Optimization and Comparison of Ultrasound and Microwave-Assisted Extraction of Phenolic Compounds from Cotton-Lavender (Santolina chamaecyparissus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Microwave-Assisted Extraction (MAE)
2.5. Determining the Total Phenolic Content (TPC)
2.6. Experimental Design and Statistical Analysis
2.7. Identification of the Phenolic Compounds by UHPLC-QToF-MS
2.8. Analysis of the Phenolic Compounds by UHPLC-PAD
3. Results and Discussion
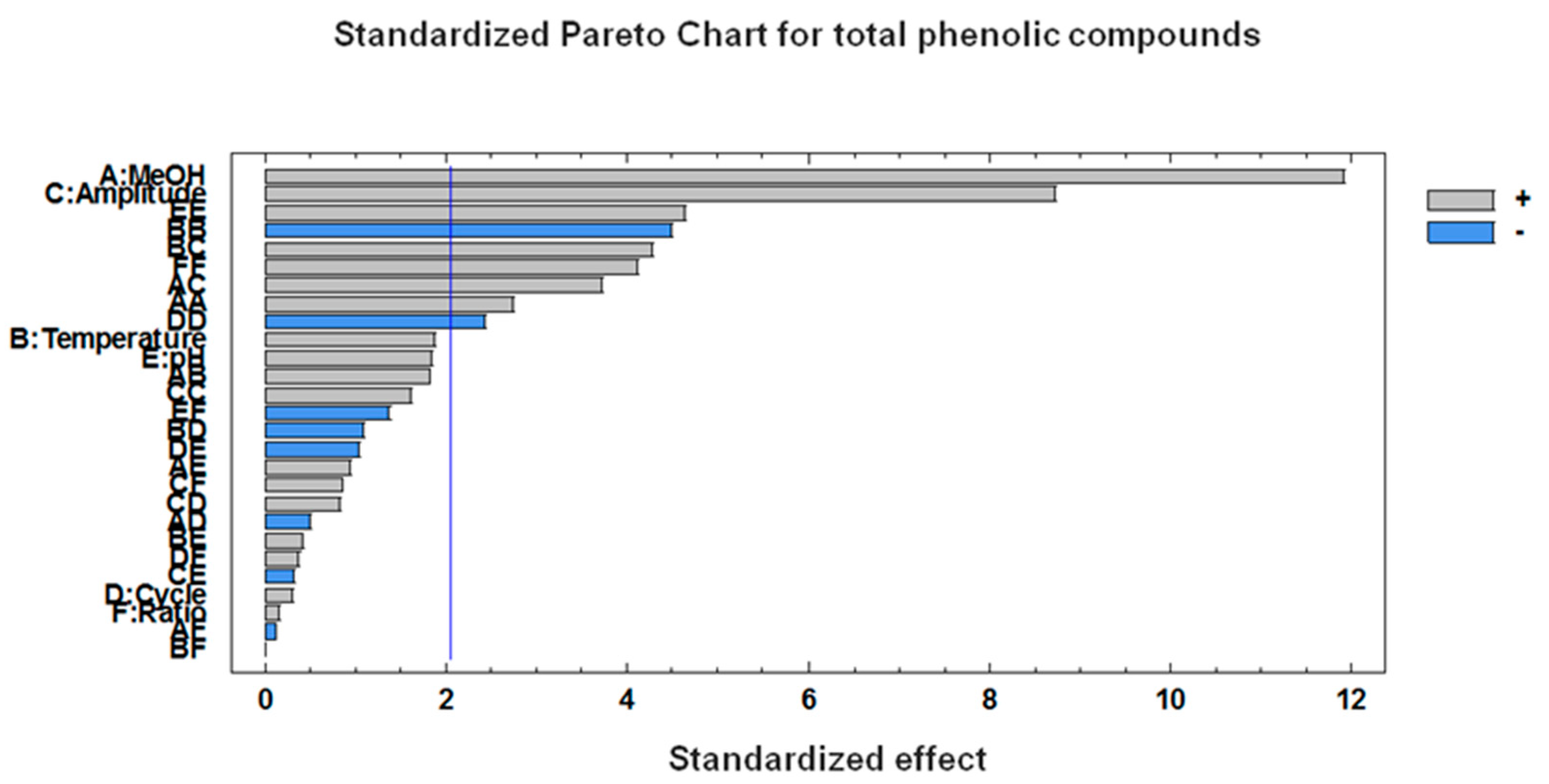
3.1. Optimization of the Ultrasound-Assisted Extraction (UAE) Conditions
+ 1.47·X1X5 − 0.17·X1X6 − 6.12·X22 + 6.61·X2 X3 − 1.69·X2X4 + 0.44·X2X5 − 0.004·X2X6 + 2.19·X32 + 1.25·X3X4 − 0.48·X3X5 +
0.93·X3X6 − 3.30·X42 − 1.60 ·X4X5 + 0.57·X4X6 + 6.31·X52 − 2.12·X5X6 + 5.59·X62
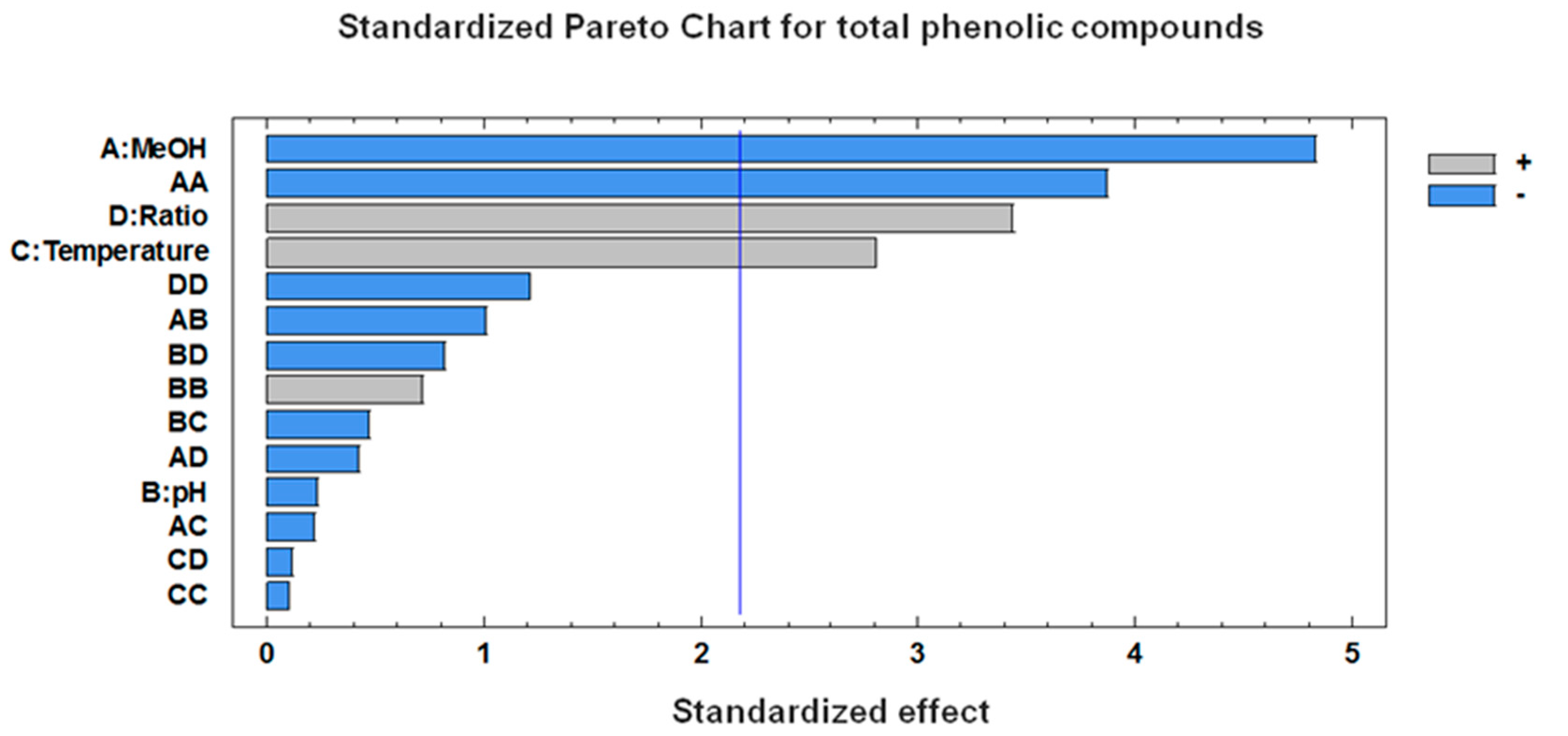
3.2. Optimization of the Microwave-Assisted Extraction (MAE) Conditions
0.36·X2X4 + 1.93·X32 − 2.56·X3X4 − 3.27·X42
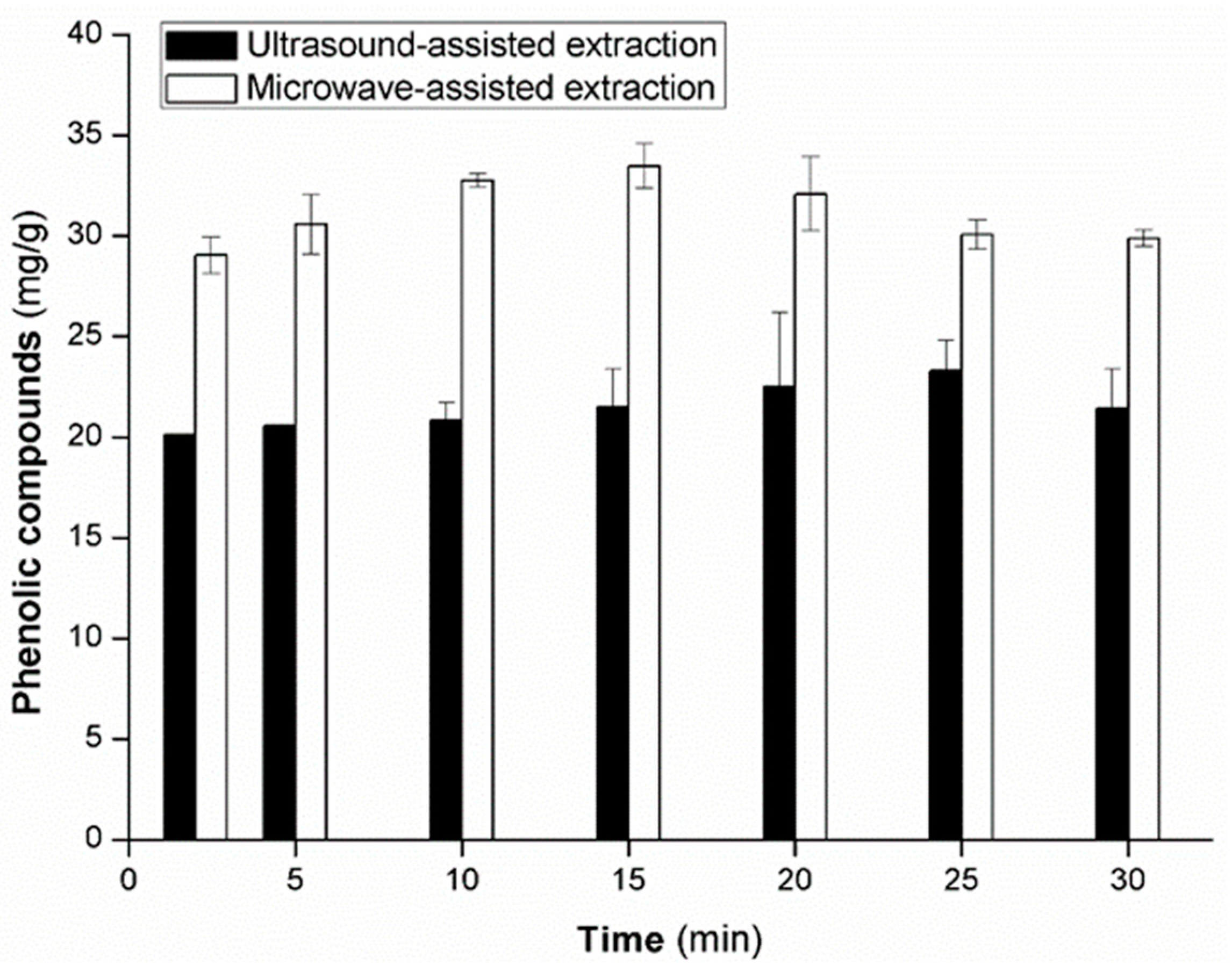
3.3. Extraction Time
3.4. Repeatability and Intermediate Precision of the Methods
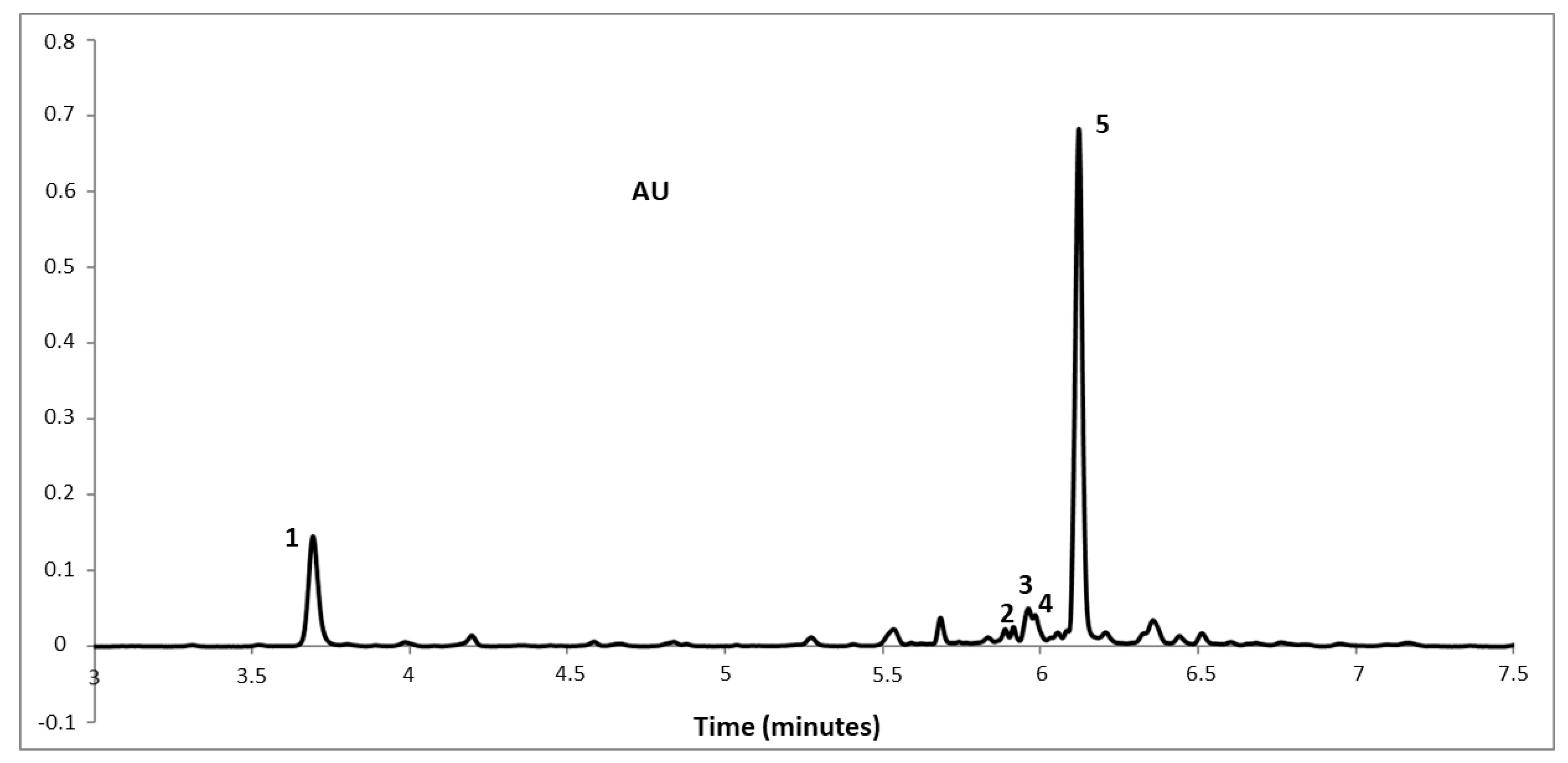
3.5. Identification and Quantification of the Phenolic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Perez-Alvarez, J.A.; Sendra, E.; Fernández-López, J. Chemical Characterization and Antibacterial Activity of Two Aromatic Herbs (Santolina chamaecyparissus and Sideritis angustifolia) Widely Used in the Folk Medicine. J. Food Saf. 2012, 32, 426–434. [Google Scholar] [CrossRef]
- Djarmouni, M.; Baghiani, A.; Adjadj, M.; Arrar, L. In vitro and in vivo Antioxidant, Antihemolytic and Anti-inflammatory Activities of Santolina chamaecyparissus Extracts. Pharmacogn. Commun. 2018, 8, 15–24. [Google Scholar] [CrossRef]
- Elguea-Culebras, G.O.; Sánchez-Vioque, R.; Berruga, M.I.; Herraiz-Peñalver, D.; González-Coloma, A.; Andrés, M.F.; Santana-Méridas, O. Biocidal Potential and Chemical Composition of Industrial Essential Oils from Hyssopus officinalis, Lavandula × intermedia var. Super, and Santolina chamaecyparissus. Chem. Biodivers. 2018, 15, e1700313. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-M.; Kim, J.; Kang, J.; Koh, S.-H.; Ahn, Y.-J.; Kang, K.-S.; Park, I.-K. Fumigant toxicity and acetylcholinesterase inhibitory activity of 4 Asteraceae plant essential oils and their constituents against Japanese termite (Reticulitermes speratus Kolbe). Pestic. Biochem. Physiol. 2014, 113, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.-J.; Jung, C.-S.; Kang, J.; Kim, J.; Lee, J.-H.; Kim, D.-S.; Kim, H.-S.; Park, P.-S.; Kang, K.-S.; Park, I.-K. Insecticidal and Acetylcholine Esterase Inhibition Activity of Asteraceae Plant Essential Oils and Their Constituents against Adults of the German Cockroach (Blattella germanica). J. Agric. Food Chem. 2015, 63, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Diánez, F.; Santos, M.; Parra, C.; Navarro, M.J.; Blanco, R.; Gea, F.J. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett. Appl. Microbiol. 2018, 67, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Salah-Fatnassi, K.B.H.; Hassayoun, F.; Cheraif, I.; Khan, S.; Jannet, H.B.; Hammami, M.; Aouni, M.; Harzallah-Skhiri, F. Chemical composition, antibacterial and antifungal activities of flowerhead and root essential oils of Santolina chamaecyparissus L., growing wild in Tunisia. Saudi J. Biol. Sci. 2017, 24, 875–882. [Google Scholar] [CrossRef]
- Djeddi, S.; Djebile, K.; Hadjbourega, G.; Achour, Z.; Argyropoulou, C.; Skaltsa, H. In vitro antimicrobial properties and chemical composition of santolina chamaecyparissus essential oil from Algeria. Nat. Prod. Commun. 2012, 7, 937–940. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G.; Sprawka, I.; Sytykiewicz, H. Aphicidal activity of selected Asteraceae essential oils and their effect on enzyme activities of the green peach aphid, Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2018, 145, 84–92. [Google Scholar] [CrossRef]
- Demirci, B.; Özek, T.; Baser, K.H.C. Chemical composition of santolina chamaecyparissus L. Essential oil. J. Essent. Oil Res. 2000, 12, 625–627. [Google Scholar] [CrossRef]
- Derbesy, M.; Touche, J.; Zola, A. The Essential Oil of Santolina chamaecyparissus L. J. Essent. Oil Res. 1989, 1, 269–275. [Google Scholar] [CrossRef]
- Garg, S.N.; Gupta, D.; Mehta, V.K.; Kumar, S. Volatile Constituents of the Essential Oil of Santolina chamaecyparissus Linn, from the Southern Hills of India. J. Essent. Oil Res. 2001, 13, 234–235. [Google Scholar] [CrossRef]
- Zaiter, L.; Benayache, F.; Beghidja, N.; Figueredo, G.; Chalard, P.; Chalchat, J.-C.; Marchioni, E.; Benayache, S. Essential oils of Santolina africana Jord. & Fourr. and Santolina chamaecyparissus L. J. Essent. Oil Bear. Plants 2015, 18, 1338–1342. [Google Scholar] [CrossRef]
- Sacchetti, G.; Romagnoli, C.; Ballero, M.; Tosi, B.; Poli, F. Internal secretory structures and preliminary phytochemical investigation on flavonoid and coumarin content in Santolina insularis (Asteraceae). Phyton 1997, 37, 219–228. [Google Scholar]
- Güneş, A.; Kordali, Ş.; Turan, M.; Bozhüyük, A.U. Determination of antioxidant enzyme activity and phenolic contents of some species of the Asteraceae family from medicanal plants. Ind. Crop. Prod. 2019, 137, 208–213. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Falé, P.D.V.; Madeira, P.J.A. Phenolic profile and biological activities of decoctions from Santolina impressa, a Portuguese endemic species. J. Herb. Med. 2020, 21, 100335. [Google Scholar] [CrossRef]
- de Castro, M.D.L.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Alupului, A.; Calinescu, I.; Lavric, V. Microwave extraction of active principles from medicinal plants. UPB Sci. Bull. Ser. B 2012, 74, 129–142. [Google Scholar]
- Chemat, F.; Tomao, V.; Virot, M. Chapter 5: Ultrasound-assisted extraction in food analysis. In Handbook of Food Analysis Instruments, 1st ed.; Otles, S., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 80–100. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Herrera, M.C.; De Castro, M.L. Ultrasound-assisted extraction of phenolic compounds from strawberries prior to liquid chromatographic separation and photodiode array ultraviolet detection. J. Chromatogr. A 2005, 1100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Kratchanova, M.; Pavlova, E.; Panchev, I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr. Polym. 2004, 56, 181–185. [Google Scholar] [CrossRef]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barbero, G.F.; Jiménez-Cantizano, A. Alternative Ultrasound-Assisted Method for the Extraction of the Bioactive Compounds Present in Myrtle (Myrtus communis L.). Molecules 2019, 24, 882. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.P.; Souza, M.C.; Sumere, B.R.; da Silva, L.C.; Cunha, D.T.; Bezerra, R.M.N.; Rostagno, M.A. Extraction of bioactive compounds from pomegranate peel (Punica granatum L.) with pressurized liquids assisted by ultrasound combined with an expansion gas. Ultrason. Sonochem. 2019, 54, 11–17. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González de Peredo, A.V.; Ferreiro-González, M.; Barbero, G.F.; Espada-Bellido, E. Assessment of ultrasound assisted extraction as an alternative method for the extraction of anthocyanins and total phenolic compounds from maqui berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González de Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Toledo-Dominguez, J.J.; Carrera, C.; Palma, M.; Barbero, G.F. Ultrasound-assisted extraction of two types of antioxidant compounds (TPC and TA) from black chokeberry (Aronia melanocarpa L.): Optimization of the individual and simultaneous extraction methods. Agronomy 2019, 9, 456. [Google Scholar] [CrossRef]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Álvarez, J.A.; Barbero, G.F.; Ayuso, J. Optimization of analytical ultrasound-assisted methods for the extraction of total phenolic compounds and anthocyanins from sloes (Prunus spinosa L.). Agronomy 2020, 10, 966. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Espada-Bellido, E.; González de Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of Microwave-Assisted Extraction for the Recovery of Bioactive Compounds from the Chilean Superfruit (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2018, 8, 240. [Google Scholar] [CrossRef]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barroso, C.G.; Barbero, G.F. Development of New Analytical Microwave-Assisted Extraction Methods for Bioactive Compounds from Myrtle (Myrtus communis L.). Molecules 2018, 23, 2992. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Ayuso, J.; Barbero, G.F.; Álvarez, J.A. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 3: Microwave-Assisted Extraction. Agronomy 2020, 10, 179. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pereira, A.L.D.; Barbero, G.F.; Martínez, J. Recovery of anthocyanins from residues of Rubus fruticosus, Vaccinium myrtillus and Eugenia brasiliensis by ultrasound assisted extraction, pressurized liquid extraction and their combination. Food Chem. 2017, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandao, G.C.; da Silva, E.P.; Portugal, L.A.; Dos Reis, P.S.; Souza, A.S. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jung, J.; Tomasino, E.; Zhao, Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT-Food Sci. Technol. 2016, 72, 229–238. [Google Scholar] [CrossRef]
- Zardo, I.; de Sobczyk, A.E.; Marczak, L.D.F.; Sarkis, J. Optimization of Ultrasound Assisted Extraction of Phenolic Compounds from Sunflower Seed Cake Using Response Surface Methodology. Waste Biomass Valori. 2019, 10, 33–44. [Google Scholar] [CrossRef]
- Ryu, D.; Koh, E. Optimization of Ultrasound-Assisted Extraction of Anthocyanins and Phenolic Compounds from Black Soybeans (Glycine max L.). Food Anal. Methods 2019, 12, 1382–1389. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Guo, D.; Chen, F.; Wheeler, J.; Winder, J.; Selman, S.; Peterson, M.; Dixon, R.A. Improvement of in-rumen digestibility of alfalfa forage by genetic manipulation of lignin O-methyltransferases. Transgenic Res. 2001, 10, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Birch, J.; Ma, Z.F.; Xie, C.; Yang, H.; Bekhit, A.E.-D.; Dias, G. Optimization of microwave-assisted extraction of bioactive compounds from New Zealand and Chinese Asparagus officinalis L. roots. J. Food Sci. Technol. 2019, 56, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Kovačević, D.B.; Penić, M.; Fegeš, M.; Dragović-Uzelac, V. Microwave-Assisted Extraction (MAE) of Dalmatian Sage Leaves for the Optimal Yield of Polyphenols: HPLC-DAD Identification and Quantification. Food Anal. Methods 2016, 9, 2385–2394. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Manual on Policies and Procedures. In Peer Verified Methods Program; AOAC International: Gaithersburg, MD, USA, 1998. [Google Scholar]
| Sample | Parameter | TPC (mg/g) | ||||
|---|---|---|---|---|---|---|
| MeOH X1 | Temp. X2 | pH X3 | Ratio X4 | Observed Value | Estimated Value | |
| 1 | −1 | −1 | 0 | 0 | 26.83999 | 30.5967 |
| 2 | 1 | −1 | 0 | 0 | 24.63146 | 25.0459 |
| 3 | −1 | 1 | 0 | 0 | 32.20955 | 33.3202 |
| 4 | 1 | 1 | 0 | 0 | 23.71069 | 21.4791 |
| 5 | 0 | 0 | −1 | −1 | 23.19759 | 24.3012 |
| 6 | 0 | 0 | 1 | −1 | 30.54152 | 29.7064 |
| 7 | 0 | 0 | −1 | 1 | 28.48667 | 30.8469 |
| 8 | 0 | 0 | 1 | 1 | 35.11375 | 35.5353 |
| 9 | 0 | 0 | 0 | 0 | 32.61005 | 31.8689 |
| 10 | −1 | 0 | 0 | −1 | 23.80272 | 25.5979 |
| 11 | 1 | 0 | 0 | −1 | 20.48519 | 18.2364 |
| 12 | −1 | 0 | 0 | 1 | 32.37091 | 33.1196 |
| 13 | 1 | 0 | 0 | 1 | 26.38459 | 23.0893 |
| 14 | 0 | −1 | −1 | 0 | 31.03386 | 29.6506 |
| 15 | 0 | 1 | −1 | 0 | 30.20629 | 30.6968 |
| 16 | 0 | −1 | 1 | 0 | 38.15586 | 36.1653 |
| 17 | 0 | 1 | 1 | 0 | 34.3926 | 34.2757 |
| 18 | 0 | 0 | 0 | 0 | 31.18951 | 31.8689 |
| 19 | 0 | −1 | 0 | −1 | 27.33226 | 27.0386 |
| 20 | 0 | 1 | 0 | −1 | 28.69945 | 29.1781 |
| 21 | 0 | −1 | 0 | 1 | 36.2908 | 35.7871 |
| 22 | 0 | 1 | 0 | 1 | 32.53565 | 32.8042 |
| 23 | −1 | 0 | −1 | 0 | 32.96712 | 27.9884 |
| 24 | 1 | 0 | −1 | 0 | 17.56914 | 19.9767 |
| 25 | −1 | 0 | 1 | 0 | 36.15198 | 33.7194 |
| 26 | 1 | 0 | 1 | 0 | 19.38559 | 24.3392 |
| 27 | 0 | 0 | 0 | 0 | 31.80706 | 31.8689 |
| Sample | Parameters | TPC (mg/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| MeOH X1 | Temp. X2 | Amplitude X3 | Cycle X4 | pH X5 | Ratio X6 | Observed Value | Estimated Value | |
| 1 | 0 | 0 | −1 | 0 | −1 | −1 | 15.8107 | 18.2751 |
| 2 | 0 | 0 | 1 | 0 | −1 | −1 | 28.1210 | 25.5952 |
| 3 | 0 | 0 | −1 | 0 | 1 | −1 | 23.2624 | 22.518 |
| 4 | 0 | 0 | 1 | 0 | 1 | −1 | 31.3854 | 28.8766 |
| 5 | 0 | 0 | −1 | 0 | −1 | 1 | 17.9978 | 19.6054 |
| 6 | 0 | 0 | 1 | 0 | −1 | 1 | 27.1499 | 28.7954 |
| 7 | 0 | 0 | −1 | 0 | 1 | 1 | 17.9788 | 19.6035 |
| 8 | 0 | 0 | 1 | 0 | 1 | 1 | 29.3954 | 27.8321 |
| 9 | 0 | −1 | 0 | −1 | −1 | 0 | 11.2657 | 12.0803 |
| 10 | 0 | 1 | 0 | −1 | −1 | 0 | 15.2585 | 14.9915 |
| 11 | 0 | −1 | 0 | 1 | −1 | 0 | 15.6285 | 15.6357 |
| 12 | 0 | 1 | 0 | 1 | −1 | 0 | 16.1609 | 15.1685 |
| 13 | 0 | −1 | 0 | −1 | 1 | 0 | 13.6044 | 14.8733 |
| 14 | 0 | 1 | 0 | −1 | 1 | 0 | 18.9555 | 18.6718 |
| 15 | 0 | −1 | 0 | 1 | 1 | 0 | 14.6914 | 15.2349 |
| 16 | 0 | 1 | 0 | 1 | 1 | 0 | 16.7463 | 15.6551 |
| 17 | −1 | 0 | −1 | −1 | 0 | 0 | 12.3622 | 12.0446 |
| 18 | 1 | 0 | −1 | −1 | 0 | 0 | 18.5369 | 17.4708 |
| 19 | −1 | 0 | 1 | −1 | 0 | 0 | 10.3544 | 12.8199 |
| 20 | 1 | 0 | 1 | −1 | 0 | 0 | 28.9242 | 29.735 |
| 21 | −1 | 0 | −1 | 1 | 0 | 0 | 11.4718 | 11.6154 |
| 22 | 1 | 0 | −1 | 1 | 0 | 0 | 19.3494 | 15.9295 |
| 23 | −1 | 0 | 1 | 1 | 0 | 0 | 12.8794 | 14.8999 |
| 24 | 1 | 0 | 1 | 1 | 0 | 0 | 31.3398 | 30.7029 |
| 25 | 0 | −1 | −1 | 0 | 0 | −1 | 17.2901 | 16.6491 |
| 26 | 0 | 1 | −1 | 0 | 0 | −1 | 12.2594 | 11.7105 |
| 27 | 0 | −1 | 1 | 0 | 0 | −1 | 16.3708 | 16.8797 |
| 28 | 0 | 1 | 1 | 0 | 0 | −1 | 24.7626 | 25.1585 |
| 29 | 0 | −1 | −1 | 0 | 0 | 1 | 15.3561 | 15.8613 |
| 30 | 0 | 1 | −1 | 0 | 0 | 1 | 10.5216 | 10.9139 |
| 31 | 0 | −1 | 1 | 0 | 0 | 1 | 18.3142 | 17.962 |
| 32 | 0 | 1 | 1 | 0 | 0 | 1 | 26.4921 | 26.232 |
| 33 | −1 | −1 | 0 | 0 | −1 | 0 | 14.4602 | 14.1986 |
| 34 | 1 | −1 | 0 | 0 | −1 | 0 | 22.7181 | 20.5516 |
| 35 | −1 | 1 | 0 | 0 | −1 | 0 | 12.4957 | 12.6284 |
| 36 | 1 | 1 | 0 | 0 | −1 | 0 | 25.0246 | 24.5659 |
| 37 | −1 | −1 | 0 | 0 | 1 | 0 | 13.7431 | 13.9253 |
| 38 | 1 | −1 | 0 | 0 | 1 | 0 | 23.6263 | 23.2171 |
| 39 | −1 | 1 | 0 | 0 | 1 | 0 | 10.7994 | 13.2424 |
| 40 | 1 | 1 | 0 | 0 | 1 | 0 | 27.5806 | 28.1187 |
| 41 | −1 | 0 | 0 | −1 | 0 | −1 | 14.4986 | 14.2599 |
| 42 | 1 | 0 | 0 | −1 | 0 | −1 | 25.2806 | 25.6064 |
| 43 | −1 | 0 | 0 | 1 | 0 | −1 | 15.8753 | 14.5097 |
| 44 | 1 | 0 | 0 | 1 | 0 | −1 | 19.8659 | 24.7441 |
| 45 | −1 | 0 | 0 | −1 | 0 | 1 | 17.9268 | 14.003 |
| 46 | 1 | 0 | 0 | −1 | 0 | 1 | 24.5866 | 24.9978 |
| 47 | −1 | 0 | 0 | 1 | 0 | 1 | 16.6842 | 15.404 |
| 48 | 1 | 0 | 0 | 1 | 0 | 1 | 24.0936 | 25.2867 |
| 49 | 0 | 0 | 0 | 0 | 0 | 0 | 18.6470 | 16.8419 |
| 50 | 0 | 0 | 0 | 0 | 0 | 0 | 16.1529 | 16.8419 |
| 51 | 0 | 0 | 0 | 0 | 0 | 0 | 17.9339 | 16.8419 |
| 52 | 0 | 0 | 0 | 0 | 0 | 0 | 15.4796 | 16.8419 |
| 53 | 0 | 0 | 0 | 0 | 0 | 0 | 16.8702 | 16.8419 |
| 54 | 0 | 0 | 0 | 0 | 0 | 0 | 15.9677 | 16.8419 |
| Source | Ultrasound-Assisted Extraction | Microwave-Assisted Extraction | ||
|---|---|---|---|---|
| Coefficient Estimate | p-Value | Coefficient Estimate | p-Value | |
| Average | 16.8419 | 31.8689 | ||
| A:MeOH | 10.6146 | 0.0000 | −8.6959 | 0.0004 |
| B:Temperature | 1.6657 | 0.0730 | 5.0467 | 0.0159 |
| C:Amplitude | 7.7743 | 0.0000 | ||
| D:Cycle | 0.2693 | 0.7649 | ||
| E:pH | 1.6398 | 0.0772 | −0.4217 | 0.8188 |
| F:Ratio | 0.1429 | 0.8739 | 6.1873 | 0.0049 |
| AA | 3.7328 | 0.0109 | −10.4496 | 0.0022 |
| AB | 2.7922 | 0.0821 | −0.6842 | 0.8300 |
| AC | 5.7445 | 0.0010 | ||
| AD | −0.5560 | 0.6148 | ||
| AE | 1.4694 | 0.3500 | −3.1451 | 0.3331 |
| AF | −0.1758 | 0.9102 | −1.3344 | 0.6763 |
| BB | −6.1191 | 0.0001 | −0.2763 | 0.9202 |
| BC | 6.6087 | 0.0002 | ||
| BD | −1.6891 | 0.2839 | ||
| BE | 0.4437 | 0.6877 | −1.4678 | 0.6463 |
| BF | −0.0044 | 0.9977 | −0.3584 | 0.9104 |
| CC | 2.1893 | 0.1199 | ||
| CD | 1.2546 | 0.4238 | ||
| CE | −0.4807 | 0.7580 | ||
| CF | 0.9350 | 0.3995 | ||
| DD | −3.3014 | 0.0226 | ||
| DE | −1.5968 | 0.3105 | ||
| DF | 0.5756 | 0.7123 | ||
| EE | 6.3145 | 0.0001 | 1.9328 | 0.4879 |
| EF | −2.1224 | 0.1809 | −2.5612 | 0.4275 |
| FF | 5.5877 | 0.0004 | −3.2665 | 0.2497 |
| Peak | Rt (min) | λmax (nm) | λquantification (nm) | Molecularion [M-H]− | Molecular Formula | Compound | Quantification (mg g–1) dw (MAE) |
|---|---|---|---|---|---|---|---|
| 1 | 2.74 | 325 | 325 | 353 | C16H18O9 | 5-O-Caffeoylquinic acid (Chlorogenic acid) | 2.66 |
| 2 | 5.25 | 353 | 350 | 463 | C21H20O12 | Quercetin 3-O-galactoside | 0.05 |
| 3 | 5.41 | 353 | 350 | 463 | C21H20O12 | Quercetin 3-O-glucoside | 0.28 |
| 4 | 5.44 | 350 | 350 | 477 | C21H20O11 | Luteolin 6-C-glucoside (isoorientin) | 0.18 |
| 5 | 5.52 | 325 | 325 | 515 | C25H24O12 | 1,5-Dicaffeoylquinic acid (Cynarin) | 8.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aourach, M.; V. González-de-Peredo, A.; Vázquez-Espinosa, M.; Essalmani, H.; Palma, M.; F. Barbero, G. Optimization and Comparison of Ultrasound and Microwave-Assisted Extraction of Phenolic Compounds from Cotton-Lavender (Santolina chamaecyparissus L.). Agronomy 2021, 11, 84. https://doi.org/10.3390/agronomy11010084
Aourach M, V. González-de-Peredo A, Vázquez-Espinosa M, Essalmani H, Palma M, F. Barbero G. Optimization and Comparison of Ultrasound and Microwave-Assisted Extraction of Phenolic Compounds from Cotton-Lavender (Santolina chamaecyparissus L.). Agronomy. 2021; 11(1):84. https://doi.org/10.3390/agronomy11010084
Chicago/Turabian StyleAourach, Mohammed, Ana V. González-de-Peredo, Mercedes Vázquez-Espinosa, Haiat Essalmani, Miguel Palma, and Gerardo F. Barbero. 2021. "Optimization and Comparison of Ultrasound and Microwave-Assisted Extraction of Phenolic Compounds from Cotton-Lavender (Santolina chamaecyparissus L.)" Agronomy 11, no. 1: 84. https://doi.org/10.3390/agronomy11010084
APA StyleAourach, M., V. González-de-Peredo, A., Vázquez-Espinosa, M., Essalmani, H., Palma, M., & F. Barbero, G. (2021). Optimization and Comparison of Ultrasound and Microwave-Assisted Extraction of Phenolic Compounds from Cotton-Lavender (Santolina chamaecyparissus L.). Agronomy, 11(1), 84. https://doi.org/10.3390/agronomy11010084









