Effect of Row Spacing, Sowing Density, and Harvest Time on Floret Yield and Yield Components of Two Safflower Cultivars Grown in Southwestern Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
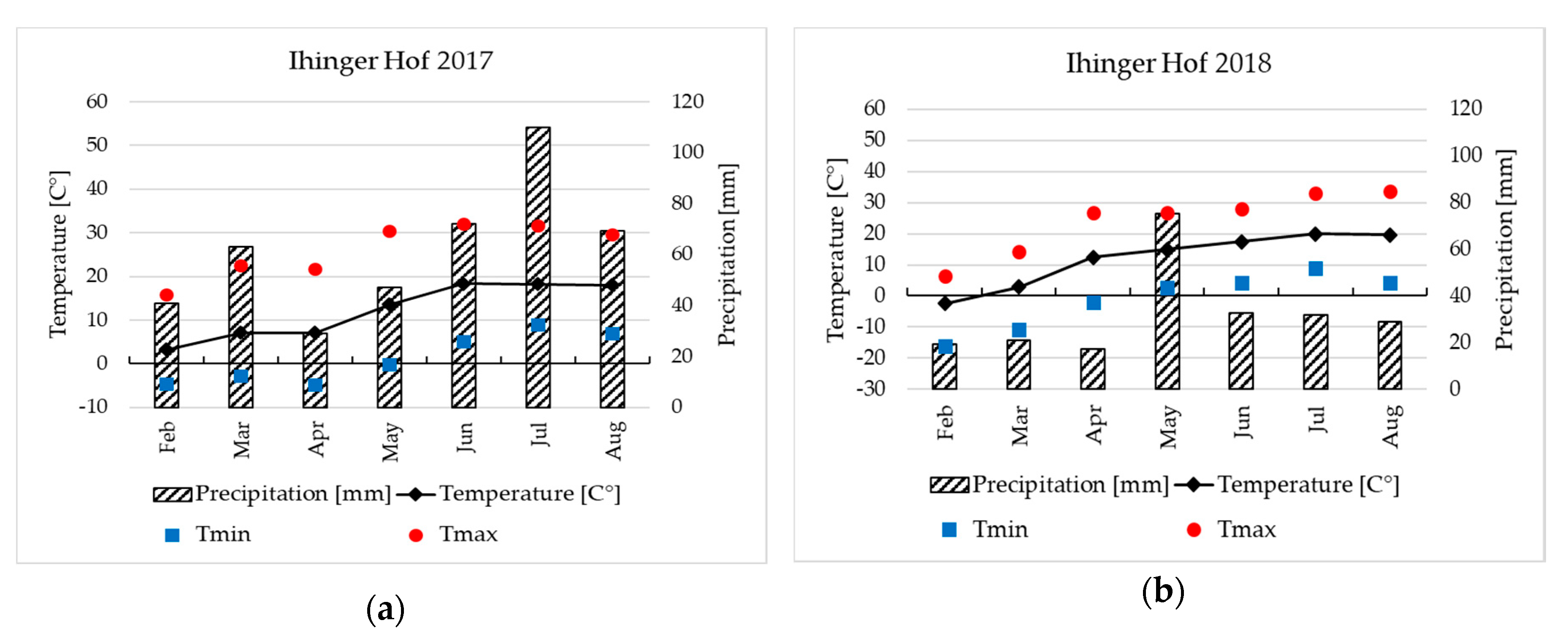
2.2. Field Site Characteristics
2.3. Field Experiments
2.4. Data Collection
2.4.1. Destructive Harvests
2.4.2. Determination of Color Content (Carthamidin Content)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Yield Parameters
3.1.1. Primary Branches per Plant
3.1.2. Number of Capitula per Plant
3.2. Yield and Quality Parameters
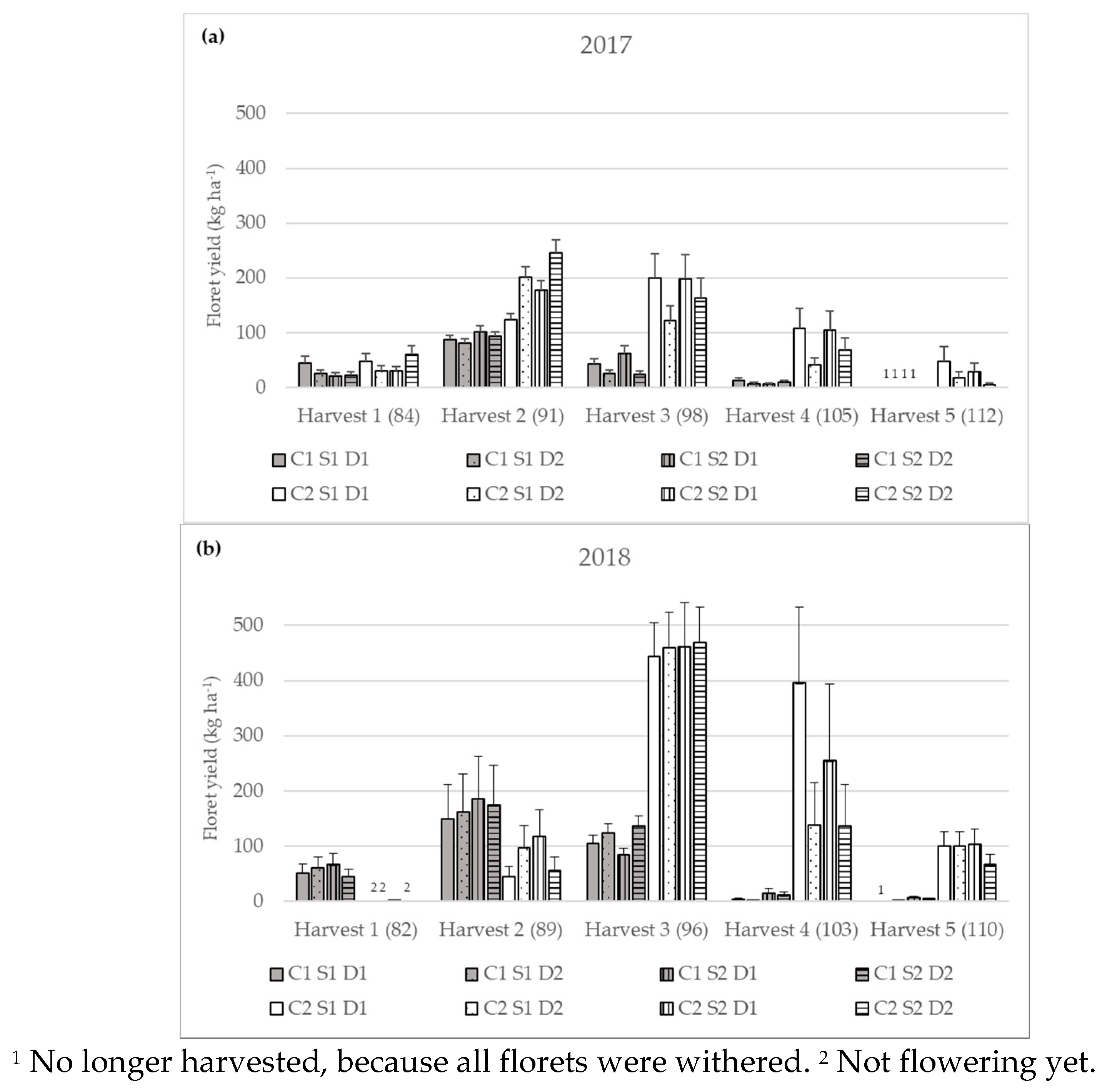
3.2.1. Yield of Flowering Florets
3.2.2. Carthamidin Content of Flowering Florets
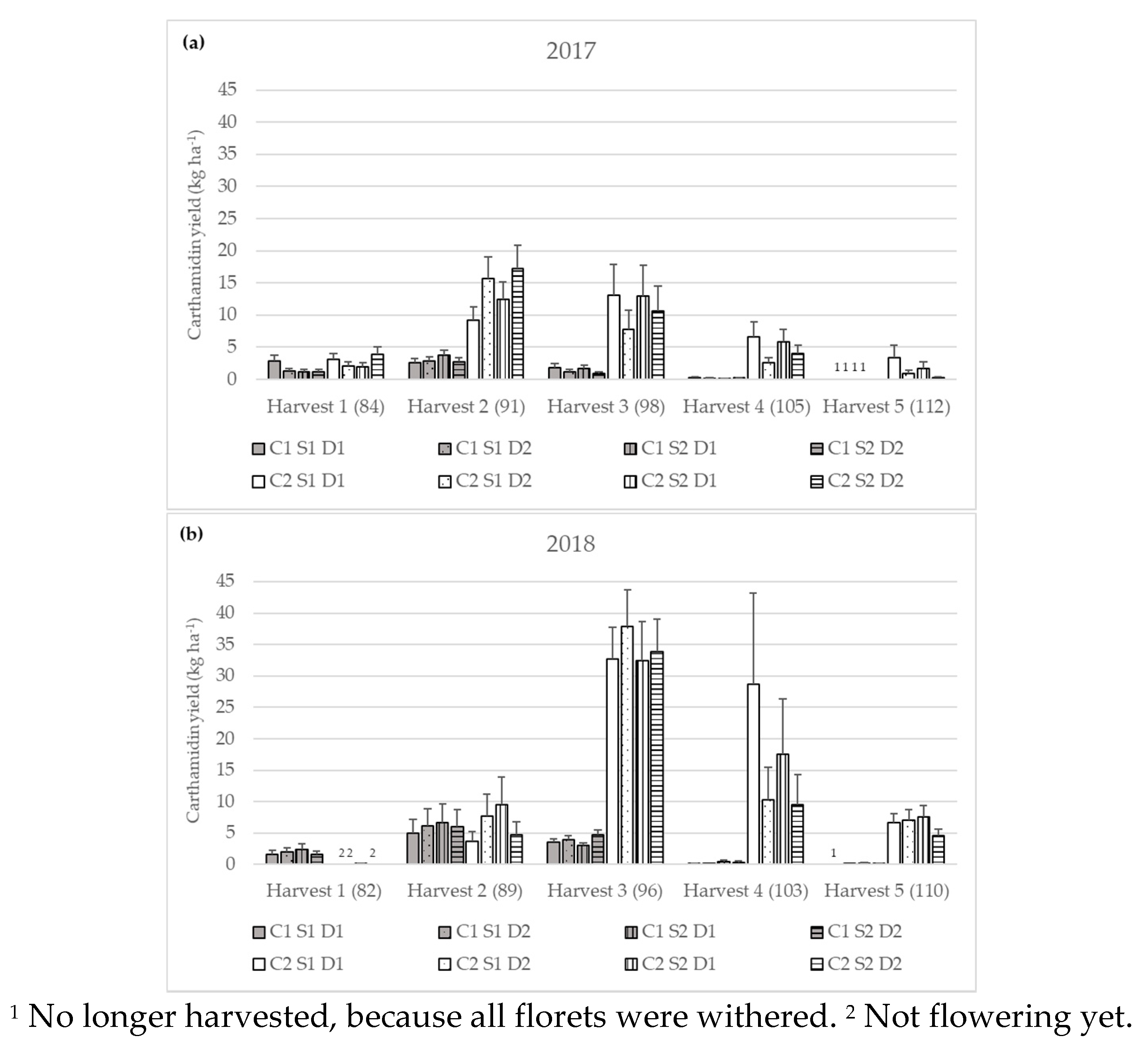
3.2.3. Carthamidin Yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Year | Harvest Number | Cultivar | |
|---|---|---|---|---|
| C1 | C2 | |||
| Yield of flowering florets (kg ha−1) | 2017 | Harvest 1 | 26.76 bA ± 3.86 | 40.30 cA ± 5.81 |
| Harvest 2 | 90.64 aB ± 4.28 | 181.82 aA ± 8.59 | ||
| Harvest 3 | 36.41 bB ± 4.06 | 167.56 aA ± 18.68 | ||
| Harvest 4 | 8.89 cB ± 1.49 | 74.99 bA ± 12.56 | ||
| Harvest 5 | n.d. | 18.98 dA ± 5.22 | ||
| 2018 | Harvest 1 | 55.38 bA ± 8.70 | 0.67 dB ± 0.11 | |
| Harvest 2 | 167.81aA ± 34.83 | 73.30 cB ± 15.21 | ||
| Harvest 3 | 110.57 aB ± 7.58 | 458.70 aA ± 33.75 | ||
| Harvest 4 | 5.47 cB ± 1.50 | 209.34 bA ± 57.27 | ||
| Harvest 5 | 2.36 dB ± 0.31 | 91.22 cA ± 12.12 | ||
| Carthamidin content of flowering florets (%) | Harvest 1 | 4.56 ± 0.17 | n.d. | |
| Harvest 2 | 3.48 B ± 0.17 | 7.71 A ± 0.17 | ||
| Harvest 3 | 3.75 B ± 0.17 | 7.03 A ± 0.17 | ||
| Harvest 4 | 2.94 B ± 0.17 | 6.56 A ± 0.17 | ||
| Harvest 5 | n.d. | 6.45 ± 0.17 | ||
| Carthamidin yield (kg ha−1) | 2017 | Harvest 1 | 1.51 bB ± 0.23 | 2.65 cA ± 0.40 |
| Harvest 2 | 2.94 aB ± 0.32 | 13.28 aA ± 1.45 | ||
| Harvest 3 | 1.30 bB ± 0.24 | 10.88 aA ± 2.00 | ||
| Harvest 4 | 0.19 cB ± 0.03 | 4.46 bA ± 0.75 | ||
| Harvest 5 | n.d. | 1.09 dA ± 0.32 | ||
| 2018 | Harvest 1 | 1.88 bA ± 0.33 | 0.04 dB ± 0.01 | |
| Harvest 2 | 5.93 aA ± 1.33 | 5.94 cA ± 1.33 | ||
| Harvest 3 | 3.76 aB ± 0.29 | 34.13 aA ± 2.81 | ||
| Harvest 4 | 0.19 cB ± 0.05 | 14.92 bA ± 3.75 | ||
| Harvest 5 | 0.08 dB ± 0.01 | 6.34 cA ± 0.73 | ||
| Parameter | Year | Harvest Number | Sowing Density | |
|---|---|---|---|---|
| D1 | D2 | |||
| Number of primary branches per plant | 13.0 A ± 0.40 | 9.54 B ± 0.40 | ||
| Number of capitula per plant | 14.35 A ± 0.36 | 8.73 B ± 0.22 | ||
| Yield of flowering florets (kg ha−1) | 2017 | Harvest 1 | 33.93 A ± 4.89 | 31.79 A ± 4.59 |
| Harvest 2 | 118.27 B ± 5.59 | 139.35 A ± 6.58 | ||
| Harvest 3 | 101.57 A ± 11.32 | 60.06 B ± 6.70 | ||
| Harvest 4 | 31.57 A ± 5.29 | 21.12 A ± 3.54 | ||
| Harvest 5 | n.d. | n.d. | ||
| 2018 | Harvest 1 | 6.87 eA ± 1.08 | 5.41dA ± 0.85 | |
| Harvest 2 | 110.19 bA ± 22.29 | 111.63 bA ± 23.17 | ||
| Harvest 3 | 206.71 aA ± 15.21 | 245.37 aA ± 16.82 | ||
| Harvest 4 | 49.57 cA ± 13.56 | 23 08 cA ± 6.31 | ||
| Harvest 5 | 14.22 dA ± 1.89 | 15.15 cA ± 2.01 | ||
| Carthamidin yield (kg ha−1) | n.d. | n.d. | ||
| Parameter | Cultivar | Year | Harvest Number | Row Spacing | |
|---|---|---|---|---|---|
| S1 | S2 | ||||
| Carthamidin yield (kg ha−1) | C1 | Harvest 1 | 1.85 A ± 0.30 | 1.53 A ± 0.25 | |
| Harvest 2 | 3.87 A ± 0.68 | 4.51 A ± 0.79 | |||
| Harvest 3 | 2.33 A ± 0.33 | 2.10 A ± 0.30 | |||
| Harvest 4 | 0.15 A ± 0.03 | 0.25 A ± 0.05 | |||
| Harvest 5 | n.d. | n.d. | |||
| C2 | Harvest 1 | 0.28 dA ± 0.05 | 0.37dA ± 0.06 | ||
| Harvest 2 | 7.95 bA ± 1.40 | 9.88 bA ± 1.74 | |||
| Harvest 3 | 18.86 aA ± 2.66 | 19.69 aA ± 2.83 | |||
| Harvest 4 | 8.42 bA ± 1.80 | 7.90 bA ± 1.68 | |||
| Harvest 5 | 3.46 cA ± 0.77 | 2.00 cA ± 0.44 | |||
| Carthamidin yield (kg ha−1) | 2017 | n.d. | n.d. | ||
| 2018 | 7.9B ± 0.7 1 | 10.9A ± 0.95 1 | |||
References
- Emongor, V. Safflower (Carthamus tinctorius L.) the underutilized and neglected crop: A review. Asian J. Plant Sci. 2010, 9, 299–306. [Google Scholar] [CrossRef]
- Weiss, E.A. Oilseed Crops, 2nd ed.; Blackwell Science: Oxford, UK, 2000; ISBN 0632052597. [Google Scholar]
- Emongor, V.; Oagile, O. Safflower Production; Impression House Publication: Gaborone, Botswana, 2017; ISBN 978-99968-0-607-0. [Google Scholar]
- Dajue, L.; Mündel, H.H. Safflower. Carthamus tinctorius L.; IPGRI, IPK, Eds.; IPGRI: Rome, Italy, 1996; ISBN 9290432977. [Google Scholar]
- Ekin, Z. Resurgence of safflower (Carthamus tinctorius L.) utilization: A global view. J. Agron. 2005, 4, 83–87. [Google Scholar]
- Corleto, A.; Alba, E.; Polignano, G.B.; Vonghio, G. Safflower: A multipurpose species with unexploited potential and world adaptability. The research in Italy. In Safflower: A Multipurpose Species with Unexploited Potential and World Adaptability. Proceedings of the Fourth International Safflower Conference, Bari, Italy, 2–7 June, 1997; Corleto, A., Mündel, H.-H., Eds.; Adruatica Editrice: Bari, Italy, 1997; pp. 23–31. [Google Scholar]
- Sirel, Z.; Aytac, Z. Relationship between the seed yield and some agronomic characteristics of safflower (Carthamus tinctorius L.) under semi-arid conditions. Turk. J. Field Crops 2016, 21, 39. [Google Scholar] [CrossRef]
- Chakradhari, S.; Perkons, I.; Mišina, I.; Sipeniece, E.; Radziejewska-Kubzdela, E.; Grygier, A.; Rudzińska, M.; Patel, K.S.; Radzimirska-Graczyk, M.; Górnaś, P. Profiling of the bioactive components of safflower seeds and seed oil: Cultivated (Carthamus tinctorius L.) vs. wild (Carthamus oxyacantha M. Bieb.). Eur. Food Res. Technol. 2020, 246, 449–459. [Google Scholar] [CrossRef]
- Singh, V.; Nimbkar, N. Safflower (Carthamus tinctorius L.), Chapter 6. In Genetic Resources, Chromosome Engineering, and Crop Improvement; Singh, R.J., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 167–194. ISBN 9780849336393. [Google Scholar]
- Garfield, S. Mauve. How One Man Invented a Color that Changed the World; W.W. Norton & Company: New York, NY, USA, 2002; ISBN 9780393323139. [Google Scholar]
- Zhaomu, W.; Lijie, D. Current situation and prospects of safflower products development in China. In Safflower: A Multipurpose Species with Unexploited Potential and World Adaptability. Proceedings of the Fifth International Safflower Conference, Williston, ND, USA, 23–27 July, 2001; Bergman, J.W., Mündel, H.H., Eds.; North Dakota State University: Fargo, ND, USA, 2001; pp. 315–319. [Google Scholar]
- Future Market Insights. Natural Food Colours Market: Significant Demand for Clean Label and Naturally Sourced Ingredients in Food Products Spurring Revenue Growth: Global Industry Analysis (2013–2017) & Opportunity Assessment (2018–2028). Available online: https://www.futuremarketinsights.com/reports/global-natural-food-colours-market (accessed on 6 May 2019).
- Grand View Research, Inc. Natural Food Colors Market Estimates & Trend Analysis by Product (Curcumin, Carotenoids, Anthocyanin, Carmine, Chlorophyllin), by Application (Bakery & Confectionery, Beverages, Dairy & Frozen Products, Meat Products), and Segment Forecasts, 2018–2025. Available online: https://www.grandviewresearch.com/industry-analysis/natural-food-colors-market (accessed on 6 May 2019).
- Křížová, H. Natural dyes: Their past, present, future and sustainability. Recent Dev. Fibrous Mater. Sci. 2015, 12, 59–71. [Google Scholar]
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural colorants: Historical, processing and sustainable prospects. Nat. Prod. Bioprospect. 2017, 7, 123–145. [Google Scholar] [CrossRef]
- European Commission. Guidance Notes on the Classification of Food Extracts with Colouring Properties. Available online: https://ec.europa.eu/food/safety/food_improvement_agents/additives/eu_rules_en (accessed on 20 November 2019).
- GNT International B.V. Europäische Union Verabschiedet Guidance Notes Zur Klassifizierung Färbender Lebensmittel. 2013. Available online: https://www.food-monitor.de/2013/12/europaeische-union-verabschiedet-giudance-notes-zur-klassifizierung-faerbender-lebensmittel/ (accessed on 20 August 2019).
- Bratinova, S. Provision of Scientific and Technical Support with Respect to the Classification of Extracts/Concentrates with Colouring Properties Either as Food Colours (Food Additives Falling under Regulation (EC) No 1333/2008) or Colouring Foods; Publications Office of the European Union: Luxembourg, 2015; ISBN 978-92-79-50607-9. [Google Scholar]
- Gao, W.Y.; Fan, L.; Paek, K.Y. Yellow and red pigment production by cell cultures of Carthamus tinctorius in a bioreactor. Plant Cell Tissue Organ Cult. 2000, 60, 95–100. [Google Scholar] [CrossRef]
- Rudolf Wild GmbH & Co., KG. EU-Leitlinien: Die Übergangsfrist für Färbende Lebensmittel Endet. 2015. Available online: https://www.lvt-web.de/topstories/fooddesign-hygiene-und-ueberwachung/eu-leitlinien-die-uebergangsfrist-fuer-faerbende-lebe (accessed on 20 August 2019).
- Dajue, L. Progress research and production in China. In Proceedings of the Third International Safflower Conference, Beijing, China, 14–18 June 1993; Dajue, L., Yuanzhou, H., Eds.; Beijing Botanical Garden, Institute of Botany, Chinese Academy of Sciences: Beijing, China, 1993; pp. 35–46. [Google Scholar]
- Food Standards Agency. Importing Confectionary. Guidance on Licensing, Labelling, Packaging, Chemical Safety When Importing Confectionery Products. Available online: https://www.food.gov.uk/business-guidance/importing-confectionery (accessed on 29 April 2020).
- Fusaro, D. When It Comes to Synthetic Food Colours: Beware the ‘Southampton Six’. Available online: https://www.foodprocessing.com/articles/2010/colorants/ (accessed on 29 April 2020).
- Shin, Y.-S.; Yoo, D.-I. Storage stability and color reproducibility of yellow and red dyes extracted from Carthamus tinctorius L. Text. Color. Finish. 2012, 24, 165–172. [Google Scholar] [CrossRef]
- Yoon, J.-M.; Cho, M.-H.; Park, J.-E.; Kim, Y.-H.; Hahn, T.-R.; Paik, Y.-S. Thermal stability of the pigments hydroxysafflor yellow A, safflor yellow B, and precarthamin from safflower (Carthamus tinctorius). J. Food Sci. 2003, 68, 839–843. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT-Crop Statistics: Safflower. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 7 May 2019).
- Rudometova, N.V.; Pasovskij, A.P.; Blohina, E.A. Method of isolation and identification of carthamin in safflower. Application´s perspectives in Russian food products. In Safflower: A Multipurpose Species with Unexploited Potential and World Adaptability. Proceedings of the Fifth International Safflower Conference, Williston, ND, USA, 23–27 July, 2001; Bergman, J.W., Mündel, H.H., Eds.; North Dakota State University: Fargo, ND, USA, 2001; pp. 309–313. [Google Scholar]
- Yu, H.; Xu, L.X. Separation and determination of flavonols in the flowers of Carthamus tinctorius by RP-HPLC. Yaoxue Xuebao (Acta Pharm. Sin.) 1997, 32, 120–122. [Google Scholar]
- Mohammadi, M.; Tavakoli, A. Effect of harvest time of spring safflower (Carthamus tinctorius L.) florets on the production of red and yellow pigments. Q. Assur. Saf. Crops Foods 2015, 7, 581–588. [Google Scholar] [CrossRef]
- Kizil, S.; Çakmak, Ö.; Kirici, S.; İnan, M. A Comprehensive Study on Safflower (Carthamus Tinctorius L.) in Semi-Arid Conditions. Biotechnol. Biotechnol. Equip. 2008, 22, 947–953. [Google Scholar] [CrossRef]
- Azari, A.; Khajehpour, M.R. Effect of planting pattern on development, growth, yield components and seed and petal yields of safflower in summer planting, local variety of Isfahan, Koseh. Person. J. Sci. Technol. Agric. Nat. Resour. 2005, 9, 131–142. [Google Scholar]
- Camaş, N.; Esendal, E. Estimates of broad-sense heritability for seed yield and yield components of safflower (Carthamus tinctorius L.). Hereditas 2006, 143, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Knowles, P.F. Centers of plant diversity and conservation of crop germ plasm: Safflower. Econ. Bot. 1969, 23, 324–329. [Google Scholar] [CrossRef]
- Omidi, A.H.; Sharifmoghaddasi, M. Study of safflower varieties for flower and grain yields and fatty acids composition. Adv. Environ. Biol. 2010, 4, 524–527. [Google Scholar]
- Sharifmoghaddasi, M.; Omidi, A.H. Determination of optimum row-spacing and plant density in Goldasht safflower variety. Adv. Environ. Biol. 2009, 3, 233–238. [Google Scholar]
- Zheng, N.; Futang, C.; Xinchun, S.; Yancai, W. Path analysis of correkated characters on flower yield of safflower individuals. In Proceedings of the Third International Safflower Conference, Beijing, China, 14–18 June 1993; Dajue, L., Yuanzhou, H., Eds.; Beijing Botanical Garden, Institute of Botany, Chinese Academy of Sciences: Beijing, China, 1993; pp. 582–588. [Google Scholar]
- Caliskan, S.; Caliskan, M.E. Row and plant spacing effects on the yield and yield components of safflower in mediterrranean-type environment. Turk. J. Field Crops 2018, 23, 85–92. [Google Scholar] [CrossRef]
- Köse, A.; Bilir, Ö. The influence of row spacing and seed rate on yield and yield components of safflower (Carthamus tinctorius L.). Tarla Bitk. Merk. Araşt. Enst. Derg. 2017, 26, 45–52. [Google Scholar] [CrossRef][Green Version]
- Salem, N.; Msaada, K.; Sriti, J.; Mejri, H.; Limam, F.; Marzouk, B.; Dhifi, W. Effect of drought on safflower natural dyes and their biological activities. EXCLI J. 2014, 13, 1–18. [Google Scholar]
- Horner, J.D. Nonlinear effects of water deficits on foliar tannin concentration. Biochem. Syst. Ecol. 1990, 18, 211–213. [Google Scholar] [CrossRef]
- Mohammadi, M.; Karimizadeh, R. Response of safflower to row spacing and intra-row plant distance in semi-warm dryland condition. Agric. For. 2013, 59, 147–155. [Google Scholar]
- Mündel, H.-H.; Morrison, R.J.; Entz, T.; Blackshaw, R.E.; Roth, B.T.; Kiehn, F.; Vandenberg, A. Row spacing and seeding rates to optimize safflower yield on the Canadian prairies. Can. J. Plant Sci. 1994, 74, 319–321. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006. A Framework for International Classification, Correlation and Communication; FAO: Rome, Italy, 2006; ISBN 9251055114. [Google Scholar]
- International Soil Reference and Information Centre; FAO. Lecture Notes on the Major Soils of the World; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; ISBN 9251046379. [Google Scholar]
- Thun, R.; Hoffmann, G. Texturanalyse des Feinbodens. Kombination von Nasssiebung und Pipettmethode nach KÖHN. In Die Untersuchung von Böden, 4th ed.; Bassler, R., Ed.; VDLUFA-Verl.: Darmstadt, Germany, 2012; ISBN 9783941273139. [Google Scholar]
- Thun, R.; Hoffmann, G. Bestimmung von mineralischem Stickstoff (Nitrat und Ammonium) in Bodenprofilen (Nmin-Labormethode). In Die Untersuchung von Böden, 4th ed.; Bassler, R., Ed.; VDLUFA-Verl.: Darmstadt, Germany, 2012; ISBN 9783941273139. [Google Scholar]
- Anderson, R.L. Broadleaf weed control in safflower (Carthamus tinctorius) with sulfonylurea herbicides. Weed Technol. 1987, 1, 242–246. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Mündel, H.-H.; Derksen, D.A. Herbicides for weed control in safflower (Carthamus tinctorius). Can. J. Plant Sci. 1990, 70, 237–245. [Google Scholar] [CrossRef]
- Flemmer, A.C.; Franchini, M.C.; Lindström, L.I. Description of safflower (Carthamus tinctorius) phenological growth stages according to the extended BBCH scale. Ann. Appl. Biol. 2015, 166, 331–339. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Carthamus yellow. In Compendium of Food Additive Specifications, Addendum 6, 52; Food and Nutrition Paper; FAO: Rome, Italy, 1998. [Google Scholar]
- McIlvaine, T.C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar]
- Jadhav, B.A.; Joshi, A.A. Extraction and quantitative estimation of bio active component (yellow and red carthamin) from dried safflower petals. Indian J. Sci. Technol. 2015, 8, 1–5. [Google Scholar] [CrossRef][Green Version]
- Clementi, C.; Basconi, G.; Pellegrino, R.; Romani, A. Carthamus tinctorius L.: A photophysical study of the main coloured species for artwork diagnostic purposes. Dyes Pigment. 2014, 103, 127–137. [Google Scholar] [CrossRef]
- Piepho, H.P.; Buchse, A.; Emrich, K. A Hitchhiker’s Guide to Mixed Models for Randomized Experiments. J. Agron. Crop Sci. 2003, 189, 310–322. [Google Scholar] [CrossRef]
- Wolfinger, R. Covariance structure selection in general mixed models. Commun. Stat. Simul. Comput. 1993, 22, 1079–1106. [Google Scholar] [CrossRef]
- Piepho, H.-P. A SAS macro for generating letter displays of pairwise mean comparisons. Commun. Biometry Crop Sci. 2012, 7, 4–13. [Google Scholar]
- Hamza, M. Influence of different plant densities on crop yield of six safflower genotypes under Egyptian newly reclaimed soils conditions. Int. J. Agric. Crop Sci. (IJACS) 2015, 8, 168–173. [Google Scholar]
- Ehsanzadeh, P.; Baghdad-Abadi, A.Z. Yield, yield components and growth characteristics of two safflower genotypes under varying plant densities. J. Sci. Technol. Agric. Nat. Resour. 2003, 7, 129–140. [Google Scholar]
- Zarei, G.; Shamsi, H.; Fazeli, F. Effect of planting density on yield and yield components of safflower cultivars in spring planting. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2011, 5, 929–931. [Google Scholar] [CrossRef]
- Usha Kiran, B.; Mukta, N.; Kadirvel, P.; Alivelu, K.; Senthilvel, S.; Kishore, P.; Varaprasad, K.S. Genetic diversity of safflower (Carthamus tinctorius L.) germplasm as revealed by SSR markers. Plant Genet. Resour. 2017, 15, 1–11. [Google Scholar] [CrossRef]
- Rampure, N.H.; Majumdar, P.N.; Badere, R.S. Genetic variability for morphological and biochemical characters in safflower (Carthamus tinctorius L.). Indian J. Genet. Plant Breed. 2014, 74, 353–361. [Google Scholar] [CrossRef]
- Oad, F.C.; Samo, M.A.; Qayyum, S.M.; Oad, N.L. Inter and intra row spacing effect in the growth, seed yield and oil content of safflower Carthamus tinctorius L. Asian J. Plant Sci. 2002, 1, 18–19. [Google Scholar]
- Houmanat, K.; Mazouz, H.; Fechtali, M.E.; Nabloussi, A. Evaluation and pooling of safflower (Carthamus tinctorius L.) accessions from different world origins using agro-morphologicla traits. IJAR 2017, 5, 926–934. [Google Scholar] [CrossRef]
- Eryigit, T.; Yilldirim, B.; Kumlay, A.M.; Sancaktaroglu, S. The effects of different row distances and nitrogen fertilizer rates on yield and yield components of safflower (Carthamus tinctorious) under micro-climate conditions of Iğdır Plain –Turkey. In Proceedings of the 3rd International Conference on Biological, Chemical and Environmental Sciences, Kuala Lumpur, Malaysia, 21–22 September 2015; Rahman, A., Ahmadi, R., Eds.; International Institute of Chemical, Biological & Environmental Engineering: Kuala Lumpur, Malaysia, 2015; pp. 17–22, ISBN 9789384422387. [Google Scholar]
- Abel, G.H. Growth and yield of safflower in three temperature regimes. Agron. J. 1975, 67, 639–642. [Google Scholar] [CrossRef]
- Mündel, H.H.; Blackshaw, R.E.; Byers, J.R.; Huang, H.C.; Johnson, D.L.; Keon, R.; Kubik, J.; McKenzie, R.; Otto, B.; Roth, B.; et al. Safflower Production on the Canadian Prairies: Revisited in 2004; Graphcom Printers Ltd.: Lethbridge, AB, Canada, 2004; ISBN 0662382099. [Google Scholar]
- Oelke, E.A.; Oplinger, E.S.; Teynor, T.M.; Putman, D.H.; Doll, J.D.; Kelling, K.A.; Durgan, B.R.; Noetzel, D.M. Safflower; University of Winsconsin-Extension, Cooperative Extendion; University of Minnisota: Center for Alternative Plant & Animal Products; Minnesota Extension Service. Available online: https://hort.purdue.edu/newcrop/afcm/safflower.html (accessed on 29 March 2020).
- Marchione, V. Effect of plant population and row spacing on the yield and some biometric characters of safflower. In Safflower: A Multipurpose Species with Unexploited Potential and World Adaptability. Proceedings of the Fourth International Safflower Conference, Bari, Italy, 2–7 June, 1997; Corleto, A., Mündel, H.-H., Eds.; Adruatica Editrice: Bari, Italy, 1997; pp. 108–111. [Google Scholar]
- Abbadi, J.; Gerendás, J.; Sattelmacher, B. Effects of nitrogen supply on growth, yield and yield components of safflower and sunflower. Plant Soil 2008, 306, 167–180. [Google Scholar] [CrossRef]
- Elfadl, E.; Reinbrecht, C.; Frick, C.; Claupein, W. Optimization of nitrogen rate and seed density for safflower (Carthamus tinctorius L.) production under low-input farming conditions in temperate climate. Field Crops Res. 2009, 114, 2–13. [Google Scholar] [CrossRef]
- Sampaio, M.C.; Santos, R.F.; Bassegio, D.; de Vasconselos, E.S.; da Silveira, L.; Barchinski Galant Lenz, N.; Lewandoski, C.F.; Tokuro, L.K. Effect of plant density on oil yield of safflower. Afr. J. Agric. Res. 2017, 12, 2147–2152. [Google Scholar] [CrossRef][Green Version]
- Bardhi, N.; Susaj, E.; Dodona, E.; Kallco, I.; Mero, G.; Susaj, L. Producticity indicators of five safflower cultivars (Carthamus tinctorius L.) grown under Lushnja, Albania, climatic conditions. Online Int. Interdiscip. Res. J. 2013, 3, 1–10. [Google Scholar]
- Pascual-Villalobos, M.J.; Alburquerque, N. Genetic variation of a safflower germplasm collection grown as a winter crop in southern Spain. Euphytica 1996, 92, 327–332. [Google Scholar] [CrossRef]
- Arslan, B. Assessing of heritability and variance components of yield and some agronomic traits in different safflower (Carthamus tinctorius L.) cultivars. Asian J. Plant Sci. 2007, 6, 554–557. [Google Scholar]
- Bina, E.; Mirhadi, M.J.; Delkhosh, B.; Omidi, A.H. Study of Agronomically Important and Physiological Traitson Quantitative and Qualitative Yield of 15 Genotypes of Domestic and Forign Safflower. Biosci. Biotechnol. Res. Asia 2014, 11, 461–467. [Google Scholar] [CrossRef]
- Nagaraj, G. Oilseeds. Properties, Processing, Products and Procedures; New India Publ. Agency: Pitam Pura, India, 2009; ISBN 9788190723756. [Google Scholar]
- Knowles, P.F. The plant geneticist’ contribution toward changing lipid and amino acid composition of safflower. J. Am. Oil Chem. Soc. 1972, 49, 27–29. [Google Scholar] [CrossRef]
- Armah-Agyeman, G.; Loiland, J.; Karow, R.; Hang, A.N. Safflower. In Dryland Cropping Systems; Oregon State University: Corvallis, OR, USA, 2002; pp. 1–7. [Google Scholar]
- Koutroubas, S.D.; Papakosta, D.K.; Doitsinis, A. Phenotypic variation in physiological determinants of yield in spring sown safflower under Mediterranean conditions. Field Crops Res. 2009, 112, 199–204. [Google Scholar] [CrossRef]
- Singh, V.; Kolekar, N.M.; Nimbkar, N. Breeding strategy for improvement of flowers and seed yields in safflower. In Safflower: Unexploited Potential and World Adaptability. Proceedings of the Seventh International Safflower Conference, Wagga Wagga, Australia, 3–6 November, 2008; Knights, S.E., Potter, T.D., Eds.; Agri-MC Marketing and Communication: Bendigo, Australia, 2008; ISBN 978-0-646-50329-5. [Google Scholar]
- Tabrizi, A.H.O. Floret removal effects on grain and oil yield and their components in spring safflower. In Sesame and Safflower Newsletter; Instituto de Agricultura Sostenible: Cordoba, Spain, 2002; pp. 71–75. [Google Scholar]
- Ghorbani, E.; Keleshteri, R.H.; Shahbazi, M.; Moradi, F.; Sadri, M. Optimization of extraction yield of carthamine and safflower yellow pigments from safflower (Carthamus tinctorious L.) under different treatments and solvent systems. Res. J. Pharm. (RJP) 2015, 2, 17–23. [Google Scholar]
- Cho, M.H.; Paik, Y.S.; Hahn, T.R. Enzymatic conversion of precarthamin to carthamin by a purified enzyme from the yellow petals of safflower. J. Agric. Food Chem. 2000, 48, 3917–3921. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. A new enzymatic method for the extraction of precarthamine from dyer’s saffron florets. Z. Lebensmittel-Unters. Forsch. 1993, 34–36. [Google Scholar] [CrossRef]
| Abbreviation Treatment | Origin and Cultivar | Row Spacing (cm) | Sowing Density (plants m−2) |
|---|---|---|---|
| C1 S1 D1 | Germany (C1) | 12 (S1) | 40 (D1) |
| C1 S1 D2 | Germany (C1) | 12 (S1) | 75 (D2) |
| C1 S2 D1 | Germany (C1) | 33 (S2) | 40 (D1) |
| C1 S2 D2 | Germany (C1) | 33 (S2) | 75 (D2) |
| C2 S1 D1 | China (C2) | 12 (S1) | 40 (D1) |
| C2 S1 D2 | China (C2) | 12 (S1) | 75 (D2) |
| C2 S2 D1 | China (C2) | 33 (S2) | 40 (D1) |
| C2 S2 D2 | China (C2) | 33 (S2) | 75 (D2) |
| Year | Sowing Date | Number of Harvest | Date of Harvest (DAS) |
|---|---|---|---|
| 2017 | 25.04.2017 | 1 | 18.07 (84) |
| 2 | 25.07 (91) | ||
| 3 | 01.08 (98) | ||
| 4 | 08.08 (105) | ||
| 5 | 15.08 (112) | ||
| 2018 | 19.04.2018 | 1 | 10.07 (82) |
| 2 | 17.07 (89) | ||
| 3 | 24.07 (96) | ||
| 4 | 31.07 (103) | ||
| 5 | 07.08 (110) |
| Year | Harvest Date | |
|---|---|---|
| 1 | 3 | |
| 2017 | 10.38 bA ± 0.53 | 9.54 aA ± 0.53 |
| 2018 | 14.15 aA ± 0.60 | 11.02 aB ± 0.60 |
| Trait | ANOVA Table of the Significant Terms and Interactions | ||
|---|---|---|---|
| Model Term | Degree of Freedom | p-Value 1 | |
| Branches | Sowing density | 1 | <0.0001 |
| Year * Harvest date | 1 | 0.0477 | |
| Capitula | Sowing density | 1 | <0.0001 |
| Year * Cultivar | 1 | <0.0001 | |
| Year | Cultivar | |
|---|---|---|
| C1 | C2 | |
| 2017 | 8.97 aB ± 0.27 | 12.46 bA ± 0.37 |
| 2018 | 8.61 aB ± 0.60 | 16.32 aA ± 0.60 |
| ANOVA Table of the Significant Terms and Interactions | ||
|---|---|---|
| Model Term | Degree of Freedom | p-Value 1 |
| Harvest date * Cultivar * Row spacing | 4 | 0.0027 |
| Year * Harvest date * Cultivar | 3 | <0.0001 |
| Year * Harvest date * Sowing density | 4 | 0.0258 |
| Cultivar | Harvest 1 | Harvest 2 | Harvest 3 | Harvest 4 | Harvest 5 | |
|---|---|---|---|---|---|---|
| 84 DAS | 91 DAS | 98 DAS | 105 DAS | 112 DAS | ||
| 2017 | C1 | 5.72 ± 0.32 | 3.43 ± 0.32 | 4.08 ± 0.32 | 2.68 ± 0.31 | 1 |
| C2 | 6.58 ± 0.32 | 7.29 ± 0.32 | 6.51 ± 0.32 | 5.97 ± 0.31 | 5.91 ± 0.34 | |
| 82 DAS | 89 DAS | 96 DAS | 103 DAS | 110 DAS | ||
| 2018 | C1 | 3.40 ± 0.14 | 3.54 ± 0.14 | 3.41 ± 0.14 | 3.19 ± 0.18 | 1 |
| C2 | 2 | 8.12 ± 0.14 | 7.55 ± 0.14 | 7.14 ± 0.14 | 6.98 ± 0.14 | |
| ANOVA table of the significant terms and interactions. | ||||||
| Model term | Degree of freedom | p-value3 | ||||
| Harvest date * Cultivar | 4 | 0.0006 | ||||
| Year * Cultivar | 1 | <0.0001 | ||||
| Year * Harvest date | 4 | 0.0007 | ||||
| ANOVA Table of the Significant Terms and Interactions | ||
|---|---|---|
| Model Term | Degree of Freedom | p-Value 1 |
| Sowing density | 1 | 0.0026 |
| Harvest date * Cultivar * Row spacing | 4 | 0.0242 |
| Year * Row spacing | 1 | 0.0299 |
| Year * Harvest date * Cultivar | 3 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steberl, K.; Hartung, J.; Munz, S.; Graeff-Hönninger, S. Effect of Row Spacing, Sowing Density, and Harvest Time on Floret Yield and Yield Components of Two Safflower Cultivars Grown in Southwestern Germany. Agronomy 2020, 10, 664. https://doi.org/10.3390/agronomy10050664
Steberl K, Hartung J, Munz S, Graeff-Hönninger S. Effect of Row Spacing, Sowing Density, and Harvest Time on Floret Yield and Yield Components of Two Safflower Cultivars Grown in Southwestern Germany. Agronomy. 2020; 10(5):664. https://doi.org/10.3390/agronomy10050664
Chicago/Turabian StyleSteberl, Kathrin, Jens Hartung, Sebastian Munz, and Simone Graeff-Hönninger. 2020. "Effect of Row Spacing, Sowing Density, and Harvest Time on Floret Yield and Yield Components of Two Safflower Cultivars Grown in Southwestern Germany" Agronomy 10, no. 5: 664. https://doi.org/10.3390/agronomy10050664
APA StyleSteberl, K., Hartung, J., Munz, S., & Graeff-Hönninger, S. (2020). Effect of Row Spacing, Sowing Density, and Harvest Time on Floret Yield and Yield Components of Two Safflower Cultivars Grown in Southwestern Germany. Agronomy, 10(5), 664. https://doi.org/10.3390/agronomy10050664





