Abstract
Soil-applied herbicides can persist in sufficient concentrations to affect the growth of crops in rotations. The sensitivity of wheat, barley, oat, lucerne and lentil to trifluralin and atrazine residues were investigated with three glasshouse experiments in 2018 and 2019. Each bioassay crop species was tested against different concentrations of trifluralin and atrazine in sandy soil using a full factorial design. Shoot and root parameters of the tested crop species were fitted in logistic equations against herbicide concentrations to calculate effective doses for 50% growth inhibition (ED50). Results revealed that both shoot and root parameters of all the test crop species were significantly affected by trifluralin and atrazine. Trifluralin delayed crop emergence at the lower concentrations examined, while higher concentrations prevented emergence entirely. Low concentrations of atrazine did not affect emergence but significantly reduced plant height, soil–plant analyses development (SPAD) index, shoot dry weight, root length, root dry weight and number of nodules of all the crop species. At high concentration, atrazine resulted in plant death. Legumes were found to be more sensitive than cereals when exposed to both trifluralin and atrazine treatments, with lucerne being the most sensitive to both herbicides, ED50 ranging from 0.01 to 0.07 mg/kg soil for trifluralin; and from 0.004 to 0.01 mg/kg for atrazine. Barley was the most tolerant species observed in terms of the two herbicides tested. Lucerne can be used to develop a simple but reliable bioassay technique to estimate herbicide residues in the soil so that a sound crop rotation strategy can be implemented.
1. Introduction
Farming systems in Australia have undergone a substantial revolution over the past 25 years with the adoption of conservation tillage. The trend towards minimum or zero till has reduced cultivation practices for weed management [1] and driven the increased adoption of herbicides as the primary mechanism for weed control [2,3]. This is a global issue, with herbicides commonly implemented to control weeds that are a persistent risk to crop production [4]. Herbicides account for approximately 60% of total pesticide expenditure across Australian farming systems, costing growers approximately $1.80 billion in 2017–2018 [5]. Herbicide adoption in farming systems has not only raised concerns about their negative impacts on the environment, human and animal health, and agricultural sustainability with the evolution of herbicide resistance, but also raised concerns about their ultimate fates in soil [6,7,8].
In systems employing conservation tillage, herbicide applications tend to leave a greater concentration of herbicide near the soil surface [9]. The presence of persistent herbicides in this concentrated zone may affect subsequent sensitive crops and compromise overall crop performance [10], thus limiting planting options for farmers. Precise assessment of the herbicide residues to gauge persistence and degradation patterns in soil is crucial for ensuring minimum risk in farming systems practicing crop rotation [11]. A survey of Australian cropping soils undertaken prior to sowing in 2016 detected residues of 23 herbicides, with trifluralin detected in more than 30% of the fields surveyed, with a maximum residue concentration of 5345 µg/kg compared to 590 µg/kg detected in 2015 [12]. The advancement of conservation tillage farming systems associated with relatively higher herbicide application rates may have contributed to the high detection frequency of trifluralin. In addition, higher concentrations of atrazine residues were also detected in cropping paddocks of New South Wales and South Australia, which was possibly due to the higher persistence of s-triazine herbicides in alkaline soils [13].
Trifluralin, developed in the 1970s, belongs to the dinitroaniline chemical group. It is a popular pre-emergent soil-incorporated herbicide used to control annual grass and broadleaf weeds in field crops [14]. Trifluralin interferes with mitosis and inhibits microtubule assembly by restricting polymerisation of tubulin [15,16,17], a structural protein of plant cells, subsequently inhibiting growth and resulting in plant death [18]. Being one of the most common pre-emergent herbicides used in conservation tillage systems [19,20], the role of trifluralin in the adoption of no-till farming systems has been acknowledged by several researchers [21,22,23]. Trifluralin is highly volatile due to high vapor pressure, and is generally incorporated into soil after application to reduce losses caused by volatilisation and photodegradation [24]. The level of persistence of trifluralin in soil is regulated by various factors, including soil moisture, temperature, soil type, and duration before incorporation [25,26,27].
Another common herbicide, atrazine, is often used alone or in combination with other herbicides to control grass and broadleaf weeds in field crops [28,29]. Introduced into the market almost 50 years ago, atrazine now ranks as the second most heavily used pesticide in the world [30]. Atrazine interferes with the photosystem II (PSII) process by affecting adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) production, ultimately reducing efficiency of the CO2 fixation process [31]. Atrazine is also responsible for the rapid accumulation of reactive oxygen species (ROS) by limiting electron transport system in chloroplasts, causing membrane injury [32,33]. Researchers suggest that atrazine is highly persistent in soil and can be detected even after 22 years of application [34], which indicates a high potential for the contamination of agricultural fields due to its low adsorption, moderate aqueous solubility, and long half-life.
Herbicide recommendation is fundamentally crop-specific. The sensitivity of different crop species to a particular herbicide is related to differences in crop morphology, physiology and phenology [35]. Some herbicides can remain in soil for weeks, months or even years. This is advantageous in regard to long term weed control. However, persistence can affect sensitive crop species by the residual activity of the herbicide in subsequent years [36]. Therefore, to minimise the negative impacts of herbicide residues in the soil on rotational crops, it is highly recommended to study the residual effects of herbicides on crops utilised in rotational systems [37]. As a result, this study was undertaken to investigate the potential impact of trifluralin and atrazine residues on the establishment and growth of selected cereals and legumes (wheat, barley, oat, lucerne and lentil). The development of a reliable and simple bioassay technique to identify sensitive crop species may allow informed decisions to be made prior to implementing a crop rotation strategy.
2. Materials and Methods
2.1. Test Crop Species, Herbicides and Soil Type
Three experiments were conducted under glasshouse conditions at Charles Sturt University, Wagga Wagga, New South Wales in 2018 and 2019. Five crops were chosen based on reported sensitivity to both trifluralin and atrazine [38], being wheat (Triticum aestivum cv. Corack), barley (Hordeum vulgare cv. Hindmarsh), oat (Avena sativa cv. Savannah), lucerne (Medicago sativa cv. Stamina) and lentil (Lens culinaris cv. Hurricane XT). Seeds of the test crop species were obtained from NSW Department of Primary Industries (DPI), Wagga Wagga, NSW and were tested for germination prior to use. Two commercial herbicides Triflur X (a.i. 480 g/L trifluralin) and Farmozine 900 WG (a.i. 900 g/kg atrazine) were used in this study. Non-autoclaved sandy soils were used to investigate the phytotoxicity associated with each herbicide because autoclaving the soil may alter the toxicity symptoms exhibited by the test crop in natural conditions due to the removal of degrading microorganisms [39]. The soil was determined to have a pH of 6.80 (water), organic matter <0.30%, organic carbon <0.20%, total N, P, and K was 4.00, 7.00 and 4.80 mg/kg, respectively. Prior to use the soil was sieved to 2 mm.
2.2. Preparation and Application of Herbicides at Different Concentrations
Based on a preliminary study, a concentration series of 0, 0.075, 0.15, 0.30, 0.60, 1.20 and 2.40 mg a.i./kg dry soil was prepared for trifluralin and a series of 0, 0.15, 0.30, 0.90, 1.50, 2.10 and 3.60 mg a.i./kg dry soil was prepared for atrazine. The experiment was repeated over time, using the above concentration series. Since legume species did not survive the minimum concentration of atrazine (0.15 mg/kg soil) used in first and second experiments (2018), a separate lower concentration series of 0, 0.006, 0.017, 0.05 and 0.15 mg/kg dry soil was prepared for the third trial in 2019.
Each herbicide concentrate was diluted with deionised water at required concentrations and applied by a hand sprayer onto the 1.0 kg dry soil equivalent while the soil was continuously mixed with a cement mixer to approximately 50% water holding capacity, according to Hasanuzzaman et al. [40]. Soils for control treatments were prepared by applying deionised water only.
2.3. Planting and Growing Test Crops
A previously published protocol on crop sensitivity to residual herbicide [41] was used in this study with modifications. Plastic pots (80 × 145 mm) were filled with each herbicide treated soil representing the concentration series. For each crop, five seeds were planted at a depth of 5 cm across the herbicide concentration series. For trifluralin, seven concentrations were tested against five crop species including control, with four replications in a factorial design. For atrazine, seven concentrations were tested against five crop species in first two trials and five lower concentrations were tested for the two legume species during the third trial only. Pots were placed on a bench and blocked by replicates. Pots were hand watered on daily basis with a hand sprayer to maintain moist conditions and allow seedling emergence but avoid overwatering and leaching of herbicides. Pots were maintained in a temperature-controlled glasshouse (30 ± 2 °C) under natural sunlight throughout the 4-week experimental period. Soon after emergence, seedlings were counted and thinned to 3 plants per pot.
2.4. Measurements
The total number of seedlings emerged was counted for each of the test crop species. Plant height and leaf chlorophyll content were measured at 28 days after sowing (DAS). Leaf chlorophyll content was calculated as a soil–plant analyses development (SPAD) index with the Minolta SPAD-502 (Konica Minolta Sensing). Measurements were carried out from the leaf lamina of the second uppermost leaves at three different points (tip, middle and base). After the chlorophyll measurements, aboveground parts were harvested by cutting the shoots approximately 2 mm above the soil surface. Shoots were labelled and bagged accordingly. Shoot dry weight (SDW) was determined after drying the samples at 70 °C for 48 h. Root samples were extracted by gently washing away the soil with tap water and then transferred to a Perspex tray containing deionised water. Samples were imaged at 600 dpi using a flatbed scanner (Epson Expression 11000XL). The scanned images were further analysed by WinRHIZO (Regent Instruments Inc., Quebec, Canada) to determine root length (RL, cm), mean root diameter (RD, mm) and specific root length. Thereafter, root samples were dehydrated at 70 °C for 48 h to determine the root dry weight (RDW). In case of legume species (lucerne and lentil), the total number of nodules were counted under magnification, using a Nikon SMZ25 motorised stereo zoom microscope.
2.5. Statistical Analysis
All the recorded data were transformed as a percent of control for each of the parameters in order to compare between different treatments of each herbicide tested in three successive trials (except mean root diameter). As there was no significant difference found between first two trials, hence the data were pooled. Statistical analysis was performed using software R operated in RStudio 3.5.3 [42] with a range of R packages including drc [43], ggplot2 [44] and cowplot [45] for explanatory data analysis. Data normality and distribution were validated by Q-Q plot and Shapiro-Wilk test of normality. The best fitted model was selected based on the Akaike information criterion (AIC) value. A non-linear two parameter log-logistic model (Equation (1)) was fitted for the emergence of test crop species (wheat, barley, oat, lucerne and lentil). Other shoot and root parameters of the test crop species were fitted in Weibull four parameter model (Equation (2)) except for trifluralin concentrations on root diameter and root dry weight of the test crop species and atrazine concentrations on the shoot dry weight of wheat, barley and oat best fitted on a four parameter log-logistic model (Equation (3)) [46]:
where d = the upper limit corresponding to the mean response of the control treatment, c = the lower limit corresponding to the mean response at the maximum herbicide dose levels, x = the herbicide dose level, e = the effective herbicide dose levels required for the 50% growth inhibition (ED50) and b = the slope of the curve around the inflection point e (ED50).
3. Results and Discussion
3.1. Effect of Trifluralin on the Test Crop Species
The development of trifluralin toxicity was monitored for each crop species from emergence to 28 DAS for each of the concentration series. From this evaluation, it was determined that trifluralin concentrations in soil had a significant effect on the emergence of the test crop species (P < 0.001). Emergence of the test species was delayed at the lowest concentration of 0.075 mg/kg. Trifluralin at the highest concentration of 2.40 mg/kg dry soil completely suppressed the emergence for all five test species (Figure 1A). Wheat and oat only emerged up to the trifluralin concentration of 0.30 mg/kg soil, while barley, lucerne and lentil had 27.98, 19.58 and 30.82% emergence at the concentration of 0.30 mg/kg, respectively. Almeida and Rodrigues [47] reported that trifluralin inhibits seed germination by interfering with cell division of meristematic tissues. It is due to adsorption primarily by the hypocotyl, followed by seedling radicles during germination [48,49].
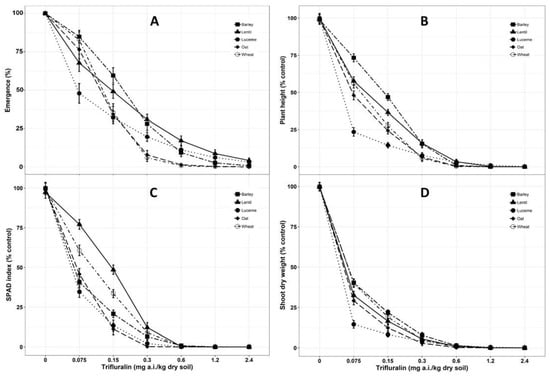
Figure 1.
Dose-response effects of trifluralin on shoot parameters (A. Emergence; B. Plant height; C. SPAD index; D. Shoot dry weight) of barley, lentil, lucerne, oat and wheat at 28 days after sowing (DAS). Lines denote predicted responses according to the equations reported in the materials and methods section. Symbols shown on the graphs are the original means of each parameter (% control) against each dose of trifluralin concentration. Mean value was pooled from eight observational units.
Toxicity symptoms associated with trifluralin residues were identified as stunted growth with twisted leaves, swollen hypocotyl and thickened primary root with no secondary roots. Similar types of toxicity symptoms were reported by Senseman [49], Deuber [50]. Trifluralin toxicity induced significant damage in respect to plant height with increasing levels of trifluralin concentration in the soil (Figure 1B). Approximately 50% inhibition was recorded in all the crop species under the lowest concentration of trifluralin (0.075 mg/kg soil), with the exception of barley (ED50 0.19 mg/kg). The ED50 value of lucerne (0.03 mg/kg) was significantly lower (P < 0.001) than the other crop species as no further elongation was observed after emergence (Table 1). Trifluralin interruption on cell mitosis has been acknowledged in literature [51] which resulted in inhibition of root and shoot cell division [52]. Khalil et al. [53] identified shoot length as the most sensitive parameter to assess trifluralin activity due to sensitivity of the coleoptile node documented in green foxtail [54,55]. Residual activity of trifluralin has been reported to reduce at least 44% plant height in sesame [56] and 80% in rice [57] as compared to control.

Table 1.
Estimated regression parameters of the two parameter log-logistic model, four parameter Weibull model (Weibull1) and four parameter log-logistic model for the shoot and root parameters of wheat, barley, oat, lucerne and lentil against various levels of trifluralin concentrations as per equations 1, 2 and 3 mentioned in materials and methods section.
In terms of leaf chlorophyll content, a significant reduction (P < 0.001) in SPAD index were recorded in all plants treated with trifluralin, with the presence of twisted and yellowing leaves. Increasing trifluralin soil concentrations from 0.075 to 0.30 mg/kg soil resulted in the gradual reduction of SPAD index (Figure 1C). Lucerne had the lowest SPAD index at 0.075 mg/kg soil trifluralin concentration compared to others (ED50 0.07 mg/kg) (Table 1).
Shoot dry weight was considerably reduced with the increase of trifluralin concentrations in soil, causing as high as 60–70% reduction at the lowest concentration (0.075 mg/kg) compared to control, while plant death occurred at the highest levels (Figure 1D). Lucerne was the most sensitive to trifluralin concentrations compared to other crops as the ED50 value was significantly lower (P < 0.001) than others (0.01 mg/kg soil) (Table 1). Chaudhari et al. [58] reported considerable shoot dry weight reduction (up to 89%) of turnip when exposed to trifluralin at concentrations of approximately 1.70 mg/kg soil. Nosratti et al. [59] identified the toxicity symptoms of trifluralin including reduction in plant height, chlorophyll content and shoot dry weight.
For the root parameters measured, root length, root dry weight and nodulation were highly affected by the presence of trifluralin in the soil. Root length of all the species was reduced by 60–80% even at minimum concentrations of trifluralin in soil (Figure 2A). The affected roots were characterised by a thickening of hypocotyls, swollen primary root and absence of lateral root and root hairs, which are similar to results previously reported [49,50]. Oat was the most sensitive crop in regards to root length (ED50 0.01 mg/kg) in both of the trials whereas lucerne performed better (ED50 0.09 mg/kg) (Table 1). Wheat, oat and lucerne roots died at 0.30 mg/kg concentration, while lentil roots exhibited their maximum mean root diameter (2.14 mm) at the trifluralin concentration of 0.30 mg/kg soil, which is four times that of the control (Figure 2B). However, trifluralin even at the lowest concentration of 0.075 mg/kg soil significantly reduced (P < 0.001) root dry weight of all the crop species, with reductions varying from 95% in oat to 69% in lucerne compared to the untreated control (Figure 2C). Root development was greatly hampered due to trifluralin toxicity as root dry weight is known to be the most sensitive and precise measures for trifluralin toxicity [60,61]. The ED50 for oat in terms of root dry weight was 0.01 mg/kg soil (Table 1), significantly lower than the other crop species (P < 0.001). Trifluralin interfered with the nodulation process of legume species even at the lowest application rate and the nodulation was completely inhibited at 0.30 mg/kg soil concentration (Figure 2D). Variable levels of plant injury and growth reduction due to trifluralin have been reported due to the differences in soil type, temperature, soil moisture and duration of incorporation [25,26,58,62]. Soil organic matter tends to reduce the bioavailability of trifluralin in soil via sorption [63], which is correlated with an increase in soil organic matter [64]. Soils containing high organic matter were reported to adsorb herbicide compounds more likely compared to those with low organic matter [65]. Thus, trifluralin injury in sandy soils was found to be prominent and logical in this experiment as the organic matter content was negligible.
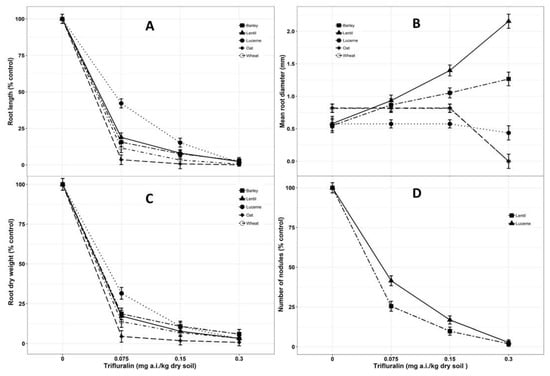
Figure 2.
Effect of various levels of trifluralin concentrations on root parameters (A. Root length; B. Mean root diameter; C. Root dry weight; D. Number of nodules) of barley, lentil, lucerne, oat and wheat at 28 days after sowing (DAS). Lines denote predicted responses according to the equations reported in the materials and methods section. Symbols shown on the graphs are the original means of each parameter (% control) against each dose of trifluralin concentration. Mean value was pooled from eight observational units.
3.2. Effect of Atrazine on the Test Crop Species
Although pre-emergence application of atrazine primarily targets germination of weed seeds, no significant effect on the emergence of the crop species was observed in all experiments. However, atrazine caused considerable damage to all tested crop species in the current study, regardless of the application rate. Initial toxicity symptoms appeared after two weeks of growth and became more prominent on the tips and edges of the mature leaves, manifested by chlorosis later spreading both upwards and downwards ultimately affecting height, chlorophyll content and shoot dry weight of all the species. Plants died over time due to the inhibition of photosynthesis. Shoots were more affected compared to roots, regardless of the crop species, even though atrazine is reported to concentrate in roots compared to shoots [66]. The plant heights of the three cereal crops were reduced significantly (P < 0.001) with the increased concentrations of atrazine (Figure 3A). In the third experiment, lucerne survived at the lowest atrazine concentration (0.006 mg/kg soil) and was identified as the most sensitive species based on plant height (Figure 3B). At the atrazine concentration 0.15 mg/kg, both lucerne and lentil did not survive, but the cereals had a plant height that was approximately 65% of the control. This study revealed that cereal crops (wheat, barley and oat) were relatively more tolerant to atrazine concentrations than the legume species (lucerne and lentil). Barley was the most tolerant species (ED50 1.21 mg/kg) as it managed to survive under the highest concentration of atrazine tested (3.60 mg/kg soil) while all other crop species died at this concentration (Table 2). Zhang et al. [67] reported 67.10% reduction in shoot length of rice when exposed to 0.40 mg/L of atrazine compared to control.
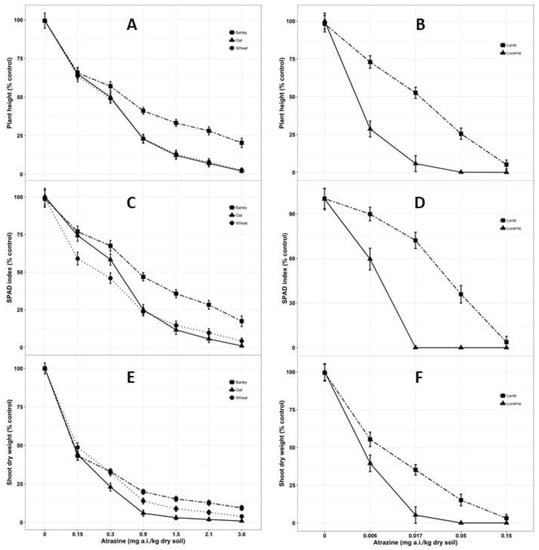
Figure 3.
Dose-response assay of atrazine concentrations on plant height (A. barley, wheat and oat; B. lentil and lucerne), SPAD index (C. barley, wheat and oat; D. lentil and lucerne) and shoot dry weight (E. barley, wheat and oat; F. lentil and lucerne) at 28 days after sowing (DAS). Lines denote predicted responses according to the equations reported in the materials and methods section. Symbols shown on the graphs are the original means of each parameters (% control) against each doses of atrazine concentration. Mean value was pooled from eight observational units for wheat, barley and oat; whereas four observational units for lentil and lucerne.

Table 2.
Estimated regression parameters of the four parameter Weibull model (Weibull1) and four parameter log-logistic model for the shoot and root parameters of wheat, barley, oat, lucerne and lentil against various levels of atrazine concentrations as per Equations (2) and (3) mentioned in materials and methods section.
Chlorophyll is regarded as a sensitive biomarker for plant growth [68]. Figure 3C revealed that the SPAD index of all the crop species followed a decreasing pattern with increasing concentration of atrazine and all the plants but barley died due to chlorosis at the highest concentrations in extreme conditions. Lucerne and lentil were the most sensitive species in terms of the SPAD index. Lentil performed better than lucerne as it did not survive the atrazine concentration of 0.017 mg/kg soil, whereas 30% reduction in SPAD index was observed in case of lentil (Figure 3D). Huiyun et al. [69] reported that chlorophyll content inhibition was positively correlated with dosage of atrazine. Barley was the only species that survived the maximum atrazine concentrations (3.60 mg/kg soil) with an ED50 value of 1.46 mg/kg, significantly higher (P < 0.001) than the other crop species, although the SPAD index decreased to one third of the control (Table 2). Chlorophyll content acts as an indicator of the growth and photosynthetic ability of the plant [70]. A decrease in chlorophyll content with the increase of atrazine concentrations in soil indicates the negative effects of atrazine on the growth of plants [69]. Chlorophyll content of rice exposed to 0.80 mg/L atrazine was reduced by 60% compared to the untreated control [67].
A reduction in shoot dry weight was observed with the increase of atrazine residue concentration in soil (Figure 3E,F). Approximately 55% reduction of shoot dry weight has been recorded in wheat, barley and oat when exposed to the lowest atrazine concentration (0.15 mg/kg soil), whereas legume species were more sensitive than cereals, having 100% reduction in shoot dry weight at the same atrazine concentration (0.15 mg/kg soil). Higher application rate of atrazine resulted in higher reduction in shoot dry weight which can be related to the higher exposure and absorption of the atrazine residues in soil accompanying root growth. Lucerne had ED50 values for shoot dry weight 0.01 mg/kg, which was significantly lower (P < 0.001) than other crop species (Table 2). Reduction in plant height and SPAD index due to exposure of different levels of atrazine might have contributed to the reduction in the shoot dry weight of all the crop species. Atrazine plays an essential role on seedling growth, inhibiting rice shoot dry weight to an extent of 39% [66] and 48.90% [67]. Phytotoxicity associated with atrazine to wheat, corn, mustard, turnip, pearl-millet and carrot has also been reported by Burhan, et al [71].
Atrazine residues had adverse effects on the root length, root dry weight and nodulation, depending on the concentrations in the soil. It did not affect the mean root diameter and specific root length. Atrazine at the lowest concentration of 0.15 mg/kg soil caused 45–65% reduction in root length in wheat, barley and oat (Figure 4A); while lentil and lucerne had 50 and 78% reduction at 0.006 mg/kg soil atrazine concentration (Figure 4B). Lentil experienced greater reduction in root length than lucerne, with the ED50 values of 0.003 mg/kg soil significantly lower (P < 0.001) compared to others. Figure 4C,D depicted similar results regarding root dry weight. Wheat, barley and oat exhibited similar type of sensitivity towards different atrazine levels with 60–75% reductions in root dry weight at 0.15 mg/kg soil atrazine concentration.
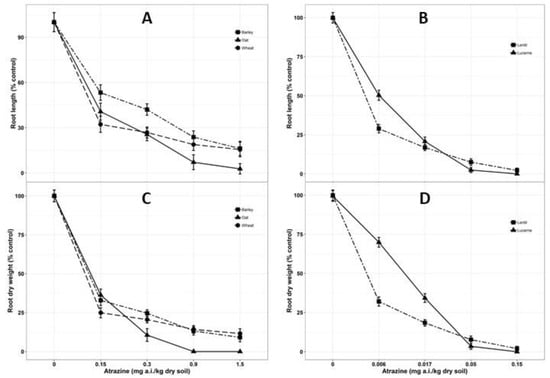
Figure 4.
Effect of different levels of atrazine concentrations on root length (A. barley, wheat and oat; B. lentil and lucerne) and root dry weight (C. barley, wheat and oat; D. lentil and lucerne) at 28 days after sowing (DAS). Lines denote predicted responses according to the equations reported in the materials and methods section. Symbols shown on the graphs are the original means of each parameter (% control) against each dose of trifluralin concentration. Mean value was pooled from eight observational units for wheat, barley and oat; whereas four observational units for lentil and lucerne.
Lucerne and lentil were more sensitive and had approximately 65–75% reductions at 0.017 mg/kg concentration. Oat recorded the highest ED50 value 0.15 mg/kg soil and lentil the least, 0.004 mg/kg soil (Table 2). Reduction of root dry weight due to various levels of atrazine exposure have been acknowledged in the literature [66,67]. Atrazine in soil had a significant effect (P < 0.001) on nodulation in legume species. During the first and second trial, no nodule was seen under minimum atrazine concentration (0.15 mg/kg soil) studied and all the plants died after emergence, whereas in the third trial, 62 and 73% inhibition of nodulation was observed in lucerne and lentil under the minimum residue concentration (0.006 mg/kg soil) when compared to the untreated control (Figure 5). No nodules were formed when legumes were exposed to atrazine concentrations more than 0.017 mg/kg of soil. Root is the primary organ by which plants absorb water, nutrient and pollutants as well [72]. Moreover, the majority of the absorbed substances are accumulated in the roots, although some of them are transported to other parts [73]. Therefore, it is apparent that accumulated atrazine in roots caused a reduction in root length, root dry weight and nodulation by damaging cell membranes through oxidative stress, as atrazine can produce reactive oxygen species [32,33].
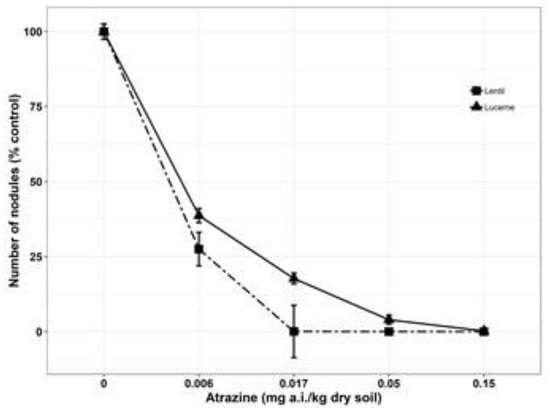
Figure 5.
Effect of different levels of atrazine concentrations on number of nodules of lucerne and lentil at 28 days after sowing (DAS). Lines denote predicted responses according to the equations reported in the materials and methods section. Symbols shown on the graphs are the original means of each parameter (% control) against each dose of trifluralin concentration. Mean value was pooled from four observational units for lentil and lucerne.
4. Conclusions
The described bioassay technique provides an indication of herbicide residues remaining in soil, especially with those herbicides having persistence potential. Phytotoxicity associated with pre-emergence herbicide depends on the extent of herbicides bounded by the organic matter of soil. To investigate the actual phytotoxicity, interference of organic matter has been minimised by using sandy soils in this study. Our study revealed that trifluralin and atrazine in the soil can affect the emergence and growth of wheat, barley, oat, lucerne and lentil with the legume species being more sensitive than the cereals. This study revealed that lucerne was the most sensitive crop species compared to others and barley was the most tolerant towards trifluralin and atrazine in the soil. Hence, farmers should be careful about the carry-over issues of trifluralin and atrazine prior to selecting legumes in crop rotation. Lucerne can be used in soil bioassays to quickly determine the levels of herbicide residues in the soil so that suitable crops can be chosen prior to sowing.
Author Contributions
Conceptualisation, H.W., I.F.C., G.S.D. and B.J.S.; Methodology, H.W., G.S.D. and B.J.S.; Software, I.F.C.; Validation, H.W., B.J.S., C.C. and G.S.D.; Formal Analysis, I.F.C.; Investigation, I.F.C.; Resources, H.W.; Data Curation, I.F.C.; Writing–Original Draft Preparation, I.F.C.; Writing–Review & Editing, H.W., B.J.S. and G.S.D.; Visualisation, I.F.C.; Supervision, H.W., B.J.S., C.C. and G.S.D.; Project Administration, I.F.C.; Funding Acquisition, I.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
Imtiaz Faruk Chowdhury acknowledges Charles Sturt University, for the award of an Australian Government Research Training Program (AGRTP) Scholarship for pursuing his PhD. Authors acknowledge the financial support provided by the Graham Centre for Agricultural Innovation for publication costs.
Acknowledgments
Imtiaz Faruk Chowdhury is thankful to New South Wales Department of Primary Industries (NSW DPI), Wagga Wagga, Australia for technical support throughout the experimentation. He is also thankful to Mr. Graeme Sandral from NSW DPI, Wagga Wagga for assistance with WinRHIZO.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Congreve, M.; Cameron, J. Soil Behaviour of Pre-Emergent Herbicides in Australian Farming Systems: A Reference Manual for Agronomic Advisers; Grains Research and Development Corporation: Canberra, Australia, 2014. [Google Scholar]
- D’Emden, F.H.; Llewellyn, R.S.; Burton, M.P. Adoption of conservation tillage in Australian cropping regions: An application of duration analysis. Technol. Forecast. Soc. Chang. 2006, 73, 630–647. [Google Scholar] [CrossRef]
- Lewis, S.E.; Silburn, D.M.; Kookana, R.S.; Shaw, M. Pesticide behavior, fate, and effects in the tropics: An overview of the current state of knowledge. J. Agric. Food Chem. 2016, 64, 3917–3924. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.G.; Kukulies, T.; Forstner, C.; Orton, T.G.; Pattison, A.B. The effects of glyphosate, glufosinate, paraquat and paraquat-diquat on soil microbial activity and bacterial, archaeal and nematode diversity. Sci. Rep. 2018, 8, 2119. [Google Scholar] [CrossRef] [PubMed]
- APVMA. Gazette of Agricultural and Veterinary Chemicals; Australian Pesticides and Veterinary Medicines Authority: Armidale, Australia, 19 December 2019; p. 16. [Google Scholar]
- Daam, M.A.; Van den Brink, P.J. Implications of differences between temperate and tropical freshwater ecosystems for the ecological risk assessment of pesticides. Ecotoxicology 2010, 19, 24–37. [Google Scholar] [CrossRef]
- Lacher, T.E., Jr.; Goldstein, M.I. Tropical ecotoxicology: Status and needs. Environ. Toxicol. Chem. 1997, 16, 100–111. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Hyne, R.V. Comparison of environmental risks of pesticides between tropical and nontropical regions. Integr. Environ. Assess. Manag. 2011, 7, 577–586. [Google Scholar] [CrossRef]
- Curran, W.S. Persistence of herbicides in soil. Crops and Soils 2016, 49, 16–21. [Google Scholar] [CrossRef]
- Yu, L.; Van Eerd, L.L.; O’Halloran, I.; Sikkema, P.H.; Robinson, D.E. Response of four spring-seeded cover crops to residues of selected herbicides. Can. J. Plant Sci. 2015, 95, 303–313. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Alebrahim, M.T.; Roushani, M. Determination of two sulfonylurea herbicides residues in soil environment using HPLC and phytotoxicity of these herbicides by lentil bioassay. Bull. Environ. Contam. Toxicol. 2017, 99, 93–99. [Google Scholar] [CrossRef]
- Rose, M.; Van Zwieten, L.; Zhang, P.; McGrath, G.; Seymour, N.; Scanlan, C.; Rose, T. Herbicide residues in soil—What is the scale and significance? GRDC project code: DAN00180; 12 Feb 2019. GRDC Grains Research Update, 2019. [Google Scholar]
- Van Zwieten, L.; Rose, M.; Zhang, P.; Nguyen, D.; Scanlan, C.; Rose, T.; McGrath, G.; Vancov, T.; Cavagnaro, T.; Seymour, N. Herbicide residues in soils–are they an issue? GRDC project code: DAN00180; 23 Feb 2016. GRDC Grains Research Update, 2016; 117. [Google Scholar]
- Chen, J.; Goggin, D.; Han, H.; Busi, R.; Yu, Q.; Powles, S. Enhanced trifluralin metabolism can confer resistance in Lolium rigidum. J. Agric. Food Chem. 2018, 66, 7589–7596. [Google Scholar] [CrossRef]
- Blume, Y.B.; Nyporko, A.Y.; Yemets, A.I.; Baird, W.V. Structural modeling of the interaction of plant α-tubulin with dinitroaniline and phosphoroamidate herbicides. Cell Biol. Int. 2003, 27, 171–174. [Google Scholar] [CrossRef]
- Nyporko, A.Y.; Blume, Y.B. Spatial distribution of tubulin mutations conferring resistance to antimicrotubular compounds. In The Plant Cytoskeleton: A Key Tool for Agro-Biotechnology; Blume, Y.B., Baird, W.V., Yemets, A.I., Breviario, D., Eds.; Springer: Cham, The Netherlands, 2009; pp. 397–417. [Google Scholar]
- Nyporko, A.Y.; Blume, Y.B. Structural mechanisms of interaction of cyanolcrylates with plant tubulin. Cytol. Genet. 2014, 48, 7–14. [Google Scholar] [CrossRef]
- Breviario, D.; Nick, P. Plant tubulins: A melting pot for basic questions and promising applications. Transgenic Res. 2000, 9, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Boutsalis, P.; Gill, G.S.; Preston, C. Incidence of herbicide resistance in rigid ryegrass (Lolium rigidum) across southeastern Australia. Weed Technol. 2012, 26, 391–398. [Google Scholar] [CrossRef]
- Saini, R.K.; Kleemann, S.G.; Preston, C.; Gill, G.S. Alternative herbicides for the management of clethodim-resistant rigid ryegrass (Lolium rigidum) in faba bean (Vicia faba L.) in Southern Australia. Weed Technol. 2015, 29, 578–586. [Google Scholar] [CrossRef]
- Chauhan, B.; Gill, G.; Preston, C. Tillage system effects on weed ecology, herbicide activity and persistence: A review. Aust. J. Exp. Agric. 2006, 46, 1557–1570. [Google Scholar] [CrossRef]
- D’Emden, F.H.; Llewellyn, R.S.; Burton, M.P. Factors influencing adoption of conservation tillage in Australian cropping regions. Aust. J. Agric. Resour. Econ. 2008, 52, 169–182. [Google Scholar] [CrossRef]
- Llewellyn, R.S.; D’Emden, F.H.; Kuehne, G. Extensive use of no-tillage in grain growing regions of Australia. Field Crops Res. 2012, 132, 204–212. [Google Scholar] [CrossRef]
- Savage, K.; Barrentine, W. Trifluralin persistence as affected by depth of soil incorporation. Weed Sci. 1969, 17, 349–352. [Google Scholar] [CrossRef]
- Horowitz, M.; Hulin, N.; Blumenfeld, T. Behaviour and persistence of trifluralin in soil. Weed Res. 1974, 14, 213–220. [Google Scholar] [CrossRef]
- Kennedy, J.M.; Talbert, R.E. Comparative persistence of dinitroaniline type herbicides on the soil surface. Weed Sci. 1977, 25, 373–381. [Google Scholar] [CrossRef]
- Messersmith, C.; Burnside, O.; Lavy, T. Biological and non-biological dissipation of trifluralin from soil. Weed Sci. 1971, 19, 285–290. [Google Scholar] [CrossRef]
- Zhang, J.J.; Lu, Y.C.; Yang, H. Chemical modification and degradation of Atrazine in Medicago sativa through multiple pathways. J. Agric. Food Chem. 2014, 62, 9657–9668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, L.; Ma, F.; Bai, S.; Yang, J.; Qi, S. Pseudomonas sp. ZXY-1, a newly isolated and highly efficient atrazine-degrading bacterium, and optimization of biodegradation using response surface methodology. J. Environ. Sci. 2017, 54, 152–159. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y.; Wan, J.; Gong, X.; Zhu, Y. Degradation of atrazine by a novel Fenton-like process and assessment the influence on the treated soil. J. Hazard. Mater. 2016, 312, 184–191. [Google Scholar] [CrossRef]
- Qian, H.; Tsuji, T.; Endo, T.; Sato, F. PGR5 and NDH pathways in photosynthetic cyclic electron transfer respond differently to sublethal treatment with photosystem-interfering herbicides. J. Agric. Food Chem. 2014, 62, 4083–4089. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Alexieva, V.S.; Karanov, E.N. Cumulative effect of low and high atrazine concentrations on Arabidopsis thaliana plants. Russ. J. Plant Physiol. 2005, 52, 213–219. [Google Scholar] [CrossRef]
- Wang, Q.; Que, X.; Zheng, R.; Pang, Z.; Li, C.; Xiao, B. Phytotoxicity assessment of atrazine on growth and physiology of three emergent plants. Environ. Sci. Pollut. Res. 2015, 22, 9646–9657. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Köppchen, S.; Hofmann, D.; Schäffer, A.; Burauel, P. Persistence of 14C-labeled atrazine and its residues in a field lysimeter soil after 22 years. Environ. Pollut. 2009, 157, 2126–2131. [Google Scholar] [CrossRef]
- Li, Z.; Wehtje, G.R.; Walker, R.H. Physiological basis for the differential tolerance of Glycine max to sulfentrazone during seed germination. Weed Sci. 2000, 48, 281–285. [Google Scholar] [CrossRef]
- Colquhoun, J. Herbicide Persistence and Carryover; University of Wisconsin Extension A: Madison, WI, USA, 2006; p. 3819. [Google Scholar]
- Yadav, A.; Malik, R.K.; Punia, S.S.; Mehta, R.; Bir, D. Studies on carry-over effects of herbicides applied in wheat on the succeeding crops in rotation. Indian J. Weed Sci. 2004, 36, 15–18. [Google Scholar]
- Frank, R.; Sirons, G.; Anderson, G. Atrazine: The impact of persistent residues in soil on susceptible crop species. Can. J. Soil Sci. 1983, 63, 315–325. [Google Scholar] [CrossRef]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Autoclaving kills soil microbes yet soil enzymes remain active. Pedobiologia 2007, 51, 295–299. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Shabala, L.; Brodribb, T.J.; Zhou, M.; Shabala, S. Assessing the suitability of various screening methods as a proxy for drought tolerance in barley. Funct. Plant Biol. 2017, 44, 253–266. [Google Scholar] [CrossRef]
- Rose, T.J.; Van Zwieten, L.; Claassens, A.; Scanlan, C.; Rose, M.T. Phytotoxicity of soilborne glyphosate residues is influenced by the method of phosphorus fertiliser application. Plant. Soil 2018, 422, 455–465. [Google Scholar] [CrossRef]
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for “ggplot2”. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 16 January 2020).
- Wild, C.J.; Seber, G.A.F. Nonlinear Regression; John Wiley & Sons: New York, NY, USA, 1989; Volume 46, pp. 86–88. [Google Scholar]
- Almeida, F.S.; Rodrigues, B.N. Guia de Herbicidas-Contribuição Para o Uso Adequado em Plantio Direto e Convencional; Iapar: Fundacão Instituto Agronoˆmico do Parana, Londrina, 1985; p. 482. [Google Scholar]
- Rodrigues, B.N.; Almeida, F.S. Guide of Herbicides; IAPAR: Londrina, Brazil, 2005. [Google Scholar]
- Senseman, S.A. Herbicide Handbook; 1891276565; Weed Science Society of America: Lawrence, KS, USA, 2007; p. 458. [Google Scholar]
- Deuber, R. Botânica das plantas daninhas. Ciência das Plantas Daninhas; Deuber, R., Ed.; FUNEP: Jaboticabal, Brazil, 1992; pp. 31–73. [Google Scholar]
- Fernandes, T.C.C.; Pizano, M.A.; Marin-Morales, M.A. Characterization, modes of action and effects of trifluralin: A review. In Herbicides-Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; IntechOpen: London, UK, 2013; pp. 489–517. [Google Scholar]
- Shaner, D.L. Herbicide Handbook; 0615989373; Weed Science Society of America: Champaign, IL, USA, 2014; p. 315. [Google Scholar]
- Khalil, Y.; Siddique, K.H.M.; Ward, P.; Piggin, C.; Bong, S.H.; Nambiar, S.; Trengove, R.; Flower, K. A bioassay for prosulfocarb, pyroxasulfone and trifluralin detection and quantification in soil and crop residues. Crop. Pasture Sci. 2018, 69, 606–616. [Google Scholar] [CrossRef]
- Appleby, A.P.; Valverde, B.E. Behavior of dinitroaniline herbicides in plants. Weed Technol. 1989, 3, 198–206. [Google Scholar] [CrossRef]
- Rahman, A.; Ashford, R. Selective action of trifluralin for control of green foxtail in wheat. Weed Sci. 1970, 18, 754–759. [Google Scholar] [CrossRef]
- Grichar, W.J.; Sestak, D.C.; Brewer, K.D.; Besler, B.A.; Stichler, C.R.; Smith, D.T. Sesame (Sesamum indicum L.) tolerance and weed control with soil-applied herbicides. Crop. Protect. 2001, 20, 389–394. [Google Scholar] [CrossRef]
- Lawrence, B.H.; Bond, J.A.; Edwards, H.M.; Golden, B.R.; Montgomery, G.B.; Eubank, T.W.; Walker, T.W. Effect of fall-applied residual herbicides on rice growth and yield. Weed Technol. 2018, 32, 526–531. [Google Scholar] [CrossRef]
- Chaudhari, S.; Jennings, K.M.; Culpepper, S.; Batts, R.B.; Bellinder, R. Turnip tolerance to preplant incorporated trifluralin. Weed Technol. 2019, 33, 123–127. [Google Scholar] [CrossRef]
- Nosratti, I.; Mahdavi-Rad, S.; Heidari, H.; Saeidi, M. Differential tolerance of pumpkin species to bentazon, metribuzin, trifluralin, and oxyfluorfen. Planta Daninha 2017, 35, 1–9. [Google Scholar] [CrossRef]
- Roggenbuck, F.C.; Penner, D. Factors influencing corn (Zea mays) tolerance to trifluralin. Weed Sci. 1987, 35, 89–94. [Google Scholar] [CrossRef]
- Vencill, W.K. Herbicide Handbook, 8th ed.; Vencill, W.K., Ed.; Weed Science Society of America: Lawrence, KS, USA, 2002; pp. 457–462. [Google Scholar]
- Savage, K. Persistence of several dinitroaniline herbicides as affected by soil moisture. Weed Sci. 1978, 26, 465–471. [Google Scholar] [CrossRef]
- Kenaga, E.E. Predicted bioconcentration factors and soil sorption coefficients of pesticides and other chemicals. Ecotoxicol. Environ. Saf. 1980, 4, 26–38. [Google Scholar] [CrossRef]
- Weber, J.B. Behavior of dinitroaniline herbicides in soils. Weed Technol. 1990, 4, 394–406. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Datta, A.; Scott, J.; Porpiglia, P.J. Dose–response curves of KIH-485 for preemergence weed control in corn. Weed Technol. 2009, 23, 34–39. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhu, Y.G.; Lin, A.J.; Zhang, X.H. Interaction between cadmium and atrazine during uptake by rice seedlings (Oryza sativa L.). Chemosphere 2005, 60, 802–809. [Google Scholar] [CrossRef]
- Zhang, J.J.; Lu, Y.C.; Zhang, J.J.; Tan, L.R.; Yang, H. Accumulation and toxicological response of atrazine in rice crops. Ecotoxicol. Environ. Saf. 2014, 102, 105–112. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Zheng, Q.; Liu, Z.P.; Yang, Z.M. Regulation of tolerance of Chlamydomonas reinhardtii to heavy metal toxicity by heme oxygenase-1 and carbon monoxide. Plant. and Cell Physiol. 2011, 52, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Huiyun, P.A.N.; Xiaolu, L.I.; Xiaohua, X.U.; Shixiang, G.A.O. Phytotoxicity of four herbicides on Ceratophyllum demersum, Vallisneria natans and Elodea nuttallii. J. Environ. Sci. 2009, 21, 307–312. [Google Scholar]
- Liu, A.; Zhang, Y.; Chen, D. Effects of salt stress on the growth and the antioxidant enzyme activity of Thellungiella halophila. Bull. Bot. Res. 2006, 26, 216–221. [Google Scholar]
- Burhan, N.; Shaukat, S.S. Effects of atrazine and phenolic compounds on germination and seedling growth of some crop plants. Pak. J. Biol. Sci. 2000, 3, 269–274. [Google Scholar]
- Islam, E.; Yang, X.; Li, T.; Liu, D.; Jin, X.; Meng, F. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2007, 147, 806–816. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235, 343–351. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).