Abstract
Some of the key components that contribute to the acceptance of high-quality fresh mangoes by consumers are its flavour, odour, texture and chemical constituents that depend mainly on level of maturity. In the European market, the demand for tree-ripened fruit has increased in recent decades. Nevertheless, the qualitative response and the marketable characteristics of tree-ripened mango fruit grown in the Mediterranean area are not yet studied. Tree-ripened fruits of cv Keitt, Glenn, Osteen, Maya, Kensington Pride and Tommy Atkins were submitted to analytical (fruit weight, transversal diameter, longitudinal diameter, flesh firmness, total soluble solid content, titratable acidity, seed weight, peel weight, percentage of flesh and fibre, ash content, fat content, carbohydrate content, riboflavin, niacin, thiamin, K, Na, Ca, Mg, Fe, Cu, Mn and Zn contents, ascorbic acid and vitamin A) and sensory evaluations. Significant differences were observed for sensory and physicochemical characteristics in a cultivar-dependent manner. The mango Keitt, in addition to its interesting physicochemical traits, content of protein and vitamin, has a higher sensory appeal. Glenn fruit showed more suitable weight, flesh firmness, soluble solids, vitamin content, total antioxidant activity and total polyphenolics content; Maya had the lowest titratable acidity and the highest soluble solid content, whereas Tommy Atkins revealed preferable ground colour, total antioxidant activity, and vitamin B2 and vitamin C contents. Tree-ripened fruits grown in the Mediterranean climate show interesting peculiarities in regard to fresh market requirements. The analytical-sensory approach established a qualitative characterization of the six observed cultivars.
1. Introduction
Mango is an evergreen tree belonging to the family Anacardiaceae and it is originated in the Indo-Burmese region. Mango is named the “king of fruits” due to its high palatability, its sweetness, richness of taste, flavour, huge variability, large production volume and exemplary nutritive value [1]. This fruit is disseminated in about 100 countries in the world with a production of over 42 Mt [2]. It is widely cultivated in many tropical and subtropical regions with the prominent production in India, China, Thailand, Indonesia, Philippines, Brazil, Pakistan and Mexico [3]. Mangoes were not commonly known among the consumers outside the tropics before 1960s, and there was virtually no trade of fresh fruit while, today, they have become well established as products in the global market, particularly in Europe, North America, Asia and Africa [4]. Mango fruit traits such as size, colour, sugar and acidity depend on cultivar [5]. Generally, mango fruit contains a high percentage of starch [6] and it is an excellent source of minerals (calcium, phosphorus, potassium and iron) and essential vitamins (A, B and C) [7]. Moreover, mango flesh has a high nutraceutical value in a genotype dependent manner [8]. Mango is consumed and appreciated as fresh fruit, and is minimally processed [9] or dried [10]. The consumption of 100 g of raw mango will cover more than 50% of the recommended daily amount of each of the important vitamins [11].
Today, mango production is spread outside the traditional geographical regions to Central and South America, Australia, Southeast Asia, Hawaii, Egypt, Israel and South Africa [12,13]. In Italy, the fruit has been especially developed for export markets and it has gained a stable place among consumer preferences [5,14]. In Italy, mango fruit was originally only cultivated in Sicily when it started to be disseminated in the 80s, but in the last decade its cultivation has quickly expanded to several coastal areas characterized by a mild climate [15].
Some of the key components that contribute to the acceptance of high-quality fresh mango by consumers are its flavour, aroma, texture, chemical constituents [16] and antioxidant properties [17] that all depend mainly on the fruit’s level of maturity [8,18]. In Europe, aroma and sweetness have always been important for consumption. Countries such as France, Spain and Switzerland offer interesting markets for airfreighted tree-ripened mango or ready-to-eat mango. In fact, generally this type of fruit reaches superior taste and aroma compared to those shipped by sea. Mangoes are usually collected at the stage of partial development when they are still hard and green, that is, before complete ripening during the mature-green stage. They are then allowed to ripen progressively post-harvest [8,14]. Moreover, tree-ripened mangoes are a specialty found in specialized shops and high-end retailers, but in large-scale retail trade too. For these reasons, the demand for tree-ripened fruit that is imported via air-shipping has increased in recent decades. Nevertheless, from an environmental point of view, locally grown (km 0) tree-ripened mangoes could reach markets in a fresher and more flavourful state, and their distribution through short supply chains could reduce food-miles and the associated transportation greenhouse gas emissions (carbon footprint) due to the shortened travel distances to refrigerated ships, airplanes or truck cargo [19]. Additionally, tree-ripened mangoes reach a more suitable pomological and sensory quality than those green-picked and post-harvested [8]. In this context, mangoes grown in Sicily (southern Italy) could be harvested as tree-ripened fruit capable of reaching neighbouring countries in 24–48 h. In this scenario, it is also necessary to evaluate the chemical-physical characteristics of this kind of harvested fruit in order to define their sensory attributes and the most important nutraceutical traits, such as antioxidant activities.
The use of analytical studies for mangoes is also significant to characterize the most important physicochemical parameters of fruit quality [20]. The developments in the field of sensory evaluation and instrumental analysis further accentuated the interface between humans (sensory science) and machines (instrumental analysis) [21] in evaluating fruit quality. Moreover, sensory profiles of the mangoes have an important commercial aspect, because colour, firmness and taste have a great impact on purchase decision [22,23]. The role of scientific methodologies on fruit quality perception by using both instrumental and sensory analyses has been affirmed and tested on many fruit tree species [24].
In the literature, to date, there is a lack of information on the physicochemical and sensory traits of tree-ripened fruit. The aim of this work is to evaluate the qualitative characteristics of tree-ripened fruit of several of the most important mango cultivars grown in Mediterranean climate. The physicochemical, antioxidant activities and sensory traits are expected to provide information about the fruit quality, as well as address all components of short supply chains to reduce food-miles in order to enhance sustainable production of European mangoes.
2. Materials and Methods
2.1. Experimental Site and Plant Material
The study was carried out in 2018 in a commercial orchard located in Acquedolci, province of Messina, Sicily, Italy (38°3′ N, 14°33′ E; 5 m a.s.l.). Temperatures average 17.9 °C and the mean rainfall is 691 mm over 77 rainy days) [25,26,27]. The station is referred to as the upper thermos-Mediterranean lower subhumid bioclimatic belt [27].
Fruits of the mango cv Keitt, Glenn, Osteen, Maya, Kensington Pride and Tommy Atkins were examined after harvest (Table 1).

Table 1.
Harvest date of the six mango cultivars in trial.
Three uniform 11-year-old trees grafted on gomera-3 rootstock and trained to a globe shape were selected for each cultivar. Trees were planted in single rows (north-south oriented), spaced at 5 × 4 m, and submitted to routine cultural cares.
Fruiting of mango was continuously observed after full bloom (when more than 60% flowers had opened). Mango fruits were collected from using shape and colour as indexes of ripening [28]. Fruit harvest took place at full ripeness directly from the same tree, and fruit analysis was performed during one productive season. Fruits of uniform size, shape, and ripening stage were selected for analysis.
2.2. Physicochemical Analyses
A sample of 15 tree-ripened fruits (5 × tree × cultivar) were collected using colour as maturity index, and they were submitted for analytical and sensory evaluations. In particular, fruit fresh weight (FW), transversal diameter (TD), longitudinal diameter (DL), flesh firmness (FF), total soluble solids (TSS), titratable acidity (TA), seed weight (SW), peel weight (PW), percentage of flesh (PF) and presence/absence of fibre (FI) were recorded. FW, SW, PW (g) were determined by a digital scale (Gibertini EU-C 2002 RS, Novate Milanese, Italy); LD and TD (mm) by a digital caliper TR53307 (Turoni, Forlì, Italy); FF (N) by a digital penetrometer TR5325 with an 8-mm diameter tip (Turoni, Forlì, Italy); TSS (in Brix°) by a digital refractometer Atago Palette PR-32 (Atago Co., Ltd., Tokyo, Japan); TA (in % citric acid) and pH using a CrisonS compact titrator (Crison Instruments, SA, Barcelona, Spain). Flesh colour was determined by a digital image analysis of five photographs per cultivar each containing 6 fruits. Digital images were analyzed using an algorithm developed with MATLAB software (The MathWorks Inc., Natick, MA, USA) that converts images from RGB values to CIE 1976 L*, a*, b* format (by lookup tables), extracts the fruit from the image (removing the image background), separates the total fruit area into two subregions—cover colour (closer to red) and ground colour (closer to green) according to an adjustable green-red threshold, and quantifies colour characteristics of each region as the weighed distance of each pixel in the image from pure green (ground colour) or pure red (cover colour) [29,30]. The output is an index for cover colour (CCI) ranging from 0 (no red) to 1 (red) and an index for the ground colour (GCI) ranging from 0 (no green) to 1 (green). Percentage of cover colour was calculated by dividing the number of pixels of the red region by the number of pixels of the entire fruit area.
2.3. Proximate Composition
Ash content was determined through the procedure described in AOAC [31,32,33,34,35,36], while the Kjeldal method was used for protein determination [37].
The fat content (FAT) was obtained through acid hydrolysis with a 1:4 HCl solution on the sample followed by filtration and dehydration in a heater (70 °C). After solvent evaporation, the extraction in Soxhlet with petroleum ether was determined through a gravimetric method of residual fat.
The carbohydrate content (TSG), either free of or present in polysaccharides, was obtained with the anthrone method reported in [38]. Carbohydrates are first hydrolysed into simple sugars using diluted hydrochloric acid. In hot acidic medium, glucose is dehydrated to hydroxymethyl furfural that with anthrone forms a green-coloured product with an absorption maximum at 630 nm.
The minerals K, Na, Ca, Mg, Fe, Cu, Mn and Zn were determined using atomic absorption spectroscopy following wet mineralization, while P was determined using colourimetry [39].
Riboflavin was extracted in an autoclave with a solution of diluted H2SO4 and later, after enzymatic treatment, was determined through HPLC (for the fluorescent spectra) [40]. Niacin was extracted from the sample in an acidic solution at 12–18 °C for 30 min and measured through a microbiological method. The titrator strain was Lactobacillus plantatarum ATCC8014. The test was carried out in a liquid culture medium with all the indispensable factors for the growth of L. plantarum present with the exclusion of the examined vitamin. The presence of niacin in the sample caused a proportional increase in growth of L. plantarum after 24 h of incubation at 37 °C. The growth was evaluated using a turbidimeter and was then compared with the values of a standard curve prepared in parallel to the test. The thiamine content was obtained through extraction in 0.1 N HCl and oxidation by thiochromium and analysis in HPLC using fluorometric detection.
The ascorbic acid (vitamin C) and the retinol (vitamin A) were determined according to procedures previously described by Barros et al. [41]. For this determination, the dried methanolic extract (100 mg) was extracted with 10 mL of 1% metaphosphoric acid for 45 min at room temperature and filtered through Whatman No. 4 filter paper. The filtrate (1 mL) was mixed with 9 mL of 2.6-dichlorophenolindophenol and the absorbance was measured within 30 min at 515 nm against a blank. Vitamin C and vitamin A were calculated on the basis of the calibration curve of authentic L-ascorbic acid (0.02–0.12 mg/100 g). Vitamin E was determined by HPLC after extraction of the lipophilic component from pulp samples. All measurements were completed in three replicates.
2.4. Antioxidant Properties
The total phenolic content (TPC) of ethanolic extracts was determined by the reduction of phosphotungstic-phosphomolybdic acid (Folin–Ciocalteu’s reagent) to blue pigments, in an alkaline solution according to Folin and Denis [42]. Quantification was performed by gallic acid (GA) calibration curve, and the results were expressed as mg GA equivalents (GAE) per 100 g FW. All measurements were done in three replicates.
The total antioxidant activity (TAA) of ethanol extracts was evaluated using the ABTS radical cation decolourization assay [43]. ABTS•+ was prepared by reacting of ABTS with potassium persulfate [44]. Samples were analyzed at five different dilutions within the linearity range of the assay. TAA was expressed as μmol Trolox equivalent (TE) per 100 FW. All measurements were repeated three times.
2.5. Sensory Analyses
For the sensory characterization of mango fruits, a subsample of 10 fruits per cultivar was submitted to the sensory profile analysis according to the UNI 10957 [45] by a trained panel consisting of ten judges (five female and five male, 22–35 years old) qualified in different kinds of fruit evaluation [46,47,48,49,50]. The judges, in preliminary sessions, using both commercial and experimental mango samples, generated 20 sensory descriptors: two for appearance (flesh colour—FC; presence of filaments—FL), one for rheological (consistency to cut—C), six for odour (sea odour—SO; peach odour—PO; exotic fruit odour—EO; medicinal odour—MO; cheese odour—CO; burned oil odour—BO), two for touch in mouth (juicy—J; mellow—M), three for tastes (acid—A; sweet—SWE; bitter—B) and six for flavour (sea flavour—SF; peach flavour—PF; exotic fruits flavour—EF; medicinal flavour—MF; cheese flavour—CF; burned oil flavour—BF) [30,51]. The evaluations were carried out from 10.00 to 12.00 a.m. in individual booths with controlled illumination and temperature. In each session, judges evaluated the samples in triplicate using three different fruits from each cultivar. The sample order for each panellist was randomized and water was provided for rinsing between mango samples. The judges evaluated samples using a discontinuous scale, assigning to each descriptor a score from 1 (absence of sensation) to 9 (maximum intensity of the descriptor) [51]. Assessments were conducted at the laboratory of sensory analysis at University of Catania, built to UNI EN ISO 8589[52] with a specific software for sensory data acquisition (FIZZ, Software Solutions for Sensory Analysis and Consumer Tests, Biosystemes, Couternon, France). The study was carried out over three months (from August to October).
2.6. Statistical Analyses
The chemical and sensory data were tested for differences between the cultivars using the one-way analysis of variance (ANOVA; general linear model). The differences between cultivars were tested with Tukey’s high significance difference (HSD) test at the 0.05 significant level using XlStat® statistical software version 9.0 (Addinsoft, Paris, France). Principal component analysis (PCA) was also applied to sensory and instrumental means data in order to interpret differences among mango cultivars using THE UNSCRAMBLER® statistical software package version 9.8 (Camo As, Trondheim, Norway).
3. Results and Discussion
3.1. Physicochemical Analyses
The examined mango cultivars showed a great variability in physicochemical traits in a cultivar-dependent manner (Table 2 and Table 3). The fruits of cv Keitt and Osteen showed the greatest fresh weight (FW), followed by Kensington Pride and Maya, whereas the smallest fruits were observed for Glenn and Tommy Atkins. Diameter values confirmed the classification of fruit on the FW basis. Weight and diameter define fruit size and are important commercial characteristics. In the case of mangoes, the preferred size ranges 350–450 g due to packaging and logistics practices [53]. According to international marketing standards for mango [54], the studied cultivars fall under the B-size group class, ranging 351–550 g. (Table 2)

Table 2.
Pomological aspects of the six mango cultivars in trial. Fruit weight (FW); longitudinal diameter (LD); transversal diameter (TD); seed weight (SW); peel weight (PW); ground colour index (GCI); flesh colour index (FCI); presence of fibre (FI); flesh percentage (FP).

Table 3.
Physicochemical parameters of the six mango cultivars in trial. Flesh firmness (FF); total soluble solid content (SSC); titratable acidity (TA).
The percentage of flesh (PF) indicates the edible part of a fruit. Our results for PF are partially in agreement with those reported by Rodríguez [55] in Spain, who recorded the highest percentage (85.5%) for Osteen and the lowest value for Kensington Pride (74.6%). As expected, the highest values of seed weight (SW) were observed in the biggest fruits. In particular, Kensington Pride showed the highest value of peel weight (68.7 g). The incidence of seed and peel weight ranged from 71% for Tommy Atkins and Kensington Pride fruits to 82% for Osteen fruits and had no influence on the quantity of edible parts. Fibres (FI) were present only in Kensington Pride, Osteen and Tommy Atkins fruits. The absence of fibre is a characteristic which is liked by European mango consumers [56,57]. In fact, the consumers, especially in Western Europe, prefer fibre-free and easy-to-slice mangoes such as Kent, Keitt and Palmer fruits.
For mango, peel colour is one of the most important quality attributes and plays a dominant role in consumer acceptability [58]. Both colouration and fruit size are determinant traits of acceptance, but often consumers show a strong preference for peel colour [59]. It is the first characteristic of quality perceived by consumers who generally prefer a well and intensely coloured fruits; thus, in the case of mangoes, a colouration ranging from pink to red [60].
In this case, Tommy Atkins fruits exhibited the lowest values of GCI (less green) followed by Kensington Pride and Maya, while Keitt fruits had the lowest values (more green). On the other hands, Glenn fruits reached the highest value of CCI (more red), whereas Keitt fruits showed the lowest value, exhibiting a dark green ground colour and a very poor extension of cover colour. Despite this, consumer preference studies conducted in Europe indicated that Tommy Atkins mango was not the preferred mango according to recent tasting reports [61]. Despite the fact Tommy Atkins is inferior in taste to Keitt or Kent, it is by far the favourite of both retailers and consumers due to its transport resistance and long shelf life [61].
For mango flesh, firmness is an important quality factor that affects not only consumers’ acceptance but also handling and transportation capability. Fruit firmness, in general, decreases as the fruits become more mature and decreases rapidly as they ripen. Overripe or injured fruit are relatively soft. Our data varied in a tight range and generally indicated a ready-to-eat fruit with a shelf-life compatible with a short supply chain.
Soluble solid content (SSC) is the most relevant quality attribute for assessing mango sweetness [62]. It is strictly linked with ripeness [63], also responsible for fruit taste and consequently for consumers’ appreciation (Table 3). Significant differences were observed among the studied cultivars. In particular, SSC values were higher in Maya, Keitt and Glenn fruits, while Osteen, Kensington Pride and Tommy Atkins fruits showed similar lower values. According to Mukherjee [64], acidity is cultivar-related. Acidity values (TA) were higher in Keitt (0.40) and Glenn (0.37) fruits compared with those of Tommy Atkins (0.16).
Carvalho et al. [65] distinguished two groups in ripe mango cultivars, one with low acidity (0.20–0.29%) and the other with high acidity (0.35–0.48%). The values measured in Tommy Atkins fruits were lower than those reported by Carvalho et al. (2004) [65] for the same cultivar (0.20%), however grown in the tropics (Brazil). Consequently, the SST/TA ratio that we observed was balanced in Keitt Keitt and Glenn mangoes, whereas Maya and Tommy Atkins fruits showed higher values, followed by Osteen fruits.
3.2. Mineral Composition
Potassium (K) was the predominant element of the mineral elements in the examined mango fruit. The highest K contents were observed in Osteen (242.15 mg 100 g−1) and Maya fruits (250.25 mg 100 g−1), and the lowest values in Tommy Atkins fruits (190.34 mg 100 g−1). Potassium, in fact, is the major mineral of mango fruit, and contributes to the major fraction of the ash [16].
Phosphorus (P) was the second element most present in the observed mango fruits, with levels that ranged from 18.44 mg 100 g−1 in Keitt to 10.05 mg 100 g−1 in Kensington Pride (Table 4). Similar trends (5.5–18.1 mg 100 g−1) have been reported [16,66]. Our results showed that mango pulp is a good source of phosphorus, with similar content to that of papaya (15.0–20.5 mg 100 g−1) or rambutan (8.8–18.8) [67], but lower values compared to that of litchi (23.7–31.1 mg 100 g−1) and longan (30.1–35.7 mg 100 g−1) [68] among tropical fruits (Table 4). Mango is a good source of calcium (Ca). Its amount in fruit ranged from 12.40 mg 100 g−1 in Keitt to 9.16 mg 100 g−1 in Tommy Atkins. This range is comparable to previous reports (14.4–6.1 mg 100 g−1) in mango fruits from Cuba, Central America, Africa and India [69], from Spain [55]; and lower to Dodo [16] and Viringe mangoes from Tanzania (20.6–23.7 mg 100 g−1) [70]. Factors that influence Ca fruit content include nutrient balance and fruit size [71,72].

Table 4.
Flesh mineral composition (mg 100 g−1) of the 6 mango cultivars in trial. Potassium (K); sodium (Na); calcium (Ca); magnesium (Mg); phosphorus (P); iron (Fe); copper (Cu); manganese (Mn); zinc (Zn).
The sodium (Na) content in mango fruit varied from 8.55 mg 100 g−1 in Keitt to 7.11 mg 100 g−1 in Tommy Atkins. The observed levels were higher than values (2.0–4.0 mg 100 g−1) reported by Othman and Mbogo [70], and lower than those (30.51 mg 100 g−1) reported by Mamiro et al. [16], probably due to different varieties and soil characteristics.
Among the nine mineral elements studied, manganese (Mn) and copper (Cu) were the lowest in all the analyzed fruits. The contents ranged from 0.02 to 0.05 mg 100 g−1 of CU and from 0.02 to 0.03 mg 100 g−1 of Mn. Our analyses ranged lower amounts of Mn (0.97–1.19 mg 100 g−1) and Cu (0.16–0.21 mg 100 g−1) compared with those of African mangoes [70].
3.3. Biochemical Composition
Fruit of the studied mango varieties showed high moisture contents that ranged from 82.65 to 85.40%. This moisture content is slightly higher than in cv. Dodo (79.06%), a local Tanzanian cultivar [70], and similar to the range (82.9–84.9%) reported by Mahattanatawee et al. [73] in cv. Keitt. Values are in line with other fresh fruit that contain more than 80% water, such as apples, bananas and guavas, [74].
The content of proteins ranged from 0.359 to 0.803%. The lowest values, measured for Kensington Pride and Maya fruits, are similar to those (0.36–0.40%) reported by Morton [69]. Mid-range values (0.49–0.62%) observed in Osteen and Tommy Atkins fruits, are comparable to the protein content recorded by Srivastava [75]. The highest protein values were found in Glenn (0.701%) and Keitt fruits (0.803%).
Crude fibre content ranged from 0.788 to 0.924%, in line with contents (0.70%) reported by Ramulu [74]. The content of fats ranged from 0.314 and 0.326 g 100 g−1 and were lower values than those recorded by Mamiro et al. [16], but higher than those for crude fat of Dodo and Viringe mangoes [68]. The fruit total sugar content of the six cultivars was within 12.93–13.55%. The ash content of mangoes was 0.257–0.453% (Table 5).

Table 5.
Mango chemistry composition (g 100 g−1) of the six mango cultivars in trial. Moisture (MST); proteins (PRO); fats (FAT), crude fibre (CRF); total sugars (TSG); ash (ASH).
Among tropical fruits, mango is one the best sources of carotenoids and provides high vitamin A content [76,77]. In this study vitamin A content ranged from 0.866 mg 100 g−1 in Tommy Atkins to 1.082 mg 100 g−1 in Keitt fruits (Table 6). This range is comparable to the range (0.135–1.872 mg 100 g−1) reported by Morton (1987) [69] with fruits grown in the tropics. Vitamin C content was higher in Tommy Atkins and Maya fruits (12.99 and 12.69 mg 100 g−1) than in Keitt fruits (10.97 mg 100 g−1) (Table 6). Mango fruit is a rich source of ascorbic acid, although the content has been reported to decrease during ripening [78,79]. Mango varieties are used to show significant differences in ascorbic acid content, with the highest contents (up to 25.2 mg 100 g−1) in indigenous accessions [70].

Table 6.
Vitamin (mg 100 g−1) of the six mango cultivars in trial. Vitamin A (Vit. A); riboflavin (Vit. B2); ascorbic acid (Vit. C); niacin (Vit. B3); thiamin (Vit. B1).
Niacin content in the six examined cultivars ranged from 0.360 to 0.841 mg 100 g−1 in Tommy Atkins and Maya fruits, respectively. Riboflavin content was 0.028–0.036 mg 100 g−1. In this case, vitamin B2 levels are lower than those reported by Quinones et al. [80]. Vitamin B1 content ranged from 0.046 mg 100 g−1 in Osteen fruits to 0.067 mg 100 g−1 in Maya fruits, in accordance with values recorded by Quinones et al. [80]. Mangoes are definitely considered an excellent source of vitamins B1 and B2 [60].
3.4. Antioxidant Properties
Bioactivity of plant chemicals has been related especially to their antioxidant properties, which are not only useful to prevent oxidative stress phenomena but also influence intracellular redox status by modulating a number of important redox-dependent cell functions [81]. Our results showed a significant variability in radical-scavenging potential of the studied genotypes (Table 7). Regardless, high values of TAA were recorded for Glenn, Tommy Atkins, Maya and Kensington Pride. Similar results were observed in mango fruits collected at the mature-ripe stage [8].

Table 7.
Bioactive compounds of the six mango cultivars in trial. Total antioxidant activity (TAA) and total polyphenolic content (TPC).
However, TAA is positively correlated with TPC (p = 0.78), which is instead comparable to what is reported in literature for other mango cv [82], and is correlated less with vitamin C (p = 0.57). These results indicate that the presence of significant amounts of phenolic compounds is a major contribution to the antioxidant activity of these fruits.
3.5. Sensory Analyses
The sensory traits recorded were significantly different between the various cultivars under sensory assessment. The twelve descriptors were used to construct the sensory profile of each mango variety (Figure 1): peel colour, presence of filaments, consistency to cut, sea odour and flavour, exotic fruit odour and flavour, medicinal odour, cheese odour, burned oil odour, acidity and sweetness.
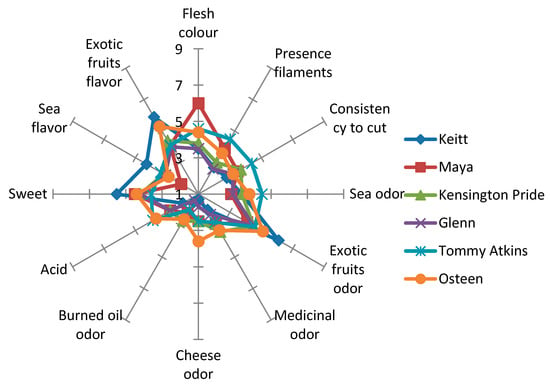
Figure 1.
Sensory profiles of the six observed mango cultivars as evaluated by a trained panel. Data are mean values ± SD (n = 30).
As highlighted by other authors [83,84], the Tommy Atkins fruit had an attractive red colour, but despite this, it is of declining interest because of the bland flavour when compared with green-skin mangoes such as Keitt.
In fact, panellists rated mangoes of cv. Keitt to be the worst for colour. Moreover, this cultivar presented the highest values for exotic fruit odour and flavour, as well as for sweetness at par with Osteen. Maya fruit was highly acceptable for flesh colour in visual examination. However, this cultivar rated lower for all other flavour attributes.
The principal component analysis applied to the significant sensory descriptors could discriminate among the samples in a multidimensional space (Figure 2).
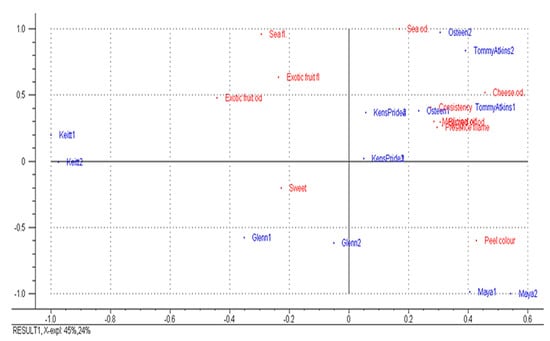
Figure 2.
Bi-plot of six mango fruit varieties from sensory descriptors during the first and the second year of study.
The variance explained by the first two principal components was 69%. Fruits of the cv Kensington Pride, Osteen and Tommy Atkins (in the first quadrant) were characterized by the presence of filaments, consistency to cut, acidity, and sea, medicinal, burned oil and cheese odours. The cv. Keitt (in the second quadrant) was characterized by a sea flavour and exotic fruit odour and flavour. The cv. Glenn (in the third quadrant) was characterized only by the sweetness, while Maya (in the fourth quadrant) was characterized by the presence of filaments, consistency to cut, acidity, and medicinal, burned oil and cheese odours.
4. Conclusions
Mangoes produced in the Mediterranean environment exhibited a high marketable value as tree-ripened fruit, but with a great variability in physicochemical traits according to the cultivar. Generally, all the analyzed cultivars of mango were transport sensitive and a good source of ascorbic acid and vitamin A, which are natural antioxidants valued by consumers . Proximate analyses of these mango fruits revealed that they are a good source of carbohydrates and proteins, which is a trait shared with tropical mangoes. Keitt fruit, in addition to its interesting FW, FP, FF, SSC, and contents of PRO, vitamin A, vitamin B3 and vitamin B2, had a higher sensory appeal. Glenn fruit had good results in terms of FW, FF, SSC, vitamin content, total antioxidant activity and total polyphenolics content, confirming the success already achieved in European markets. Other cultivars showed specific interesting fruit characteristics; for example, Maya had a low acidity and a high SSC, while Tommy Atkins had high GCI, vitamin B2, vitamin C contents and total antioxidant activity. Tree-ripened fruit of these cultivars have great commercial potential as high-quality fresh fruit in the EU and neighbouring countries’ short supply chains, and their commercial implementation would allow for a reduction in food-miles and enhance sustainable production.
Author Contributions
Conceptualization, V.F., A.M. and G.S.; methodology V.F., G.S., E.P. and C.G.; validation, V.F., A.M. and C.G.; formal analysis E.P., G.S., C.G., G.G. and V.F.; investigation, C.G., G.S., and V.F.; resources, C.G., V.F., G.S. and A.M.; software, A.M., G.G., and V.F.; data curation, A.M., G.G., C.G. and V.F.; writing—original draft preparation, A.M., G.S., G.G., C.G. and V.F.; writing—review and editing, G.S., A.M., C.G. and V.F.; visualization G.G., G.S. A.M. and V.F. Supervision V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge MedicAir Industry and Orved S.p.A. for its collaboration in the research activity and for the availability of the instrumentation needed to carry out the research. The authors would like to thank Giuseppe Marinaro for the supply of plant material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, N.K.; Mahato, A.K.; Sharma, N. A draft genome of the king of fruit, mango (Mangifera indica L.). In Proceedings of the Plant and Animal Genome XXII Conference, San Diego, CA, USA, 11–15 January 2014. [Google Scholar]
- Food and Agriculture Organisation of the United Nations. World Production of Mango (Mangoes, MangOsteens, Guavas) 2018. Available online: http://faostat.fao.org/ (accessed on 7 March 2019).
- Sortino, G.; Caviglia, V.; Liguori, G.; De Pasquale, C.; Gianguzzi, G.; Farina, V. Quality changes of tropical and subtropical fresh-cut fruits mix in modified atmosphere packaging. Chem. Eng. Trans. 2017, 58, 397–402. [Google Scholar]
- Mitra, S. Mango production in the world—Present situation and future prospect. Acta Hortic. 2016, 287–296. [Google Scholar] [CrossRef]
- Liguori, G.; Gentile, C.; Sortino, G.; Inglese, P.; Farina, V. Food quality, sensory attributes and nutraceutical value of fresh “Osteen” mango fruit grown under mediterranean subtropical climate compared to imported fruit. Agriculture 2020, 10, 103. [Google Scholar] [CrossRef]
- Fuchs, Y.; Pesis, E.; Zauberman, G. Changes in amylase activity, starch and sugars contents in mango fruit pulp. Sci. Hortic. 1980, 13, 155–160. [Google Scholar] [CrossRef]
- Jiménez, G.E.; Sánchez, P.M.; Velázquez, P.J. Getting Liqueur of Mango as a Preservation Model; National Polytechnic Institute: Mexico City, Mexico, 2004. [Google Scholar]
- Gentile, C.; Di Gregorio, E.; Di Stefano, V.; Mannino, G.; Perrone, A.; Avellone, G.; Sortino, G.; Inglese, P.; Farina, V. Food quality and nutraceutical value of nine cultivars of mango (Mangifera indica L.) fruits grown in Mediterranean subtropical environment. Food Chem. 2019, 277, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sortino, G.; Allegra, A.; Farina, V.; Inglese, P. Postharvest quality and sensory attributes of ‘Pesca di Bivona’ peaches (Prunus persica L.) during storage. Bulg. J. Agric. Sci. 2017, 23, 939–946. [Google Scholar]
- Russo, P.; Adiletta, G.; Di Matteo, M.; Farina, V.; Corona, O.; Cinquanta, L. Drying kinetics and physico-chemical quality of mango slices. Chem. Eng. 2019, 75, 109–114. [Google Scholar]
- Proserco (Promotora de Servicios Comerciales del Estado de Campeche). Diagnostic of System Mango Product; Campeche Goverment: Campeche, Messico, 2004.
- Elsheshetawy, H.; Mossad, A.; Elhelew, W.; Farina, V. Comparative study on the quality characteristics of some Egyptian mango cultivars used for food processing. Ann. Agric. Sci. 2016, 61, 49–56. [Google Scholar] [CrossRef]
- Saúco, V.G. Trends in world mango production and marketing. Acta Hortic. 2017, 351–364. [Google Scholar] [CrossRef]
- Farina, V.; D’Asaro, A.; Mazzaglia, A.; Gianguzzi, G.; Palazzolo, E. Chemical-physical and nutritional characteristics of mature-green and mature-ripe ‘Kensington Pride’ mango fruit cultivated in Mediterranean area during cold storage. Fruits 2017, 72, 221–229. [Google Scholar] [CrossRef]
- Farina, V.; Tripodo, L.; Gianguzzi, G.; Sortino, G.; Giuffre, D.; Lo Cicero, U.; Candia, R.; Collura, A. Innovative techniques to reduce chilling injuries in mango (Mangifera indica L.) trees under Mediterranean climate. Chem. Eng. Trans. 2017, 58, 823–828. [Google Scholar]
- Mamiro, P.; Fweja, L.; Chove, B.; Kinabo, J.; George, V.; Mtebe, K.; Mamiro, P.; Leonard, F.; Bernard, C.; Joyce, K.; et al. Physical and chemical characteristics of off vine ripened mango (Mangifera indica L.) fruit (Dodo). Afr. J. Biotechnol. 2007, 6, 2477–2483. [Google Scholar] [CrossRef]
- Rymbai, H.; Srivastav, M.; Sharma, R.R.; Patel, C.R.; Singh, A.K. Bio-active compounds in mango (Mangifera indica L.) and their roles in human health and plant defence—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 369–379. [Google Scholar] [CrossRef]
- Dick, E.; N’DaAdopo, A.; Camara, B.; Moudioh, E. Influence of maturity stage of mango at harvest on its ripening quality. Fruits 2009, 64, 13–18. [Google Scholar] [CrossRef]
- Lee, G.E.; Miller, S.R.; Loveridge, S. Modelling local food policy and greenhouse gas emission due to transportation. J. Reg. Anal. Policy 2017, 47, 75–87. [Google Scholar]
- Rymbai, H.; Laxman, R.H.; Dinesh, M.R.; Sunoj, V.J.; Ravishankar, K.; Jha, A. Diversity in leaf morphology and physiological characteristics among mango (Mangifera indica) cultivars popular in different agro-climatic regions of India. Sci. Hortic. 2014, 176, 189–193. [Google Scholar] [CrossRef]
- Ross, C.F. Sensory science at the human–machine interface. Trends Food Sci. Technol. 2009, 20, 63–72. [Google Scholar] [CrossRef]
- Jha, S.N.; Jaiswal, P.; Narsaiah, K.; Singh, A.K.; Kaur, P.P.; Sharma, R.; Kumar, R.; Bhardwaj, R. Prediction of sensory profile of mango using textural attributes during ripening. Food Bioprocess Technol. 2011, 6, 734–745. [Google Scholar] [CrossRef]
- Gössinger, M.; Mayer, F.; Radocha, N.; Höfler, M.; Boner, A.; Groll, E.; Nosko, E.; Bauer, R.; Berghofer, E. Consumer’s color acceptance of strawberry nectars from puree. J. Sens. Stud. 2009, 24, 78–92. [Google Scholar] [CrossRef]
- Farina, V.; Passafiume, R.; Tinebra, I.; Scuderi, D.; Saletta, F.; Gugliuzza, G.; Gallotta, G.; Sortino, .G. Postharvest Application of Aloe vera Gel-Based Edible Coating to Improve the Quality and Storage Stability of Fresh-Cut Papaya. J. Food Qual. 2020, 2020, 8303140. [Google Scholar] [CrossRef]
- Duro, A.; Piccione, V.; Scalia, C.; Zampino, D. Precipitazioni e temperature medie mensili in Sicilia relative al sessantennio 1926–1985. In Proceedings of the 5 Workshop Progetto Strategico Clima, Ambiente e Territorio nel Mezzogiorno CNR, Amalfi, Italy, 28–30 April 1993. [Google Scholar]
- Drago, A.; Cartabellotta, D.; Lo Bianco, B.; Monterosso, I. Atlante climatologico della Sicilia. Seconda edizione. Riv. Ital. Agrometeorol. 2005, 2, 67–83. [Google Scholar]
- Gianguzzi, L.; Papini, F.; Cusimano, D. Phytosociological survey vegetation map of Sicily (Mediterranean region). J. Maps 2015, 12, 845–851. [Google Scholar] [CrossRef]
- Lal, S.; Singh, S.K.; Singh, A.K.; Srivastav, M.; Singh, A.; Singh, N.K. Character association and path analysis for fruit chromatic, physicochemical and yield attributes in mango (Mangifera indica). Indian J. Agric. Sci. 2017, 87, 122–131. [Google Scholar]
- Francaviglia, D.; Farina, V.; Avellone, G.; Bianco, R.L. Fruit yield and quality responses of apple cvs Gala and Fuji to partial rootzone drying under Mediterranean conditions. J. Agric. Sci. 2013, 151, 556–569. [Google Scholar] [CrossRef]
- Liguori, G.; Sortino, G.; Inglese, P.; Farina, V. Quality changes during postharvest life in white fleshed peach (Prunus Persica L. Batsch) fruits: Preliminary observations. Bulg J. Agric. Sci. 2016, 22, 497–504. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1985; Volume 22, p. 362. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1985; Volume 3, p. 50. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1985; Volume 3, p. 69. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1985; Volume 22, p. 369. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1985; Volume 22, p. 360. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1985; Volume 22, p. 736. [Google Scholar]
- Farina, V.; Tinebra, I.; Perrone, A.; Sortino, G.; Palazzolo, E.; Mannino, G.; Gentile, C. Physicochemical, nutraceutical and sensory traits of six papaya (Carica papaya L.) cultivars grown in greenhouse conditions in the mediterranean climate. Agronomy 2020, 10, 501. [Google Scholar] [CrossRef]
- Loews, F.A. Improvement in anthrone method for the determination of carbohydrates. Anal. Chem. 1952, 24, 219–220. [Google Scholar] [CrossRef]
- Morand, P.; Gullo, J.L. Minéralisation des tissus végétaux en vue du dosage de P, K, Ca, Mg, Na. Ann. Agronomy 1970, 21, 229–236. [Google Scholar]
- Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of aloe vera gel-based edible coating with natural anti-browning and anti-oxidant additives to improve post-harvest quality of fresh-cut ‘fuji’ apple. Agronomy 2020, 10, 515. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with hosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric determination of antioxidant activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ente Nazionale Italiano di Unificazione. Sensory Analysis and Method for Establishing a Sensory Profile in Foodstuffs and Beverages; Ente Nazionale Italiano di Unificazione: Milan, Italy, 2003. [Google Scholar]
- Farina, V.; Barone, F.; Mazzaglia, A.; Lanza, C.M. Evaluation of Fruit Quality in Loquat Using Both Chemical and Sensory Analyses. Acta Hortic. 2011, 887, 345–350. [Google Scholar] [CrossRef]
- Farina, V.; Gianguzzi, G.; Mazzaglia, A. Fruit quality evaluation of affirmed and local loquat (Eriobotrya japonicaLindl) cultivars using instrumental and sensory analyses. Fruits 2016, 71, 105–113. [Google Scholar] [CrossRef]
- Farina, V.; Gianguzzi, G.; Mazzaglia, A. Fruit quality traits of six ancient apple (Malus domestica Borkh) cultivars grown in the mediterranean area. Int. J. Fruit Sci. 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Farina, V.; Volpe, G.; Mazzaglia, A.; Lanza, C. Fruit quality traits of two apricot cultivars. Acta Hortic. 2010, 862, 593–598. [Google Scholar] [CrossRef]
- Gallotta, A.; Allegra, A.; Inglese, P.; Sortino, G. Fresh-cut storage of fruit and fresh-cuts affects the behaviour of minimally processed Big Bang nectarines (Prunus persica L. Batsch) during shelf life. Food Packag. Shelf Life 2018, 15, 62–68. [Google Scholar] [CrossRef]
- Sortino, G.; Saletta, F.; Puccio, S.; Scuderi, D.; Allegra, A.; Inglese, P.; Farina, V. Extending the shelf life of white peach fruit with 1-methylcyclopropene and aloe arborescens edible coating. Agriculture 2020, 10, 151. [Google Scholar] [CrossRef]
- Ente Nazionale Italiano di Unificazione (UNI). Sensory Analysis—General Guidance for the Design of Test Rooms; Ente Nazionale Italiano di Unificazione: Milano, Italy, 2014. [Google Scholar]
- Bally, I.S.; Lu, P.; Johnson, P.R. Mango Breeding. In Breeding Plantation Tree Crops: Tropical Species; Springer Science and Business Media LLC: New York, NY, USA, 2009; pp. 51–82. [Google Scholar]
- Food and Agriculture Organization/World Health Organization. Codex Alimentarius, 1993—Codex Standard for Mangoes (Codex Stan 184-2003, Amended 2005); FAO: Rome, Italy, 2018. [Google Scholar]
- Rodríguez Pleguezuelo, C.R.; Durán Zuazo, V.H.; Muriel Fernández, J.L.; Franco Tarifa, D. Physico-chemical quality parameters of mango (Mangifera indica L.) fruits grown in a Mediterranean subtropical climate (SE Spain). J. Agric. Sci. Technol. 2011, 14, 365–374. [Google Scholar]
- Dos Santos Ribeiro, I.C.N.; Santos, C.A.F.; Neto, F.P.L. Morphological characterization of mango (Mangifera indica) accessions based on Brazilian adapted descriptors. J. Agric. Sci. Technol. B 2013, 3, 798–806. [Google Scholar]
- Pinto, A.C.Q.; Costa, J.G.; Santos, C.A.F. Principais variedades. In A Cultura da Mangueira; Embrapa Informação Tecnológica: Brasília, Brazil, 2002. [Google Scholar]
- Chaplin, G.R.; McBride, R.L.; Abdullah, A.; Nuevo, P.A. Sensory and physico-chemical quality of ‘Kensington’ mangoes after storage at low temperature. ASEAN Food J. 1991, 6, 109–113. [Google Scholar]
- Mattern, F.; Pennock, W. Potential market for improved varieties of mangos in Puerto Rican supermarkets. J. Agric. Univ. P. R. 1971, 55, 153–160. [Google Scholar]
- Litz, R.E. The Mango: Botany, Production and Uses; CAB International: Wallingford, UK, 2009. [Google Scholar]
- Sauco, V.G. Achieving Sustainable Cultivation of Mangoes; Informa UK Limited: Cambridge, UK, 2018; p. 420. [Google Scholar]
- Kane, O.; Boulet, M.; Castaigne, F. Effect of chilling-injury on texture and fungal rot of mangoes (Mangifera indica L.). J. Food Sci. 1982, 47, 992–995. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Mekwatanakarn, W.; Wang, C.Y. Soluble solids and simple sugars measurement in intact mango using near infrared spectroscopy. HortTechnol. 2008, 18, 410–416. [Google Scholar] [CrossRef]
- Mukherjee, S.K. Introduction: Botany and importance. In The Mango: Botany, Production and Uses; CABI Publishing: Wallingford, Oxfordshire, UK, 2009; pp. 1–18. [Google Scholar]
- Carvalho, C.R.L.; Rossetto, C.J.; Mantovani, D.M.B.; Morgano, M.A.; De Castro, J.V.; Bortoletto, N. Avaliação de cultivares de mangueira selecionadas pelo Instituto Agronômico de Campinas comparadas a outras de importância comercial. Rev. Bras. Frutic. 2004, 26, 264–271. [Google Scholar] [CrossRef]
- Galán Saúco, V. El Cultivo del Mango; Edicion Mundi-Prensa: Madrid, Spain, 2009. [Google Scholar]
- Hardisson, A.; Rubio, C.; Baez, A.; Martin, M.M.; Álvarez, R. Mineral composition of the papaya (Carica papaya variety sunrise) from Tenerife island. Eur. Food Res. Technol. 2001, 212, 175–181. [Google Scholar] [CrossRef]
- Wall, M.M. Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. J. Food Compos. Anal. 2006, 19, 434–445. [Google Scholar] [CrossRef]
- Morton, J.F.; Dowling, C.F. Fruits of Warm Climates; JF Morton: Miami, FL, USA, 1987; pp. 221–239. [Google Scholar]
- Othman, O.C.; Mbogo, G.P. Physico-chemical characteristics of storage-ripened mango (Mangifera indica L.) fruits varieties of eastern Tanzania. Tanzan. J. Sci. 2009, 35, 57–66. [Google Scholar]
- Van Eeden, S.J. Calcium infiltration as a possible postharvest treatment to increase storage potential of mango fruit. S. Afr. Mango Grow. Assoc. Yearb. 1992, 12, 26–27. [Google Scholar]
- Qiu, Y.; Nishina, M.S.; Paull, R.E. Papaya fruit growth, calcium uptake, and fruit ripening. J. Am. Soc. Hortic. Sci. 1995, 120, 246–253. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Manthey, J.A.; Luzio, G.; Talcott, S.T.; Goodner, K.; Baldwin, E.A. Total antioxidant activity and fiber content of select florida-grown tropical fruits. J. Agric. Food Chem. 2006, 54, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Punna, R.; Rao, P.U. Total, insoluble and soluble dietary fiber contents of Indian fruits. J. Food Compos. Anal. 2003, 16, 677–685. [Google Scholar] [CrossRef]
- Srivastava, H.; Narasimhan, P. Physiological studies during the growth and development of different varieties of guavas (Psidium Guajava L.). J. Hortic. Sci. 1967, 42, 97–104. [Google Scholar] [CrossRef]
- Yahia, E.M.; Ornelas-Paz, J.; Gardea, A. Extraction, separation and partial identification of ´ataulfo´ mango fruit carotenoids. Acta Hortic. 2006, 712, 333–338. [Google Scholar] [CrossRef]
- Pott, I.; Marx, M.; Neidhard, S.; Mulhbauer, W.; Carle, R. Quantitative determination of β-carotene stereo-isomers in fresh, dried, and solar dried mangoes (Mangifera indica L.). J. Agric. Food Chem. 2003, 51, 4527–4531. [Google Scholar] [CrossRef]
- Thomas, P. Effect of postharvest temperatures on quality, carotenoids and ascorbic acid contents in “Alphonso” mangos on ripening. J. Food Sci. 1975, 40, 704–706. [Google Scholar] [CrossRef]
- Carradori, S.; Botrè, F.; Mele, G.; Ruggieri, G. Ascorbic acid in exotic fruits: A liquid chromatographic investigation. Food Chem. 1995, 53, 211–214. [Google Scholar] [CrossRef]
- Quinones, V.L.; Guerrant, N.B.; Dutcher, R.A. Vitamin content of some tropical fruits, their juices and nectars. J. Food Sci. 1944, 9, 415–417. [Google Scholar] [CrossRef]
- Gentile, C.; Allegra, M.; Angileri, F.; Pintaudi, A.M.; Livrea, M.A.; Tesoriere, L. Polymeric proanthocyanidins from Sicilian pistachio (Pistacia vera L.) nut extract inhibit lipopolysaccharide-induced inflammatory response in RAW 264.7 cells. Eur. J. Nutr. 2011, 51, 353–363. [Google Scholar] [CrossRef]
- Shivashankara, K.S.; Isobe, S.; Al-Haq, M.I.; Takenaka, M.; Shiina, T. Fruit antioxidant activity, ascorbic acid, total phenol, quercetin, and carotene of irwin mango fruits stored at low temperature after high electric field pretreatment. J. Agric. Food Chem. 2004, 52, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Le Lagadec, M.D.; Köhne, J. Mango cultivar evaluation program in south africa. Acta Hortic. 2004, 645, 337–342. [Google Scholar] [CrossRef]
- Ngamchuachit, P.; Sivertsen, H.K.; Mitcham, E.J.; Barrett, D.M. Influence of cultivar and ripeness stage at the time of fresh-cut processing on instrumental and sensory qualities of fresh-cut mangos. Postharvest Boil. Technol. 2015, 106, 11–20. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).