An Analysis of the Genetic Diversity of Bread Wheat x Spelt Breeding Lines in Terms of Their Resistance to Powdery Mildew and Leaf Rust
Abstract
1. Introduction
2. Materials and Methods
2.1. An Evaluation of the Health Status of Wheat-Spelt Breeding Lines and Their Parental Forms
2.2. Identification of Resistance Genes
3. Results
3.1. Evaluation of Plant Health in a Field Experiment
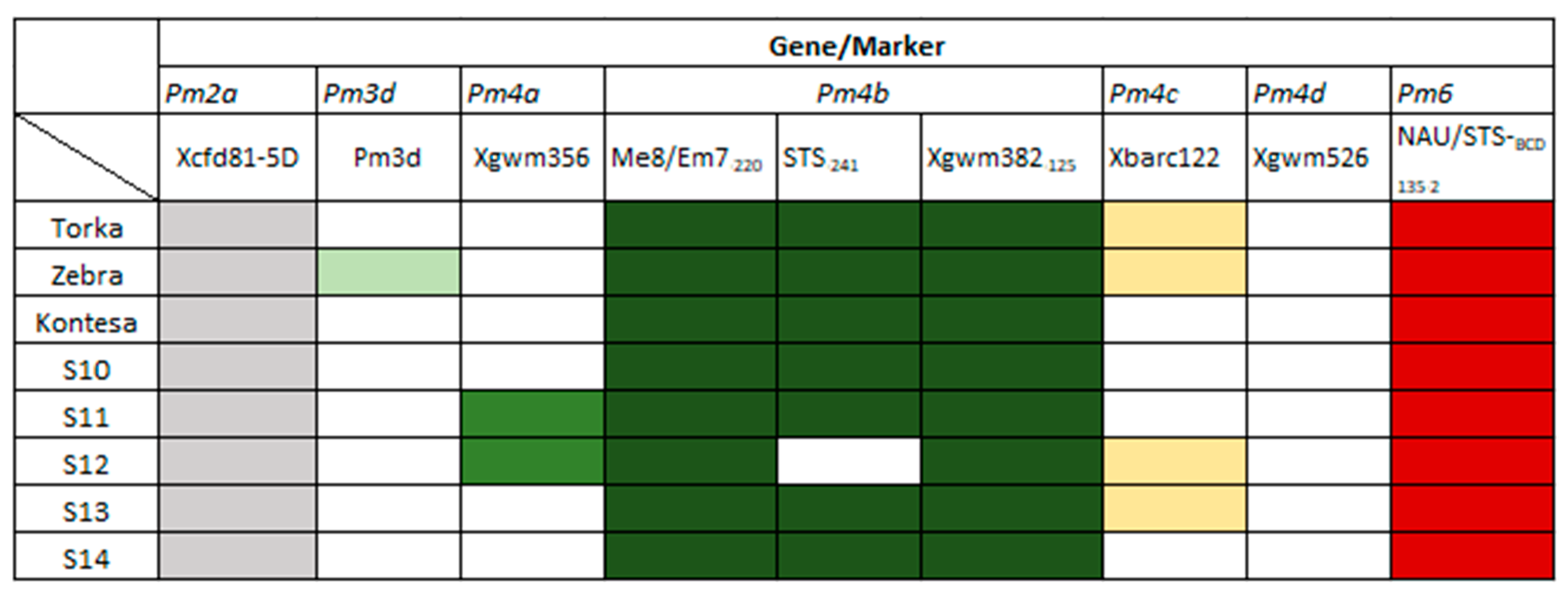
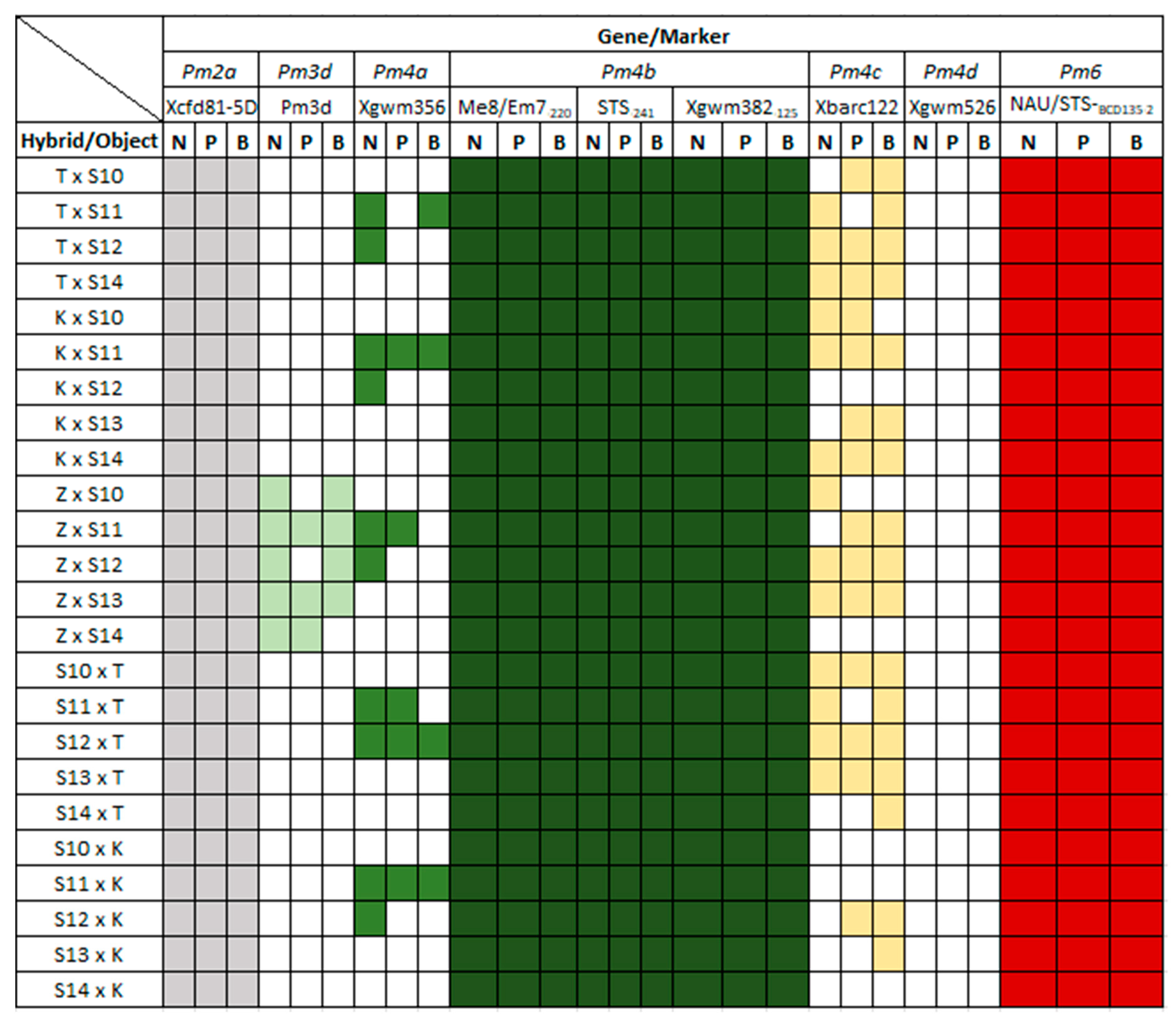
3.2. Identification of Pm Resistance Genes
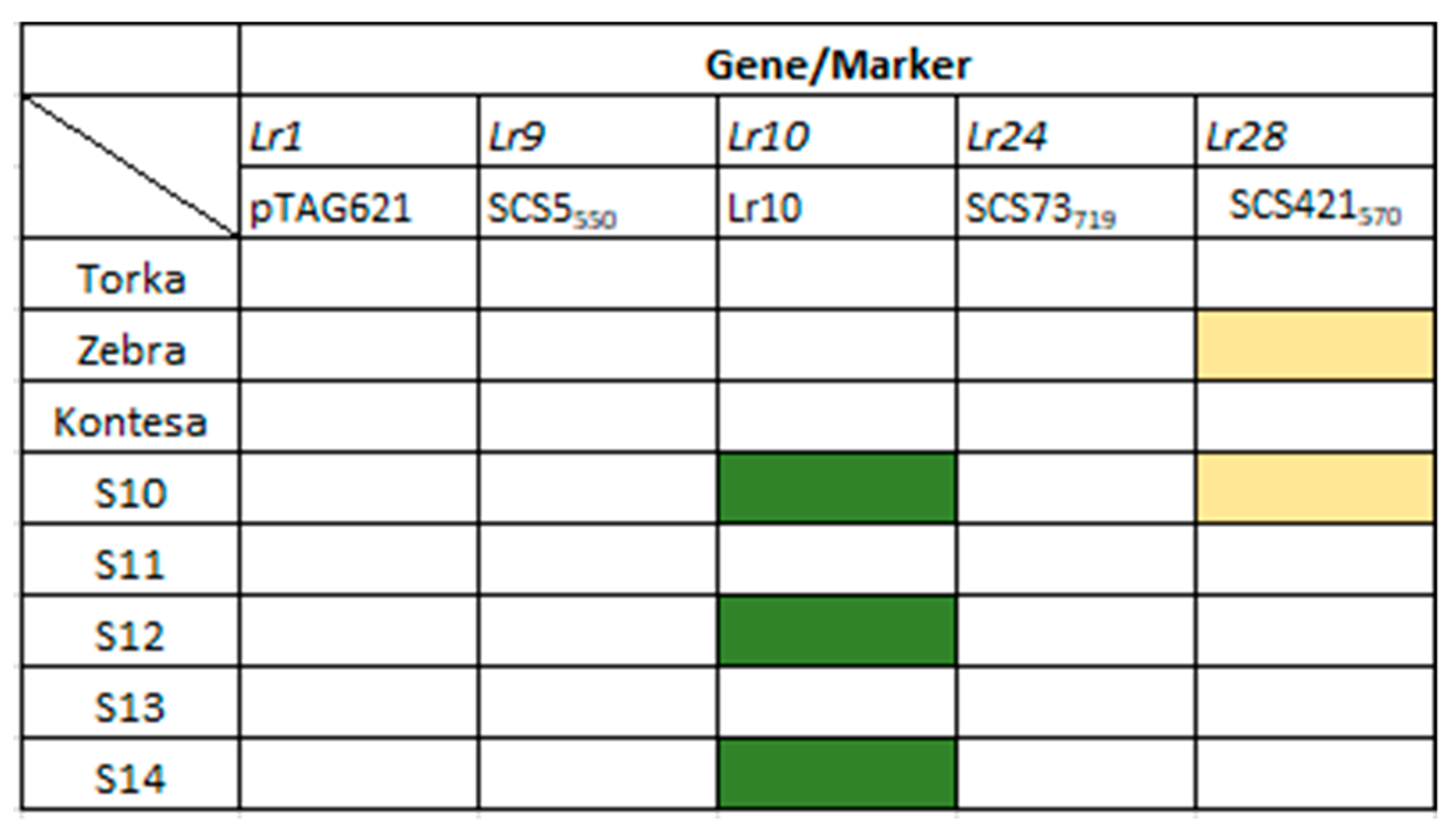
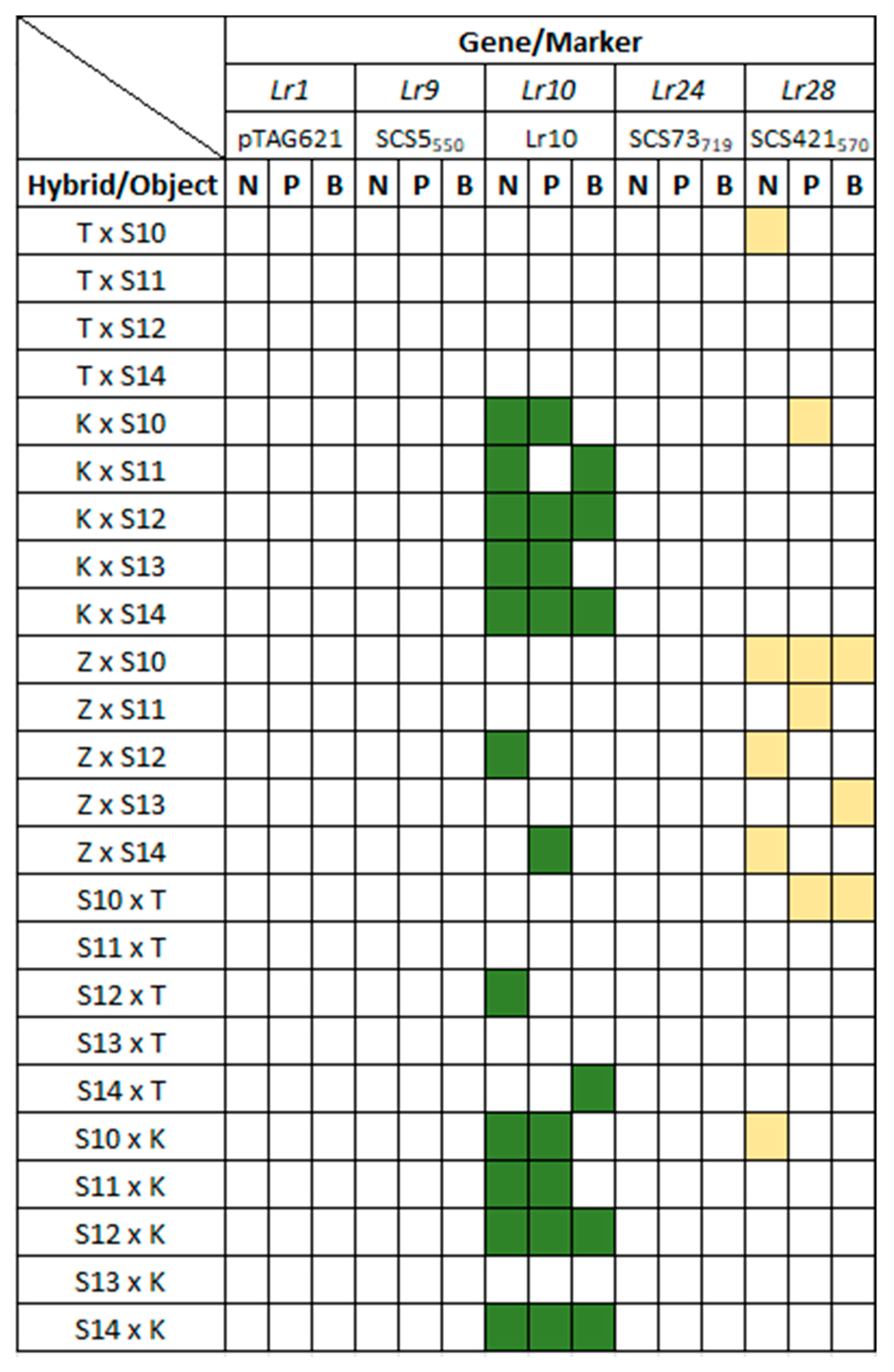
3.3. Identification of Lr Resistance Genes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Maich, R.H.; Steffolani, M.E.; Di Rienzo, J.A.; Leon, A.E. Association between grain yield, grain quality and morpho-physiological traits along ten cycles of recurrent selection in bread wheat (Triticum aestivum L.). Cereal Res. Commun. 2017, 45, 146–153. [Google Scholar] [CrossRef][Green Version]
- Tayyar, S. Variation in grain yield and quality of Romanian bread wheat varieties compared to local varieties in northwestern Turkey. Rom. Biotechnol. Lett. 2010, 15, 5189–5196. [Google Scholar]
- Smale, M.; Reynolds, M.P.; Warburton, M.; Skovmand, B.; Trethowan, R.; Singh, R.P.; Ortiz-Monasterio, I.; Crossa, J. Dimensions of diversity in modern spring bread wheat in developing countries from 1965. Crop Sci. 2002, 42, 1766–1779. [Google Scholar] [CrossRef]
- Reif, J.C.; Zhang, P.; Dreisigacker, S.; Warburton, M.L.; van Ginkel, M.; Hoisington, D.; Bohn, M.; Melchinger, A.E. Wheat genetic diversity trends during domestication and breeding. Theor. Appl. Genet. 2005, 110, 859–864. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Cultivated ancient wheats (Triticum spp.): A potential source of health-beneficial food products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef]
- Dvorak, J.; Deal, K.R.; Luo, M.C.; You, F.M.; von Borstel, K.; Dehghani, H. The origin of spelt and free-threshing hexaploid wheat. J. Hered. 2012, 103, 426–441. [Google Scholar] [CrossRef]
- Wiwart, M.; Perkowski, J.; Jackowiak, H.; Packa, D.; Borusiewicz, A.; Musko, M. Response of some cultivars of spring spelt (Triticum spelta) to Fusarium culmorum infection. Die Bodenkultur 2004, 103, 3. [Google Scholar]
- Mankevičienė, A.; Jablonskytė-Raščė, D.; Maikštėnienė, S. Occurrence of mycotoxins in spelt and common wheat grain and their products. Food Addit. Contam. Part A 2014, 31, 132–138. [Google Scholar] [CrossRef]
- Draz, I.S.; Abou-Elseoud, M.S.; Kamara, A.E.M.; Alaa-Eldein, O.A.E.; El-Bebany, A.F. Screening of wheat genotypes for leaf rust resistance along with grain yield. Ann. Agric. Sci. 2015, 60, 29–39. [Google Scholar] [CrossRef]
- Huang, X.Q.; Röder, M.S. Molecular mapping of powdery mildew resistance genes in wheat: A review. Euphytica 2004, 137, 203–223. [Google Scholar] [CrossRef]
- Revathi, P.; Tomar, S.M.S.; Singh, N.K. Marker assisted gene pyramiding of leaf rust resistance genes Lr24, Lr28 along with stripe rust resistance gene Yr15 in wheat (Triticum aestivum L.). Indian J. Genet. Pl. Br. 2010, 70, 349–354. [Google Scholar]
- Jin, Y.; Xu, H.; Ma, P.; Fu, X.; Song, L.; Xu, Y.; Zhang, X.; An, D. Characterization of a new Pm2 allele associated with broad-spectrum powdery mildew resistance in wheat line Subtil. Sci. Rep. 2018, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Hu, Y.; Zhong, S.; Luo, P. The Potential Role of Powdery Mildew-Resistance Gene Pm40 in Chinese Wheat-Breeding Programs in the Post-Pm21 Era. Engineering 2018, 4, 500–506. [Google Scholar] [CrossRef]
- Ma, P.; Xu, H.; Li, L.; Zhang, H.; Han, G.; Xu, Y.; Fu, X.; Zhang, X.; An, D. Characterization of a new Pm2 allele conferring powdery mildew resistance in the wheat germplasm line FG-1. Front. Plant Sci. 2016, 7, 546. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Morris, C.F.; Xia, X.C. Catalogue of gene symbols for wheat. Suppl. Annu. Wheat Newsl. 2017, 53, 1–20. [Google Scholar]
- Miedaner, T.; Flath, K. Effectiveness and environmental stability of quantitative powdery mildew (Blumeria graminis) resistance among winter wheat cultivars. Plant Breed. 2007, 126, 553–558. [Google Scholar] [CrossRef]
- Tommasini, L.; Yahiaoui, N.; Srichumpa, P.; Keller, B. Development of functional markers specific for seven Pm3 resistance alleles and their validation in the bread wheat gene pool. Theor. Appl. Genet. 2006, 114, 165–175. [Google Scholar] [CrossRef]
- Peusha, H.; Enno, T.; Jakobson, I.; Ts[otilde]mbalova, J.; Ingver, A.; Järve, K. Powdery mildew resistance of Nordic spring wheat cultivars grown in Estonia. Acta Agric. Scand. Sect. B–Soil Plant Sci. 2008, 58, 289–296. [Google Scholar] [CrossRef]
- Li, G.; Xu, X.; Bai, G.; Carver, B.F.; Hunger, R.; Bonman, J.M. Identification of novel powdery mildew resistance sources in wheat. Crop Sci. 2016, 56, 1817–1830. [Google Scholar] [CrossRef]
- Huang, X.Q.; Hsam, S.L.K.; Zeller, F.J. Identification of powdery mildew resistance genes in common wheat (Triticum aestivum L. em Thell.). IX. Cultivars, land races and breeding lines grown in China. Plant Breed. 1997, 116, 233–238. [Google Scholar] [CrossRef]
- Li, N.; Jia, H.; Kong, Z.; Tang, W.; Ding, Y.; Liang, J.; Ma, H.; Ma, Z. Identification and marker-assisted transfer of a new powdery mildew resistance gene at the Pm4 locus in common wheat. Mol. Breed. 2017, 37, 79. [Google Scholar] [CrossRef]
- Sharma, C.; Saripalli, G.; Kumar, S.; Gautam, T.; Kumar, A.; Rani, S.; Jain, N.; Prasad, P.; Raghuvanshi, S.; Jain, M.; et al. A study of transcriptome in leaf rust infected bread wheat involving seedling resistance gene Lr28. Funct. Plant Biol. 2018, 45, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Hao, Y.; Johnson, J.; Lopez, B.; Bland, D.; Chen, Z.; Sutton, S.; Buck, J.; Youmans, J.; Mergoum, M. Genetic mapping of a major gene for leaf rust resistance in soft red winter wheat cultivar AGS 2000. Mol. Breed. 2019, 39, 8. [Google Scholar] [CrossRef]
- Pinto da Silva, G.B.; Zanella, C.M.; Martinelli, J.A.; Chaves, M.S.; Hiebert, C.W.; McCallum, B.D.; Boyd, L.A. Quantitative Trait Loci Conferring Leaf Rust Resistance in Hexaploid Wheat. Phytopathology 2018, 108, 1344–1354. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Yamazaki, Y.; Dubcovsky, J.; Rogers, W.; Morris, C.; Appels, R.; Xia, X.C. Catalogue of gene symbols for wheat. In Proceedings of the 12th International Wheat Genetics Symposium, Yokohama, Japan, 8–14 September 2013; Ogihara, Y., Takumi, S., Handa, H., Eds.; Springer: Yokohama, Japan, 2015. [Google Scholar]
- Goriewa-Duba, K.; Duba, A.; Kwiatek, M.; Wiśniewska, H.; Wachowska, U.; Wiwart, M. Chromosomal distribution of pTa-535, pTa-86, pTa-713, 35S rDNA repetitive sequences in interspecific hexaploid hybrids of common wheat (Triticum aestivum L.) and spelt (Triticum spelta L.). PLoS ONE 2018, 13, e0192862. [Google Scholar] [CrossRef] [PubMed]
- List of Agriculture Cultivars Research Center for Cultivar Testing; COBORU: Słupia Wielka, Poland, 2008.
- Suchowilska, E.; Wiwart, M.; Krska, R.; Kandler, W. Do Triticum aestivum L. and Triticum spelta L. Hybrids Constitute a Promising Source Material for Quality Breeding of New Wheat Varieties? Agronomy 2020, 10, 43. [Google Scholar] [CrossRef]
- Witzenberger, A.; Hack, H. Explanations of the BBCH decimal code for the growth stages of cereals-with illustrations. Gesunde Pflanzen 1990, 42, 308–321. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; WEBER, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- EPPO. European and mediterranean plant protection organization. Guideline for the efficacy evaluation of fungicides, foliar diseases of cereals Bulletin. Oepp/Eppo 1998, 28, 279–290. [Google Scholar]
- Qiu, Y.; Sun, X.; Zhou, R.; Kong, X.; Zhang, S.; Jia, J. Identification of microsatellite markers linked to powdery mildew resistance gene Pm2 in wheat. Cereal Res. Commun. 2006, 34, 1267–1273. [Google Scholar] [CrossRef]
- Röder, M.S.; Korzun, V.; Wendehake, K.; Plaschke, J.; Tixier, M.H.; Leroy, P.; Ganal, M.W. A microsatellite map of wheat. Genetics 1998, 149, 2007–2023. [Google Scholar] [PubMed]
- Ji, J.; Qin, B.; Wang, H.; Cao, A.; Wang, S.; Chen, P.; Zhuang, L.; Du, Y.; Liu, D.; Wang, X. STS markers for powdery mildew resistance gene Pm6 in wheat. Euphytica 2008, 163, 159–165. [Google Scholar] [CrossRef]
- Song, Q.J.; Shi, J.R.; Singh, S.; Fickus, E.W.; Costa, J.M.; Lewis, J.; Gill, B.S.; Ward, R.; Cregan, P.B. Development and mapping of microsatellite (SSR) markers in wheat. Theor. Appl. Genet. 2005, 110, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Feuillet, C.; Messmer, M.; Schachermayr, G.; Keller, B. Genetic and physical characterization of the LR1 leaf rust resistance locus in wheat (Triticum aestivum L.). Mol. Gen. Genet. MGG 1995, 248, 553–562. [Google Scholar] [CrossRef]
- Sohail, Y. Molecular Assessment of Puccinia triticina and Available Wheat Genetic Resources to Combat Leaf Rust in Pakistan. Doctoral Dissertation, Quaid-I-Azam University Islamabad Pakistan, Islamabad, Pakistan, 2015. [Google Scholar]
- Schachermayr, G.; Feuillet, C.; Keller, B. Molecular markers for the detection of the wheat leaf rust resistance gene Lr10 in diverse genetic backgrounds. Mol. Breed. 1997, 3, 65–74. [Google Scholar] [CrossRef]
- Gupta, S.K.; Charpe, A.; Koul, S.; Prabhu, K.V.; Haq, Q.M.R. Development and validation of molecular markers linked to an Aegilops umbellulata–derived leaf-rust-resistance gene, Lr9, for marker-assisted selection in bread wheat. Genome 2005, 48, 823–830. [Google Scholar] [CrossRef]
- Cherukuri, D.P.; Gupta, S.K.; Charpe, A.; Koul, S.; Prabhu, K.V.; Singh, R.B.; Haq, Q.M.R. Molecular mapping of Aegilops speltoides derived leaf rust resistance gene Lr28 in wheat. Euphytica 2005, 143, 19–26. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Hsam, S.L.; Zeller, F.J. Identification of powdery mildew resistance genes in common wheat (Triticum aestivum L. em. Thell.). XI. Cultivars grown in Poland. J. Appl. Genet. 1998, 3, 225–236. [Google Scholar]
- Svec, M.; Szunics, L.; Miklovicova, M.; Slovakova, T.; Tisova, V.; Hauptvogel, P. Identification of genes for resistance to wheat powdery mildew in Hungarian, Polish and Slovak wheat cultivars. Plant Prot. Sci. -Prague 2002, 38, 64–72. [Google Scholar] [CrossRef]
- Czembor, H.J.; Domeradzka, O.; Czembor, J.H.; Mańkowski, D.R. Virulence structure of the powdery mildew (Blumeria graminis) population occurring on triticale (x Triticosecale) in Poland. J. Phytopathol. 2014, 162, 499–512. [Google Scholar] [CrossRef]
- Walker, A.S.; Bouguennec, A.; Confais, J.; Morgant, G.; Leroux, P. Evidence of host-range expansion from new powdery mildew (Blumeria graminis) infections of triticale (× Triticosecale) in France. Plant Pathol. 2011, 60, 207–220. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Gruszecka, D.; Nowak, M.; Leśniowska-Nowak, J. Resistance of triticale hybrids with Pm4b and Pm6 genes to powdery mildew. Acta Biol. Ser. Bot. 2011, 53, 57–62. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, Z.; Kong, X.; Huo, N.; Tian, Q.; Li, P.; Jin, C.; Dong, J.; Jia, J. Development of wheat near-isogenic lines for powdery mildew resistance. Theor. Appl. Genet. 2005, 110, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Bennett, F.G. Resistance to powdery mildew in wheat: A review of its use in agriculture and breeding programmes. Plant Pathol. 1984, 33, 279–300. [Google Scholar] [CrossRef]
- Shah, L.; Rehman, S.; Ali, A.; Yahya, M.; Riaz, M.W.; Si, H.; Ma, C.; Lu, J. Genes responsible for powdery mildew resistance and improvement in wheat using molecular marker-assisted selection. J. Plant Dis. Prot. 2018, 125, 145–158. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Würschum, T. Genetic variability, heritability and correlation among agronomic and disease resistance traits in a diversity panel and elite breeding material of spelt wheat. Plant Breed. 2014, 133, 459–464. [Google Scholar] [CrossRef]
- Nocente, F.; Gazza, L.; Pasquini, M. Evaluation of leaf rust resistance genes Lr1, Lr9, Lr24, Lr47 and their introgression into common wheat cultivars by marker-assisted selection. Euphytica 2007, 155, 329–336. [Google Scholar] [CrossRef]
- Tomkowiak, A.; Kurasiak-Popowska, D.; Mikołajczyk, S.; Weigt, D.; Niemann, J.; Kiel, A.; Lisewska, A.; Nawracała, J.; Matysik, P.; Rokicki, M.; et al. Identyfikacja genu Lr19 warunkującego odporność na rdzę brunatną powodowaną przez Puccinia recondita f. sp. tritici w zagranicznych odmianach pszenicy ozimej Triticum aestivum L. Prog. Plant Prot. 2016, 56, 318–323. [Google Scholar] [CrossRef]
- Leśniowska-Nowak, J.; Grądzielewska, A.; Majek, M. Identification of the gene resistant to leaf rust in selected European wheat cultivars and Multiplex PCR development. Ann. Univ. Mariae Curie-Skłodowska Sect. E Agric. 2013, 68, 20–28. [Google Scholar] [CrossRef]



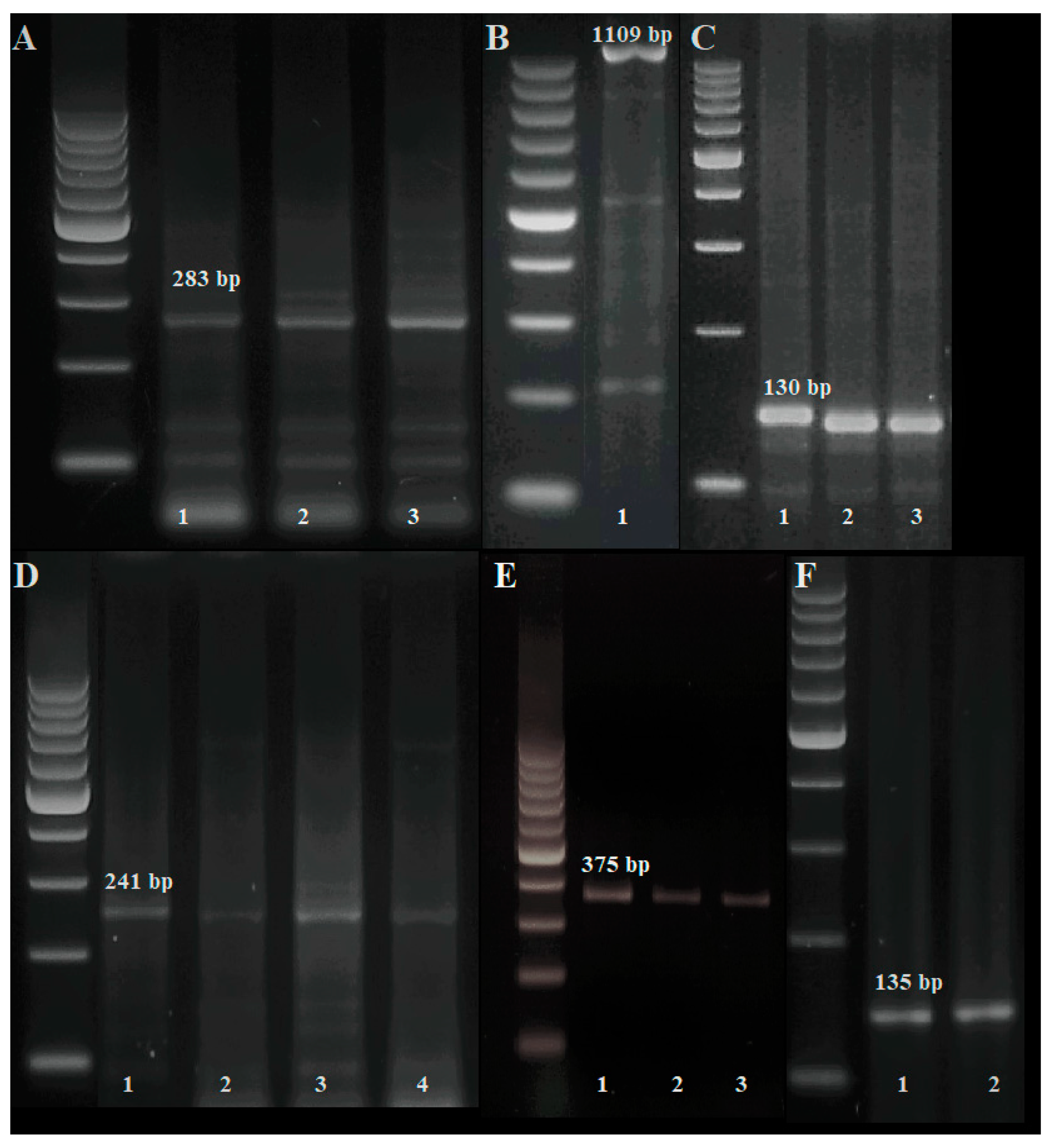

| Object | Origin | Breeding Line |
|---|---|---|
| 1 | T × S10 | 1, 1′, 1″ |
| 2 | T × S11 | 2, 2′, 2″ |
| 3 | T × S12 | 3, 3′, 3″ |
| 4 | T × S14 | 4, 4′, 4″ |
| 5 | K × S10 | 5, 5′, 5″ |
| 6 | K × S11 | 6, 6′, 6″ |
| 7 | K × S12 | 7, 7′, 7″ |
| 8 | K × S13 | 8, 8′, 8″ |
| 9 | K × S14 | 9, 9′, 9″ |
| 10 | Z × S10 | 10, 10′, 10″ |
| 11 | Z × S11 | 11, 11′, 11″ |
| 12 | Z × S12 | 12, 12′, 12″ |
| 13 | Z × S13 | 13, 13′, 13″ |
| 14 | Z × S14 | 14, 14′, 14″ |
| 15 | S10 × T | 15, 15′, 15″ |
| 16 | S11 × T | 16, 16′, 16″ |
| 17 | S12 × T | 17, 17′, 17″ |
| 18 | S13 × T | 18, 18′, 18″ |
| 19 | S14 × T | 19, 19′, 19″ |
| 20 | S10 × K | 20, 20′, 20″ |
| 21 | S11 × K | 21, 21′, 21″ |
| 22 | S12 × K | 22, 22′, 22″ |
| 23 | S13 × K | 23, 23′, 23″ |
| 24 | S14 × K | 24, 24′, 24″ |
| 25 | N/A | T |
| 26 | N/A | K |
| 27 | N/A | Z |
| 28 | N/A | S10 |
| 29 | N/A | S11 |
| 30 | N/A | S12 |
| 31 | N/A | S13 |
| 32 | N/A | S14 |
| Disease | Gene | Marker | Sequence 5’->3’ (F) | Sequence 5’->3’ (R) | Product Size (bp) |
|---|---|---|---|---|---|
| Powdery mildew | Pm2 | Xcfd81-5D | TATCCCCAATCCCCTCTTTC | GTCAATTGTGGCTTGTCCCT | 283 |
| Pm3d | Pm3d | TGACTATTCGTGGGTGCA | GACTGCGGCACAGTTCAGC | 1109 | |
| Pm4a | Xgwm356 | AGCGTTCTTGGGAATTAGAGA | CCAATCAGCCTGCAACAAC | 130 | |
| Pm4b | Me8/Em7-220 | TGAGTCCAAACCGGTGC | GACTGCGTACGAATTCAA | 220 | |
| STS-241 | CTCATTCTTGTTTTACTTCCTTCAGT | GTCTCGTCTTCAGCATCCTATACA | 241 | ||
| Xgwm382-125 | GTCAGATAACGCCGTCCAAT | CTACGTGCACCACCATTTTG | 125 | ||
| Pm4c | Xbarc122 | CCCGTGTATATCCAGGAGTG | CAGCCCTTGTGATGTGATG | 375 | |
| Pm4d | Xgwm526 | CAATAGTTCTGTGAGAGCTGCG | CCAACCCAAATACACATTCTCA | 140–160 * | |
| Pm6 | NAU/STSBCD135-2 | GCTCCGAAGCAAGAGAAGAA | TCTGCTGGTCCTCTGATGTG | 135 | |
| Leaf rust | Lr1 | pTAG621 | GGGTCACGTACTACTATA | CCTTGCCAGCCCAAAAG | 560 |
| Lr9 | SCS5550 | TGCGCCCTTCAAAGGAAG | TGCGCCCTTCTGAACTGTAT | 550 | |
| Lr10 | Lr10 | GTGTAATGCATGCAGGTTCC | AGGTGTGAGTGAGTTATG TT | 310 | |
| Lr24 | SCS73719 | TCGTCCAGATCAGAATGTG | CTCGTCGATTAGCAGTGAG | 719 | |
| Lr28 | SCS421570 | ACAAGGTAAGTCTCCAACCA | AGTCGACCGAGATTTTAACC | 570 |
| Disease | Gene | Marker | Cycle Conditions |
|---|---|---|---|
| Powdery mildew | Pm2 | Xcfd81-5D | 94 °C–5 min; 35 cycles (94 °C–30 s; 60 °C–30 s; 72 °C–1 min); 72 °C–10 min |
| Pm3d | Pm3d | 94 °C–3 min; 35 cycles (94 °C–1 min; 58 °C–1 min; 72 °C–1 min); 72 °C–5 min | |
| Pm4a | Xgwm356 | 94 °C–3 min; 45 cycles (94 °C–1 min; 55 °C–1 min; 72 °C–2 min); 72 °C–10 min | |
| Pm4b | Me8/Em7-220 | 94 °C–3 min; 35 cycles (94 °C–40 s; 50 °C–1 min; 72 °C–1 min); 72 °C–5 min | |
| STS-241 | 94 °C–3 min; 35 cycles (94 °C–40 s; 50 °C–1 min; 72 °C–1 min); 72 °C–5 min | ||
| Xgwm382-125 | 94 °C–3 min; 35 cycles (94 °C–40 s; 60 °C–1 min; 72 °C–1 min); 72 °C–5 min | ||
| Pm4c | Xbarc122 | 94 °C–3 min; 35 cycles (94 °C–40 s; 52 °C–1 min; 72 °C–1 min); 72 °C–5 min | |
| Pm4d | Xgwm526 | 94 °C–3 min; 45 cycles (94 °C–1 min; 55 °C–1 min; 72 °C–2 min); 72 °C–10 min | |
| Pm6 | NAU/STSBCD135-2 | 94 °C–4 min; 31 cycles (94 °C–30 s; 55 °C–1 min; 72 °C–1 min); 72 °C–1 min | |
| Leaf rust | Lr1 | pTAG621 | 94 °C–5 min; 30 cycles (94 °C–1 min; 55 °C–1 min; 72 °C–2 min); 72 °C–10 min |
| Lr9 | SCS5550 | 94 °C–2 min; 30 cycles (94 °C–1 min; 60 °C–1 min; 72 °C–1 min); 72 °C–7 min | |
| Lr10 | Lr10 | 94 °C–3 min; 35 cycles (94 °C–45 s; 57 °C–45 s; 72 °C–30 s); 72 °C–3 min | |
| Lr24 | SCS73719 | 94 °C–2 min; 35 cycles (94 °C–1 min; 55 °C–1 min; 72 °C–1 min); 72 °C–7 min | |
| Lr28 | SCS421570 | 94 °C–2 min; 35 cycles (94 °C–1 min; 60 °C–1 min; 72 °C–1 min); 72 °C–5 min |
| Powdery Mildew | Leaf Rust | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2017 | 2018 | |||||||
| Object | Origin | Breeding Line | Category | Average Score | Category | Average Score | Category | Average Score | Category | Average Score |
| 1 | T × S10 | 1 | None | 0% | None | 0% | None | 0% | Low | 5% |
| 1’ | 1% | 1% | 1% | 10% | ||||||
| 1’’ | 0% | 1% | 1% | 10% | ||||||
| 2 | T × S11 | 2 | None | 0% | None | 0% | None | 0% | Low | 5% |
| 2′ | 0% | 0% | 0% | 5% | ||||||
| 2″ | 0% | 0% | 0% | 10% | ||||||
| 3 | T × S12 | 3 | None | 0% | None | 1% | Low | 10% | Low | 10% |
| 3′ | 0% | 0% | None | 0% | 15% | |||||
| 3″ | 0% | 0% | None | 0% | 10% | |||||
| 4 | T × S14 | 4 | None | 1% | None | 0% | None | 1% | Low | 5% |
| 4′ | 1% | 1% | 1% | 5% | ||||||
| 4″ | 0% | 0% | 1% | 5% | ||||||
| 5 | K × S10 | 5 | None | 0% | None | 0% | Low | 5% | None | 0% |
| 5′ | 0% | 0% | None | 1% | 1% | |||||
| 5″ | 0% | 0% | None | 0% | 0% | |||||
| 6 | K × S11 | 6 | None | 0% | None | 0% | None | 1% | None | 1% |
| 6′ | 0% | 0% | Low | 10% | 1% | |||||
| 6″ | 0% | 1% | None | 1% | 0% | |||||
| 7 | K × S12 | 7 | None | 0% | None | 0% | None | 1% | None | 0% |
| 7′ | Low | 10% | 0% | None | 1% | 0% | ||||
| 7″ | Low | 10% | 0% | Low | 5% | 0% | ||||
| 8 | K × S13 | 8 | None | 0% | None | 0% | None | 0% | None | 0% |
| 8′ | 1% | 0% | Low | 10% | 0% | |||||
| 8″ | 0% | 1% | Low | 5% | 0% | |||||
| 9 | K × S14 | 9 | None | 0% | None | 0% | None | 0% | Low | 5% |
| 9′ | 1% | 0% | None | 0% | None | 0% | ||||
| 9″ | 1% | 0% | High | 35% | Low | 15% | ||||
| 10 | Z × S10 | 10 | None | 0% | None | 0% | Low | 5% | None | 0% |
| 10′ | 0% | 0% | 10% | 0% | ||||||
| 10″ | 0% | 0% | 10% | 0% | ||||||
| 11 | Z × S11 | 11 | None | 0% | None | 0% | Low | 5% | None | 1% |
| 11′ | 1% | 0% | 5% | 1% | ||||||
| 11″ | 0% | 0% | 5% | 1% | ||||||
| 12 | Z × S12 | 12 | None | 0% | None | 0% | Low | 5% | None | 0% |
| 12′ | 0% | Low | 10% | 5% | 0% | |||||
| 12″ | 0% | None | 1% | 5% | 1% | |||||
| 13 | Z × S13 | 13 | None | 0% | None | 0% | None | 0% | Low | 10% |
| 13′ | 0% | 0% | 0% | Low | 10% | |||||
| 13″ | 1% | 0% | 1% | None | 0% | |||||
| 14 | Z × S14 | 14 | None | 0% | None | 0% | None | 0% | None | 1% |
| 14′ | 1% | 1% | 0% | 0% | ||||||
| 14″ | 0% | 1% | 0% | 1% | ||||||
| 15 | S10 × T | 15 | None | 0% | Low | 10% | None | 0% | None | 0% |
| 15′ | 0% | Low | 5% | 1% | 0% | |||||
| 15″ | 0% | None | 0% | 0% | 1% | |||||
| 16 | S11 × T | 16 | None | 0% | None | 1% | None | 1% | None | 0% |
| 16′ | 1% | 0% | 1% | 1% | ||||||
| 16″ | 0% | 0% | 1% | 1% | ||||||
| 17 | S12 × T | 17 | None | 0% | None | 0% | None | 1% | None | 0% |
| 17′ | 0% | 1% | 0% | 0% | ||||||
| 17″ | 0% | 0% | 0% | 1% | ||||||
| 18 | S13 × T | 18 | Low | 5% | None | 1% | None | 1% | None | 1% |
| 18′ | 5% | 1% | High | 35% | High | 60% | ||||
| 18″ | 10% | 1% | None | 1% | Low | 10% | ||||
| 19 | S14 × T | 19 | Low | 10% | None | 0% | None | 0% | Low | 10% |
| 19′ | 15% | 1% | Low | 5% | 15% | |||||
| 19″ | 10% | 1% | None | 1% | 5% | |||||
| 20 | S10 × K | 20 | Low | 5% | None | 1% | None | 0% | None | 0% |
| 20′ | 5% | 1% | 1% | 0% | ||||||
| 20″ | 5% | 1% | 1% | 1% | ||||||
| 21 | S11 × K | 21 | None | 0% | None | 0% | None | 1% | None | 1% |
| 21′ | 0% | 1% | 1% | 1% | ||||||
| 21″ | 0% | 0% | 1% | 1% | ||||||
| 22 | S12 × K | 22 | None | 0% | None | 0% | Low | 5% | None | 1% |
| 22′ | 1% | 0% | None | 0% | 1% | |||||
| 22″ | 0% | 1% | Low | 10% | 1% | |||||
| 23 | S13 × K | 23 | High | 40% | Low | 5% | High | 30% | Low | 15% |
| 23′ | 35% | 15% | 40% | 15% | ||||||
| 23″ | 40% | 5% | 35% | 20% | ||||||
| 24 | S14 × K | 24 | None | 1% | Low | 5% | High | 30% | Low | 10% |
| 24′ | 0% | 15% | None | 1% | None | 1% | ||||
| 24″ | 0% | 20% | None | 1% | None | 1% | ||||
| 25 | N/A | T | None | 1% | None | 1% | High | 30% | Low | 5% |
| 26 | Z | None | 0% | None | 0% | Low | 5% | Low | 10% | |
| 27 | K | Low | 10% | Low | 5% | Low | 10% | Low | 15% | |
| 28 | S10 | Low | 10% | None | 0% | None | 0% | None | 0% | |
| 29 | S11 | None | 0% | None | 0% | None | 0% | None | 0% | |
| 30 | S12 | None | 0% | None | 0% | None | 0% | None | 0% | |
| 31 | S13 | None | 0% | None | 0% | None | 0% | Low | 10% | |
| 32 | S14 | Low | 15% | None | 0% | None | 0% | None | 0% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goriewa-Duba, K.; Duba, A.; Suchowilska, E.; Wiwart, M. An Analysis of the Genetic Diversity of Bread Wheat x Spelt Breeding Lines in Terms of Their Resistance to Powdery Mildew and Leaf Rust. Agronomy 2020, 10, 658. https://doi.org/10.3390/agronomy10050658
Goriewa-Duba K, Duba A, Suchowilska E, Wiwart M. An Analysis of the Genetic Diversity of Bread Wheat x Spelt Breeding Lines in Terms of Their Resistance to Powdery Mildew and Leaf Rust. Agronomy. 2020; 10(5):658. https://doi.org/10.3390/agronomy10050658
Chicago/Turabian StyleGoriewa-Duba, Klaudia, Adrian Duba, Elżbieta Suchowilska, and Marian Wiwart. 2020. "An Analysis of the Genetic Diversity of Bread Wheat x Spelt Breeding Lines in Terms of Their Resistance to Powdery Mildew and Leaf Rust" Agronomy 10, no. 5: 658. https://doi.org/10.3390/agronomy10050658
APA StyleGoriewa-Duba, K., Duba, A., Suchowilska, E., & Wiwart, M. (2020). An Analysis of the Genetic Diversity of Bread Wheat x Spelt Breeding Lines in Terms of Their Resistance to Powdery Mildew and Leaf Rust. Agronomy, 10(5), 658. https://doi.org/10.3390/agronomy10050658





