Phenolic Content, Color Development, and Pigment−Related Gene Expression: A Comparative Analysis in Different Cultivars of Strawberry during the Ripening Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetal Material
2.2. Skin Color Determination
2.3. RNA Isolation, Reverse Transcription, and qRT−PCR Analysis
2.4. Total Phenolic, Flavonoid and Anthocyanin Content
2.5. Correlation Analysis
3. Results and Discussion
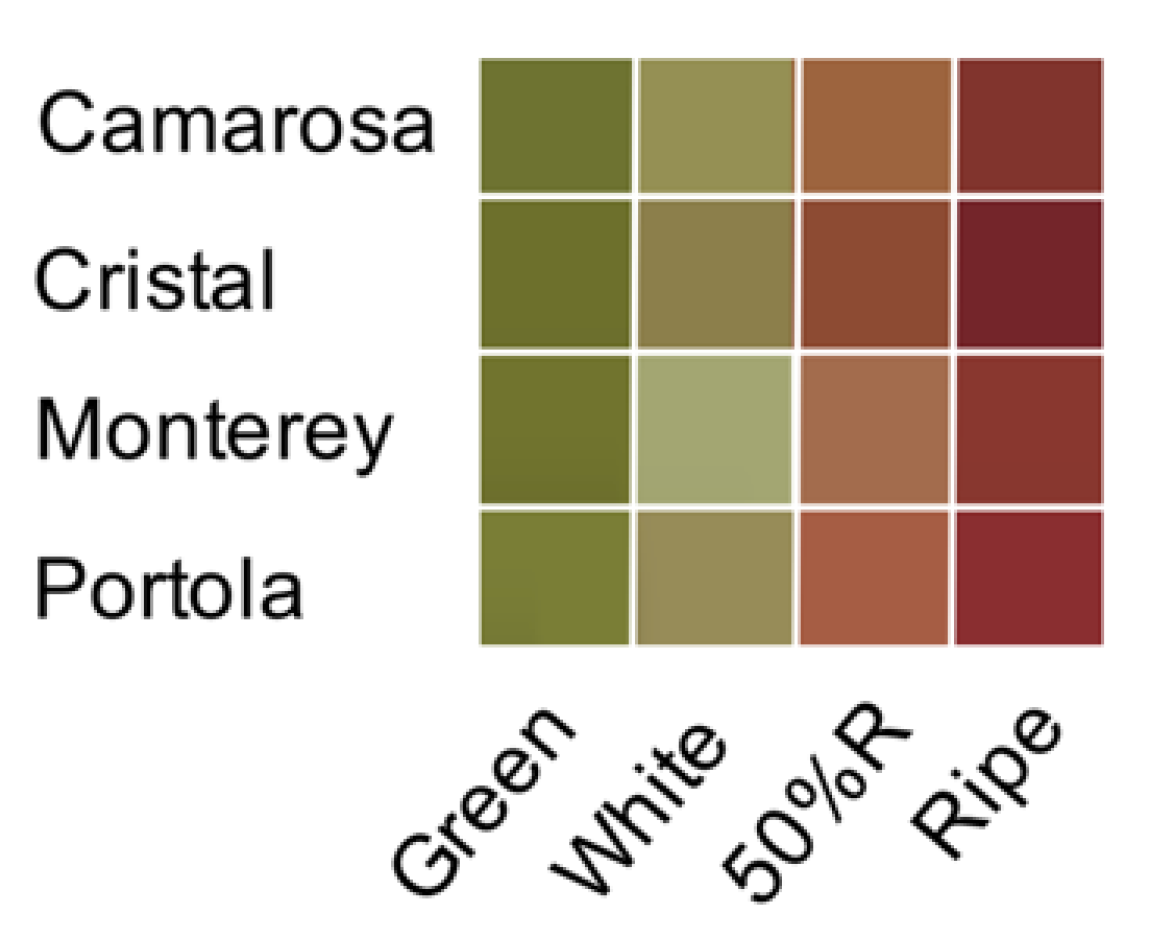
3.1. Color Development and Color Quality
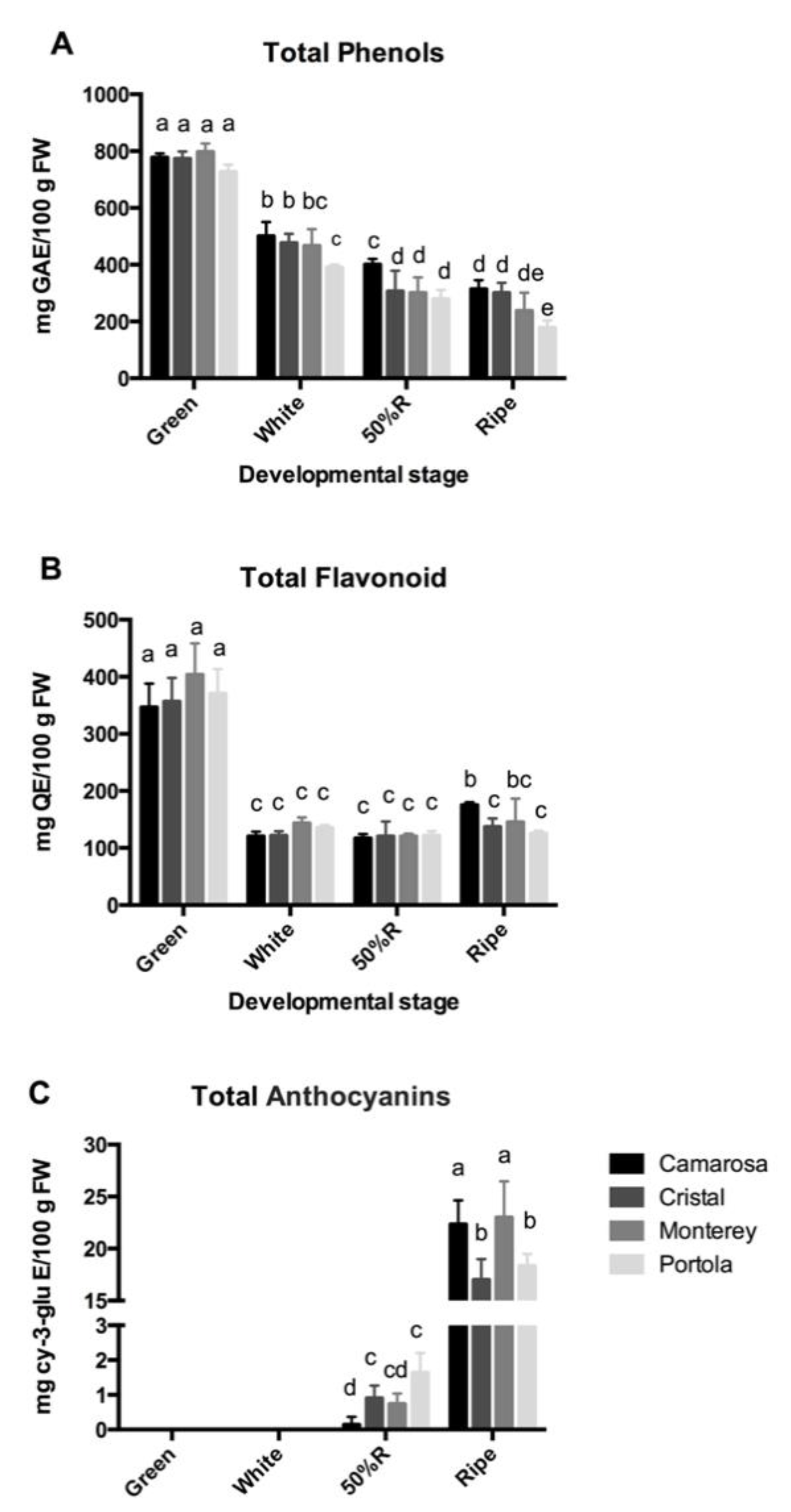
3.2. Total Phenolic, Flavonoid, and Anthocyanin Content
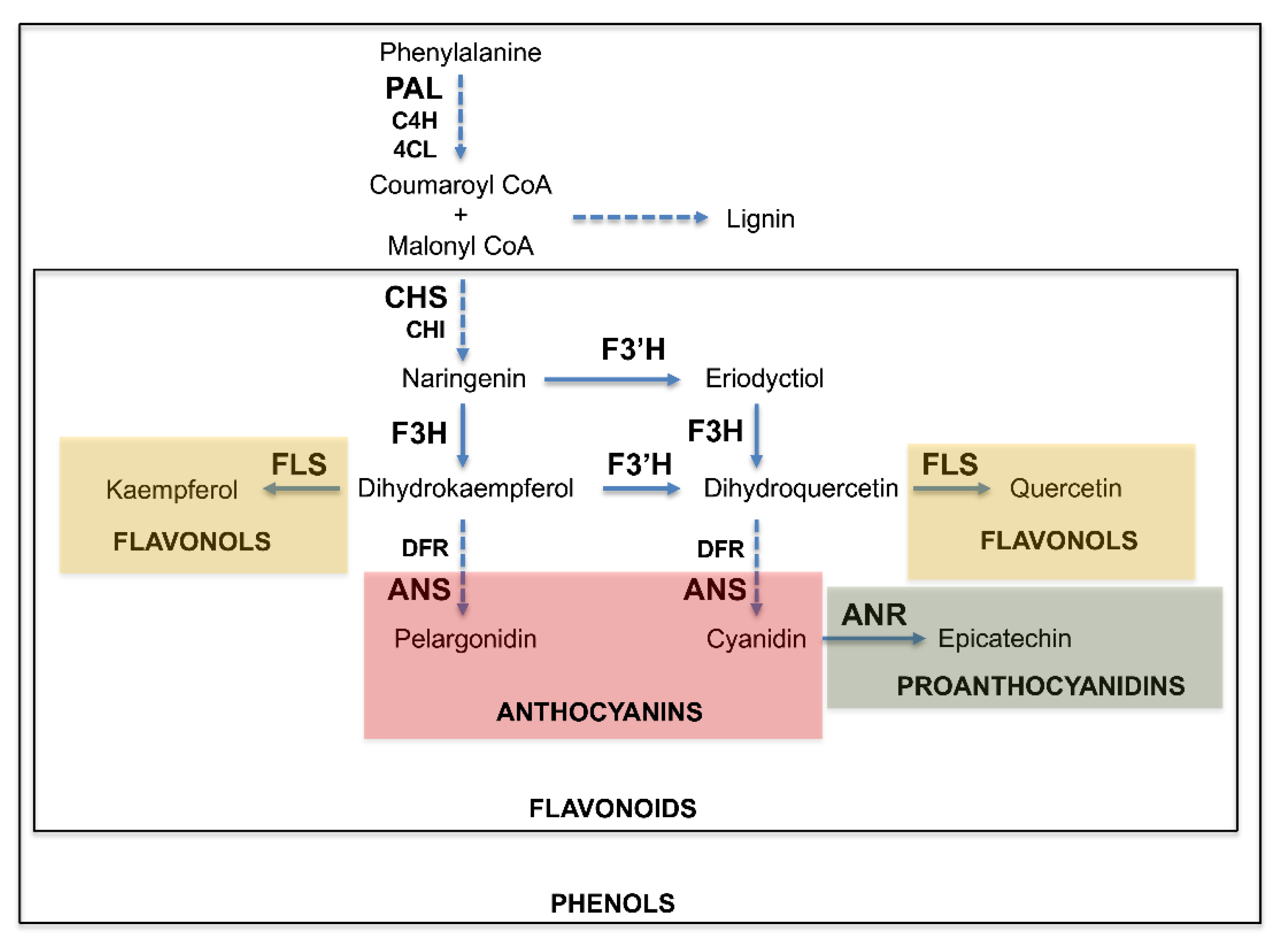
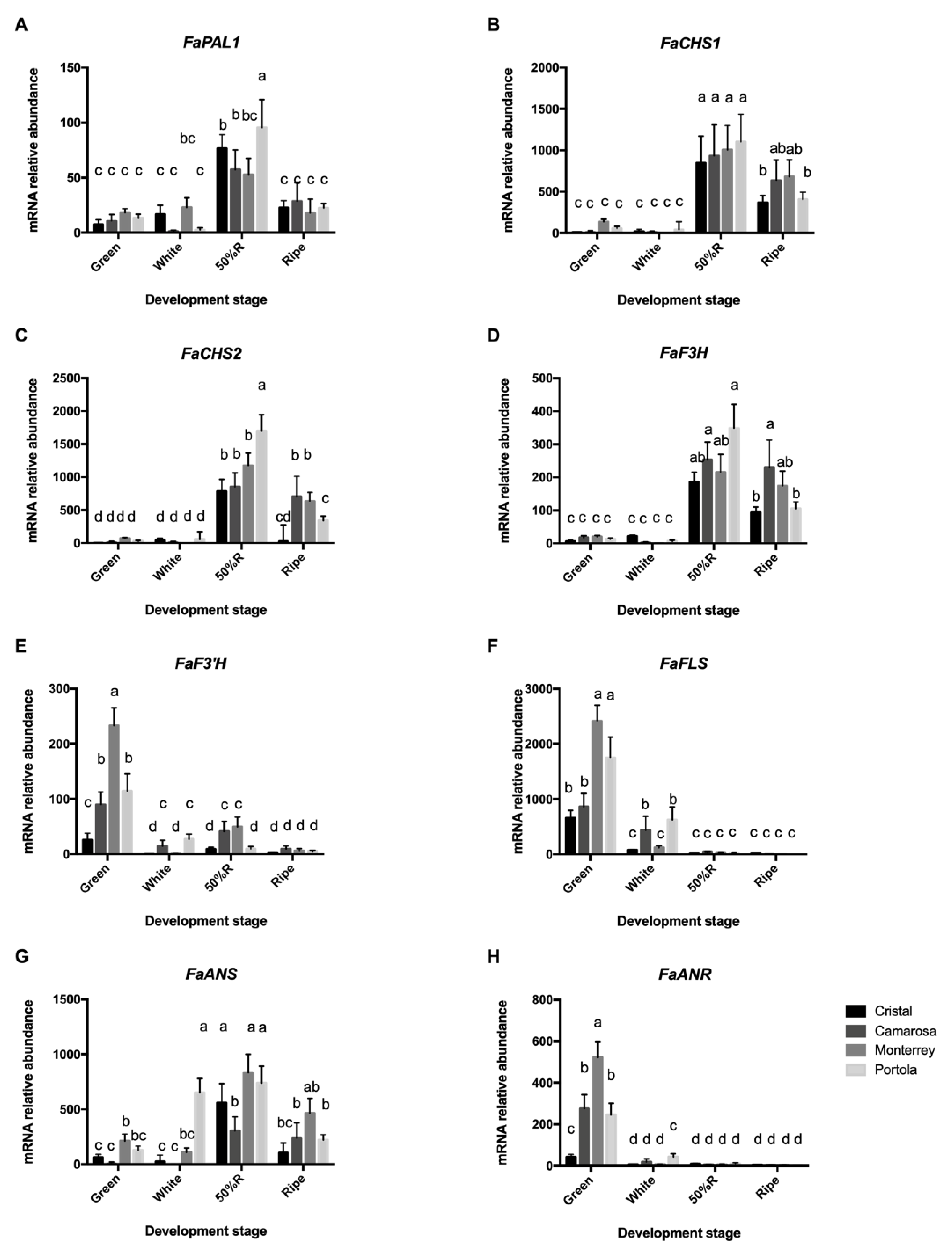
3.3. Expression of Phenylpropanoid Pathway Genes
3.4. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoberg, E.; Ulrich, D. Comparison of sensory perception and instrumental analysis. Acta Hortic. 2000, 538, 439–442. [Google Scholar] [CrossRef]
- Brummell, D.; Howie, W.J.; Ma, C.; Dunsmuir, P. Postharvest fruit quality of transgenic tomatoes suppressed in expression of a ripening-related expansin. Postharvest Boil. Technol. 2002, 25, 209–220. [Google Scholar] [CrossRef]
- Aaby, K.; Wrolstad, R.E.; Ekeberg, D.; Skrede, G. Polyphenol Composition and Antioxidant Activity in Strawberry Purees; Impact of Achene Level and Storage. J. Agric. Food Chem. 2007, 55, 5156–5166. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Parra-Palma, C.; Fuentes, E.; Palomo, I.; Torres, C.A.; Moya-León, M.A.; Ramos, P. Linking the platelet antiaggregation effect of different strawberries species with antioxidants: Metabolomic and transcript profiling of polyphenols. Bol. Latinoam. Caribe Plant Med. Aromat. 2018, 17, 36–52. [Google Scholar]
- Kosar, M.; Kafkas, N.E.; Paydas, S.; Baser, K.H.C. Phenolic Composition of Strawberry Genotypes at Different Maturation Stages. J. Agric. Food Chem. 2004, 52, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Parra-Palma, C.; Figueroa, C.R.; Zúñiga, P.E.; Valenzuela-Riffo, F.; Gonzàlez, J.; Gaete, C.; Morales-Quintana, L. Cell wall-related enzymatic activities and transcriptional profiles in four strawberry (Fragaria × ananassa) cultivars during fruit development and ripening. Sci. Hortic. 2018, 238, 325–332. [Google Scholar] [CrossRef]
- Morales-Quintana, L.; Ramos, P. Chilean strawberry (Fragaria chiloensis): An integrative and comprenhensive review. Food Res. Int. 2018, 119, 769–776. [Google Scholar] [CrossRef]
- Staudt, G. The origin and history of the large-fruited garden strawberry, Fragaria × ananassa. Theor. Appl. Genet. 1961, 31, 212–218. [Google Scholar]
- Ulrich, D.; Komes, D.; Olbricht, K.; Hoberg, E. Diversity of aroma patterns in wild and cultivated Fragaria accessions. Genet. Resour. Crop. Evol. 2006, 54, 1185–1196. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, S.N.; Guevara-González, R.G.; Miranda-López, R.; Feregrino-Pérez, A.; Torres-Pacheco, I.; Vazquez-Cruz, M.A. Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology, and genomics. Food Res. Int. 2013, 54, 1195–1207. [Google Scholar] [CrossRef]
- Kayesh, E.; Shangguan, L.; Korir, N.K.; Sun, X.; Bilkish, N.; Zhang, Y.; Han, J.; Song, C.; Cheng, Z.-M.; Fang, J. Fruit skin color and the role of anthocyanin. Acta Physiol. Plant. 2013, 35, 2879–2890. [Google Scholar] [CrossRef]
- Lin-Wang, K.; Liu, Y.; Espley, R.; Karunairetnam, S.; McGhie, T.; Hellens, R.P.; Allan, A.C. Regulation of anthocyanin biosynthesis in strawberry (Fragaria sp.) By over-expression of a key transcription factor. Acta Hortic. 2014, 137–142. [Google Scholar] [CrossRef]
- Hossain, M.R.; Kim, H.-T.; Shanmugam, A.; Nath, U.K.; Goswami, G.; Song, J.-Y.; Park, J.-I.; Nou, I.-S. Expression Profiling of Regulatory and Biosynthetic Genes in Contrastingly Anthocyanin Rich Strawberry (Fragaria × ananassa) Cultivars Reveals Key Genetic Determinants of Fruit Color. Int. J. Mol. Sci. 2018, 19, 656. [Google Scholar] [CrossRef]
- Cheel, J.; Theoduloz, C.; Rodriguez, J.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Free radical scavenging activity and phenolic content in achenes and thalamus from Fragaria chiloensis subsp. chiloensis, F. vesca and F. × ananassa cv. Chandler. Food Chem. 2007, 102, 36–44. [Google Scholar] [CrossRef]
- Baldi, P.; Orsucci, S.; Moser, M.; Brilli, M.; Giongo, L.; Si-Ammour, A. Gene expression and metabolite accumulation during strawberry (Fragaria × ananassa) fruit development and ripening. Planta 2018, 248, 1143–1157. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Theoduloz, C.; Caligari, P.D.; Schmeda-Hirschmann, G. Comparison of phenolic composition and antioxidant properties of two native Chilean and one domestic strawberry genotypes. Food Chem. 2009, 113, 377–385. [Google Scholar] [CrossRef]
- Olivares, D.; Contreras, C.; Muñoz, V.; Rivera, S.; González-Agüero, M.; Retamales, J.; Defilippi, B.G. Relationship among color development, anthocyanin and pigment-related gene expression in ‘Crimson Seedless’ grapes treated with abscisic acid and sucrose. Plant Physiol. Biochem. 2017, 115, 286–297. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A.; Matarrese, A.; Pacucci, C.; Punzi, R.; Faccia, M.; Trani, A.; Gambacorta, G. Application of abscisic acid (S-ABA) and sucrose to improve colour, anthocyanin content and antioxidant activity of cv. Crimson Seedless grape berries. Aust. J. Grape Wine Res. 2014, 21, 18–29. [Google Scholar] [CrossRef]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Muñoz, C.; Sevilla, J.F.S.; Botella, J.R.; Hoffmann, T.; Schwab, W.; Valpuesta, V. Polyphenol Composition in the Ripe Fruits of Fragaria Species and Transcriptional Analyses of Key Genes in the Pathway. J. Agric. Food Chem. 2011, 59, 12598–12604. [Google Scholar] [CrossRef] [PubMed]
- Schaart, J.G.; Dubos, C.; De La Fuente, I.R.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; Jonker, H.H.; Xu, W.; Routaboul, J.M.; Lepiniec, L.; Bovy, A.G. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Hannum, S.M. Potential Impact of Strawberries on Human Health: A Review of the Science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Andersen, O.M.; Øvstedal, D.O.; Pedersen, A.T.; Raknes, Å. Characteristic Anthocyanin Pattern from Onions and other Allium spp. J. Food Sci. 1996, 61, 703–706. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; De Vos, C.H.R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, Phenolic Compounds, and Nutritional Quality of Different Strawberry Genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Concha, C.M.; Figueroa, N.E.; Poblete, L.A.; Oñate, F.J.; Schwab, W.; Figueroa, C.R. Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 2013, 70, 433–444. [Google Scholar] [CrossRef]
- Rosli, H.G.; Civello, P.; Martínez, G.A. Changes in cell wall composition of three Fragaria × ananassa cultivars with different softening rate during ripening. Plant Physiol. Biochem. 2004, 42, 823–831. [Google Scholar] [CrossRef]
- Parra-Palma, C.; Úbeda, C.; Gil, M.; Ramos, P.; Castro, R.; Morales-Quintana, L. Comparative study of the volatile organic compounds of four strawberry cultivars and it relation to alcohol acyltransferase enzymatic activity. Sci. Hortic. 2019, 251, 65–72. [Google Scholar] [CrossRef]
- Pillet, J.; Yu, H.-W.; Chambers, A.H.; Whitaker, V.M.; Folta, K.M. Identification of candidate flavonoid pathway genes using transcriptome correlation network analysis in ripe strawberry (Fragaria × ananassa) fruits. J. Exp. Bot. 2015, 66, 4455–4467. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.F.; Villarreal, N.M.; Opazo, M.C.; Martínez, G.A.; Moya-León, M.A.; Civello, P. Expression of FaXTH1 and FaXTH2 genes in strawberry fruit. Cloning of promoter regions and effect of plant growth regulators. Sci. Hortic. 2014, 165, 111–122. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.Y.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug. Anal. 2002, 10, 178–182. [Google Scholar]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Correlation of two anthocyanin quantification methods: HPLC and spectrophotometric methods. Food Chem. 2008, 110, 782–786. [Google Scholar] [CrossRef]
- Castro, R.; Morales-Quintana, L. Study of the cell wall components produced during different ripening stages through thermogravimetric analysis. Cellulose 2019, 26, 3009–3020. [Google Scholar] [CrossRef]
- Hertog, M.L.; Lammertyn, J.; Desmet, M.; Scheerlinck, N.; Nicolaï, B. The impact of biological variation on postharvest behaviour of tomato fruit. Postharvest Boil. Technol. 2004, 34, 271–284. [Google Scholar] [CrossRef]
- Rababah, T.M.; Ereifej, K.I.; Howard, L. Effect of Ascorbic Acid and Dehydration on Concentrations of Total Phenolics, Antioxidant Capacity, Anthocyanins, and Color in Fruits. J. Agric. Food Chem. 2005, 53, 4444–4447. [Google Scholar] [CrossRef]
- Ganhão, R.; Pinheiro, J.; Tino, C.; Faria, H.; Gil, M.M. Characterization of Nutritional, Physicochemical, and Phytochemical Composition and Antioxidant Capacity of Three Strawberry “Fragaria × ananassa Duch.” Cultivars (“Primoris”, “Endurance”, and “Portola”) from Western Region of Portugal. Foods 2019, 8, 682. [Google Scholar]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria × ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef] [PubMed]
- García-Viguera, C.; Zafrilla, P.; Tomas-Barberan, F. The use of acetone as an extraction solvent for anthocyanins from strawberry fruit. Phytochem. Anal. 1998, 9, 274–277. [Google Scholar] [CrossRef]
- Clifford, M. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Castro, I.; Goncalves, O.; Teixeira, J.A.; Vicente, A.A. Comparative study of Selva and Camarosa strawberries from the commercial market. J. Food Sci. 2002, 67, 2132–2137. [Google Scholar] [CrossRef]
- Edger, P.P.; Poorten, T.J.; VanBuren, R.; Hardigan, M.A.; Colle, M.; McKain, M.R.; Smith, R.D.; Teresi, S.J.; Nelson, A.; Wai, C.M.; et al. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 2019, 51, 541–547. [Google Scholar] [CrossRef]

| “Camarosa” | FaPAL | FaCHS1 | FaCHS2 | FaF3H | FaFLS | FaF3‘H | FaANS | FaANR | Total Phenols | Total Flavonoids | Total Anthocyanin | Skin Color (a*) |
| FaPAL | ||||||||||||
| FaCHS1 | 0.959 * | |||||||||||
| FaCHS2 | 0.922 * | 0.992 ** | ||||||||||
| FaF3H | 0.903 * | 0.981 ** | 0.997 ** | |||||||||
| FaFLS | −0.697 | −0.863 | −0.890 | −0.879 | ||||||||
| FaF3‘H | −0.062 | −0.315 | −0.368 | −0.359 | 0.750 | |||||||
| FaANS | 0.934 * | 0.995 ** | 0.999 *** | 0.995 ** | −0.881 | −0.350 | ||||||
| FaANR | −0.407 | −0.597 | −0.613 | −0.584 | 0.897 | 0.919 * | −0.604 | |||||
| Total phenols | −0.526 | −0.735 | −0.776 | −0.769 | 0.976 * | 0.872 | −0.763 | 0.944 * | ||||
| Total flavonoids | −0.370 | −0.508 | −0.491 | −0.444 | 0.781 | 0.833 | −0.489 | 0.959 * | 0.820 | |||
| Total anthocyanin | 0.117 | 0.349 | 0.466 | 0.522 | −0.552 | −0.535 | 0.439 | −0.370 | −0.614 | −0.093 | ||
| Skin color (a*) | 0.699 | 0.864 | 0.920 * | 0.938 * | −0.919 * | −0.576 | 0.907 * | −0.676 | −0.877 * | −0.477 | 0.765 | |
| “Cristal” | FaPAL | FaCHS1 | FaCHS2 | FaF3H | FaFLS | FaF3‘H | FaANS | FaANR | Total Phenols | Total Flavonoids | Total Anthocyanin | Skin Color (a*) |
| FaPAL | ||||||||||||
| FaCHS1 | 0.958 * | |||||||||||
| FaCHS2 | 0.984 ** | 0.910 * | ||||||||||
| FaF3H | 0.951 * | 0.997 ** | 0.891 | |||||||||
| FaFLS | −0.545 | −0.568 | −0.408 | −0.629 | ||||||||
| FaF3‘H | −0.203 | −0.218 | −0.060 | −0.293 | 0.927 * | |||||||
| FaANS | 0.983 ** | 0.949 * | 0.989 ** | 0.928 * | −0.389 | −0.023 | ||||||
| FaANR | −0.380 | −0.417 | −0.232 | −0.486 | 0.983 ** | 0.977 * | −0.213 | |||||
| Total phenols | −0.643 | −0.724 | −0.500 | −0.776 | 0.959 * | 0.812 | −0.520 | 0.918 * | ||||
| Total flavonoids | −0.530 | −0.516 | −0.405 | −0.578 | 0.991 ** | 0.937 * | −0.366 | 0.975 * | 0.917 * | |||
| Total anthocyanin | −0.123 | 0.144 | −0.279 | 0.188 | −0.403 | −0.434 | −0.169 | −0.478 | −0.527 | −0.296 | ||
| Skin color (a*) | 0.528 | 0.716 | 0.377 | 0.755 | −0.759 | −0.587 | 0.459 | −0.725 | −0.902 * | −0.666 | 0.772 | |
| “Monterey” | FaPAL | FaCHS1 | FaCHS2 | FaF3H | FaFLS | FaF3‘H | FaANS | FaANR | Total Phenols | Total Flavonoids | Total Anthocyanin | Skin Color (a*) |
| FaPAL | ||||||||||||
| FaCHS1 | 0.710 | |||||||||||
| FaCHS2 | 0.801 | 0.990 ** | ||||||||||
| FaF3H | 0.625 | 0.991 ** | 0.967 * | |||||||||
| FaFLS | −0.399 | −0.488 | −0.516 ns | −0.545 | ||||||||
| FaF3‘H | −0.205 | −0.303 | −0.323 ns | −0.378 | 0.976 * | |||||||
| FaANS | 0.828 | 0.979 * | 0.994 ** | 0.943 * | −0.429 | −0.225 | ||||||
| FaANR | −0.389 | −0.457 | −0.488 | −0.514 | 0.999 *** | 0.980 ** | −0.401 | |||||
| Total phenols | −0.402 | −0.705 | −0.697 | −0.770 | 0.938 * | 0.878 | −0.614 | 0.924 * | ||||
| Total flavonoids | −0.469 | −0.507 | −0.545 | −0.553 | 0.996 ** | 0.959 * | −0.464 | 0.996 ** | 0.925 * | |||
| Total anthocyanin | −0.375 | 0.348 | 0.230 | 0.469 | −0.374 | −0.414 | 0.152 | −0.354 | −0.586 | −0.303 | ||
| Skin color (a*) | 0.069 | 0.717 | 0.631 | 0.805 | −0.572 | −0.522 | 0.561 | −0.545 | −0.809 * | −0.529 | 0.899 * | |
| “Portola” | FaPAL | FaCHS1 | FaCHS2 | FaF3H | FaFLS | FaF3‘H | FaANS | FaANR | Total Phenols | Total Flavonoids | Total Anthocyanin | Skin Color (a*) |
| FaPAL | ||||||||||||
| FaCHS1 | 0.980 * | |||||||||||
| FaCHS2 | 0.993 ** | 0.986 ** | ||||||||||
| FaF3H | 0.989 ** | 0.998 *** | 0.994 ** | |||||||||
| FaFLS | −0.514 | −0.666 | −0.593 | −0.636 | ||||||||
| FaF3‘H | −0.402 | −0.557 | −0.496 | −0.530 | 0.984 ** | |||||||
| FaANS | 0.535 | 0.526 | 0.617 | 0.555 | −0.527 | −0.583 | ||||||
| FaANR | −0.384 | −0.538 | −0.481 | −0.512 | 0.978 * | 0.999 *** | −0.599 | |||||
| Total phenols | −0.377 | −0.549 | −0.458 | −0.511 | 0.986 ** | 0.981 ** | −0.422 | 0.975 * | ||||
| Total flavonoids | −0.353 | −0.497 | −0.456 | −0.476 | 0.950 * | 0.989 ** | −0.666 | 0.993 ** | 0.944 * | |||
| Total anthocyanin | −0.0878 | 0.093 | −0.074 | 0.031 | −0.543 | −0.493 | −0.419 | −0.474 | −0.647 | −0.388 | ||
| Skin color (a*) | 0.470 | 0.634 | 0.504 | 0.586 | −0.881 | −0.804 | 0.0955 | −0.783 | −0.896 * | −0.706 | 0.811 * |
| “Camarosa” | Total Anthocyanin | Total Flavonoids | Skin Color (a*) | Total Phenols | Firmness a | Weight a | pH a | SSC/TA a | Total Esters b |
| Total anthocyanin | |||||||||
| Total Flavonoids | −0.093 | ||||||||
| Skin color (a*) | 0.765 | −0.477 | |||||||
| Total phenols | −0.614 | −0.820 | −0.877 * | ||||||
| Firmness | −0.603 | 0.798 | −0.610 | 0.820 | |||||
| Weight | 0.799 | −0.663 | 0.779 | −0.964 * | −0.953 * | ||||
| pH | −0.918 * | 0.750 | −0.918 * | 0.866 | 0.867 | −0.960 | |||
| SSC/TA | 0.727 | −0.454 | 0.599 | −0.853 | −0.889 | 0.871 | −0.911 * | ||
| Total esters | 0.999 *** | −0.090 | 0.998 *** | −0.610 | −0.599 | 0.797 | −0.916 * | 0.723 | |
| “Cristal” | Total Anthocyanin | Total Flavonoids | Skin Color (a*) | Total Phenols | Firmness a | Weight a | pH a | SSC/TA a | Total Esters b |
| Total anthocyanin | |||||||||
| Total Flavonoids | −0.296 | ||||||||
| Skin color (a*) | 0.772 | −0.666 | |||||||
| Total phenols | −0.527 | 0.917 * | 0.902 * | ||||||
| Firmness | −0.680 | 0.888 | 0.638 | 0.974 * | |||||
| Weight | 0.903 * | −0.677 | 0.904 * | −0.823 | −0.99 ** | ||||
| pH | −0.851 | 0.750 | 0.840 | 0.883 | 0.963 * | 0.993 ** | |||
| SSC/TA | 0.912 | −0.538 | 0.911 * | −0.793 | −0.865 | 0.949 * | −0.941 * | ||
| Total esters | 0.998 *** | −0.272 | 0.998 *** | −0.494 | −0.655 | −0.891 | −0.834 | 0.890 | |
| “Monterey” | Total Anthocyanin | Total Flavonoids | Skin Color (a*) | Total Phenols | Firmness a | Weight a | pH a | SSC/TA a | Total Esters b |
| Total anthocyanin | |||||||||
| Total Flavonoids | −0.303 | ||||||||
| skin color (a*) | 0.899 * | −0.529 | |||||||
| Total phenols | −0.586 | 0.925 * | −0.809 * | ||||||
| Firmness | −0.731 | 0.843 | −0.738 | 0.981 ** | |||||
| Weight | 0.861 | 0.032 | 0.877 | −0.977 * | −0.925 * | ||||
| pH | −0.912 * | 0.657 | −0.922 * | 0.8667 | 0.946 * | −0.934 * | |||
| SSC/TA | 0.935 * | −0.577 | 0.931 * | −0.8254 | −0.920 * | 0.960 * | −0.992 ** | ||
| Total esters | 0.999 *** | −0.286 | 0.998 *** | 0.567 | −0.715 | 0.846 | −0.903 * | 0.925 * | |
| “Portola” | Total anthocyanin | Total Flavonoids | skin color (a*) | Total phenols | Firmness a | Weight a | pH a | SSC/TA a | Total esters b |
| Total anthocyanin | |||||||||
| Total Flavonoids | −0.388 | ||||||||
| skin color (a*) | 0.811 * | −0−706 | |||||||
| Total phenols | −0.647 | 0.944 * | − 0.896 * | ||||||
| Firmness | −0.7133 | 0.898 | −0.7313 | 0.993 * | |||||
| Weight | 0.792 | −0.827 | 0.772 | −0.967 * | −0.987 ** | ||||
| pH | −0.932 * | 0.695 | −0.920 * | 0.875 | 0.910 * | −0.943 * | |||
| SSC/TA | 0.899 | −0.667 | 0.829 | −0.876 | −0.926 * | 0.970 * | −0.964 * | ||
| Total esters | 0.996 ** | −0.345 | 0.976 * | −0.601 | −0.664 | 0.746 | −0.912 | 0.865 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Palma, C.; Morales-Quintana, L.; Ramos, P. Phenolic Content, Color Development, and Pigment−Related Gene Expression: A Comparative Analysis in Different Cultivars of Strawberry during the Ripening Process. Agronomy 2020, 10, 588. https://doi.org/10.3390/agronomy10040588
Parra-Palma C, Morales-Quintana L, Ramos P. Phenolic Content, Color Development, and Pigment−Related Gene Expression: A Comparative Analysis in Different Cultivars of Strawberry during the Ripening Process. Agronomy. 2020; 10(4):588. https://doi.org/10.3390/agronomy10040588
Chicago/Turabian StyleParra-Palma, Carolina, Luis Morales-Quintana, and Patricio Ramos. 2020. "Phenolic Content, Color Development, and Pigment−Related Gene Expression: A Comparative Analysis in Different Cultivars of Strawberry during the Ripening Process" Agronomy 10, no. 4: 588. https://doi.org/10.3390/agronomy10040588
APA StyleParra-Palma, C., Morales-Quintana, L., & Ramos, P. (2020). Phenolic Content, Color Development, and Pigment−Related Gene Expression: A Comparative Analysis in Different Cultivars of Strawberry during the Ripening Process. Agronomy, 10(4), 588. https://doi.org/10.3390/agronomy10040588







