Soil Nutrients Effects on the Performance of Durum Wheat Inoculated with Entomopathogenic Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Crop
2.3. Soils and Soil Properties
2.4. Fungi
2.4.1. Fungal Isolates
2.4.2. Fungal Preparation
2.4.3. Fungal Treatment: Seed Dressing
2.5. Pot Experiment
2.6. Plant Growth, Gas Exchange, Water Use Efficiency (WUE) and Nutrients Analyses
2.7. Statistical Analysis
3. Results
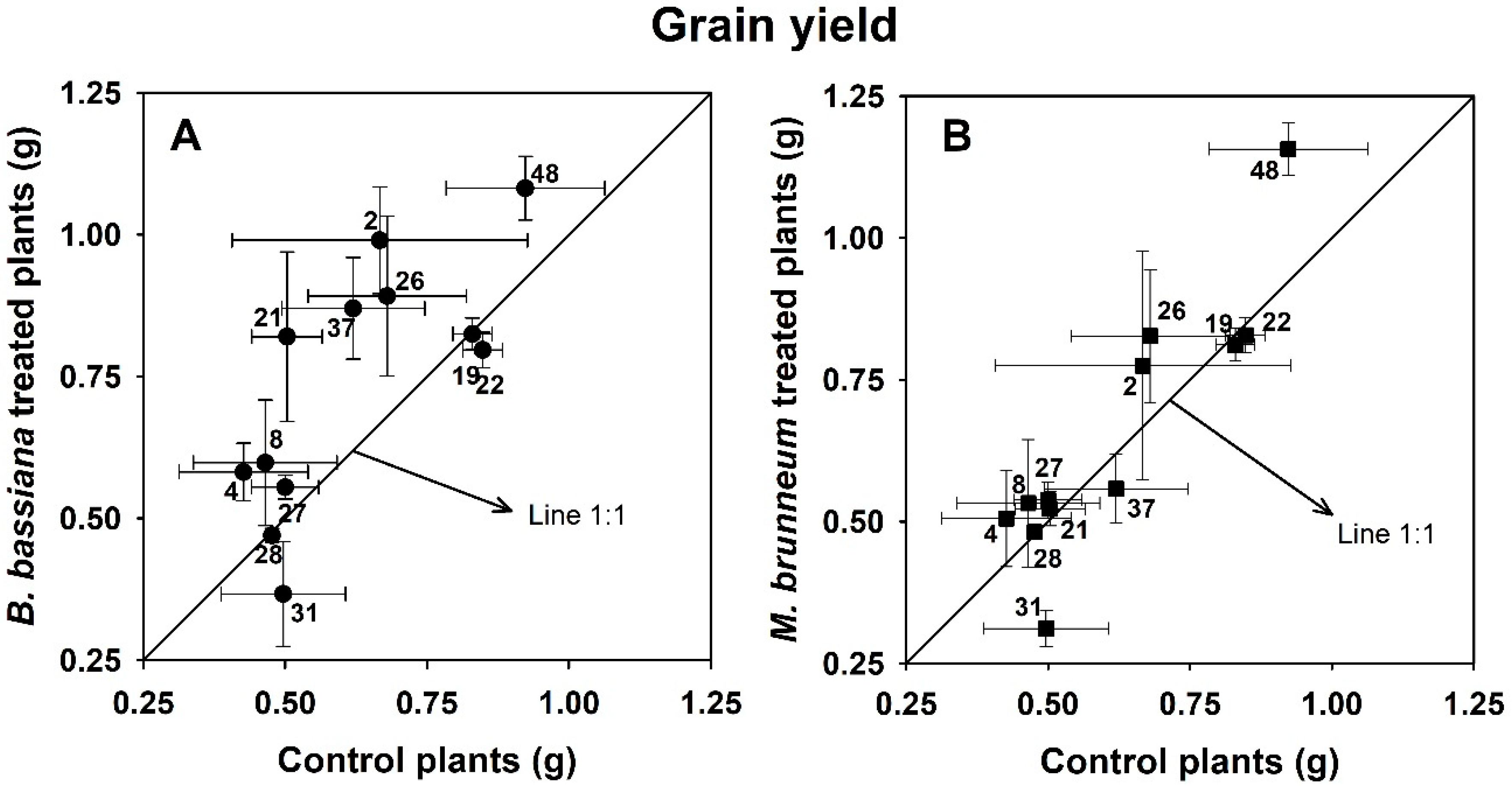
3.1. Plant Growth and Yield
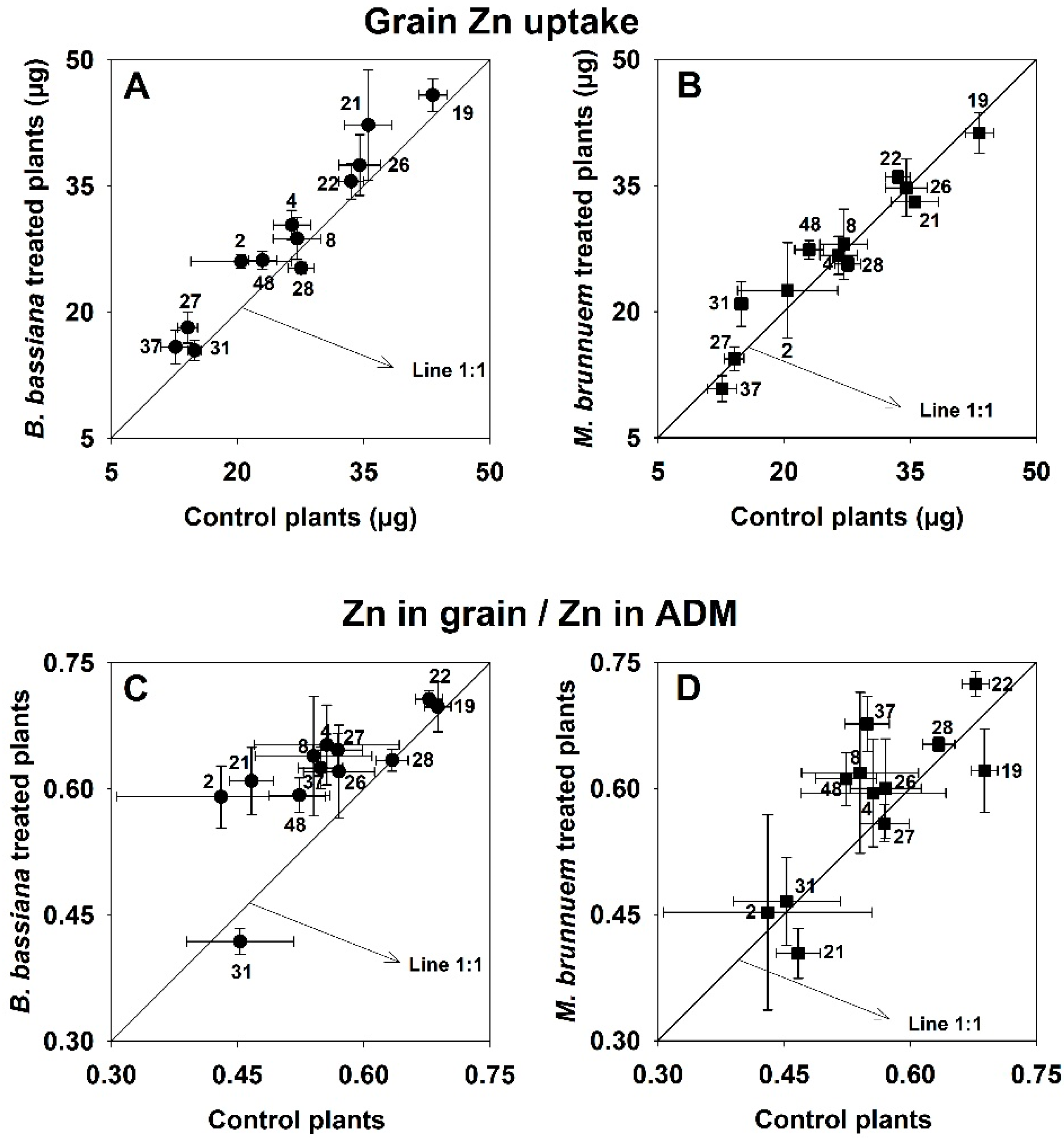
3.2. Nutrient Uptake and Grain Nutrient Concentration
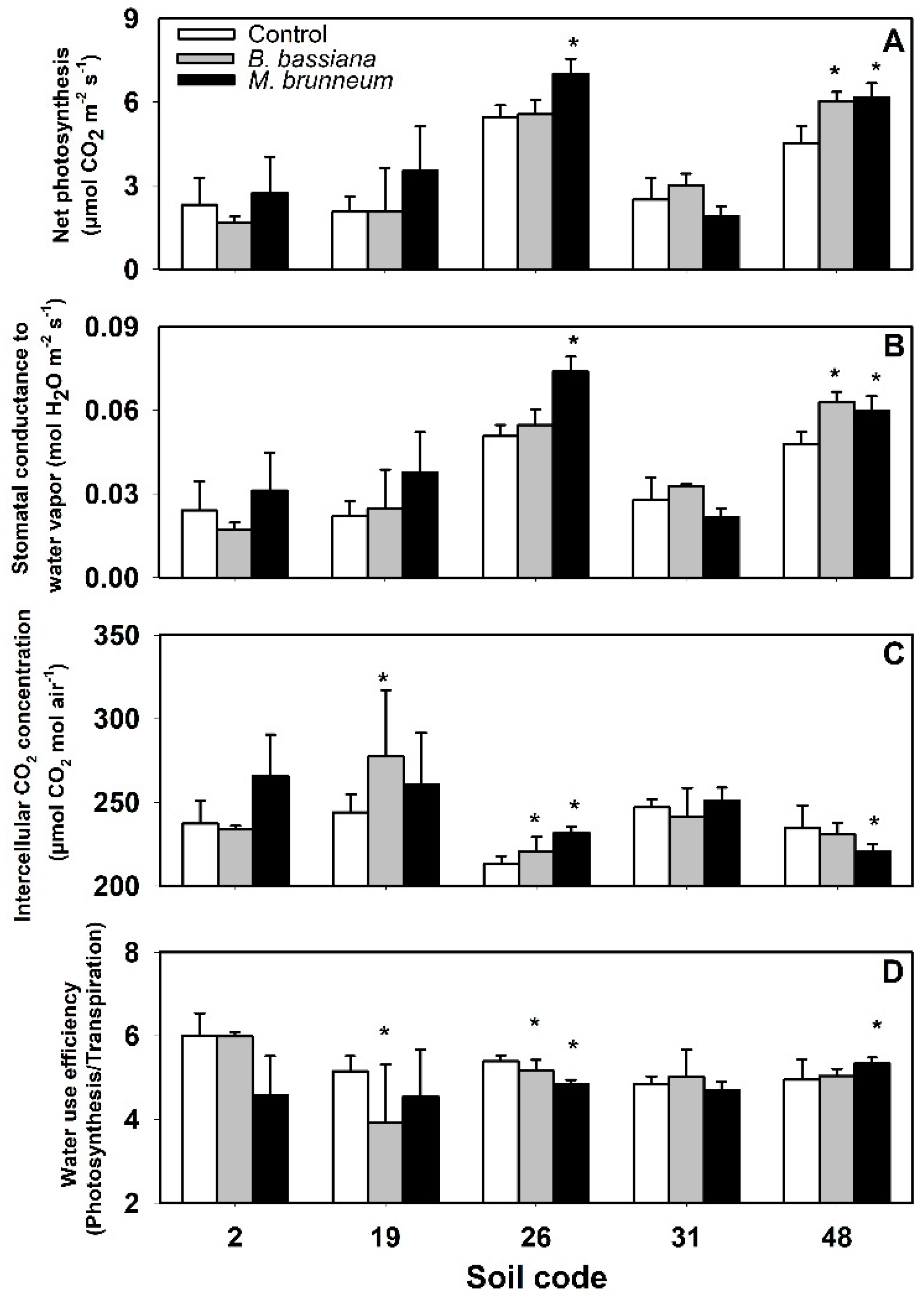
3.3. Gas Exchange Variables and Water Use Efficiency
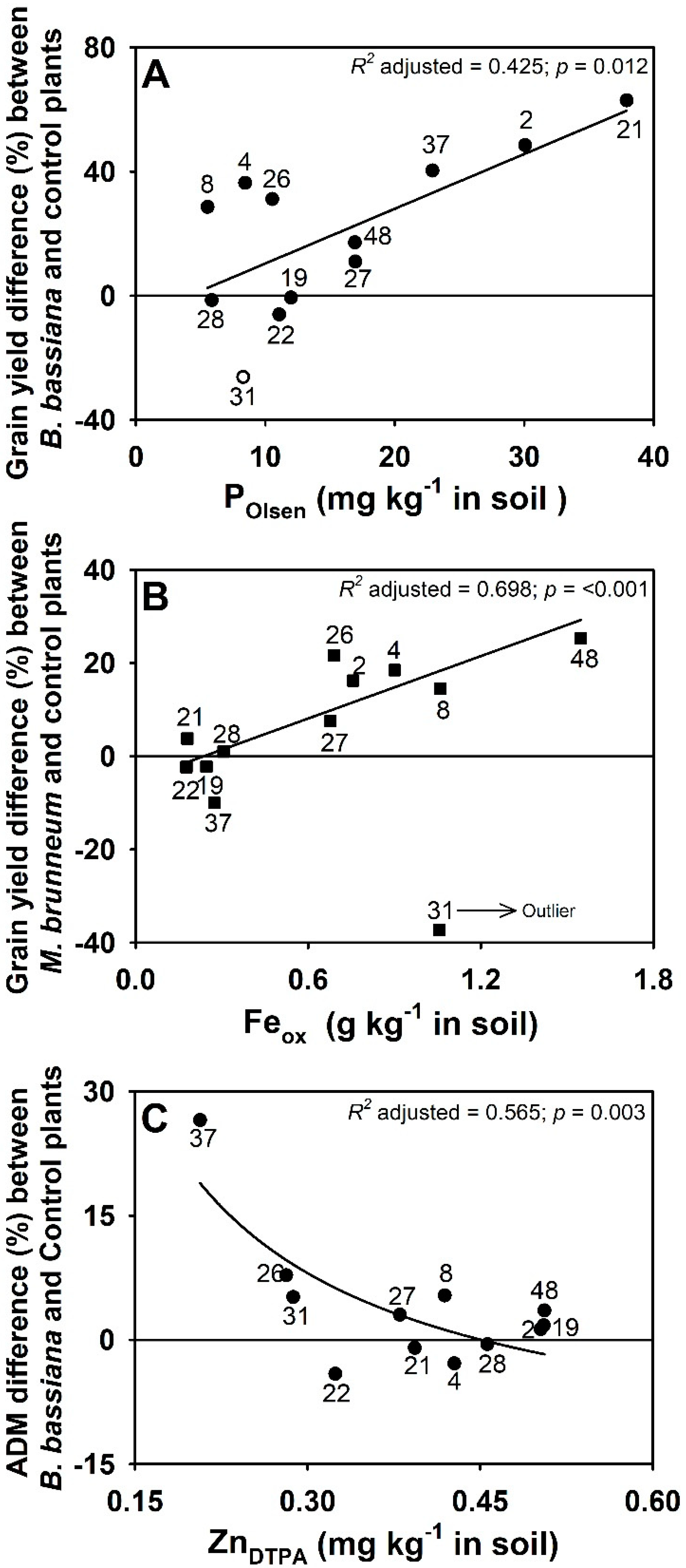
3.4. Effect of Fungal Inoculation on Plant Performance in Relation to Soil Properties
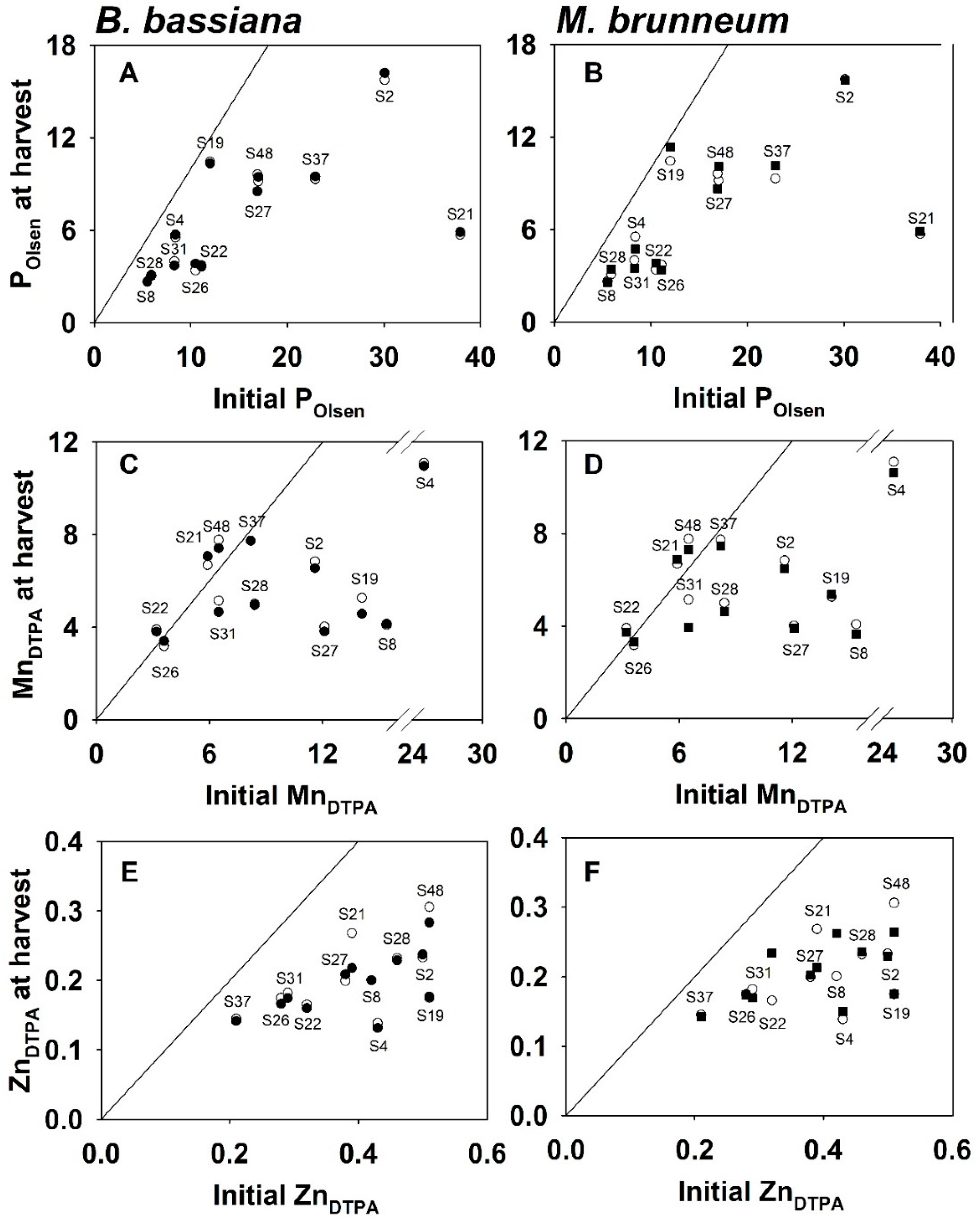
3.5. Changes in Nutrient Availability
4. Discussion
4.1. Plant Growth in Relation with Soil Nutrients
4.2. Effect of Fungal Inoculation on Plant Growth, Grain Yield, Photosynthesis Rate and Water Use Efficiency
4.3. Effect of Fungal Inoculation on Plant Nutrition and Grain Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Partida-Martínez, L.P.; Heil, M. The microbe-free plant: Fact or artifact. Front. Plant Sci. 2011, 2, 16. [Google Scholar] [CrossRef]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Yousef, M.; Lozano-Tovar, M.D.; Garrido-Jurado, I.; Quesada-Moraga, E. Biocontrol of Bactrocera oleae (Diptera: Tephritidae) with Metarhizium brunneum and its extracts. Biol. Microb. Control 2013, 106, 1118–1125. [Google Scholar] [CrossRef]
- Ownley, B.H.; Griffin, M.R.; Klingeman, W.E.; Gwinn, K.D.; Moulton, J.K.; Pereira, R.M. Beauveria bassiana: Endophytic colonization and plant disease control. J. Invertebr. Pathol. 2008, 98, 267–270. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Afolabi, O.G.; Hussain, M.; Qasim, M.; Wang, L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018, 217, 34–50. [Google Scholar] [CrossRef]
- Resquín-Romero, G.; Garrido-Jurado, I.; Delso, C.; Ríos-Moreno, A.; Quesada-Moraga, E. Transient endophytic colonizations of plants improve the outcome of foliar applications of mycoinsecticides against chewing insects. J. Invertebr. Pathol. 2016, 136, 23–31. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; López-Díaz, C.; Landa, B.B. The hidden habit of the entomopathogenic fungus Beauveria bassiana: First demonstration of vertical plant transmission. PLoS ONE 2014, 9, 8–13. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzón, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Raya-Díaz, S.; Quesada–Moraga, E.; Barrón, V.; del Campillo, M.C.; Sánchez–Rodríguez, A.R. Redefining the dose of the entomopathogenic fungus Metarhizium brunneum (Ascomycota, Hypocreales) to increase Fe bioavailability and promote plant growth in calcareous and sandy soils. Plant Soil 2017, 418, 387–404. [Google Scholar] [CrossRef]
- Jaber, L.R. Seed inoculation with endophytic fungal entomopathogens promotes plant growth and reduces crown and root rot (CRR) caused by Fusarium culmorum in wheat. Planta 2018, 248, 1525. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, A.R.; Raya-Díaz, S.; Zamarreños, Á.M.; García-Mina, J.M.; del Campillo, M.C.; Quesada-Moraga, E. An endophytic Beauveria bassiana strain increases spike production in bread and durum wheat plants and effectively controls cotton leafworm (Spodoptera littoralis) larvae. Biol. Control 2018, 116, 90–102. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; del Campillo, M.C.; Quesada-Moraga, E. Beauveria bassiana: An entomopathogenic fungus alleviates Fe chlorosis symptoms in plants grown on calcareous substrates. Sci. Hortic. (Amsterdam) 2015, 197, 193–202. [Google Scholar]
- Sánchez-Rodríguez, A.R.; Barrón, V.; del Campillo, M.C. The entomopathogenic fungus Metarhizium brunneum: A tool to alleviate Fe chlorosis. Plant Soil 2016, 406, 295–310. [Google Scholar] [CrossRef]
- Krell, V.; Unger, S.; Jakobs-Schoenwandt, D.; Patel, A.V. Endophytic Metarhizium brunneum mitigates nutrient deficits in potato and improves plant productivity and vitality. Fungal Ecol. 2018, 34, 43–49. [Google Scholar] [CrossRef]
- Raya-Díaz, S.; Sánchez-Rodríguez, A.R.; Segura-Fernández, J.M.; del Campillo, M.C.; Quesada-Moraga, E. Entomopathogenic fungi-based mechanisms for improved Fe nutrition in sorghum plants grown on calcareous substrates. PLoS ONE 2017, 12, 1–28. [Google Scholar]
- Garrido-Jurado, I.; Torrent, J.; Barrón, V.; Corpas, A.; Quesada-Moraga, E. Soil properties affect the availability, movement, and virulence of entomopathogenic fungi conidia against puparia of Ceratitis capitata (Diptera: Tephritidae). Biol. Control 2011, 58, 277–285. [Google Scholar] [CrossRef]
- Ryan, J.; Rashid, A.; Torrent, J.; Yau, S.K.; Ibrikci, H.; Sommer, R.; Erenoglu, E.B. Micronutrient constraints to crop production in the Middle East–West Asia region: Significance, research, and management. In Advances in Agronomy; Academic Press: San Diego, CA, USA, 2013; Volume 122, pp. 1–75. ISBN 9780124076853. [Google Scholar]
- Ryan, J.; Ibrikci, H.; Delgado, A.; Torrent, J.; Sommer, R.; Rashid, A. Significance of phosphorus for agriculture and the environment in the West Asia and North Africa region. In Advances in Agronomy; Elsevier Inc.: San Diego, CA, USA, 2012; Volume 114, pp. 91–153. ISBN 9780123942753. [Google Scholar]
- Matar, A.; Torrent, J.; Ryan, J. Soil and fertilizer phosphorus and crop responses in the dryland mediterranean zone. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1992; pp. 81–146. [Google Scholar]
- Rashid, A.; Ryan, J. Micronutrient constraints to crop production in soils with Mediterranean-type characteristics: A review. J. Plant Nutr. 2004, 27, 959–975. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Liu, Y.; Cui, Z.; Chen, X.; Zou, C. Zinc uptake and accumulation in winter wheat relative to changes in root morphology and mycorrhizal colonization following varying phosphorus application on calcareous soil. Field Crop. Res. 2016, 197, 74–82. [Google Scholar] [CrossRef]
- Sacristán, D.; González–Guzmán, A.; Barrón, V.; Torrent, J.; del Campillo, M.C. Phosphorus-induced zinc deficiency in wheat pot-grown on noncalcareous and calcareous soils of different properties. Arch. Agron. Soil Sci. 2019, 65, 208–223. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In USDA Circular No. 939; US Gov. Print. Office: Washington, DC, USA, 1954; p. 18. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Evans, L.T.; Rawson, H.M. Photosynthesis and respiration by the flag leaf and components of the ear during grain development in wheat. Aust. J. Biol. Sci. 1970, 23, 245–254. [Google Scholar] [CrossRef]
- Zasoski, R.J.; Burau, R.G. A rapid nitric-perchloric acid digestion method for multi-element tissue analysis. Commun. Soil Sci. Plant Anal. 1977, 8, 425–436. [Google Scholar] [CrossRef]
- Fan, M.S.; Zhao, F.J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef]
- Garvin, D.F.; Welch, R.M.; Finley, J.W. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm†‡. J. Sci. Food Agric. 2006, 87, 2213–2220. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Behie, S.W.; Moreira, C.C.; Sementchoukova, I.; Barelli, L.; Zelisko, P.M.; Bidochka, M.J. Carbon translocation from a plant to an insect-pathogenic endophytic fungus. Nat. Commun. 2017, 8, 5. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C. Agronomy for sustainable development. Ital. J. Agron. 2008, 3, 77–78. [Google Scholar]
- Huber, D.M.; Wilhelm, N.S. The role of manganese in resistance to plant diseases. In Manganese in Soils and Plants; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; Springer: Dordrecht, The Netherlands, 1988; Volume 33, pp. 155–173. ISBN 978-94-010-7768-2. [Google Scholar]
- Crush, J.R. Occurrence of endomycorrhizas in soils of the mackenzie basin, canterbury, New Zealand. N. Zeal. J. Agric. Res. 1975, 18, 361–364. [Google Scholar] [CrossRef]
- St Leger, R.J. Studies on adaptations of Metarhizium anisopliae to life in the soil. J. Invertebr. Pathol. 2008, 98, 271–276. [Google Scholar] [CrossRef]
- Rubio, M.B.; Hermosa, R.; Vicente, R.; Gómez-Acosta, F.A.; Morcuende, R.; Monte, E.; Bettiol, W. The combination of Trichoderma harzianum and chemical fertilization leads to the deregulation of phytohormone networking, preventing the adaptive responses of tomato plants to salt stress. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J. Parasitic and mutualistic associations between a mycorrhizal fungus and soybean: Development of the endophyte interactions. Physiol. Plant 1983, 57, 543–548. [Google Scholar] [CrossRef]
- Römer, W.; Schilling, G. Phosphorus requirements of the wheat plant in various stages of its life cycle. Plant Soil 1986, 91, 221–229. [Google Scholar] [CrossRef]
- Jeschke, W.D. Effects of transpiration on potassium and sodium fluxes in root cells and the regulation of ion distribution between roots and shoots of barley seedlings. J. Plant Physiol. 1984, 117, 267–285. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J. Plant Nutritional Genomics; Broadley, M.R., White, P.J., Eds.; Willey-Blackwell: Oxford, UK, 2005; ISBN 10 1-4051-2114-9. [Google Scholar]
- Bahmanyar, M.A.; Ranjbar, G.A. The role of potassium in improving growth indices and increasing amount of grain nutrient elements of wheat cultivars. J. Appl. Sci. 2008, 8, 1280–1285. [Google Scholar]
| Soil Code | Clay | OC | CCE | pH | EC | CEC | Feox | FeDTPA | CuDTPA | MnDTPA | ZnDTPA | Kavailable | POlsen | PCaCl2 | POlsen/ ZnDTPA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | µS cm−1 | cmolc kg−1 | g kg−1 | mg kg−1 | mg L−1 | ||||||||||
| Non-calcareous soils | |||||||||||||||
| 2 | 286 | 5 | 0 | 8.8 | 176 | 17 | 0.76 | 5.3 | 1.45 | 11.6 | 0.50 | 148 | 30.1 | 0.03 | 60 |
| 4 | 62 | 13 | 0 | 6.6 | 30 | 11 | 0.90 | 36.7 | 0.18 | 25.0 | 0.43 | 78 | 8.4 | 0.01 | 20 |
| 8 | 645 | 8 | 0 | 8.1 | 158 | 43 | 1.06 | 4.9 | 0.64 | 15.4 | 0.42 | 293 | 5.5 | 0.00 | 13 |
| Calcareous soils | |||||||||||||||
| 19 | 200 | 11 | 596 | 8.3 | 213 | 22 | 0.25 | 4.5 | 6.11 | 14.1 | 0.51 | 382 | 12.0 | 0.00 | 24 |
| 21 | 108 | 5 | 231 | 8.0 | 397 | 14 | 0.18 | 3.7 | 3.03 | 5.9 | 0.39 | 101 | 37.9 | 0.04 | 96 |
| 22 | 116 | 5 | 153 | 8.4 | 132 | 15 | 0.18 | 3.6 | 1.69 | 3.2 | 0.32 | 86 | 11.1 | 0.00 | 34 |
| 26 | 371 | 8 | 346 | 8.5 | 156 | 34 | 0.69 | 15.4 | 2.07 | 3.6 | 0.28 | 585 | 10.5 | 0.01 | 37 |
| 27 | 362 | 6 | 330 | 8.3 | 284 | 34 | 0.68 | 9.8 | 1.80 | 12.1 | 0.38 | 663 | 17.0 | 0.00 | 45 |
| 28 | 163 | 8 | 587 | 8.6 | 170 | 23 | 0.31 | 4.1 | 6.30 | 8.4 | 0.46 | 254 | 5.9 | 0.01 | 13 |
| 31 | 385 | 7 | 32 | 8.0 | 163 | 25 | 1.06 | 5.6 | 5.13 | 6.5 | 0.29 | 546 | 8.3 | 0.00 | 29 |
| 37 | 185 | 10 | 61 | 8.1 | 245 | 17 | 0.27 | 5.3 | 0.40 | 8.2 | 0.21 | 164 | 22.9 | 0.01 | 111 |
| 48 | 120 | 12 | 43 | 8.3 | 173 | 29 | 1.55 | 8.6 | 0.95 | 6.5 | 0.51 | 585 | 16.9 | 0.07 | 33 |
| Treatments | Grain Yield | Straw | Harvest Index ‡ | Grains | Tillers |
|---|---|---|---|---|---|
| g Plant‒1 | No. Plant‒1 | ||||
| Control | 0.634 ± 0.034 | 1.20 ± 0.06 | 0.35 ± 0.02 | 17.3 ± 0.96 | 1.29 ± 0.16 |
| B. bassiana | 0.741 ± 0.034 | 1.14 ± 0.05 | 0.40 ± 0.01 | 19.8 ± 0.86 | 1.19 ± 0.15 |
| p | 0.018 | 0.314 | 0.034 | <0.001 | 0.011 |
| M. brunneum | 0.670 ± 0.039 | 1.17 ± 0.06 | 0.37 ± 0.02 | 18.4 ± 1.03 | 1.11 ± 0.14 |
| p | 0.288 | 0.668 | 0.243 | <0.001 | <0.001 |
| Treatment | Aerial Dry Matter | Nutrient Uptake | Grain Nutrient Concentration | Grain P/Zn Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | Straw | P | K | Na | Zn | P | K | Na | Zn | ||
| g plant–1 | g plant−1 | µg plant−1 | g kg−1 | mg kg−1 | |||||||
| Control | 0.63 ± 0.05 | 1.45 ± 0.08 | 3.89 ± 0.36 | 46.2 ± 2.5 | 939 ± 146 | 52.2 ± 2.9 | 3.98 ± 0.24 | 4.73 ± 0.12 | 50.6 ± 2.9 | 51.6 ± 4.5 | 100 ± 11 |
| B. bassiana | 0.83 ± 0.05 | 1.31 ± 0.07 | 3.42 ± 0.32 | 50.5 ± 2.7 | 1102 ± 163 | 49.6 ± 2.3 | 3.19 ± 0.23 | 5.07 ± 0.16 | 56.3 ±4.4 | 42.5 ± 3.5 | 91 ± 10 |
| pFT§ | 0.002 | 0.04 | 0.022 | 0.011 | 0.042 | 0.306 | 0.000 | 0.067 | 0.549 | 0.027 | 0.776 |
| pinteraction | 0.977 | 0.295 | 0.181 | 0.049 | 0.217 | 0.707 | 0.496 | 0.332 | † | 0.871 | † |
| Control | 0.66 ± 0.07 | 1.38 ± 0.09 | 3.60 ± 0.44 | 44.8 ± 3.1 | 1077 ± 201 | 51.2 ± 1.84 | 3.78 ± 0.29 | 4.64 ± 0.09 | 54.2 ± 3.9 | 51.2 ± 5.2 | 99 ± 15 |
| M. brunneum | 0.78 ± 0.07 | 1.30 ± 0.08 | 3.62 ± 0.42 | 51.5 ± 3.9 | 1206 ± 238 | 49.2 ± 1.4 | 3.54 ± 0.25 | 5.00 ± 0.13 | 54.6 ± 4.2 | 46.4 ± 5.1 | 101 ± 14 |
| pFT§ | 0.187 | 0.205 | 0.882 | 0.023 | 0.399 | 0.319 | 0.46 | 0.041 | 0.788 | 0.468 | 0.650 |
| pinteraction | 0.970 | 0.978 | 0.976 | 0.186 | 0.524 | 0.522 | 1.000 | 0.227 | 0.264 | 0.978 | 0.982 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Guzmán, A.; Sacristán, D.; Sánchez-Rodríguez, A.R.; Barrón, V.; Torrent, J.; del Campillo, M.C. Soil Nutrients Effects on the Performance of Durum Wheat Inoculated with Entomopathogenic Fungi. Agronomy 2020, 10, 589. https://doi.org/10.3390/agronomy10040589
González-Guzmán A, Sacristán D, Sánchez-Rodríguez AR, Barrón V, Torrent J, del Campillo MC. Soil Nutrients Effects on the Performance of Durum Wheat Inoculated with Entomopathogenic Fungi. Agronomy. 2020; 10(4):589. https://doi.org/10.3390/agronomy10040589
Chicago/Turabian StyleGonzález-Guzmán, Adrián, Daniel Sacristán, Antonio Rafael Sánchez-Rodríguez, Vidal Barrón, José Torrent, and María Carmen del Campillo. 2020. "Soil Nutrients Effects on the Performance of Durum Wheat Inoculated with Entomopathogenic Fungi" Agronomy 10, no. 4: 589. https://doi.org/10.3390/agronomy10040589
APA StyleGonzález-Guzmán, A., Sacristán, D., Sánchez-Rodríguez, A. R., Barrón, V., Torrent, J., & del Campillo, M. C. (2020). Soil Nutrients Effects on the Performance of Durum Wheat Inoculated with Entomopathogenic Fungi. Agronomy, 10(4), 589. https://doi.org/10.3390/agronomy10040589





