Bioactive Compounds and Antiradical Activity of the Rosa canina L. Leaf and Twig Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Extracts Preparation
2.3. Total Phenolic Content and HPLC Analysis
2.4. Water-Soluble Vitamins and Tocopherol Isomers
2.5. Amino Acids
2.6. Interaction with Human Serum Albumin: Circular Dichroism
2.7. Interaction with Biomembranes: Hemolysis Test
2.8. Antioxidant and Antiradical Activity: Oxidative Stress Markers
2.9. Cytotoxicity
2.10. ROS Inhibition in Human BJ Cell Line
2.11. Statistical Analysis
2.12. Compliance with Ethical Standards
3. Results
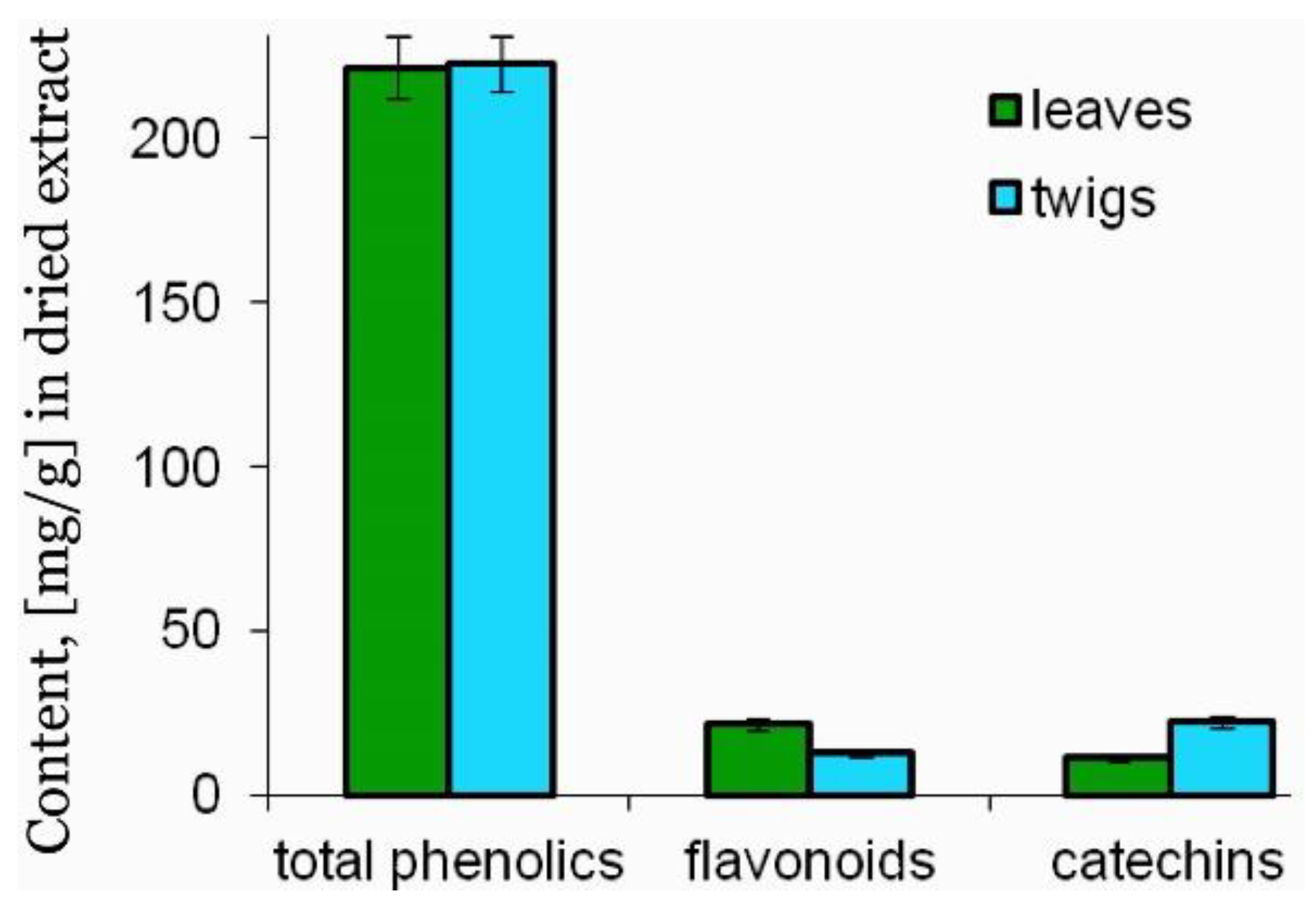
3.1. Phenolic Content and HPLC Analysis
3.2. Water-Soluble Vitamins and Content of Vitamin E
3.3. Amino Acid Content
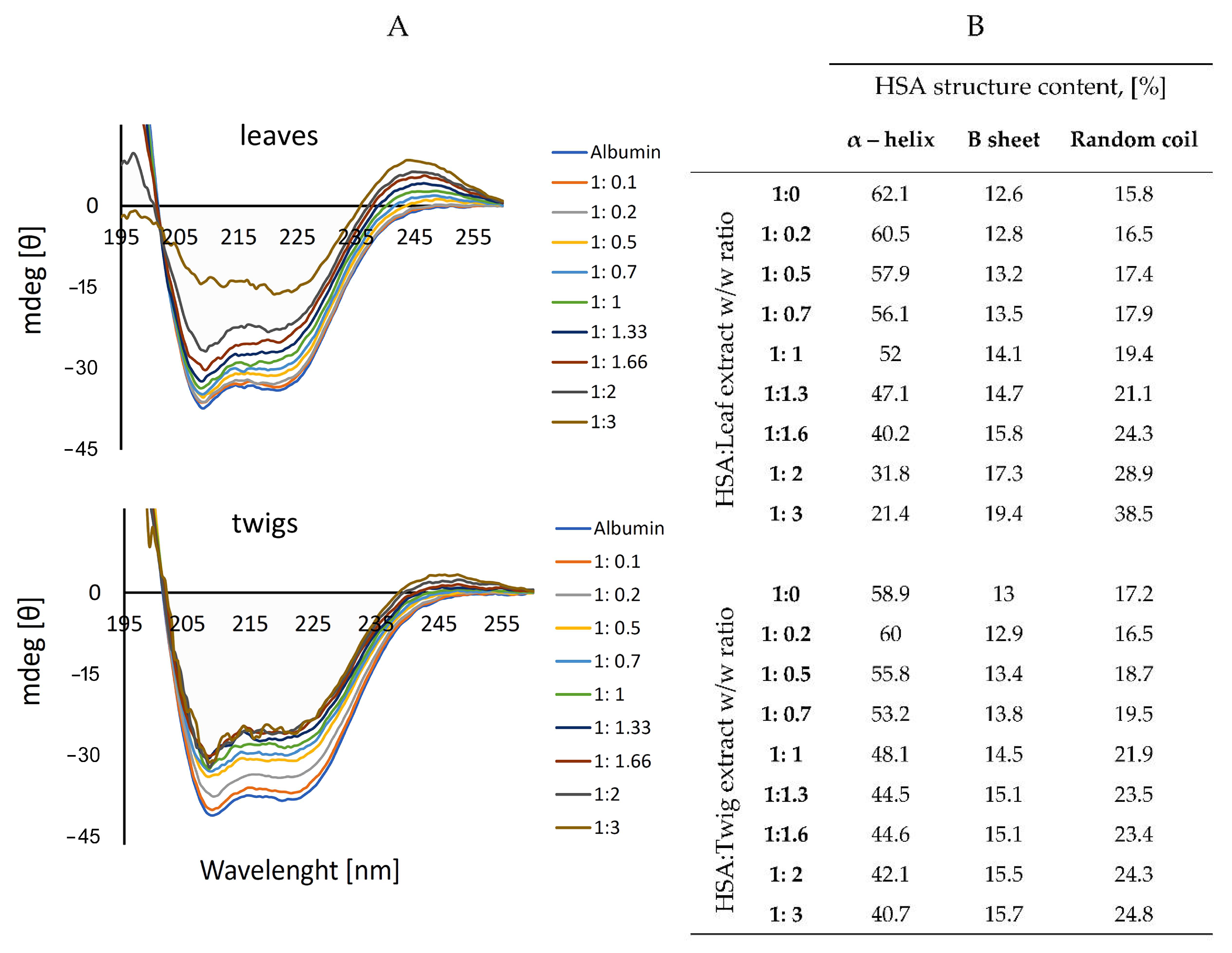
3.4. Interaction with Human Serum Albumin: Circular Dichroism
3.5. Hematoxicity and Cytotoxicity
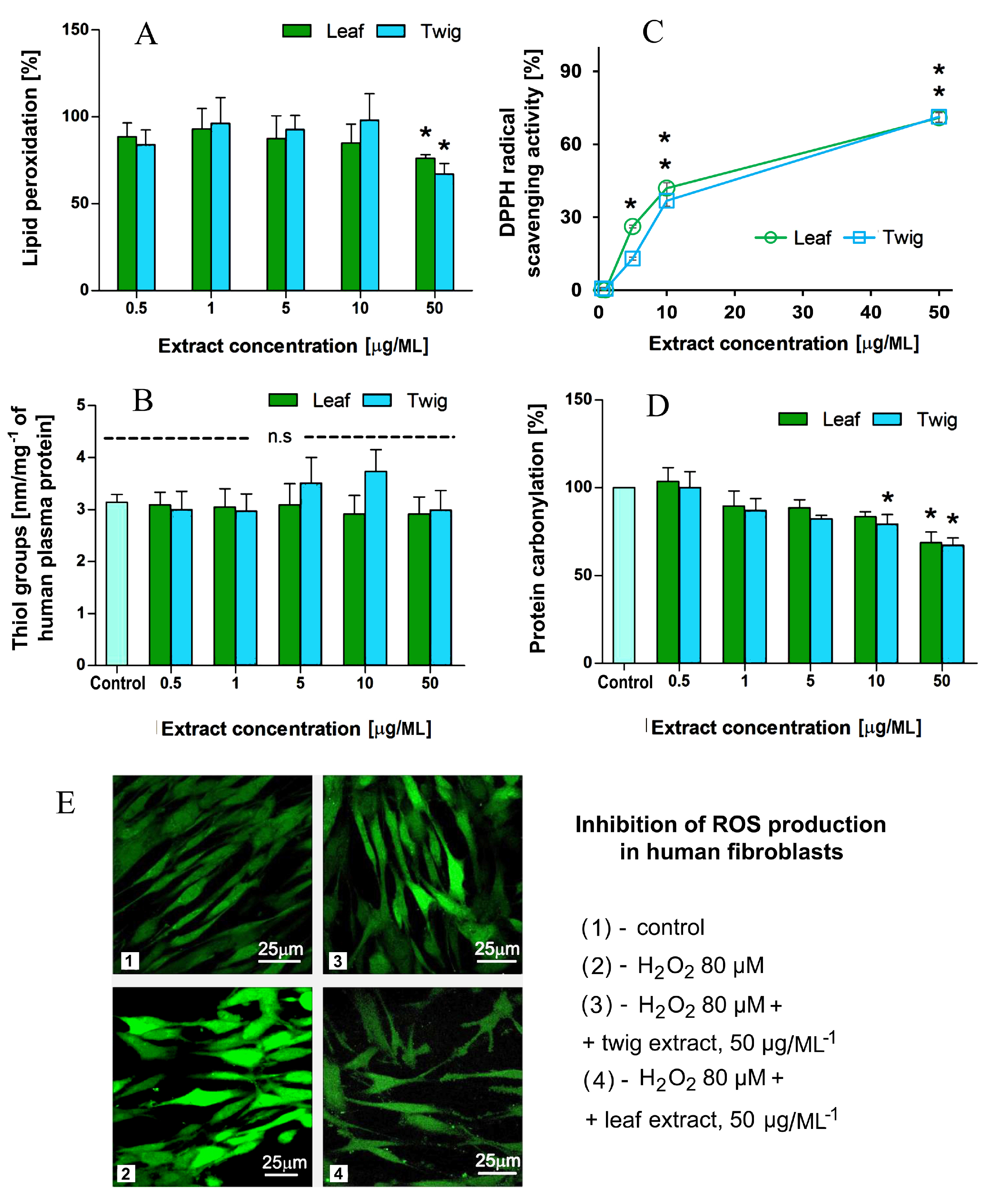
3.6. Markers of Oxidative Stress, Free Radical Scavenging and ROS Inhibition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval
References
- Lesjak, M.M.; Šibul, F.S.; Anac, G.T.; Beara, I.N.; Mimica-dukic, N.M. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar]
- Williams, P. Consumer Understanding and Use of Health Claims for Foods. Nutr. Rev. 2005, 63, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M. Plants: An alternative source for antimicrobials. J. Appl. Pharm. Sci. 2011, 1, 16–20. [Google Scholar]
- Oguntibeju, O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018, 11, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Abu-darwish, M.S.; Efferth, T. Medicinal Plants from Near East for Cancer Therapy. Front. Pharm. 2018, 9, 56. [Google Scholar] [CrossRef]
- Perez, R.M. Antidiabetic effect of compounds isolated from plants. Phytomedicine 1998, 5, 55–75. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Fascella, G.; Angiolillo, F.D.; Massimo, M.; Amenta, M.; Romeo, F.V.; Rapisarda, P.; Ballistreri, G. Bioactive compounds and antioxidant activity of four rose hip species from spontaneous Sicilian flora. Food Chem. 2019, 289, 56–64. [Google Scholar] [CrossRef]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Ogah, O.; Watkins, S.C.; Ubi, B.E.; Oraguzie, N. Phenolic compounds in Rosaceae fruit and nut crops—A review Department of Horticulture, Washington State University-Irrigated Agriculture and Extension. J. Agric. Food Chem. 2014, 62, 9369–9386. [Google Scholar] [CrossRef]
- Sardarodiyan, M.; Mohamadi, S.A. Natural antioxidants: Sources, extraction and application in food systems. Nutr. Food Sci. 2016, 46, 363–373. [Google Scholar] [CrossRef]
- Jiménez, S.; Jiménez-moreno, N.; Luquin, A.; Laguna, M. Chemical composition of rosehips from different Rosa species: An alternative source of antioxidants for food industry. Food Addit. Contam. Part A 2017, 34, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Al-yafeai, A.; Malarski, A.; Böhm, V. Characterization of carotenoids and vitamin E in R. rugosa and R. canina: Comparative analysis. Food Chem. 2018, 242, 435–442. [Google Scholar] [CrossRef]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.E.; Anderson, A.N.; Duan, R.D. Inhibition of Cancer Cell Proliferation in Vitro by Fruit and Berry Extracts and Correlations with Antioxidant Levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef] [PubMed]
- Genç, N.; Dölek, Ü.; Günes, M. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 2017, 235, 154–159. [Google Scholar]
- Hosni, K.; Chrif, R.; Zahed, N.; Abid, I.; Medfei, W.; Sebei, H.; Ben Brahim, N. Fatty acid and phenolic constituents of leaves, flowers and fruits of tunisian dog rose (Rosa canina L.). Riv. Ital. Delle Sostanze Grasse 2010, 87, 117–123. [Google Scholar]
- Olsson, M.E.; Andersson, S.; Werlemark, G.; Uggla, M.; Gustavsson, K.E. Carotenoids and phenolics in rose hips. Acta Hortic. 2005, 690, 249–252. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Ciz, M.; Lojek, A.; Vasicek, O.; Nedelcheva, P.; Blazheva, D.; Toshkova, R.; Gardeva, E.; Yossifova, L.; et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014, 157, 37–44. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Banyai, E.; Ombodi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef]
- Patel, S. Rose hips as complementary and alternative medicine: Overview of the present status and prospects. Med. J. Nutr. Metab. 2012, 6, 89–97. [Google Scholar] [CrossRef]
- Tumbas, V.T.; Canadanovic-Brunet, J.M.; Cetkovic-Simin, D.D.; Cetkovic, G.S.; Dilas, S.M.; Gille, L. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J. Sci. Food Agric. 2012, 92, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Chawla, R.; Arora, R.; Singh, S.; Sagar, R.K.; Sharma, R.K.; Kumar, R.; Sharma, A.; Gupta, M.L.; Singh, S.; Prasad, J.; et al. Radioprotective and antioxidant activity of fractionated extracts of berries of Hippophae rhamnoides. J. Med. Food 2007, 10, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Watanabe, S.; Miyake, N.; Kohno, H.; Osawa, T. Dihydrochalcones: Evaluation as novel radical scavenging antioxidants. J. Agric. Food Chem. 2003, 51, 3309–3312. [Google Scholar] [CrossRef] [PubMed]
- Nogala-Kałucka, M.; Dwiecki, K.; Siger, A.; Górnaś, P.; Polewski, K.; Ciosek, S. Antioxidant synergism and antagonism between tocotrienols, quercetin and rutin in model system. Acta Aliment. 2013, 42, 360–370. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, Traditional Uses and Pharmacological Profile of Rose Hip: A Review. Curr. Pharm. Des. 2018, 24, 4101–4124. [Google Scholar] [CrossRef]
- Wenzig, E.M.; Widowitz, U.; Kunert, O.; Chrubasik, S. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine 2008, 15, 826–835. [Google Scholar] [CrossRef]
- Marino, T.; Galano, A.; Russo, N. Radical scavenging ability of gallic acid toward OH and OOH radicals-reaction mechanism and rate constants from the density functional theory. J. Phys. Chem. B 2014, 118, 10380–10389. [Google Scholar] [CrossRef]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Koulocheri, S.D.; Haroutounian, S.A.; Kouretas, D.; Stagos, D. Polyphenolic Composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea Extracts and Assessment of Their Antioxidant Activity in human endothelial cells. Antioxidants 2019, 8, 92. [Google Scholar] [CrossRef]
- Nađpal, J.D.; Lesjak, M.M.; Mrkonjić, Z.O.; Majkić, T.M.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Beara, I.N. Phytochemical composition and in vitro functional properties of three wild rose hips and their traditional preserves. Food Chem. 2018, 241, 290–300. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Exotic fruits as a source of important phytochemicals: Improving the traditional use of Rosa canina fruits in Portugal. Food Res. Int. 2011, 44, 2233–2236. [Google Scholar] [CrossRef]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound Identification of Selected Rose Species and Cultivars: An Insight to Petal and Leaf Phenolic Profiles. J. Am. Soc. Hortic. Sci. 2014, 139, 157–166. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Ieri, F.; Innocenti, M.; Possieri, L.; Gallori, S.; Mulinacci, N. Phenolic composition of “bud extracts” of Ribes nigrum L., Rosa canina L. and Tilia tomentosa M. J. Pharm. Biomed. Anal. 2015, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, S.; Saija, A.; Siracusa, L.; Ruberto, G.; Dhaouadi, K.; Cimino, F.; Cristani, M. LC-DAD-ESI-MS and HPLC-DAD phytochemical investigation and in vitro antioxidant assessment of Rosa sp. stem pruning products from different northern areas in Tunisia. Phytochem. Anal. 2020, 31, 98–111. [Google Scholar] [CrossRef]
- Riffault, L.; Destandau, E.; Pasquier, L.; André, P.; Elfakir, C. Phytochemical analysis of Rosa hybrida cv. ‘Jardin de Granville’ by HPTLC, HPLC-DAD and HPLC-ESI-HRMS: Polyphenolic fingerprints of six plant organs. Phytochemistry 2014, 99, 127–134. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Bakkalbaşı, E.; Yemiş, O.; Aslanova, D.; Artık, N. Major flavan-3-ol composition and antioxidant activity of seeds from different grape cultivars grown in Turkey. Eur. Food Res. Technol. 2005, 221, 792–797. [Google Scholar] [CrossRef]
- Whitaker, J.R.; Granum, P.E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal. Biochem. 1980, 109, 156–159. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Sebei, H.; Siracusa, L.; Ruberto, G.; Saija, A.; Cimino, F.; Cristani, M. Comparative study of phenolic composition and antioxidant activity of leaf extracts from three wild Rosa species grown in different Tunisia regions: Rosa canina L., Rosa moschata Herrm. and Rosa sempervirens L. Ind. Crop. Prod. 2016, 94, 167–177. [Google Scholar] [CrossRef]
- Collakova, E.; DellaPenna, D. The Role of Homogentisate Phytyltransferase and Other Tocopherol Pathway Enzymes in the Regulation of Tocopherol Synthesis during Abiotic Stress. Plant Physiol. 2003, 133, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Colinas, M.; Eisenhut, M.; Tohge, T.; Pesquera, M.; Fernie, A.R.; Weber, A.P.M.; Fitzpatrick, T.B. Balancing of B 6 vitamers is essential for plant development and metabolism in Arabidopsis. Plant Cell 2016, 28, 439–453. [Google Scholar] [CrossRef]
- Asensi-fabado, M.A.; Munne-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Igwenyi, I.O.; Elekwa, A.E. Phytochemical Analysis and Determination of Vitamin Contents of Geranium Robertianum. J. Dent. Med. Sci. 2014, 13, 44–47. [Google Scholar]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. L-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef]
- Yang, M.; Vousden, K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef]
- Kim, J.; Jang, H.; Cho, W.; Yeon, S.; Lee, C. In vitro antioxidant actions of sulfur-containing amino acids. Arab. J. Chem. 2020, 13, 1678–1684. [Google Scholar] [CrossRef]
- Prasad, K. HPLC Analysis of Amino Acid and Antioxidant Composition of Three Medicinal Plants of (Pithoragarh) Uttarakhand Himalayas. J. Anal. Pharm. Res. 2017, 6, 00816. [Google Scholar] [CrossRef]
- Das, P.; Chaudhari, S.K.; Das, A.; Kundu, S. Interaction of Flavonols with Human Serum Albumin: A biophysical study showing structure activity relationship and enhancement when coated on silver nanoparticles. J. Biomol. Struct. Dyn. 2019, 37, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yotnda, P. Production and detection of reactive oxygen species (ROS) in cancers. J. Vis. Exp. 2011, 57, e3357. [Google Scholar] [CrossRef] [PubMed]
- Grauzdytė, D.; Pukalskas, A.; Viranaicken, W.; El Kalamouni, C.; Venskutonis, P.R. Protective effects of Phyllanthus phillyreifolius extracts against hydrogen peroxide induced oxidative stress in HEK293 cells. PLoS ONE 2018, 13, e0207672. [Google Scholar] [CrossRef] [PubMed]
- Ladokun, O.; Ojezele, M.; Arojojoye, O. Comparative study on the effects of aqueous extracts of viscum album (Mistletoe) from three host plants on hematological parameters in albino rats. Afr. Health Sci. 2015, 15, 606–612. [Google Scholar] [PubMed]
- De Oliveira, V.M.A.; Carneiro, A.L.B.; Cauper, G.S.D.B.; Pohlit, A.M. In vitro screening of amazonian plants for hemolytic activity and inhibition of platelet aggregation in human blood. Acta Amaz. 2009, 39, 973–980. [Google Scholar] [CrossRef][Green Version]
- Nayebi, N.; Khalili, N.; Kamalinejad, M.; Emtiazy, M. A systematic review of the efficacy and safety of Rosa damascena Mill. with an overview on its phytopharmacological properties. Complement. Ther. Med. 2017, 34, 129–140. [Google Scholar] [CrossRef]
- Liu, C.M.; Kao, C.L.; Wu, H.M.; Li, W.J.; Huang, C.T.; Li, H.T.; Chen, C.Y. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef]
- Zarei, O.; Yaghoobi, M.M. Cytotoxic and anti-proliferative effects of Rosa beggeriana Schrenk extracts on human liver and breast cancer cells. Avicenna J. Phytomed. 2019, 9, 386–395. [Google Scholar]

| No. | Phenolic Compounds | Synonyms | Quantification Wavelengths: A 235; 280; 325; 375 nm Em 420 nm (Ex 270 nm) | RT | Content (mg/g) in Dry Matter of Extract | |
|---|---|---|---|---|---|---|
| Leaf | Twig | |||||
| 1 | Gallic acid | 3,4,5-Trihydroxybenzoic acid | 280 | 6992 | 0.805 ± 0.075 | 0.357 ± 0.033 |
| 2 | p-Benzoquinone | Quinone | 235 | 11,642 | 0.252 ± 0.022 | 0.989 ± 0.087 |
| 3 | α-Resorcylic acid | 3,5-Dihydroxybenzoic acid | 420 | 11,841 | 0.138 ± 0.013 | 0.117 ± 0.012 |
| 4 | Pyrocatechol | 1,2-Dihydroxybenzene; Catechol | 280 | 12,283 | 0.226 ± 0.014 | 0.493 ± 0.049 |
| 5 | Protocatechuic acid | 3,4-Dihydroxybenzoic acid | 420 | 12,415 | 0.153 ± 0.015 | 13.911 ± 1.303 |
| 6 | Neochlorogenic acid | trans-5-O-Caffeoylquinic acid | 325 | 12,833 | 57.148 ± 5.031 | 0.258 ± 0.0262 |
| 7 | (−)-Epigallocatechin | Monomeric flavan-3-ol | 235 | 14,992 | 0.207 ± 0.022 | 1.680 ± 0.076 |
| 8 | (+)-Catechin | Flavan-3-ol; monomeric flavan-3-ol | 235 | 15,308 | 2.804 ± 0.206 | 17.798 ± 1.544 |
| 9 | 4-Hydroxybenzoic acid | - | 235 | 15,700 | 1.182 ± 0.111 | 0.323 ± 0.0361 |
| 10 | Gentisic acid | 2,5-Dihydroxybenzoic acid | 325 | 16,267 | 1.577 ± 0.164 | 0.340 ± 0.033 |
| 11 | Procyanidin B2 | Polymeric flavan-3-ol; pentahydroxyflavane (cis,cis″-4,8″-Bi(3,3′,4′,5,7-pentahydroxyflavane) | 280 | 16,500 | 22.473 ± 2.019 | 3.222 ± 0.317 |
| 12 | 4-Hydroxybenzaldehyde | - | 280 | 16,733 | 1.111 ± 0.095 | 0.263 ± 0.0277 |
| 13 | Chlorogenic acid | trans-3-O-Caffeoylquinic acid | 325 | 17,417 | 4.609 ± 0.408 | 0.934 ± 0.0955 |
| 14 | Vanillic acid | 4-Hydroxy-3-methoxybenzoic acid | 420 | 18,062 | 0.102 ± 0.014 | 0.379 ± 0.007 |
| 15 | Caffeic acid | trans-3,4-Dihydroxycinnamic acid | 325 | 18,492 | 0.035 ± 0.003 | 0.203 ± 0.021 |
| 16 | β-Resorcylic acid | 2,4-Dihydroxybenzoic acid | 420 | 18,618 | 0.017 ± 0.002 | 0.015 ± 0.001 |
| 17 | (−)-Epicatechin | Monomeric flavan-3-ol ((−)-cis-3,3′,4′,5,7-pentahydroxyflavane) | 235 | 18,950 | 1.822 ± 0.115 | 1.379 ± 0.125 |
| 18 | Syringic acid | 4-Hydroxy-3,5-dimethoxybenzoic acid | 420 | 19,420 | 0.613 ± 0.057 | 0.134 ± 0.011 |
| 19 | 1.3-Dicaffeoylquinic acid | 1,5-Dicaffeoylquinic acid | 325 | 19,675 | 0.826 ± 0.078 | 0.189 ± 0.019 |
| 20 | Cyanidin | 3,3′,4,5,7-Pentahydroxyflavone (3,3′,4,5,7-pentahydroxyflavylium chloride) | 280 | 19,708 | 47.448 ± 4.461 | 4.453 ± 0.406 |
| 21 | Syringaldehyde | 4-Hydroxy-3,5-dimethoxybenzaldehyde | 280 | 20,825 | 0.402 ± 0.007 | 0.262 ± 0.016 |
| 22 | p-Coumaric acid | trans-4-Hydroxycinnamic acid | 325 | 22,567 | 0.520 ± 0.058 | 0.247 ±0.028 |
| 23 | Ferulic acid | 4-Hydroxy-3-methoxy-cinnamic acid | 420 | 23,737 | 0.439 ± 0.041 | 0.081 ± 0.007 |
| 24 | Coumarin | 1,2-Benzopyrone | 280 | 24,600 | 1.285 ± 0.116 | 0.170 ± 0.016 |
| 25 | Sinapic acid | 4-Hydroxy-3,5-dimethoxy-cinnamic acid | 420 | 24,731 | 0.214 ± 0.011 | 0.140 ± 0.014 |
| 26 | trans-3-Hydroxycinnamic acid | m-Coumaric acid | 280 | 25,067 | 0.241 ± 0.022 | 0.119 ± 0.012 |
| 27 | Luteolin 7-O- β -D-glucoside | Glucoluteolin; luteoloside | 325 | 26,133 | 1.614 ± 0.171 | 1.417 ± 0.118 |
| 28 | Rutin | quercetin-3-O-rutinoside | 375 | 26,765 | 26.66 ± 2.481 | 4.431 ± 0.433 |
| 29 | Ellagic acid | 4,4′,5,5′,6,6′-Hexahydroxydiphenic acid 2,6,2′,6′-dilactone | 235 | 26,783 | 35.881 ± 3.346 | 14.448 ± 1.451 |
| 30 | Hesperidin | Hesperetin-7-rutinoside | 280 | 27,058 | 4.013 ± 0.303 | 0.633 ± 0.006 |
| 31 | o-Coumaric acid | trans-2-Hydroxycinnamic acid | 420 | 27,483 | 0.711 ± 0.66 | 0.149 ± 0.015 |
| 32 | Rosmarinic acid | 3,4-Dihydroxycinnamic acid (R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl ester | 420 | 27,970 | 4.406 ± 0.411 | 1.851 ± 0.0182 |
| 33 | Salicylic acid | 2-Hydroxybenzoic acid | 420 | 28,158 | 0.457 ± 0.052 | 0.474 ± 0.045 |
| 34 | Myricetin (flavonol) | 3,3′,4′,5,5′,7-Hexahydroxyflavone | 375 | 28,308 | 3.928 ± 0.265 | 7.175 ± 0.711 |
| 35 | Quercetin | Flavonol (3,3′,4′,5,7-pentahydroxyflavone) | 375 | 32,142 | 0.156 ± 0.016 | 0.241 ± 0.025 |
| 36 | trans-Cinnamic acid | Cinnamic acid | 280 | 32,552 | 0.167 ± 0.017 | 0.034 ± 0.003 |
| 37 | Naringenin | 4′,5,7-Trihydroxyflavanone | 280 | 33,142 | 0.461 ± 0.037 | 0.924 ± 0.091 |
| 38 | Luteolin | 3′,4′,5,7-Tetrahydroxyflavone | 325 | 33,420 | 0.911 ± 0.087 | 0.364 ± 0.036 |
| 39 | Kaempferol | 3,4′,5,7-Tetrahydroxyflavone | 375 | 35,375 | 0.148 ± 0.015 | 0.167 ± 0.017 |
| 40 | 3-Hydroxyflavone | Flavonol | 235 | 46,958 | 0.103 ± 0.011 | 0.128 ± 0.006 |
| (A) Water Soluble Vitamins | ||
| Content (mg/g) in Dry Matter of the Extracts | ||
| Leaf | Twig | |
| B1 (thiaminechloride) # | 1.12 ± 0.05 | 0.77 ± 0.05 |
| B2 (riboflavin) # | 0.48 ± 0.07 | 0.51 ± 0.06 |
| B3 (pantothenic acid) # | 2.10 ± 0.02 | 2.70 ± 0.12 |
| B5 (nicotinicacid) # | 3.30 ± 0.18 | 3.10 ± 0.10 |
| B6 (pyridoxine) # | 5.70 ± 0.10 | 6.20 ± 0.10 |
| Bc (folic acid) # | 0.97 ± 0.11 | 0.86 ± 0.12 |
| (B) Vitamin E Isomers | ||
| Isomers | Content (mg/g) in Dry Matter of the Extracts | |
| Leaf | α—tocopherol # | 0.54 ± 0.07 |
| ß—tocopherol # | 0.13 ± 0.05 | |
| γ—tocopherol # | 0.18 ± 0.03 | |
| Twig | α—tocopherol | 0.31 ± 0.05 |
| ß—tocopherol | 0.15 ± 0.02 | |
| γ—tocopherol | 0.09 ± 0.02 | |
| Amino Acids | Content (mg/g) in Dry Matter of the Extracts | |
|---|---|---|
| Leaf | Twig | |
| Arginine # | 4.979 ± 0.009 | - |
| Lysine # | 3.378 ± 0.008 | 1.426 ± 0.008 |
| Tyrosine # | 3.023 ± 0.002 | 0.713 ± 0.023 |
| Phenylalanine # | 6.045 ± 0.032 | 2.262 ± 0.003 |
| Histidine # | 0.960 ± 0.035 | 0.565 ± 0.005 |
| Leucine + isoleucine# | 6.900 ± 0.020 | 1.819 ± 0.020 |
| Methionine # | 1.494 ± 0.041 | 0.787 ± 0.063 |
| Valine # | 0.460 ± 0.010 | 0.713 ± 0.003 |
| Proline # | 10.491 ± 0.004 | 3.196 ± 0.040 |
| Threonine # | 4.445 ± 0.005 | 1.770 ± 0.010 |
| Serine # | 4.801 ± 0.020 | 2.040 ± 0.053 |
| Alanine # | 5.334 ± 0.019 | 1.573 ± 0.020 |
| Glycine # | 4.801 ± 0.001 | - |
| Total content | 61.487 | 16.864 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubczak, M.; Khassenova, A.B.; Skalski, B.; Michlewska, S.; Wielanek, M.; Aralbayeva, A.N.; Murzakhmetova, M.K.; Zamaraeva, M.; Skłodowska, M.; Bryszewska, M.; et al. Bioactive Compounds and Antiradical Activity of the Rosa canina L. Leaf and Twig Extracts. Agronomy 2020, 10, 1897. https://doi.org/10.3390/agronomy10121897
Kubczak M, Khassenova AB, Skalski B, Michlewska S, Wielanek M, Aralbayeva AN, Murzakhmetova MK, Zamaraeva M, Skłodowska M, Bryszewska M, et al. Bioactive Compounds and Antiradical Activity of the Rosa canina L. Leaf and Twig Extracts. Agronomy. 2020; 10(12):1897. https://doi.org/10.3390/agronomy10121897
Chicago/Turabian StyleKubczak, Małgorzata, Ainur B. Khassenova, Bartosz Skalski, Sylwia Michlewska, Marzena Wielanek, Araylim N. Aralbayeva, Maira K. Murzakhmetova, Maria Zamaraeva, Maria Skłodowska, Maria Bryszewska, and et al. 2020. "Bioactive Compounds and Antiradical Activity of the Rosa canina L. Leaf and Twig Extracts" Agronomy 10, no. 12: 1897. https://doi.org/10.3390/agronomy10121897
APA StyleKubczak, M., Khassenova, A. B., Skalski, B., Michlewska, S., Wielanek, M., Aralbayeva, A. N., Murzakhmetova, M. K., Zamaraeva, M., Skłodowska, M., Bryszewska, M., & Ionov, M. (2020). Bioactive Compounds and Antiradical Activity of the Rosa canina L. Leaf and Twig Extracts. Agronomy, 10(12), 1897. https://doi.org/10.3390/agronomy10121897







