Advances in Understanding the Molecular Mechanisms and Potential Genetic Improvement for Nitrogen Use Efficiency in Barley
Abstract
1. Introduction
2. Effect of N Fertilizers on Crop Growth and Yield
3. Nitrogen Uptake, Assimilation and Use Efficiency in Crops
4. NUE Screening and Phenotyping
5. QTL Mapping and the Major Loci Controlling NUE
6. Functional Genes for NUE
7. Candidate Genes for NUE in Barley
8. CRISPR/Cas9 Genome Editing for Barley NUE Improvement
9. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cai, J.; Xia, X.; Chen, H.; Wang, T.; Zhang, H. Decomposition of fertilizer use intensity and its environmental risk in China’s grain production process. Sustainability 2018, 10, 498. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, X.; Li, J. A 1961-2010 record of fertilizer use, pesticide application and cereal yields: A review. Agron. Sustain. Dev. 2015, 35, 83–93. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2017, 10, 51. [Google Scholar] [CrossRef]
- IFA. Annual Conference, Berlin, “Fertilizer Outlook 2018–2022” PIT and Agriculture Services, IFA. Available online: https://www.fertilizer.org/Public/About_fertilizers/Public/About_Fertilizers/About_Fertilizers.aspx?hkey=c35de5b6-2f79-4db3-93cc-d2cef45ae5d4 (accessed on 5 April 2019).
- Chien, S.H.; Teixeirab, L.A.; Cantarellab, H.; Rehmc, G.W.; Grantd, C.A.; Gearhart, M.M. Agronomic effectiveness of granular nitrogen/phosphorus fertilizers containing elemental sulfur with and without ammonium sulfate: A review. Agron. J. 2016, 108, 1203–1213. [Google Scholar] [CrossRef]
- Anbessa, Y.; Juskiw, P. Review: Strategies to increase nitrogen use efficiency of spring barley. Can. J. Plant Sci. 2012, 92, 617–625. [Google Scholar] [CrossRef]
- Glass, A.D.M. Nitrogen use efficiency of crop plants: Physiological constraints upon nitrogen absorption. CRC Crit. Rev. Plant Sci. 2010, 22, 453–470. [Google Scholar] [CrossRef]
- Chen, Z.C.; Ma, J.F. Improving nitrogen use efficiency in rice through enhancing root nitrate uptake mediated by a nitrate transporter, NRT1.1B. J. Genet. Genom. 2015, 42, 463–465. [Google Scholar] [CrossRef]
- Ding, W.; Xu, X.; He, P.; Ullah, S.; Zhang, J.; Cui, Z.; Zhou, W. Improving yield and nitrogen use efficiency through alternative fertilization options for rice in China: A meta-analysis. Field Crops Res. 2018, 227, 11–18. [Google Scholar] [CrossRef]
- Presterl, T.; Seitz, G.; Landbeck, M.; Thiemt, E.M.; Schmidt, W.; Geiger, H.H. Improving nitrogen use efficiency in European maize. Crop Sci. 2003, 43, 1259–1265. [Google Scholar] [CrossRef]
- Wang, R.F.; An, D.G.; Hu, C.S.; Li, L.H.; Zhang, Y.M.; Jia, Y.G.; Tong, Y.P. Relationship between nitrogen uptake and use efficiency of winter wheat grown in North China plain. Crop Pasture Sci. 2011, 62, 504–514. [Google Scholar] [CrossRef]
- Ranjitha, K.M.S.; Biradar, S.; Desai, S.A.; Naik, V.R.; Bhat, S.; Satisha, T.N.; Hiremath, G.; Kumar, K.J.Y.; Chethana, C.K.; Venkatesh, K. Media standardization for hydroponic culture to screen wheat genotypes for nitrogen use efficiency. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2814–2820. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Improving plant nitrogen use efficiency through alteration of amino acid transport processes. Plant Physiol. 2017, 175, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Shrawat, A.K.; Carroll, R.T.; DePauw, M.; Taylor, G.J.; Good, A.G. Genetic engineering of improved nitrogen use efficiency in rice by the tissue-specific expression of alanine aminotransferase. Plant Biotechnol. J. 2008, 6, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, B.; Yuan, D.; Liu, Y.; Che, R.; Hu, Y.; Ou, S.; Liu, Y.; Zhang, Z.; Wang, H.; et al. Expression of the Nitrate Transporter Gene OsNRT1.1A/OsNPF6.3 Confers High Yield and Early Maturation in Rice. Plant Cell 2018, 30, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Nian, J.; Xie, X.; Yu, H.; Zhang, J.; Bai, J.; Dong, G.; Hu, J.; Bai, B.; Chen, L.; et al. Genetic variations in ARE1 mediate grain yield by modulating nitrogen utilization in rice. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Good, A.G.; Johnson, S.J.; Pauw, M.D.; Carroll, R.T.; Savidov, N.; Vidmar, J.; Lu, Z.; Taylor, G.; Stroeher, V. Engineering nitrogen use efficiency with alanine aminotransferase. Can. J. Bot. 2017, 85, 252–262. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, C.; Zhang, Y.; Wang, C. Nitrogen use efficiency in rice. In Nitrogen in Agriculture-Updates; Amanulla, K., Fahad, S., Eds.; IntechOpen: London, UK, 2017; pp. 187–208. [Google Scholar]
- Han, M.; Wong, J.; Su, T.; Beatty, P.H.; Good, A.G. Identification of nitrogen use efficiency genes in barley: Searching for QTLs controlling complex physiological traits. Front. Plant Sci. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Kindu, G.A.; Tang, J.; Yin, X.; Struik, P.C. Quantitative trait locus analysis of nitrogen use efficiency in barley (Hordeum vulgare L.). Euphytica 2014, 199, 207–221. [Google Scholar] [CrossRef]
- Li, P.; Chen, F.; Cai, H.; Liu, J.; Pan, Q.; Liu, Z.; Gu, R.; Mi, G.; Zhang, F.; Yuan, L. A genetic relationship between nitrogen use efficiency and seedling root traits in maize as revealed by QTL analysis. J. Exp. Bot. 2015, 66, 3175–3188. [Google Scholar] [CrossRef]
- Loudet, O.; Chaillou, S.; Merigout, P.; Talbotec, J.; Daniel-Vedele, F. Quantitative trait loci analysis of nitrogen use efficiency in Arabidopsis. Plant Physiol. 2003, 131, 345–359. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Tong, Y.; Zhao, H.; Xie, Q.; Liu, D.; Zhang, A.; Li, B.; Xu, H.; An, D. Mapping QTLs for yield and nitrogen related traits in wheat: Influence of nitrogen and phosphorus fertilization on QTL expression. Theor. Appl. Genet. 2014, 127, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, Y.; Tang, D.; Wang, J.; Zhong, J.; Wang, Y.; Yuan, Q.; Yu, X.; Zhang, Y.; Wang, Y.; et al. Identification of QTL associated with nitrogen uptake and nitrogen use efficiency using high throughput genotyped CSSLs in rice (Oryza sativa L.). Front. Plant Sci. 2003, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.P.; Marshall, P. Growth, yield and grain quality of barley (Hordeum vulgare L.) in response to nitrogen uptake: II. Plant development and rate of germination. J. Exp. Bot. 1998, 49, 1021–1029. [Google Scholar] [CrossRef]
- Basu, C.P. Nitrogen nutrition in rice. Indian J. Plant Sci. 2015, 4, 28–37. Available online: http://www.cibtech.org/jps.htm (accessed on 27 December 2019).
- Narolia, G.P.; Yadav, R.S. Effect of nitrogen levels and its scheduling on growth, yield and grain quality of malt barley (Hordeum vulgare L.) under normal and late sown conditions in North-West Rajasthan. Ann. Arid Zone 2013, 52, 95–99. [Google Scholar]
- Liu, X.; Wang, H.; Zhou, J.; Hu, F.; Zhu, D.; Chen, Z.; Liu, Y. Effect of N fertilization pattern on rice yield, nitrogen use efficiency and fertilizer N fate in the Yangtze river basin, China. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef]
- Beatty, P.H.; Anbessa, Y.; Juskiw, P.; Carroll, R.T.; Wang, J.; Good, A.G. Nitrogen use efficiencies of spring barley grown under varying nitrogen conditions in the field and growth chamber. Ann. Bot. 2010, 105, 1171–1182. [Google Scholar] [CrossRef]
- Ghoneim, A.M.; Gewaily, E.E.; Osman, M.M.A. Effects of nitrogen levels on growth, yield and nitrogen use efficiency of some newly released Egyptian rice genotypes. Open Agric. 2018, 3, 310–318. [Google Scholar] [CrossRef]
- Safina, S.A. Effect of nitrogen levels on grain yield and quality of some barley genotypes grown on sandy soil and salinity irrigation. Egypt J. Agron. 2010, 32, 207–222. Available online: https://www.researchgate.net/publication/279197907 (accessed on 6 January 2020).
- Shah, J.M.; Asgher, Z.; Zeng, J.; Quan, X.; Ali, E.; Shamsi, I.H.; Zhang, G. Growth and physiological characterization of low nitrogen responses in Tibetan wild barley (Hordeum spontaneum) and cultivated barley (Hordeum vulgare). J. Plant Nutr. 2016, 40, 861–868. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, F.; Zhi, Y.; Chen, F.; Xiao, K. The yields, agronomic, and nitrogen use efficiency traits of wheat cultivars in north China under N-sufficient and deficient conditions. J. Plant Nutr. 2017, 40, 1053–1065. [Google Scholar] [CrossRef]
- Yoneyama, T.; Tanno, F.; Tatsumi, J.; Mae, T. Whole plant dynamic system of nitrogen use for vegetative growth and grain filling in rice plants (Oryza sativa L.) as revealed through the production of 350 grains from a germinated seed over 150 days: A review and synthesis. Front. Plant Sci 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Janković, S.; Glamočlija, D.; Maletić, R.; Rakić, S.; Hristov, N.; Ikanović, J. Effects of nitrogen fertilization on yield and grain quality in malting barley. Afr. J. Biotechnol. 2011, 10, 19534–19541. [Google Scholar] [CrossRef]
- Kılıç, H.; Akar, T.; Kendal, E.; Sayim, I. Evaluation of grain yield and quality of barley varieties under rainfed conditions. Afr. J. Biotechnol. 2010, 9, 7617–7628. Available online: http://www.academicjournals.org/AJB (accessed on 5 January 2020).
- Magliano, P.N.; Prystupa, P.; Gutiérrez-Boem, F.H. Protein content of grains of different size fractions in malting barley. J. Inst. Brew. 2014, 120, 347–352. [Google Scholar] [CrossRef]
- Gondwe, B.M.; Mweetwa, A.M.; Munyinda, K.; Phiri, E.; Lungu, D. Evaluation of maize genotypes for nitrogen use efficiency. Zambian J. Agric. Sci. 2014, 10, 55–63. Available online: www.researchgate.net/publication/273004258 (accessed on 5 November 2019).
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Lin, S.; Kuo, H.; Canivenc, G.; Lin, C.; Lepetit, M.; Hsu, P.; Tillard, P.; Lin, H.; Wang, Y.; Tsai, C.; et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 2008, 20, 2514–2528. [Google Scholar] [CrossRef]
- Williams, L.E.; Miller, A.J. Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 659–688. [Google Scholar] [CrossRef]
- Lezhneva, L.; Kiba, T.; Feria-Bourrellier, A.; Lafouge, F.; Boutete-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrogen acquisition and remobilization in nitrogen-starved plants. Plant J. 2004, 80, 230–241. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Noulas, C.; Stamp, P.; Soldati, A.; Liedgens, M. Nitrogen use efficiency of spring wheat genotypes under field and lysimeter conditions. J. Agron. Crop Sci. 2004, 190, 111–118. [Google Scholar] [CrossRef]
- Salon, C.; Munier-Jolain, N.G.; Duc, G.; Voisin, A.; Grandgirard, D.; Larmure, A.; Emery, R.J.N.; Ney, B. Grain legume seed filling in relation to nitrogen acquisition: A review and prospects with particular reference to pea. Agronomie 2001, 21, 539–552. [Google Scholar] [CrossRef]
- Glass, A.D.M.; Britto, D.T.; Kaiser, B.N.; Kinghorn, J.R.; Kronzucker, H.J.; Kumar, A.; Okamoto, M.; Rawat, S.; Siddiqi, M.Y.E.S.; Joseph, U.; et al. The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 2002, 53, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, E.; Araki, T.; Hamaoka, N. Ammonia emission from rice leaves in relation to photorespiration and genotypic differences in glutamine synthetase activity. Ann. Bot. 2011, 8, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, B.; Chu, C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef]
- Have, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence: Lessons from Arabidopsis to crops. J. Exp. Bot. 2016, 68, 2513–2529. [Google Scholar] [CrossRef]
- Diaz, C.; Lemaitre, T.; Christ, A.; Azzopardi, M.; Kato, Y.; Sato, F.; Morot-Gaudry, J.F.; Le-Dily, F.; Masclaux-Daubresse, C. Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition. Plant. Physiol. 2008, 147, 1437–1449. [Google Scholar] [CrossRef]
- Malagoli, P.; Laine, P.; Rossato, L.; Ourry, A. Dynamics of nitrogen uptake and mobilization in field-grown winter oilseed rape (Brassica napus) from stem extension to harvest. II.An 15N-labelling-based simulation model of N partitioning between vegetative and reproductive tissues. Ann. Bot. 2005, 95, 1187–1198. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Anbessa, Y.; Juskiw, P.; Good, A.; Nyachiro, J.; Helm, J. Genetic variability in nitrogen use efficiency of spring barley. Crop. Sci. 2009, 49, 1259–1269. [Google Scholar] [CrossRef]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Alves, B.; Aulakh, M.; Bekunda, M.; ZuCong, C.; Drinkwater, L.; Mugendi, D.; van Kessel, C.; Oenema, O. Crop, environmental and management factors affecting nitrogen use efficiency. In Agriculture and Nitrogen Cycle: Assessing the Impact of Fertilizer Use on Food Production and the Environment; Mosier, A.R., Syers, K.J., Freny, J.R., Eds.; Island Press: Washington, DC, USA, 2004; pp. 19–33. [Google Scholar]
- Ju, C.; Buresh, R.J.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crops Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Swamy, K.N.; Kondamudi, R.; Vijayalakshmi, P.; Jaldhani, V.; Suchandranath, B.M.; Kiran, T.V.; Srikanth, B.; Subhakar, R.I.; Sailaja, N.; Surekha, K.; et al. A comparative study on nitrogen response among Upland, IRHTN, DRR and other released rice groups. Afr. J. Agric. Res. 2015, 10, 4364–4369. [Google Scholar] [CrossRef]
- Moose, S.; Below, F.E. Biotechnology approaches to improving maize nitrogen use efficiency. In Molecular Genetic Approaches to Maize Improvement. Biotechnology in Agriculture and Forestry; Kriz, A.L., Larkins, B.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 65–77. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Methodology for evaluation of lowland rice genotypes for nitrogen use efficiency. J. Plant Nutr. 2003, 26, 1315–1333. [Google Scholar] [CrossRef]
- Mickelson, S.; See, D.; Meyer, F.D.; Garner, J.P.; Foster, C.R.; Blake, T.K.; Fischer, A.M. Mapping of QTL associated with nitrogen storage and remobilization in barley (Hordeum vulgare L.) leaves. J. Exp. Bot. 2003, 54, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Pasam, R.K.; Sharma, R.; Malosetti, M.; Eeuwijk, F.A.V.; Haseneyer, G.; Kilian, B.; Graner, A. Genome-wide association studies for agronomical traits in a worldwide spring barley collection. BMC Plant. Biol. 2012, 12, 1–22. [Google Scholar] [CrossRef]
- Garnett, T.; Conn, V.; Kaiser, B.N. Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ. 2009, 32, 1272–1283. [Google Scholar] [CrossRef]
- An, D.; Su, J.; Liu, Q.; Zhu, Y.; Tong, Y.; Li, J.; Jing, R.; Li, B.; Li, Z. Mapping QTLs for nitrogen uptake in relation to the early growth of wheat (Triticum aestivum L.). Plant. Soil 2006, 284, 73–84. [Google Scholar] [CrossRef]
- Yang, L.; Hu, H.; Zhu, B.; Jin, X.; Wu, F.; Zhang, G. Genotypic variations of nitrogen use efficiency in Tibetan wild and cultivated barleys. J. Zhejiang Univ. 2014, 40, 155–164. [Google Scholar] [CrossRef]
- Yang, X.; Xia, X.; Zhang, Z.; Nong, B.; Zeng, Y.; Xiong, F.; Wu, Y.; Gao, J.; Deng, G.; Li, D. QTL mapping by whole genome resequencing and analysis of candidate genes for nitrogen use efficiency in rice. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Agrama, H.A.S.; Zakaria, A.G.; Said, F.B.; Tuinstra, M. Identification of quantitative trait loci for nitrogen use efficiency in maize. Mol. Breed. 1999, 5, 187–195. [Google Scholar] [CrossRef]
- Gallais, A.; Hirel, B. An approach to the genetics of nitrogen use efficiency in maize. J. Exp. Bot. 2004, 55, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hirel, B.; Bertin, P.; Quillere’, I.; Bourdoncle, W.; Attagnant, C.I.; Dellay, C.; Gouy, A.I.; Cadiou, S.; Retailliau, C.; Flaque, M.; et al. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Ribaut, J.M.; Fracheboud, Y.; Monneveux, P.; Banziger, M. Quantitative trait loci for yield and correlated traits under high and low soil nitrogen conditions in tropical maize. Mol. Breed. 2007, 20, 15–29. [Google Scholar] [CrossRef]
- Pauli, D.; Muehlbauer, G.J.; Smith, K.P.; Cooper, B.; Hole, D.; Obert, D.E.; Ullrich, S.E.; Blake, T.K. Association mapping of agronomic QTLs in U.S. spring barley breeding germplasm. Plant Genome 2014, 7, 1–15. [Google Scholar] [CrossRef]
- Jiang, W.; Yongbo, D.; Chin, J.H.; Mccouch, S. Identification of QTLs associated with physiological nitrogen use efficiency in rice. Mol. Cells 2007, 3, 72–79. Available online: https://www.researchgate.net/publication/6365528 (accessed on 6 January 2020).
- Ye, G.; Huang, J.; Pan, J.; Nie, L. QTL mapping for nitrogen use efficiency and nitrogen deficiency tolerance traits in rice. Plant Soil 2012, 359, 281–295. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, M.; Zheng, H.; Yuan, Y.; Zhou, X.; Guo, Y.; Zhang, G.; Zhao, Y.; Kong, F.; An, Y.; et al. QTL mapping for nitrogen use efficiency and agronomic traits at the seedling and maturity stages in wheat. Mol. Breed. 2019, 39, 1–17. [Google Scholar] [CrossRef]
- Lei, L.; Li, G.; Zhang, H.; Powers, C.; Fang, T.; Chen, Y.; Wang, S.; Zhu, X.; Carver, B.F.; Yan, L. Nitrogen use efficiency is regulated by interacting proteins relevant to development in wheat. Plant. Biotech. J. 2017, 16, 1214–1226. [Google Scholar] [CrossRef]
- Mandolino, C.I.; D’Andrea, K.E.; Olmos, S.E.; Otegui, M.E.; Eyherabide, G.H. Maize nitrogen use efficiency: QTL mapping in a U.S. Dent×Argentine Caribbean Flint RILs population. Maydica 2018, 63, 1–17. Available online: https://www.researchgate.net/publication/324706705 (accessed on 10 January 2020).
- Wang, M.; Jiang, N.; Jia, T.; Leach, L.; Cockram, J.; Comadran, J.; Shaw, P.; Waugh, R.; Luo, Z. Genome-wide association mapping of agronomic and morphologic traits in highly structured populations of barley cultivars. Theor. Appl. Genet. 2012, 124, 233–246. [Google Scholar] [CrossRef]
- Mansour, E.; Casas, A.M.; Gracia, M.P.; Molina-Cano, J.L.; Moralejo, M.; Cattivelli, L.; Thomas, W.T.B.; Igartua, E. Quantitative trait loci for agronomic traits in an elite barley population for Mediterranean conditions. Mol. Breed. 2013, 33, 249–265. [Google Scholar] [CrossRef]
- Comadran, J.; Russell, J.R.; Booth, A.; Pswarayi, A.; Ceccarelli, S.; Grando, S.; Stanca, A.M.; Pecchioni, N.; Akar, T.; Al-Yassin, A.; et al. Mixed model association scans of multi-environmental trial data reveal major loci controlling yield and yield related traits in Hordeum vulgare in Mediterranean environments. Theor. Appl. Genet. 2011, 122, 1363–1373. [Google Scholar] [CrossRef]
- Berger, G.L.; Liu, S.; Hall, M.D.; Brooks, W.S.; Chao, S.; Muehlbauer, G.J.; Baik, B.K.; Steffenson, B.; Griffey, C.A. Marker-trait associations in Virginia Tech winter barley identified using genome-wide mapping. Theor. Appl. Genet. 2013, 126, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jiang, Z.; Wang, W.; Qiu, Y.; Zhang, Z.; Liu, Y.; Li, A.; Gao, X.; Liu, L.; Qian, Y.; et al. Nitrate–NRT1.1B–SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat. Plants 2019, 5, 401–413. [Google Scholar] [CrossRef]
- Li, S.; Li, B.; Shi, W. Expression Patterns of Nine Ammonium Transporters in Rice in Response to N Status. Pedosphere 2012, 22, 860–869. [Google Scholar] [CrossRef]
- Bao, A.; Liang, Z.; Zhao, Z.; Cai, H. Overexpressing of OsAMT1-3, a high affinity ammonium transporter gene, modifies rice growth and carbon-nitrogen metabolic status. Int. J. Mol. Sci. 2015, 16, 9037–9063. [Google Scholar] [CrossRef]
- Shelden, M.; Dong, B.; de Bruxelles, G.L.; Trevaskis, B.; Whelan, J.; Ryan, P.R.; Howitt, S.M.; Udvardi, M.K. Arabidopsis ammonium transporters, AtAMT1;1 and AtAMT1;2, have different biochemical properties and functional roles. Plant. Soil 2001, 231, 151–160. [Google Scholar] [CrossRef]
- Pathak, R.R.; Ahmad, A.; Lochab, S.; Raghuram, N. Molecular physiology of plant nitrogen use efficiency and biotechnological options for its enhancement. Curr. Sci. 2008, 94, 1394–1403. Available online: https://www.researchgate.net/publication/216085652 (accessed on 3 January 2020).
- Pathak, R.R.; Lochab, S.; Raghuram, N. Plant systems: Improving plant nitrogen-use efficiency. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 209–218. [Google Scholar]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Sugiyama, K.; Ishiyama, K.; Inoue, E.; Sato, T.; Takahashi, H.; Yamaya, T. Severe reduction in growth rate and grain filling of rice mutants lacking OsGS1;1, a cytosolic glutamine synthetase1;1. Plant J. 2005, 42, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhao, X.; Liu, Q.; Hong, X.; Zhang, W.; Zhang, Y.; Sun, L.; Li, H.; Tong, Y. Transgenic expression of plastidic glutamine synthetase increases nitrogen uptake and yield in wheat. Plant Biotechnol. J. 2018, 16, 1858–1867. [Google Scholar] [CrossRef]
- Yamaya, T.; Obara, M.; Nakajima, M.; Sasaki, S.; Hayakawa, T.A.; Sato, T. Genetic manipulation and quantitative-trait loci mapping for nitrogen recycling in rice. J. Exp. Bot. 2002, 53, 917–925. [Google Scholar] [CrossRef]
- Tamura, W.; Kojima, S.; Toyokawa, A.; Watanabe, H.; Tabuchi-Kobayashi, M.; Hayakawa, T.; Yamaya, T. Disruption of a novel NADH-glutamate synthase2 gene caused marked reduction in spikelet number of rice. Front. Plant Sci. 2011, 2, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Nian, J.; Xie, Q.; Feng, J.; Zhang, F.; Dong, G.; Liang, Y.; Peng, J.; Wang, G.; Qian, Q.; et al. Rice ferredoxin-dependent glutamate synthase regulates nitrogen–carbon metabolomes and is genetically differentiated between japonica and indica subspecies. Mol. Plant 2016, 9, 1520–1534. [Google Scholar] [CrossRef]
- Selvaraj, M.G.; Valencia, M.O.; Ogawa, S.; Lu, Y.; Wu, L.; Downs, C.; Skinner, W.; Lu, Z.; Kridl, J.C.; Ishitani, M.; et al. Development and field performance of nitrogen use efficient rice lines for Africa. Plant. Biotechnol. J. 2017, 15, 775–787. [Google Scholar] [CrossRef]
- Górny, A.G.; Banaszak, Z.; Ługowska, B.; Ratajczak, D. Inheritance of the efficiency of nitrogen uptake and utilization in winter wheat (Triticum aestivum L.) under diverse nutrition levels. Euphytica 2010, 177, 191–206. [Google Scholar] [CrossRef]
- He, X.; Qu, B.; Li, W. The Nitrate-Inducible NAC Transcription Factor TaNAC2-5A Controls Nitrate Response and Increases Wheat Yield. Plant Physiol. 2015, 169, 1991–2005. [Google Scholar] [CrossRef]
- Fan, X.; Feng, H.; Tan, Y.; Xu, Y.; Miao, Q.; Xu, G. A putative 6-transmembrane nitrate transporter OsNRT1.1b plays a key role in rice under low nitrogen. J. Integr. Plant Biol. 2016, 58, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.Y.; Glass, A.D.M.; Ruth, T.J.A.; Rufty, J.T.W. Studies of the uptake of nitrate in barley. Plant Physiol. 1990, 93, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, M.; Kumar, S.; Tyagi, P.; Wani, S.H.; Gajula, M.N.V.P.; Singh, K.P. Functional and structural insights in to candidate genes associated with nitrogen and phosphorus nutrition in wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2018, 118, 76–91. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, H.; Zhou, C.; Guo, X.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Liu, L. A combined association mapping and t-test analysis of SNP loci and candidate genes involving in resistance to low nitrogen traits by a wheat mutant population. PLoS ONE 2019, 14, 1–15. [Google Scholar] [CrossRef]
- He, J.; Zhao, X.; Laroche, A.; Lu, Z.X.; Liu, H.; Li, Z. Genotyping-by-sequencing (GBS), an ultimate marker-assisted selection (MAS) tool to accelerate plant breeding. Front. Plant Sci. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Ashkani, S.; Rafii, M.Y.; Shabanimofrad, M.; Miah, G.; Sahebi, M.; Azizi, P.; Tanweer, F.A.; Akhtar, M.S.; Nasehi, A. Molecular breeding strategy and challenges towards improvement of blast disease resistance in rice crop. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bortesi, L.; Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015, 33, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, C.; Si, H.; Yang, J. CRISPR/Cas9-mediated genome editing in plants. Methods 2017, 121–122, 94–102. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, Q.; Chen, Y.; Liu, Y. CRISPR/Cas9 platforms for genome editing in plants: Developments and applications. Mol. Plant. 2016, 9, 961–974. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Arora, L.; Narula, A. Gene editing and crop improvement using CRISPR-Cas9 system. Front. Plant Sci. 2017, 8, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Guo, D.; Gao, W.; Yang, W.; Hou, L.; Ma, X.; Miao, Y.; Botella, J.R.; Song, C. Optimization of CRISPR/Cas9 genome editing in cotton by improved sgRNA expression. Plant Methods 2018, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Lawrenson, T.; Shorinola, O.; Stacey, N.; Li, C.; Ostergaard, L.; Patron, N.; Uauy, C.; Harwood, W. Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol. 2015, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; Cigan, A.M. Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, B.; Weeks, D.P.; Spalding, M.H.; Yang, B. Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res. 2014, 42, 10903–10914. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, K.; Chen, K.; Gao, C. Targeted Mutagenesis in Zea mays Using TALENs and the CRISPR/Cas System. J. Genet. Genom. 2014, 41, 63–68. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, K.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the erf transcription factor gene OsERF922. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant. Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.A.A. Exploring Rice Genetic Resources to Improve Nutrient Use Efficiency. Ph.D. Thesis, University of York, York, UK, 2018. [Google Scholar]
- Hirel, B.; Le Gouis, J.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
| Abbreviation | Term | Definition |
|---|---|---|
| NUE | N Use Efficiency | NUpE × NUtE = Yield/N supplied |
| NUpE | N Uptake Efficiency | NUp/N(soil + fertilizer) = Acquired N/N available |
| NUtE | N Utilization Efficiency | Yield/NUp |
| NUEg | N Use Efficiency Grain | Grain production/Available N |
| UI | Utilization Index | Total plant biomass/Total plant N |
| Chr | QTL | Trait | Genes Co-Localized | Population | Parent with Positive Allele | Reference |
|---|---|---|---|---|---|---|
| 1H | qYld | Yield | HvIPT1 | Morex × Barke | Barke | [19] |
| qYld | Yield | HvIPT1 | Orria × Plaisant | Orria | [77] | |
| qGPC | Grain protein content | HvCKX5 | Morex × Barke | Barke | [19,61] | |
| qGW | Grain weight | [76] | ||||
| 2H | qYld | Yield | HvCKX7, HvGDH3 | Morex × Barke | Barke | [19] |
| qYld | Yield | HvPKABA7 | [70] | |||
| qYld | Yield | HvCKX7 | Multiple varieties | n/a | [78] | |
| qGPC | Grain protein content | HvAMT1.2, HvGS3, HvGOX1, HvIPT2, HvGOX2, HvGOGAT2 | Morex × Barke | Barke | [19] | |
| qGPC | Grain protein content | HvCIN2, HvAMT1.2 HvNAM-2, HvGOX1 | Lewis × Karl | Lewis | [60] | |
| qGPC | Grain protein content | HvIPT2, HvGOX2, HvGOGAT2, HvPKABA5, HvAlaAT2-2, HvCIN2 | Barley CAP spring lines | n/a | [70] | |
| qNUEg | NUE of grains | - | Apex × Prisma | Prisma | [20] | |
| qNutEg | NUtE of grains | - | Apex × Prisma | Prisma | [20] | |
| qNHI | N harvest index of grains | - | Apex × Prisma | Prisma | [20] | |
| 3H | qYld | Yield | HvCKX3 | Morex × Barke | Barke | [19] |
| qYld | Yield | HvASP4, HvCKX3 | [70] | |||
| qNUEb | NUE of above- ground biomass | - | Apex × Prisma | Prisma | [20] | |
| qNupEb | NUpE of grains | - | Apex × Prisma | Prisma | [20] | |
| 4H | qGPC | Grain protein content | HvCIN1, HvGS4 | Morex × Barke | Barke | [19] |
| qGPC | Grain protein content | HvCIN1 | Barley CAP spring lines | n/a | [70] | |
| qGPC | Grain protein content | HvGS4 | Multiple varieties | n/a | [61] | |
| qGW | Grain weight | HvGS4 | Morex × Barke | Barke | [19] | |
| qGW | Grain weight | HvGS4 | 615 UK barley genotypes | n/a | [76] | |
| 5H | qYld | Yield | Lewis × Karl | Lewis | [60] | |
| qGPC | Grain protein content | HvPKABA6, HvFNR2 | Morex × Barke | Barke | [19,70] | |
| Multiple varieties | n/a | [61,70] | ||||
| qNUEb | NUE of above- ground biomass | - | Apex × Prisma | Prisma | [20] | |
| qNUEg | NUE of grains | - | Apex × Prisma | Prisma | [20] | |
| 6H | qYld | Yield | Morex × Barke | Barke | [19] | |
| qYld | Yield | HvNR3, HvASP5 | Multiple varieties | n/a | [79] | |
| Lewis × Karl | Lewis | [60] | ||||
| qGPC | Grain protein content | HvNR1, HvGS1 | Morex × Barke | Barke | [19] | |
| qGPC | Grain protein content | HvNAM1 | Barley CAP spring lines | n/a | [70] | |
| qGPC | Grain protein content | HvNAM1, HvNAR2.1, HvAMT1.1 | Lewis × Karl | Lewis | [60] | |
| qGHI | Harvest index | Apex × Prisma | Prisma | [20] | ||
| 7H | qYld | Yield | HvNRT2.7, HvLHT2, HvLHT3 | Morex × Barke | Barke | [19] |
| qYld | Yield | HvLHT2, HvLHT3 | Multiple varieties | n/a | [78] | |
| qYld | Yield | HvNRT2.7 | Multiple varieties | n/a | [79] | |
| qGN | Grain N | Morex × Barke | Barke | [19] | ||
| qNHI | N harvest index of grains | - | Apex × Prisma | Prisma | [20] |
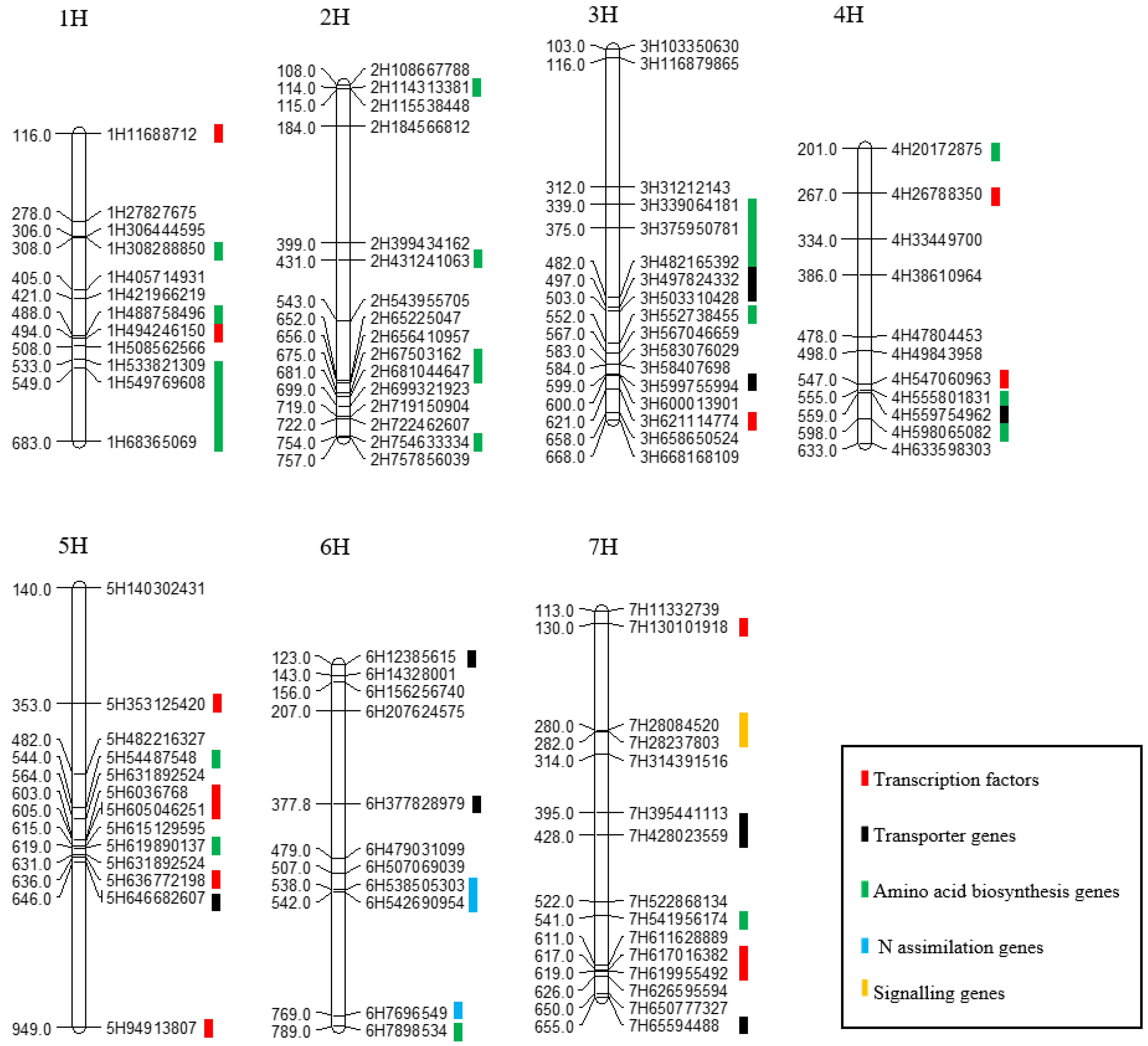
| Gene | Origin | Homolog in Barley | Chr | Start | End | Annotation |
|---|---|---|---|---|---|---|
| AtNRT1.1 | Arabidopsis | HORVU7Hr1G071600 | 7H | 395441113 | 395447440 | Protein NRT1/ PTR FAMILY |
| AtAMT1;1, AtAMT1;3 | Arabidopsis | HORVU6Hr1G057870 | 6H | 377828979 | 377831011 | Ammonium Transporter 1 |
| AtAMT2 | Arabidopsis | HORVU3Hr1G082610 | 3H | 599755994 | 599757436 | Ammonium Transporter 2 |
| AtSTP13 | Arabidopsis | HORVU4Hr1G067450 | 4H | 559754962 | 559760152 | Sugar Transporter Protein 7 |
| AtNF-YB1-2 | Arabidopsis | HORVU1Hr1G071620 | 1H | 494246150 | 494250406 | Nuclear Transcription Factor Y Subunit B |
| AtAMT1;3 | Arabidopsis | HORVU3Hr1G065320 | 3H | 497824332 | 497833404 | ABC Transporter B Family Member 4 |
| OsDEP1 | Rice | HORVU3Hr1G051800 | 3H | 375950781 | 375954891 | Grain Length Protein |
| OsRGA1 | Rice | HORVU7Hr1G008720 | 7H | 11332739 | 11337421 | Guanine Nucleotide-Binding Protein Alpha-1 Subunit |
| OsSAPK1 | Rice | HORVU2Hr1G110230 | 2H | 719150904 | 719161174 | Protein Kinase Superfamily Protein |
| OsSAPK2 | Rice | HORVU2Hr1G029900 | 2H | 108667788 | 108672779 | Protein Kinase Superfamily Protein |
| OsSAPK3 | Rice | HORVU5Hr1G097630 | 5H | 605102179 | 605108556 | Protein Kinase Superfamily Protein |
| OsSAPK4 | Rice | HORVU3Hr1G082690 | 3H | 600013901 | 600018673 | Protein Kinase Superfamily Protein |
| OsSAPK5, OsPAK7 | Rice | HORVU2Hr1G075470 | 2H | 543955705 | 543960490 | Protein Kinase Superfamily Protein |
| OsSAPK6 | Rice | HORVU1Hr1G055340 | 1H | 405714931 | 405718538 | Protein Kinase Superfamily Protein |
| OsSAPK8 | Rice | HORVU4Hr1G013540 | 4H | 47804453 | 47807197 | Protein Kinase Superfamily Protein |
| OsEND93-1 *, OsEND93-3 | Rice | HORVU7Hr1G020850 | 7H | 28237803 | 28241820 | Early Nodulin-Related |
| OsEND93-2 | Rice | HORVU7Hr1G020760 | 7H | 28084520 | 28085738 | Early Nodulin-Related |
| OsAlaAT10-1, OsAlaAT4 | Rice | HORVU1Hr1G018540 | 1H | 68365069 | 68370382 | Alanine Aminotransferase 2 |
| OsAlaAT10-2 | Rice | HORVU5Hr1G014730 | 5H | 54487548 | 54492982 | Alanine Aminotransferase 2 |
| OsAlaAT3-1 | Rice | HORVU2Hr1G063740 | 2H | 431241063 | 431250440 | Alanine Aminotransferase 2 |
| OsAlaAT3-2 | Rice | HORVU2Hr1G030820 | 2H | 114313381 | 114319007 | Alanine Aminotransferase 2 |
| OsGGT1, OsGGT3 | Rice | HORVU1Hr1G070220 | 1H | 488758496 | 488762295 | Alanine:Glyoxylate Aminotransferase 3 |
| OsGGT2 | Rice | HORVU4Hr1G075360 | 4H | 598065082 | 598068656 | Alanine:Glyoxylate Aminotransferase 2 |
| OsASNase1 | Rice | HORVU2Hr1G097890 | 2H | 681044647 | 681050401 | N(4)-(Beta-N-acetylglucosaminyl)-L-Asparaginase |
| OsASNase2 | Rice | HORVU2Hr1G123070 | 2H | 754633334 | 754644513 | Isoaspartyl Peptidase/L-Asparaginase |
| OsASP2 | Rice | HORVU7Hr1G089290 | 7H | 541956174 | 541961050 | Aspartate Aminotransferase 1 |
| OsASP3 | Rice | HORVU6Hr1G003470 | 6H | 7898534 | 7902987 | Aspartate Aminotransferase 1 |
| OsASP4 | Rice | HORVU3Hr1G073220 | 3H | 552738455 | 552750250 | Aspartate Aminotransferase 3 |
| OsASP5 | Rice | HORVU1Hr1G074590 | 1H | 508562566 | 508569749 | Aspartate Aminotransferase |
| OsASP6 | Rice | HORVU1Hr1G042490 | 1H | 308288850 | 308292215 | Aspartate Aminotransferase |
| OsAS | Rice | HORVU5Hr1G020510 | 5H | 94913807 | 94917732 | Transcription Initiation Factor TFIID Subunit 8 |
| OsGDH2-3 | Rice | HORVU2Hr1G093020 | 2H | 656410957 | 656417166 | Undescribed Protein |
| OsGDH4 | Rice | HORVU3Hr1G048870 | 3H | 339064181 | 339071356 | Glutamate Dehydrogenase |
| OsGS3 | Rice | HORVU4Hr1G007610 | 4H | 20172875 | 20175861 | Glutamine Synthetase 1.3 |
| OsGS4 | Rice | HORVU2Hr1G111300 | 2H | 722462607 | 722470196 | Bifunctional Lysine-Specific Demethylase and histidyl-hydroxylase NO66 |
| OsGOGAT1, OsGOGAT3 | Rice | HORVU3Hr1G063050 | 3H | 482165392 | 482176766 | Glutamate Synthase 2 |
| OsGOGAT2 | Rice | HORVU2Hr1G022920 | 2H | 67503162 | 67520099 | Glutamate Synthase 1 |
| OsGOX2-3 | Rice | HORVU2Hr1G103180 | 2H | 699321923 | 699325619 | L-Lactate Dehydrogenase |
| OsGOX4 | Rice | HORVU2Hr1G060010 | 2H | 399434162 | 399565758 | L-Lactate Dehydrogenase |
| OsGOX5 | Rice | HORVU2Hr1G030930 | 2H | 115538448 | 115548113 | L-Lactate Dehydrogenase |
| OsNR1, OsNR3-4 | Rice | HORVU6Hr1G003300 | 6H | 7696549 | 7701423 | Nitrate Reductase 1 |
| OsNR2 | Rice | HORVU6Hr1G079700 | 6H | 538505303 | 538508978 | Nitrate Reductase 1 |
| OsNiR1-3 | Rice | HORVU6Hr1G080750 | 6H | 542690954 | 542694406 | Sulfite Reductase |
| OsDOF1 | Rice | HORVU7Hr1G043250 | 7H | 130101918 | 130103443 | DOF Zinc Finger Protein 1 |
| OsDOF2 | Rice | HORVU4Hr1G013890 | 4H | 49843958 | 49845261 | DOF Zinc Finger Protein 1 |
| OsDOF3 | Rice | HORVU5Hr1G097620 | 5H | 605046251 | 605048334 | DOF Zinc Finger Protein 1 |
| OsDOF4 | Rice | HORVU6Hr1G069190 | 6H | 479031099 | 479167490 | Monodehydroascorbate Reductase 4 |
| OsDOF5 | Rice | HORVU1Hr1G005390 | 1H | 11688712 | 11691059 | DOF Zinc Finger Protein 1 |
| OsNF-YB2.1-2.2 | Rice | HORVU3Hr1G087390 | 3H | 621114774 | 621118012 | Nuclear Transcription Factor Y Subunit B |
| OsNF-YB2.3 | Rice | HORVU7Hr1G105460 | 7H | 617016382 | 617017035 | Nuclear Transcription Factor Y Subunit B-2 |
| OsHLHm1 | Rice | HORVU4Hr1G065640 | 4H | 547060963 | 547062633 | Basic Helix-Loop-Helix (bHLH) DNA-Binding Superfamily Protein |
| OsHLHm2 | Rice | HORVU4Hr1G009440 | 4H | 26788350 | 26791410 | Basic Helix-Loop-Helix (bHLH) DNA-Binding Superfamily Protein |
| OsHLHm3 | Rice | HORVU3Hr1G079340 | 3H | 583076029 | 583165960 | Leucine-Rich Repeat Protein Kinase Family Protein |
| OsHLHm4 | Rice | HORVU5Hr1G002090 | 5H | 6036768 | 6041581 | Basic Helix-Loop-Helix (bHLH) DNA-Binding Superfamily Protein |
| OsNAC006 | Rice | HORVU4Hr1G012030 | 4H | 38610964 | 38613054 | NAC Domain Protein |
| OsNAC6 | Rice | HORVU7Hr1G106480 | 7H | 619955492 | 619960319 | NAC Domain Containing Protein 1 |
| OsNAC9/OsSNAC1 | Rice | HORVU5Hr1G111590 | 5H | 636772198 | 636774461 | NAC Domain Protein |
| OsNAC10 | Rice | HORVU5Hr1G045650 | 5H | 353125420 | 353127305 | NAC Domain Protein |
| OsAPO1/OsFBX202 | Rice | HORVU7Hr1G108970 | 7H | 626595594 | 626597285 | Aberrant Panicle Organization 1 Protein |
| OsFBX94 | Rice | HORVU5Hr1G025530 | 5H | 140302431 | 140306350 | F-Box Only Protein 13 |
| OsNRT2.3a-2.3b | Rice | HORVU3Hr1G066090 | 3H | 503310428 | 503312717 | High-Affinity Nitrate Transporter 2.6 |
| OsNAR2.1-2.2 | Rice | HORVU5Hr1G115500 | 5H | 646682607 | 646686179 | High-Affinity Nitrate Transporter 3.1 |
| OsLHT1 | Rice | HORVU7Hr1G032060 | 7H | 65594488 | 65596772 | Lysine Histidine Transporter 2 |
| OsLHT2 | Rice | HORVU7Hr1G074660 | 7H | 428023559 | 428028502 | Transmembrane Amino Acid Transporter Family Protein |
| OsCKX2/Gn1a | Rice | HORVU3Hr1G027430 | 3H | 116879865 | 16883601 | Cytokinin Dehydrogenase 2 |
| OsCKX5 | Rice | HORVU3Hr1G075920 | 3H | 567046659 | 567052020 | Cytokinin Dehydrogenase 5 |
| OsCKX4 | Rice | HORVU3Hr1G105360 | 3H | 668168109 | 668176192 | Cytokinin Oxidase/Dehydrogenase 1 |
| OsCKX3 | Rice | HORVU1Hr1G042360 | 1H | 306444595 | 306450221 | Cytokinin Dehydrogenase 3 |
| OsCKX1 | Rice | HORVU3Hr1G019850 | 3H | 58407698 | 58410314 | Cytokinin Oxidase/Dehydrogenase 6 |
| OsCKX7 | Rice | HORVU7Hr1G086710 | 7H | 522868134 | 522870101 | Cytokinin Dehydrogenase 10 |
| OsCKX8 | Rice | HORVU1Hr1G057860 | 1H | 421966219 | 421973332 | Cytokinin Oxidase/Dehydrogenase 1 |
| OsCKX9 | Rice | HORVU6Hr1G039680 | 6H | 207624575 | 207626177 | Cytokinin Oxidase/Dehydrogenase 1 |
| OsIPT1-2 | Rice | HORVU1Hr1G011480 | 1H | 27827675 | 27830691 | tRNA Dimethylallyltransferase |
| OsIPT3 | Rice | HORVU3Hr1G025950 | 3H | 103350630 | 103351969 | tRNA Dimethylallyltransferase |
| OsIPT4-5 | Rice | HORVU5Hr1G110100 | 5H | 631892524 | 631893928 | tRNA Dimethylallyltransferase 2 |
| OsCIN1-2 | Rice | HORVU4Hr1G086300 | 4H | 633598303 | 633602296 | Beta-Fructofuranosidase, Insoluble Isoenzyme 1 |
| OsCIN3 | Rice | HORVU4Hr1G011000 | 4H | 33449700 | 33451633 | Beta-Fructofuranosidase, Insoluble Isoenzyme 3 |
| OsSGR1 | Rice | HORVU5Hr1G081500 | 5H | 564845582 | 564848348 | Protein STAY-GREEN Chloroplastic |
| OsFNR1 | Rice | HORVU2Hr1G038830 | 2H | 184566812 | 184570474 | Ferredoxin--NADP Reductase |
| OsFNR2 | Rice | HORVU5Hr1G103180 | 5H | 615129595 | 615133117 | Ferredoxin--NADP Reductase |
| OsARE1 | Rice | HORVU7Hr1G063720 | 7H | 314391516 | 314425666 | Chloroplast envelope membrane protein |
| TaAS1-3A | Wheat | HORVU3Hr1G013910 | 3H | 31212143 | 31216892 | Asparagine synthetase [glutamine-hydrolyzing] |
| TaASN2-1A | Wheat | HORVU1Hr1G084370 | 1H | 533821309 | 533827604 | Asparagine synthetase [glutamine-hydrolyzing] 2 |
| TaASN2-1B | Wheat | HORVU1Hr1G092110 | 1H | 549769608 | 549775894 | Asparagine synthetase [glutamine-hydrolyzing] 2 |
| TaANR1-6A | Wheat | HORVU6Hr1G073040 | 6H | 507069039 | 507080622 | MADS-box transcription factor 57 |
| TaGS1.1-4A | Wheat | HORVU4Hr1G066860 | 4H | 555801831 | 555805679 | Glutamine synthetase 1 |
| TaGDH1-5A | Wheat | HORVU5Hr1G104700 | 5H | 619890137 | 619895338 | Glutamate dehydrogenase 1 |
| TaNRT2.1, TaNRT2.4-6A | Wheat | HORVU6Hr1G005600 | 6H | 12385615 | 12387964 | High-affinity nitrate transporter 2.6 |
| TraesCS6B01G041800 | Wheat | HORVU7Hr1G120020 | 7H | 650777327 | 650785628 | Disease resistance protein |
| TraesCS6B01G043500 | Wheat | HORVU6Hr1G005690 | 6H | 12565857 | 12569544 | Disease resistance protein |
| TraesCS6B01G051000 | Wheat | HORVU3Hr1G098450 | 3H | 658650524 | 658656351 | Receptor kinase 3 |
| TraesCS2A01G128200 | Wheat | HORVU0Hr1G002520 | Un | 11160951 | 11162387 | UDP-Glycosyltransferase |
| TraesCS2A01G127800 | Wheat | HORVU2Hr1G124210 | 2H | 757856039 | 758101641 | Glutathione-regulated |
| TraesCS2A01G128400 | Wheat | HORVU2Hr1G022450 | 2H | 65225047 | 65230215 | Chromodomain-helicase-DNA-binding |
| TraesCS6B01G194500 | Wheat | HORVU6Hr1G033850 | 6H | 156256740 | 156263950 | Chaperone protein DnaJ |
| TraesCS2A01G130100LC | Wheat | HORVU7Hr1G102500 | 7H | 611628889 | 611629721 | Phosphoinositide phospholipase C |
| TraesCS6B01G050700 | Wheat | HORVU6Hr1G006880 | 6H | 14328001 | 14332255 | Carboxypeptidase Y homolog A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunarathne, S.D.; Han, Y.; Zhang, X.-Q.; Li, C. Advances in Understanding the Molecular Mechanisms and Potential Genetic Improvement for Nitrogen Use Efficiency in Barley. Agronomy 2020, 10, 662. https://doi.org/10.3390/agronomy10050662
Karunarathne SD, Han Y, Zhang X-Q, Li C. Advances in Understanding the Molecular Mechanisms and Potential Genetic Improvement for Nitrogen Use Efficiency in Barley. Agronomy. 2020; 10(5):662. https://doi.org/10.3390/agronomy10050662
Chicago/Turabian StyleKarunarathne, Sakura D., Yong Han, Xiao-Qi Zhang, and Chengdao Li. 2020. "Advances in Understanding the Molecular Mechanisms and Potential Genetic Improvement for Nitrogen Use Efficiency in Barley" Agronomy 10, no. 5: 662. https://doi.org/10.3390/agronomy10050662
APA StyleKarunarathne, S. D., Han, Y., Zhang, X.-Q., & Li, C. (2020). Advances in Understanding the Molecular Mechanisms and Potential Genetic Improvement for Nitrogen Use Efficiency in Barley. Agronomy, 10(5), 662. https://doi.org/10.3390/agronomy10050662







