Agron. Basel 2020, 10, 329. [Google Scholar] [CrossRef]
- García-Serrano, P.; Criado, S.R.; Marotta, J.J.L.; García, M.N. Guía Práctica de la Fertilización Racional de los Cultivos en Españai; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2010; pp. 1–119. ISBN 978-84-491-0997-3. [Google Scholar]
- Braun-Blanquet, J. Plant Sociology—The Study of Plant Communities; Hafner Publishing Company: New York, NY, USA, 1965; pp. 1–439. ISBN 9783874292085. [Google Scholar]
- Maabel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Chapman & Hall: London, UK, 1988; ISBN 978-94-015-7360-3. [Google Scholar]
- Castroviejo, S. Flora Iberica. Plantas Vasculares de la Península Ibérica e Islas Baleares; Volumes I–XV, XVI (I), XVII–XVIII y XX–XXI; Real Jardín Botánico C.S.I.C.: Madrid, Spain, 1986–2015; Available online: http://www.floraiberica.es/ (accessed on 9 May 2020).
- Reiné, R.; Ascaso, J.; Ferrer, C.; Yera, J.; Chocarro, C. 6510 Prados de Siega de Montaña (Arrhenatherion). In Bases Ecológicas Preliminares Para la Conservación de los Tipos de Hábitat de Interés Comunitario en España; Hidalgo, R., Ed.; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2009; pp. 1–60. Available online: https://www.miteco.gob.es/es/biodiversidad/temas/espacios-protegidos/6510_tcm30-196853.pdf (accessed on 9 May 2020).
- Chocarro, C.; Reiné, R.; Ascaso, J.; Yera, J.; Ferrer, C. 6520 Prados de Siega de Montaña (Triseto-Polygonion bistortae). In Bases Ecológicas Preliminares Para la Conservación de los Tipos de Hábitat de Interés Comunitario en España; Hidalgo, R., Ed.; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2009; pp. 1–48. Available online: https://www.miteco.gob.es/es/biodiversidad/temas/espacios-protegidos/6520_tcm30-196854.pdf (accessed on 9 May 2020).
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Linn, J.G.; Martin, N.P. Forage Quality Tests and Interpretations (Revised 1989); Univ. Minnesota, Agricultural Extension Service: 1989. Retrieved from the University of Minnesota Digital Conservancy. Available online: https://conservancy.umn.edu/handle/11299/207442 (accessed on 9 May 2020).
- INRA. Alimentation des Ruminants; Éditions Quæ: Versailles, France, 2018; pp. 1–728. ISBN 9782759228683. [Google Scholar]
- Roggero, P.P.; Bagella, S.; Farina, R. Un archivio dati di Indici specifici per la valutazione integrata del valore pastorale. Riv. Agron. 2002, 36, 149–156. Available online: http://eprints.uniss.it/4121/ (accessed on 9 May 2020).
- Daget, P.H.; Poissonet, T. Analyse Phytologique des Prairies. Applications Agronomiques; Centre d’Etudes Phytosociologiques et Écologiques (CNRS), Document 48: Montpellier, France, 1969; pp. 1–134. [Google Scholar]
- Farruggia, A.; Pompanon, F.; Ginane, C.; Vazeille, K.; Niderkorn, V.; Hulin, S. Reconstituer la composition du régime alimentaire des herbivores domestiques au pâturage: l’approche par métabarcoding. Fourrages 2012, 209, 43–51. Available online: https://afpf-asso.fr/article/reconstituer-la-composition-du-regime-alimentaire-des-herbivores-domestiques-au-paturage-l-approche-par-metabarcoding (accessed on 9 May 2020).
- National Research Council (NRC). Nutrient Requirements of Beef Cattle, 7th ed.; National Academic Science: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Grunes, D.L.; Welch, R.M. Plant Contents of Magnesium, Calcium and Potassium in Relation to Ruminant Nutrition. J. Anim. Sci. 1989, 67, 3485. [Google Scholar] [CrossRef] [PubMed]
- Koerselman, W.; Meuleman, A.F.M. The Vegetation N:P Ratio: A New Tool to Detect the Nature of Nutrient Limitation. J. Appl. Ecol. 1996, 33, 1441. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.; Chapin, F.S. The Mineral Nutrition of Wild Plants Revisited: A Re-evaluation of Processes and Patterns. In Advances in Ecological Research; Fitter, A.H., Raffaelli, D.G., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2000; pp. 1–66. [Google Scholar] [CrossRef]
- Bosworth, S.C.; Hoveland, C.S.; Buchanan, G.A.; Anthony, W.B. Forage Quality of selected Warm−Season Weed Species1. Agron. J. 1907, 72, 1050–1054. [Google Scholar] [CrossRef]
- Bosworth, S.C.; Hoveland, C.S.; Buchanan, G.A. Forage Quality of Selected Cool-Season Weed Species. Weed Sci. 1986, 34, 150–154. [Google Scholar] [CrossRef]
- Salt, C.A.; Mayes, R.W.; Elston, D.A. Effects of Season, Grazing Intensity and Diet Composition on the Radiocaesium Intake by Sheep on Re-Seeded Hill Pasture. J. Appl. Ecol. 1992, 29, 378. [Google Scholar] [CrossRef]
- Marinas, A.; González, R.G.; Fondevila, M. The nutritive value of five pasture species occurring in the summer grazing ranges of the Pyrenees. Anim. Sci. 2003, 76, 461–469. [Google Scholar] [CrossRef]
- Magda, D.; Theau, J.P.; Duru, M.; Coléno, F.-C. Hay-Meadows Production and Weed Dynamics as Influenced by Management. J. Range Manag. 2003, 56, 127. [Google Scholar] [CrossRef]
- Cortinovis, C.; Caloni, F. Epidemiology of intoxication of domestic animals by plants in Europe. Vet. J. 2013, 197, 163–168. [Google Scholar] [CrossRef]
- Cortinovis, C.; Caloni, F. Alkaloid-Containing Plants Poisonous to Cattle and Horses in Europe. Toxins 2015, 7, 5301–5307. [Google Scholar] [CrossRef]
- Ramos, G.; Frutos, P.; Giráldez, F.J.; Mantecón, A.R. Los compuestos secundarios de las plantas en la nutrición de los herbívoros. Arch. Zootec. 1998, 47, 597–620. Available online: https://digital.csic.es/bitstream/10261/8179/1/Mantecon%20et%20al.%201998%20Regs.%20198.pdf (accessed on 9 May 2020).
- Moldt, P.; Smitt, U.W.; Christensen, S.B. A New Sesquiterpene from Laserpitium latifolium. J. Nat. Prod. 1987, 50, 974–975. [Google Scholar] [CrossRef]
- Sostaric, K.; Kovacevic, J. La méthode “Complexe” pour la determination de la qualité et de la valeur globale des herbages et des prairies temporaires. Fourrages 1974, 60, 3–25. Available online: https://afpf-asso.fr/index.php?secured_download=2408&token=1c7eb1b12c5ed7189d725ef997c06504 (accessed on 9 May 2020).
- Benton, J.J.; Wolf, B.; Mills, H.A. Plant Analysis Handbook; Micro-Macro Publishing Inc.: Georgia, USA, 1991; p. 213. ISBN 1-878148-001. [Google Scholar]
- Vázquez-De-Aldana, B.R.; Ciudad, G.; Corona, M.E.P.; Criado, G. Nutritional quality of semi-arid grassland in western Spain over a 10-year period: changes in chemical composition of grasses, legumes and forbs. Grass Forage Sci. 2000, 55, 209–220. [Google Scholar] [CrossRef]
- Daccord, R.; Arrigo, Y.; Jeangros, B.; Scehovic, J.; Schubiger, F.X.; Lehmann, J. Valeur nutritive des plantes de prairies. 2: Teneurs en constituants pariétaux. Rev. Suisse Agric. 2001, 33, 81–86. Available online: https://www.agrarforschungschweiz.ch/fr/2001/04/valeur-nutritive-des-plantes-des-prairies-teneurs-en-constituants-parietaux/ (accessed on 9 May 2020).
- Daccord, R.; Arrigo, Y.; Kessler, J.; Jeangros, B.; Scehovic, J. Valeur nutritive des plantes de prairies. 3. Teneurs en calcium, phosphore, magnésium et potassium. Agrarforschung 2001, 8, 264–269. Available online: https://www.agrarforschungschweiz.ch/fr/2001/07/valeur-nutritive-des-plantes-des-prairies-teneurs-en-calcium-phosphore-magnesium-et-potassium/ (accessed on 9 May 2020).
- Jeangros, B.; Scehovic, J.; Schubiger, F.X.; Lehmann, J.; Daccord, R.; Arrigo, Y. Valeur nutritive des plantes de prairies. 1. Teneurs en matière sèche, matière azotée et sucres. Rev. Suisse Agric. 2001, 33, 73–80. Available online: https://www.agrarforschungschweiz.ch/fr/2001/02/valeur-nutritive-des-plantes-des-prairies-teneurs-en-matiere-seche-matiere-azotee-et-sucres/ (accessed on 9 May 2020).
- Marinas, A.; García González, R. Preliminary data on nutritional value or abundant species in supraforestal Pyrenean pastures. Pirineos 2006, 161, 85–109. Available online: http://pirineos.revistas.csic.es/index.php/pirineos/article/view/4/4 (accessed on 9 May 2020). [CrossRef]
- Juknevičius, S.; Sabienė, N. The content of mineral elements in some grasses and legumes. Ekologija 2007, 53, 44–52. Available online: http://www.gamtostyrimai.lt/uploads/publications/docs/651_05583b61a61c467113d137f303ecf9c1.pdf (accessed on 9 May 2020).
- Pontes, L.; Carrère, P.; Andueza, D.; Louault, F.; Soussana, J.-F. Seasonal productivity and nutritive value of temperate grasses found in semi-natural pastures in Europe: responses to cutting frequency and N supply. Grass Forage Sci. 2007, 62, 485–496. [Google Scholar] [CrossRef]
- Vázquez de Aldana, B.R.; García-Ciudad, A.; García-Criado, B. Relación Entre Compuestos Fenólicos Y Calidad Nutritiva en Especies Pratenses. In La Multifuncionalidad de Los Pastos: Producción Ganadera Sostenible Y Gestión de Los Ecosistemas; Reiné, R., Barrantes, O., Broca, A., Ferrer, C., Eds.; Sociedad Española para el Estudio de los Pastos: Huesca, Spain, 2009; pp. 273–278. ISBN 978-84-612-9337-7. [Google Scholar]
- Peláez, R.; Andrés, S.; Valdés, C.; García, R.; Calleja, A. Valor Alimenticio de Especies Productivas en Prados de Montaña. In Pastos, Paisajes Culturales Entre Tradición Y Nuevos Paradigmas del Siglo XXI; López-Carrasco, C., Rodríguez Rojo, M.P., San Miguel Ayanz, A., Fernández González, F., Roig Gómez, S., Eds.; Sociedad Española para el Estudio de los Pastos: Toledo, Spain, 2011; pp. 325–330. ISBN 978-84-614-8713-4. [Google Scholar]
- Vondrášková, B.; Čermák, B.; Martinkova, L.; Brouček, J. Examination of the nutritional quality of forbs from mountainous pastures in the Southwestern Bohemia region. Ekológia (Bratislava) 2012, 31, 231–237. [Google Scholar] [CrossRef]
- Grzegorczyk, S.; Alberski, J.; Olszewska, M. Accumulation of potassium, calcium and magnesium by selected species of grassland legumes and herbs. J. Elem. 2012, 18, 69–78. [Google Scholar] [CrossRef]
- Grzegorczyk, S.; Alberski, J.; Olszewska, M.; Grabowski, K.; Bałuch-Małecka, A. Content of calcium and phosphorus and the Ca:P ratio is selected species of leguminous and herbaceous plants. J. Elem. 2017, 22, 663–669. [Google Scholar] [CrossRef]
- Calsamiglia, A.; Ferret, A.; Bach, A. Tablas FEDNA de Valor Nutritivo de Forrajes y Subproductos Fibrosos Húmedos; Fundación para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2016; Available online: http://www.fundacionfedna.org/forrajes/introducci%C3%B3n-forrajes (accessed on 9 May 2020).
- Kacorzyk, P.; Głąb, T. Effect of ten years of mineral and organic fertilization on the herbage production of a mountain meadow. J. Elem. 2016, 22, 219–233. [Google Scholar] [CrossRef]
- Duru, M. Leaf and Stem In Vitro Digestibility for Grasses and Dicotyledons of Meadow Plant Communities in Spring. J. Sci. Food Agric. 1997, 74, 175–185. [Google Scholar] [CrossRef]
- Elgersma, A.; Søegaard, K.; Marker, S. Herbage dry-matter production and forage quality of three legumes and four non-leguminous forbs grown in single-species stands. Grass Forage Sci. 2013, 69, 705–716. [Google Scholar] [CrossRef]
- Wyłupek, T.; Harkot, W.; Czarnecki, Z. The content of selected macroelements in the dry weight of permanent grassland sward, grass yields and its agricultural value. J. Elem. 2014, 19, 853–864. [Google Scholar] [CrossRef]
- Kuusela, E. Annual and seasonal changes in mineral contents (Ca, Mg, P, K and Na) of grazed clover-grass mixtures in organic farming. Agric. Food Sci. 2008, 15, 23–34. [Google Scholar] [CrossRef]
- Grzegorczyk, S.; Olszewska, M.; Grabowski, K. Content of potassium, calcium, magnesium, phosphorus and sodium in meadow sward irrigated with wastewater. J. Elem. 2019, 25, 249–257. [Google Scholar] [CrossRef]
- Bowman, W.D.; Bahn, L.; Damm, M. Alpine Landscape Variation in Foliar Nitrogen and Phosphorus Concentrations and the Relation to Soil Nitrogen and Phosphorus Availability. Arct. Antarct. Alp. Res. 2003, 35, 144–149. [Google Scholar] [CrossRef]
- García-Ciudad, A.; Ruano-Ramos, A.; Vázquez-De-Aldana, B.R.; García-Criado, B. Interannual variations of nutrient concentrations in botanical fractions from extensively managed grasslands. Anim. Feed. Sci. Technol. 1997, 66, 257–269. [Google Scholar] [CrossRef]
- Gao, X.; Hao, X.; Marchbank, D.H.; Beck, R.; Willms, W.D.; Zhao, M. Responses of herbage P, Ca, K and Mg content and Ca/P and K/(Ca + Mg) ratios to long-term continuous and discontinued cattle grazing on a rough fescue grassland. Grass Forage Sci. 2016, 72, 581–589. [Google Scholar] [CrossRef]
- Ceulemans, T.; Stevens, C.J.; Duchateau, L.; Jacquemyn, H.; Gowing, D.J.G.; Merckx, R.; Wallace, H.; Van Rooijen, N.; Goethem, T.; Bobbink, R.; et al. Soil phosphorus constrains biodiversity across European grasslands. Glob. Chang. Boil. 2014, 20, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, T.; Bodé, S.; Bollyn, J.; Harpole, W.S.; Coorevits, K.; Peeters, G.; Van Acker, K.; Smolders, E.; Boeckx, P.; Honnay, O.; et al. Phosphorus resource partitioning shapes phosphorus acquisition and plant species abundance in grasslands. Nat. Plants 2017, 3, 16224. [Google Scholar] [CrossRef] [PubMed]
- Venterink, H.O.; Wassen, M.J.; Verkroost, A.W.M.; De Ruiter, P.C. Species richness-productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- Pavlů, L.; Pavlů, V.; Gaisler, J.; Hejcman, M. Relationship between soil and biomass chemical properties, herbage yield and sward height in cut and unmanaged mountain hay meadow (Polygono–Trisetion). Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 599–608. [Google Scholar] [CrossRef]
- Wassen, M.J.; Venterink, H.O.; Lapshina, E.; Tanneberger, F. Endangered plants persist under phosphorus limitation. Nature 2005, 437, 547–550. [Google Scholar] [CrossRef]
| Soil Parameters | Meadow | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Sand (%) | 53.7 | 46.0 | 36.7 | 44.9 |
| Fine Silt (%) | 26.1 | 32.5 | 39.1 | 36.0 |
| Coarse Silt (%) | 14.7 | 13.4 | 14.5 | 11.4 |
| Clay (%) | 5.5 | 8.1 | 9.7 | 7.7 |
| pH (H2O 1:2.5) | 6.6 | 6.5 | 6.3 | 6.0 |
| Salinity (dS m−1) | 0.22 | 0.22 | 0.20 | 0.22 |
| OM (%) | >6.69 | >6.69 | >6.69 | >6.69 |
| POlsen (mg kg−1) | 3.75 | 3.25 | 2.75 | 2.75 |
| KAmmonium acetate (mg kg−1) | 125.5 | 122.5 | 112.0 | 139.5 |
| N-NO3- (mg kg−1) | 37.0 | 50.0 | 47.7 | 38.2 |
| Mg (mg kg−1) | 53.5 | 113.0 | 104.5 | 136.5 |
| Species | Botanical Family | Development Stage | Meadows | Is (VP) | Intake | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| Achillea millefolium | Compositae | 1–2 | 8.7 | 1.7 | 1.7 | 1.4 | 2–2 | yes |
| Agrostis capillaris | Gramineae | 2–3 | 8.7 | 11.6 | 11.6 | 9.8 | 2–3 | yes |
| Anthyllis vulneraria | Leguminosae | 2 | 0.1 | 0.1 | 0.1 | 0.1 | 2–5 | - |
| Arrhenatherum elatius | Gramineae | 2–3 | 1.2 | 11.6 | 1.7 | 9.8 | 3–4 | yes |
| Centaurea nigra | Compositae | 2 | 18.5 | 1.7 | 1.7 | 9.8 | 0–1 | yes |
| Centaurea scabiosa | Compositae | 2 | 0.1 | 11.6 | 11.6 | 1.4 | 0–0 | yes |
| Cerastium fontanum | Caryophyllaceae | 2–3 | 0.1 | 0.1 | 0.1 | 0.1 | 0–0 | - |
| Chaerophyllum aureum | Umbelliferae | 2–3 | 1.2 | 1.7 | 1.7 | 1.4 | 0–0 | - |
| Crepis pyrenaica | Compositae | 2 | 1.2 | 1.7 | 1.7 | 9.8 | - | - |
| Dactylis glomerata | Gramineae | 2 | 1.2 | 11.6 | 1.7 | 9.8 | 4–5 | yes |
| Festuca arundinacea | Gramineae | 2–3 | 0.1 | 0.1 | 0.1 | 0.1 | 3–5 | yes |
| Galium verum | Rubiaceae | 2 | 1.2 | 0.1 | 0.1 | 0.1 | 0–1 | yes |
| Heracleum sphondylium | Umbelliferae | 2 | 8.7 | 0.1 | 1.7 | 1.4 | 0–2 | - |
| Knautia nevadensis | Dipsacaceae | 2 | 1.2 | 0.1 | 0.1 | 0.1 | 0–2 | yes |
| Laserpitium latifolium | Umbelliferae | 2 | 8.7 | 0.1 | 0.1 | 0.1 | - | - |
| Leucanthemum vulgare | Compositae | 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0–1 | - |
| Lolium perenne | Gramineae | 2 | 0.1 | 0.1 | 0.1 | 0.1 | 5–5 | yes |
| Lotus corniculatus | Leguminosae | 1–2 | 1.2 | 0.1 | 0.1 | 0.1 | 3–4 | yes |
| Onobrychis viciifolia | Leguminosae | 2–3 | 1.2 | 0.1 | 1.7 | 1.4 | 2–5 | yes |
| Phleum pratense | Gramineae | 2 | 1.2 | 11.6 | 0.1 | 0.1 | 4–5 | yes |
| Picris hieracioides | Compositae | 2 | 0.1 | 0.1 | 1.7 | 0.1 | 0–2 | - |
| Plantago lanceolata | Plantaginaceae | 1–2 | 1.2 | 1.7 | 1.7 | 1.4 | 2–3 | yes |
| Ranunculus acris | Ranunculaceae | 2–3 | 1.2 | 0.1 | 0.1 | 0.1 | 0–0 | yes |
| Rhinanthus pumilus | Scrophulariaceae | 1–2 | 1.2 | 1.7 | 0.1 | 1.4 | 0–0 | yes |
| Rumex acetosa | Polygonaceae | 1–2 | 1.2 | 0.1 | 1.7 | 0.1 | 0–1 | yes |
| Salvia pratensis | Labiatae | 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0–1 | - |
| Sanguisorba minor | Rosaceae | 2 | 0.1 | 0.1 | 1.7 | 0.1 | 1–5 | yes |
| Scabiosa columbaria | Dipsacaceae | 1–2 | 1.2 | 0.1 | 1.7 | 0.1 | 0–1 | yes |
| Silene vulgaris | Caryophyllaceae | 2 | 0.1 | 0.1 | 1.7 | 0.1 | 0–2 | yes |
| Taraxacum officinale | Compositae | 2–3 | 0.1 | 1.7 | 0.1 | 1.4 | 2–3 | yes |
| Tragopogon dubius | Compositae | 2–3 | 0.1 | 0.1 | 0.1 | 0.1 | 1–2 | yes |
| Trifolium pratense | Leguminosae | 2 | 8.7 | 11.6 | 1.7 | 9.8 | 4–4 | yes |
| Trisetum flavescens | Gramineae | 1–2 | 1.2 | 11.6 | 11.6 | 1.4 | 3–4 | yes |
| Vicia cracca | Leguminosae | 2 | 0.1 | 1.7 | 1.7 | 1.4 | 2–4 | yes |
| Total Cover of Selected Species | 81.0 | 95.9 | 62.5 | 74.0 | ||||
| Rest of species | 19.0 | 4.1 | 37.5 | 26.0 | ||||
| Total Cover of Botanical Groups | ||||||||
| Grasses | 16.5 | 60.1 | 55.1 | 42.4 | ||||
| Legumes | 11.3 | 15.2 | 6.8 | 14.2 | ||||
| Other forbs | 72.2 | 24.7 | 38.1 | 43.4 | ||||
| Diversity of Vegetal Species | ||||||||
| Shannon Index | 2.96 | 2.58 | 2.80 | 2.79 | ||||
| Number of species | 77 | 47 | 56 | 52 | ||||
| Species | DM | CP | Ash | CF | NDF | ADF | ADL | DDM | P | K | Mg | Ca | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Achillea millefolium | 31.1 | 10.1 | 6.5 | 2.2 | 55.9 | 36.5 | 9.0 | 60.4 | 0.16 | 1.61 | 0.22 | 0.90 | 0.11 |
| Agrostis capillaris | 38.5 | 8.9 | 5.3 | 1.8 | 68.1 | 33.2 | 3.3 | 63.0 | 0.14 | 0.80 | 0.22 | 0.51 | 0.08 |
| Anthyllis vulneraria | 26.7 | 13.0 | 11.8 | 1.4 | 40.9 | 26.2 | 5.4 | 68.5 | 0.14 | 0.55 | 0.17 | 3.32 | 0.06 |
| Arrhenatherum elatius | 42.1 | 7.6 | 4.5 | 1.6 | 66.5 | 35.2 | 3.0 | 61.5 | 0.13 | 0.74 | 0.18 | 0.50 | 0.08 |
| Centaurea nigra | 32.4 | 9.6 | 6.2 | 1.5 | 46.2 | 28.4 | 5.2 | 66.7 | 0.14 | 1.15 | 0.25 | 1.37 | 0.10 |
| Centaurea scabiosa | 28.6 | 8.7 | 7.4 | 1.3 | 45.9 | 29.0 | 4.8 | 66.3 | 0.14 | 1.13 | 0.25 | 1.67 | 0.09 |
| Cerastium fontanum | 33.9 | 6.6 | 6.1 | 1.6 | 59.6 | 31.2 | 4.6 | 64.6 | 0.11 | 1.65 | 0.29 | 0.77 | 0.13 |
| Chaerophyllum aureum | 26.1 | 12.7 | 10.8 | 2.0 | 39.2 | 24.1 | 5.5 | 70.1 | 0.16 | 1.49 | 0.54 | 2.39 | 0.10 |
| Crepis pyrenaica | 25.6 | 10.4 | 7.5 | 2.7 | 43.7 | 26.7 | 4.9 | 68.1 | 0.16 | 1.27 | 0.41 | 1.57 | 0.11 |
| Dactylis glomerata | 40.5 | 8.3 | 4.7 | 2.2 | 69.0 | 38.8 | 5.0 | 58.6 | 0.13 | 1.16 | 0.16 | 0.49 | 0.09 |
| Festuca arundinacea | 45.5 | 7.2 | 4.4 | 2.0 | 73.3 | 41.1 | 4.0 | 56.8 | 0.12 | 1.00 | 0.13 | 0.35 | 0.09 |
| Galium verum | 31.9 | 12.1 | 6.7 | 3.0 | 47.8 | 33.4 | 12.6 | 62.9 | 0.22 | 1.65 | 0.26 | 1.39 | 0.10 |
| Heracleum sphondylium | 18.5 | 12.9 | 9.2 | 2.2 | 43.9 | 30.2 | 4.4 | 65.4 | 0.23 | 1.89 | 0.47 | 1.60 | 0.13 |
| Knautia nevadensis | 22.3 | 11.0 | 7.8 | 2.1 | 43.8 | 26.1 | 4.3 | 68.5 | 0.18 | 1.68 | 0.37 | 1.24 | 0.12 |
| Laserpitum latifolium | 25.5 | 12.1 | 8.5 | 2.9 | 43.7 | 26.8 | 3.9 | 68.0 | 0.20 | 1.63 | 0.37 | 2.12 | 0.11 |
| Leucanthemum vulgare | 27.1 | 8.1 | 5.8 | 2.5 | 47.6 | 31.7 | 6.6 | 64.2 | 0.16 | 1.14 | 0.36 | 1.09 | 0.10 |
| Lolium perenne | 37.1 | 6.8 | 5.8 | 1.6 | 63.6 | 32.7 | 2.8 | 63.4 | 0.16 | 0.83 | 0.14 | 0.44 | 0.11 |
| Lotus corniculatus | 24.4 | 17.6 | 7.0 | 2.3 | 39.3 | 26.2 | 8.0 | 68.5 | 0.19 | 1.05 | 0.28 | 1.94 | 0.09 |
| Onobrychis viciifolia | 30.4 | 15.6 | 4.8 | 1.8 | 49.3 | 35.2 | 10.1 | 61.5 | 0.20 | 0.63 | 0.22 | 1.24 | 0.06 |
| Phleum pratense | 37.7 | 7.6 | 3.9 | 2.1 | 68.5 | 34.0 | 4.0 | 62.4 | 0.13 | 0.96 | 0.11 | 0.37 | 0.08 |
| Picris hieracioides | 26.9 | 10.3 | 7.6 | 3.0 | 49.9 | 30.6 | 4.3 | 65.0 | 0.17 | 1.09 | 0.46 | 1.74 | 0.14 |
| Plantago lanceolata | 25.5 | 9.8 | 9.3 | 1.7 | 42.3 | 28.8 | 10.1 | 66.5 | 0.14 | 1.29 | 0.40 | 2.36 | 0.10 |
| Ranunculus acris | 25.7 | 10.2 | 6.1 | 2.6 | 47.6 | 30.4 | 6.0 | 65.2 | 0.16 | 1.36 | 0.29 | 1.30 | 0.10 |
| Rhinantus pumilus | 25.5 | 12.3 | 9.3 | 4.0 | 45.7 | 28.6 | 8.8 | 66.6 | 0.29 | 1.41 | 0.39 | 1.79 | 0.11 |
| Rumex acetosa | 27.8 | 8.1 | 4.4 | 1.8 | 61.6 | 42.3 | 10.7 | 55.9 | 0.16 | 1.08 | 0.27 | 0.68 | 0.10 |
| Salvia pratensis | 21.7 | 12.4 | 8.4 | 2.7 | 45.0 | 28.0 | 6.1 | 67.1 | 0.19 | 1.50 | 0.53 | 1.79 | 0.11 |
| Sanguisorba minor | 32.7 | 9.6 | 8.6 | 3.3 | 36.2 | 19.6 | 3.7 | 73.7 | 0.16 | 0.92 | 0.48 | 1.67 | 0.08 |
| Scabiosa columbaria | 32.2 | 8.8 | 6.7 | 1.8 | 43.6 | 25.7 | 4.8 | 68.9 | 0.14 | 0.82 | 0.32 | 1.88 | 0.08 |
| Silene vulgaris | 24.1 | 8.4 | 9.3 | 2.5 | 56.3 | 32.6 | 4.8 | 63.5 | 0.20 | 2.92 | 0.32 | 1.03 | 0.17 |
| Taraxacum officinale | 19.4 | 14.1 | 12.8 | 3.1 | 30.5 | 17.6 | 5.2 | 75.2 | 0.20 | 2.27 | 0.61 | 2.38 | 0.21 |
| Tragopogon dubius | 27.5 | 8.4 | 6.4 | 4.9 | 57.5 | 37.2 | 4.0 | 60.0 | 0.15 | 0.96 | 0.26 | 1.70 | 0.08 |
| Trifolium pratense | 25.9 | 14.2 | 7.5 | 1.6 | 51.1 | 29.7 | 7.1 | 65.7 | 0.16 | 0.99 | 0.39 | 1.99 | 0.10 |
| Trisetum flavescens | 44.5 | 7.9 | 4.7 | 1.6 | 69.1 | 36.8 | 4.1 | 60.2 | 0.13 | 0.96 | 0.12 | 0.49 | 0.08 |
| Vicia cracca | 25.8 | 18.2 | 7.1 | 1.7 | 49.7 | 33.1 | 6.9 | 63.1 | 0.19 | 0.92 | 0.27 | 1.64 | 0.10 |
| Species | DM | CP | Ash | CF | NDF | ADF | ADL | DDM | P | K | Mg | Ca | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Achillea millefolium | 17 | 22 | 10 | 21 | 6 | 10 | 12 | 5 | 18 | 28 | 91 | 43 | 51 |
| Agrostis capillaris | 22 | 45 | 49 | 11 | 23 | 18 | 22 | 7 | 17 | 72 | 144 | 32 | 18 |
| Anthyllis vulneraria | 24 | 9 | 20 | 29 | 10 | 8 | 21 | 2 | 39 | 152 | 152 | 19 | 53 |
| Arrhenatherum elatius | 9 | 24 | 30 | 3 | 9 | 14 | 34 | 6 | 57 | 39 | 86 | 75 | 28 |
| Centaurea nigra | 12 | 41 | 16 | 27 | 12 | 16 | 17 | 5 | 63 | 60 | 35 | 58 | 57 |
| Centaurea scabiosa | 29 | 31 | 30 | 13 | 8 | 13 | 21 | 4 | 115 | 63 | 52 | 31 | 42 |
| Cerastium fontanum | 21 | 26 | 18 | 21 | 4 | 4 | 13 | 2 | 43 | 77 | 68 | 32 | 40 |
| Chaerophyllum aureum | 16 | 18 | 35 | 22 | 12 | 9 | 34 | 2 | 39 | 108 | 134 | 24 | 81 |
| Crepis pyrenaica | 12 | 14 | 52 | 20 | 8 | 15 | 17 | 4 | 29 | 75 | 130 | 134 | 83 |
| Dactylis glomerata | 28 | 29 | 41 | 37 | 1 | 8 | 39 | 4 | 35 | 210 | 41 | 23 | 19 |
| Festuca arundinacea | 23 | 72 | 34 | 86 | 18 | 35 | 59 | 16 | 29 | 88 | 159 | 113 | 61 |
| Galium verum | 25 | 39 | 10 | 18 | 14 | 18 | 49 | 7 | 79 | 235 | 87 | 34 | 54 |
| Heracleum sphondylium | 11 | 66 | 24 | 46 | 20 | 18 | 43 | 6 | 28 | 55 | 99 | 15 | 32 |
| Knautia nevadensis | 52 | 25 | 29 | 87 | 25 | 22 | 43 | 6 | 40 | 12 | 25 | 39 | 15 |
| Laserpitum latifolium | 25 | 34 | 15 | 74 | 20 | 30 | 50 | 8 | 26 | 69 | 84 | 80 | 40 |
| Leucanthemum vulgare | 21 | 33 | 38 | 6 | 5 | 3 | 18 | 1 | 48 | 98 | 38 | 38 | 14 |
| Lolium perenne | 48 | 13 | 66 | 71 | 2 | 10 | 41 | 4 | 203 | 137 | 208 | 112 | 49 |
| Lotus corniculatus | 31 | 31 | 18 | 22 | 6 | 16 | 33 | 4 | 18 | 169 | 35 | 71 | 45 |
| Onobrychis viciifolia | 16 | 7 | 15 | 29 | 12 | 5 | 8 | 2 | 34 | 182 | 96 | 51 | 45 |
| Phleum pratense | 25 | 34 | 26 | 70 | 7 | 2 | 15 | 1 | 72 | 99 | 24 | 119 | 21 |
| Picris hieracioides | 21 | 23 | 15 | 15 | 4 | 7 | 43 | 2 | 46 | 40 | 59 | 19 | 112 |
| Plantago lanceolata | 36 | 49 | 46 | 40 | 12 | 13 | 27 | 4 | 80 | 88 | 134 | 109 | 40 |
| Ranunculus acris | 14 | 37 | 35 | 30 | 17 | 14 | 21 | 5 | 107 | 69 | 54 | 21 | 35 |
| Rhinantus pumilus | 24 | 30 | 41 | 70 | 14 | 13 | 14 | 4 | 86 | 85 | 62 | 63 | 60 |
| Rumex acetosa | 14 | 47 | 44 | 15 | 5 | 6 | 21 | 3 | 18 | 108 | 49 | 165 | 12 |
| Salvia pratensis | 8 | 37 | 27 | 18 | 17 | 23 | 70 | 7 | 46 | 86 | 39 | 9 | 26 |
| Sanguisorba minor | 8 | 54 | 8 | 11 | 14 | 19 | 21 | 4 | 120 | 56 | 24 | 108 | 33 |
| Scabiosa columbaria | 5 | 16 | 40 | 17 | 13 | 21 | 5 | 6 | 20 | 90 | 21 | 32 | 42 |
| Silene vulgaris | 31 | 67 | 33 | 62 | 10 | 19 | 102 | 8 | 48 | 35 | 53 | 60 | 11 |
| Taraxacum officinale | 25 | 7 | 38 | 16 | 23 | 26 | 60 | 5 | 68 | 61 | 68 | 58 | 57 |
| Tragopogon dubius | 8 | 31 | 47 | 47 | 5 | 13 | 42 | 6 | 67 | 204 | 49 | 48 | 51 |
| Trifolium pratense | 62 | 28 | 13 | 68 | 13 | 20 | 35 | 7 | 32 | 66 | 164 | 21 | 65 |
| Trisetum flavescens | 8 | 11 | 7 | 22 | 11 | 10 | 24 | 5 | 48 | 123 | 59 | 61 | 25 |
| Vicia cracca | 37 | 29 | 15 | 20 | 9 | 10 | 21 | 4 | 47 | 24 | 37 | 209 | 12 |
| Botanical Groups | Meadows | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chemical Variables | Grasses (n = 28) | Legumes (n = 20) | Other Forbs (n = 88) | Sig | 1 (n = 34) | 2 (n = 34) | 3 (n = 34) | 4 (n = 34) | Sig |
| DM | 40.2 b | 26.6 a | 26.8 a | *** | 33.2 | 30.9 | 29.6 | 31.1 | ns |
| CP | 7.9 a | 15.6 c | 10.2 b | *** | 9.8 | 10.5 | 11.1 | 10.7 | ns |
| Ash | 4.8 a | 7.6 b | 7.8 b | *** | 6.8 | 7.1 | 7.5 | 7.3 | ns |
| CF | 1.9 a | 1.8 a | 2.5 b | ***1 | 2.3 | 2.5 | 2.3 | 2.2 | ns1 |
| Hem. | 32.0 b | 15.8 a | 17.5 a | *** | 20.0 | 20.4 | 19.9 | 20.7 | ns |
| Cel. | 32.0 b | 22.7 a | 23.3 a | *** | 25.4 | 25.2 | 24.6 | 24.8 | ns |
| NDF | 67.8 b | 46.1 a | 47.0 a | *** | 51.1 | 51.6 | 50.5 | 51.3 | ns |
| ADF | 35.7 b | 30.2 a | 29.5 a | ***1 | 31.1 | 31.2 | 30.7 | 30.6 | ns1 |
| ADL | 3.7 a | 7.5 c | 6.1 b | *** | 5.7 | 5.9 | 6.0 | 5.7 | ns |
| P | 0.13 a | 0.18 b | 0.18 b | *** | 0.19 b | 0.18 b | 0.14 a | 0.15 a | *** |
| K | 0.92 a | 0.87 a | 1.49 b | *** | 1.21 | 1.44 | 1.28 | 1.19 | ns |
| Mg | 0.17 a | 0.27 b | 0.36 c | *** | 0.25 | 0.33 | 0.32 | 0.33 | ns |
| Ca | 0.47 a | 2.01 c | 1.55 b | ***1 | 1.44 | 1.46 | 1.35 | 1.34 | ns1 |
| Na | 0.09 a | 0,09 a | 0,12 b | *** | 0,11 | 0,10 | 0,10 | 0.10 | ns |
| Botanical Groups | Meadows | Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nutritive Variables | Grasses (n = 28) | Legumes (n = 20) | Other Forbs (n = 88) | Sig | 1 (n = 34) | 2 (n = 34) | 3 (n = 34) | 4 (n = 34) | Sig | Sig |
| DDM | 61.1 a | 65.3 b | 65.9 b | *** | 64.7 | 64.6 | 65.0 | 65.1 | ns | ns |
| RFV | 84.3 a | 134.5 b | 135.5 b | ***1 | 124.6 | 123.5 | 125.8 | 125.4 | ns1 | ns1 |
| UFL (kg DM)−1 | 0.77 a | 0.91 b | 0.88 b | *** | 0.85 | 0.84 | 0.86 | 0.86 | ns | ns |
| PDI | 6.9 a | 8.7 c | 7.7 b | *** | 7.6 | 7.7 | 7.9 | 7.8 | ns | ns |
| Ca P−1 | 3.8 a | 11.8 c | 9.3 b | ***1 | 7.6 | 8.0 | 9.8 | 8.8 | ns1 | ns1 |
| K (Ca + Mg)−1 | 1.6 c | 0.4 a | 0.9 b | *** | 1.0 | 1.0 | 0.9 | 1.0 | ns | ns |
| N P−1 | 10.3 a | 14.1 b | 9.8 a | *** | 8.5 a | 9.3 a | 13.0 c | 11.3 b | *** | ns |
| N K−1 | 1.5 a | 3.3 b | 1.2 a | ***1 | 2.2 ab | 1.6 a | 1.9 ab | 2.4 b | ***1 | ***1 |
| K P−1 | 7.6 b | 4.9 a | 8.8 b | *** | 5.7 a | 6.9 ab | 8.5 b | 7.2 ab | * | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiné, R.; Ascaso, J.; Barrantes, O. Nutritional Quality of Plant Species in Pyrenean Hay Meadows of High Diversity. Agronomy 2020, 10, 883. https://doi.org/10.3390/agronomy10060883
Reiné R, Ascaso J, Barrantes O. Nutritional Quality of Plant Species in Pyrenean Hay Meadows of High Diversity. Agronomy. 2020; 10(6):883. https://doi.org/10.3390/agronomy10060883
Chicago/Turabian StyleReiné, Ramón, Joaquín Ascaso, and Olivia Barrantes. 2020. "Nutritional Quality of Plant Species in Pyrenean Hay Meadows of High Diversity" Agronomy 10, no. 6: 883. https://doi.org/10.3390/agronomy10060883
APA StyleReiné, R., Ascaso, J., & Barrantes, O. (2020). Nutritional Quality of Plant Species in Pyrenean Hay Meadows of High Diversity. Agronomy, 10(6), 883. https://doi.org/10.3390/agronomy10060883





