1. Introduction
Glyphosate is a broad-spectrum, foliar, non-selective and systemic herbicide acting as an inhibitor of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) [
1,
2,
3]. EPSPS is an enzyme that plays an important role in the shikimate pathway for the biosynthesis of aromatic amino acids: phenylalanine (Phe), tyrosine (Tyr) and tryptophan (Trp) [
4,
5]. Following foliar application, this herbicide is absorbed and translocated via the phloem to the apical meristematic tissues [
4,
6]. This mode of action leads to lethal injuries to susceptible plants [
2]. Thus, this herbicide has been successfully and extensively used in a broad range of agricultural and non-agricultural areas both against monocot and dicot weeds [
7]. Glyphosate in its various formulations has been continuously applied, especially in recent decades, in orchards, vineyards, olive groves and cereal fields in many countries of the Mediterranean basin [
3,
8]. As a weak acid, glyphosate needs to be applied in a form of salt, with the isopropylamine, potassium and ammonium salts among the common formulations [
1].
Herbicide efficacy can be significantly enhanced by the addition of adjuvants, leading to increased uptake and translocation of the active ingredient [
9]. In relation to targeting efficiency, the addition of adjuvants either in the formulated products or in the tank mixtures has been reported to improve glyphosate absorption and translocation in the plant tissues [
1,
10]. Surfactants, a common form of adjuvants, increase the deposition and the wetting behavior of the pesticide spray liquid on the leaf tissues, increasing, in this way, the permeability of the active ingredient through the plant surfaces [
11].
The overreliance on glyphosate has led to the evolution of resistance in populations of many weed species and, therefore, reduced efficacy of glyphosate-based weed management programs [
2,
12,
13]. Currently, there are 48 unique cases of glyphosate-resistant (GR) weeds globally [
14]. The evolution of resistance to glyphosate is the outcome of different mechanisms that do not allow the herbicide to block the shikimate pathway [
15,
16]. Overall, glyphosate resistance occurs from minor and major GR mechanisms that can be divided into two principal groups: non-target-site resistance (NTSR) and target-site resistance (TSR) mechanisms [
5,
17,
18,
19]. NTSR mechanisms include reduced uptake of the herbicide, diminished translocation to plant tissues, biochemical degradation and metabolism of the herbicide active compound, as well as altered sequestration of glyphosate to vacuoles [
12,
18]. On the contrary, TSR mechanisms refer to target-site mutations on the EPSPS enzyme, overexpression of ATP-binding cassette (ABC)-type transporters and
EPSPS gene amplification [
12,
19,
20]. Reduced translocation of glyphosate has been characterized as the main resistance mechanism in several weed species [
6]. There are many reported cases of reduced glyphosate translocation in various weeds, as reviewed by Shaner et al. [
21] and Dayan et al. [
16].
Glyphosate resistance poses a major threat for integrated management of problematic weeds, such as the species belonging to the genus
Conyza [
12].
Conyza species appear in many orchards and perennial crops in Southern Europe [
22].
Conyza sumatrensis (Retz.) E. Walker and
Conyza canadensis (L.) Cronq. are two weed species that have evolved resistance to multiple herbicide sites of action, including glyphosate [
14,
18]. Sumatran Fleabane (
C. sumatrensis) is an annual dicot species belonging to the family Asteraceae, native to Central and South America and is highly invasive in different warm-temperate and tropical areas of the Old World. High invasiveness is promoted by large achene production and extensive seed dispersal [
6,
23,
24]. This species has previously been reported to evolve both TSR and NTSR mechanisms to EPSPS inhibitors [
2]. However, glyphosate remains a very effective tool for the control of Sumatran fleabane [
2]. The need for mitigation of glyphosate resistance and better control of this species is imperative.
In this context, the objectives of this study were as follows: (1) to evaluate the efficacy of different glyphosate formulations in the control of glyphosate susceptible and resistant populations of C. sumatrensis from vineyards with different records of glyphosate applications, (2) to determine the susceptibility response to glyphosate of fleabane populations when an adjuvant is added to the formulations, and (3) to characterize physical (foliar retention) and physiological (uptake and translocation of [14C] glyphosate) factors that could explain the differential sensitivity to glyphosate formulations plus the adjuvants. The main hypothesis of this study was that the type of herbicide formulation and the addition of adjuvants may affect glyphosate retention, uptake and/or translocation in C. sumatrensis in a manner dependent on the glyphosate resistance mechanism.
2. Materials and Methods
2.1. Herbicide and Adjuvant Used
Three different glyphosate formulations were used: Rotundo Green (G1, glyphosate as isopropylamine salt 360 g L−1), MON79991 (G2, glyphosate as ammonium salt 720 g L−1) and MON79351 (G3, glyphosate as potassium salt 480 g L−1).
Two adjuvants have been tested: a non-ionic adjuvant of plant origin based on pine resins (adj1; Retenol, Daymsa Spain), used to decrease the surface tension of droplets and, thus, intended to increase wettability, foliar retention and persistence of the active substances; and a general-purpose adjuvant consisting of a mixture of ethodyl alcohols, polyglycol and aril polyethoxyethanol of non-ionic nature (adj2; INEX-A, COSMOAGRO S.A, Colombia), favoring humidification (penetration) and droplet dispersion. It is a product designed to ensure and/or improve the effectiveness of sprayed agrochemicals where greater humidification and coverage is required. Both adjuvants do not show any phytotoxic activity when they are applied alone on glyphosate resistant or susceptible C. sumatrensis plants (data not shown).
2.2. Plant Material
Seeds from 25 plants of C. sumatrensis, which had survived annual applications of glyphosate (3 L ha−1 of glyphosate as salt isopropylamine 360 g L−1), were collected from two vineyards in Southern France separated by approximately 100 km and with a different history of glyphosate applications. The first sample (R1) was collected in an old vineyard with 20 years of glyphosate use as the first control agent of C. sumatrensis, while the second sample (R2) was collected in an approximately 12 year-old vineyard with 10 years of glyphosate usage. In addition, seeds were collected from 25 plants of a C. sumatrensis population from an organic vineyard with only mechanical weed control, located in between the above vineyards. This population was putatively considered as susceptible (S).
During 5 consecutive days, seeds from the R1, R2 and S populations were sown in pots filled with moistened peat and covered with a transparent film. After emergence, seedlings were transplanted into 21 × 21 × 19 cm pots with a 1:1 (v/v) mixture of peat–sandy soil and placed in a greenhouse at 26 °C/18 °C (day/night), under 16 h photoperiod, 850 μmol m−2 s−1 photosynthetic photon flux density and 80% relative humidity. For each population, four same-age seedlings, equidistantly arranged, were placed per pot.
2.3. Investigating Molecular EPSPS
Samples of 100 mg of young leaves of
C. sumatrensis R1, R2 and S populations were used to obtain total RNA using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA was purified with TURBO DNase (RNase-Free; Ambion, Warrington, UK) and stored at −80 °C [
2]. Complimentary DNA was obtained from 2 μg of total RNA following the methodology described by González-Torralva et al. [
6] and Amaro-Blanco et al. [
2]. For
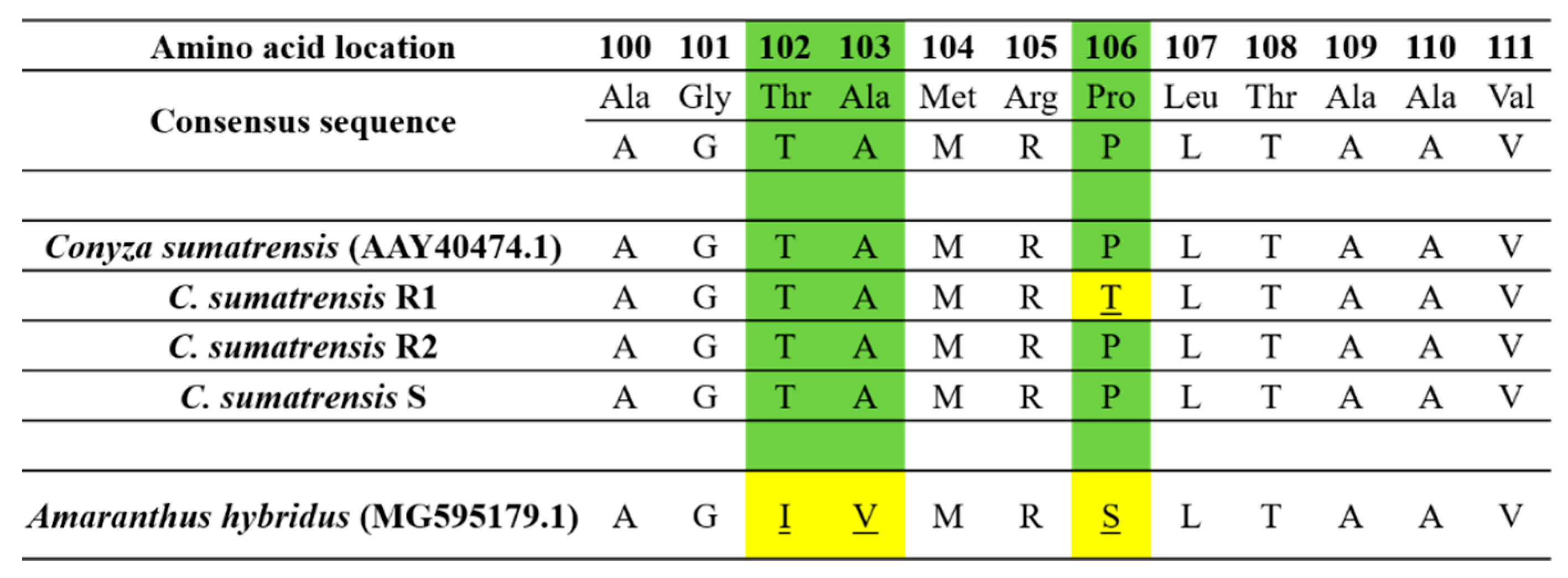
C. sumatransis, the primers were designed based on the EPSPS2 gene sequence: F3-EPSPS2 (5′-TCTAAAGCTCCAGAAGAAATTGTG-3′) and R3-EPSPS2 (5′-GAAACCCCAAACCGTYCC-3′). PCR products were sequenced (STAB VIDA, Caparica, Portugal) searching for mutations at positions 102, 103 and 106, which are commonly reported for this species [
6,
25].
2.4. Dose–Response Assays
Herbicide treatments were applied at the rosette stage (Bundesanstalt Bundessortenamt und Chemische Industrie (BBCH) 16–18) [
26]. Glyphosate formulations with or without adjuvant added (adj1 at 2 mL L
−1 and adj2 at 4 mL L
−1) were applied within a laboratory chamber (SBS-060 De Vries Manufacturing, Hollandale, MN) equipped with 8002 flat fan nozzles delivering 200 L ha
−1 at 250 kPa at 50 cm height. The following glyphosate rates were used: 0, 31.25, 62.50, 125, 250, 500, 1000, 2000, and 4000 g ae ha
−1. Two pots (i.e., two sets of four seedlings) per population were subjected to each treatment and the treatment order in the chamber was randomized. Due to differences in emergence date, plant size slightly differed between pots, so pots were grouped according to plant age (plant-size groups) in order to assure that plants of every size group received the full series of glyphosate rates. Dry weight was individually measured for the aboveground parts of R1, R2 and S plants 21 days after application.
2.5. Spray Retention Assays
The methodology described by Gauvrit [
27] was followed.
C. sumatrensis (R1, R2 and S populations) plants at the rosette stage (BBCH 16–18) were sprayed with individual glyphosate formulations (G1, G2 and G3) with or without adjuvants (adj1 at 2 mL L
−1 and adj2 at 4 mL L
−1) using the spray chamber as described above. Treatment solutions contained glyphosate formulations at 360 g ae ha
−1 in a volume of 200 L with 100 mg L
−1 of Na-fluorescein. After the solution had dried on the foliage, one randomly selected plant per pot was cut off at ground level and immersed for 30 s in 50 mL of 5 mM NaOH. Readings were made with a spectrofluorometer at 490/510 nm. Plants were then placed at 80 °C for 48 h to register dry matter. Five replications were used for each treatment.
2.6. 14C-Glyphosate Absorption and Translocation
The methodology described by Amaro-Blanco et al. [
2], with some modifications, was followed. Three glyphosate formulations (G1, G2 and G3) with or without adjuvants (adj1 at 2 mL L
−1 and adj2 at 4 mL L
−1), at the rate of 360 g ae ha
−1 and in a volume of 200 L, were mixed with radiolabeled
14C-glyphosate to obtain a solution with an activity of 0.834 kBq µL
−1. The R1, R2 and S plants were treated with the radiolabeled solution when they reached the 4–6 leaf stage, by applying one droplet of 1.0 µL on the third leaf of one randomly selected plant per pot. Plants were kept outside the growth chamber until droplets dried on the leaf surface, then they were returned to the chamber. At different time intervals after application (24, 48, 72 or 96 h), the treated leaf was washed in batches with 3 mL of a water:acetone solution (9:1
v/
v) in order to quantify the unabsorbed
14C-glyphosate. Then, 7 mL of scintillation liquid was added to each rinse, and the radioactivity measured by liquid scintillation spectrometry using a Scintillation Counter LS 6500 (Beckman Coulter, Brea, CA, USA) instrument. Finally, the plant was divided into treated leaf, rest of shoot and roots, and placed into cellulose cones. After cellulose cones with the different fresh tissues were dried at 60 °C for 72 h, samples were combusted in a biological sample oxidizer (307, PerkinElmer, Waltham, MA, USA). The
14CO
2 was trapped and mixed with 18 mL of a 9:9
v/
v mixture of Carbo-Sorb
® E and Permaf1uor
® (PerkinElmer), then quantified by liquid scintillation spectrometry as described above. The experiment was arranged in a completely randomized design with three replicates.
2.7. Data Analysis
The effects of
C. sumatrensis population, glyphosate formulation and adjuvant type on GR
50 values (i.e., treatment doses causing 50% reduction in growth) were tested using a non-linear mixed-effects model based on the following log-logistic curve:
where fw is the mean above ground fresh weight (g) per plant in each treated pot; fwc is the above ground fresh weight (g) of untreated plants, estimated as the mean value of the eight untreated plants within each plant-size group; x (explanatory variable) is the herbicide rate; and b and e are parameters. Parameter b is directly related to the slope around the inflection point and parameter e is the natural logarithm of GR
50. Population, glyphosate formulation and adjuvant type were included as fixed effects. An additional fixed factor, weight of untreated plants (fwc), which varied according to plant-size group, was included as a covariate. Plant-size group and pot replicate within each plant-size group were included as random factors. Initially, a maximal model was fitted, explaining parameters b and e according to all of the fixed factors, their interactions and the random factors. A model simplification procedure was thereafter carried out to establish the optimal model structure. Non-significant fixed terms, starting with interactions, of models fitted by maximum likelihood were dropped one at a time. Highly correlated random effects were also removed [
28]. Each simpler model was compared with the previous model using the likelihood ratio test (LRT). When two levels of a fixed factor did not differ statistically, they were grouped together. The model reduction process stopped when the LRT indicated a significant departure in likelihood from the previous model. Parameters of the final, minimal adequate model were then estimated by restricted maximum likelihood. Normality and homogeneity of residuals were explored graphically, and assumptions were met in the final model (
Supplementary Material). Analyses were performed using the package nlme (Linear and Nonlinear Mixed Effects Models) [
29] for the statistical environment R (R 3.4.3) [
30].
The effects of population, glyphosate formulation and adjuvant on leaf retention of spraying solution in C. sumatrensis leaves and of post-treatment time, population, glyphosate formulation and adjuvant on leaf uptake of 14C-glyphosate were tested in R using analysis of variance (ANOVA). For leaf retention, no transformation of the response variable was needed to satisfy the assumption of normality of residuals. However, to meet assumptions of homogeneity of variance and normality of residuals, percentage uptake was arcsine-square root transformed and post-treatment time was log transformed. For each analysis, a simplification procedure of the complete model associated to the full factorial design was carried out by progressively dropping non-significant terms, starting with higher order interactions, and testing every reduced model for loss of explanatory power using an F test. When two levels of a factor did not differ statistically, they were grouped together. When significant interactions were retained in final, most adequate models, one-way ANOVA or the Kruskal-Wallis test, depending on normality or lack of normality of residuals, respectively, were performed to compare levels of a factor at particular levels of interacting factors. When significance was declared, post hoc level separation was done either using contrasts (one-way ANOVA) or using the Wilcoxon test with Bonferroni correction to adjust for multiple comparisons (Kruskal-Wallis test).
The effects of C. sumatrensis population, glyphosate formulation and adjuvant type on translocation of the absorbed 14C-glyphosate from the treated leaf to the remaining shoot and roots at 96 h after treatment were analyzed in R using multivariate ANOVA (MANOVA). A multivariate response variable including percentage glyphosate remaining in the treated leaf and translocated to shoot and roots was defined as a measure of allocation of glyphosate among plant compartments. Percentage data were arcsine-square root transformed to meet the assumption of homogeneity of variance. As above, a simplification procedure starting with the complete model based on the initial full factorial design was carried out to select the minimal adequate model. The method based on the Pillai’s trace statistic was used for testing for significant effects.
4. Discussion
C. sumatrensis infestations concern farmers, advisors, plant protection companies and policy makers, because worldwide this species has evolved resistance to herbicides of many different modes of action, such as photosystem II (PSI) electron diverters, acetolactate synthase (ALS) inhibitors, protoporphyrinogen oxidase (PPO) inhibitors, synthetic auxins and EPSPS inhibitors, leading to common treatment failures [
14]. The recent herbicide resistance cases in Sumatran fleabane are ever-increasing, with the species demonstrating high levels of resistance to active ingredients such as paraquat [
31] and glyphosate [
5]. Thus, extensive research should be conducted regarding alternatives and additives to glyphosate in order to moderate glyphosate resistance evolution in
C. sumatrensis as in other noxious weeds.
The proper use of adjuvants is imperative in cases of glyphosate applications, since they reduce the surface tension of the spray liquid and may improve the efficacy of the treatments [
32]. Sumatran fleabane has been reported to be more sensitive to glyphosate in earlier rather than more advanced growth stages, due to altered morphological characteristics of the leaf tissues and subsequently lower deposition of the herbicide [
13,
31,
32]. The dose–response assays in the present study revealed that the susceptibility of
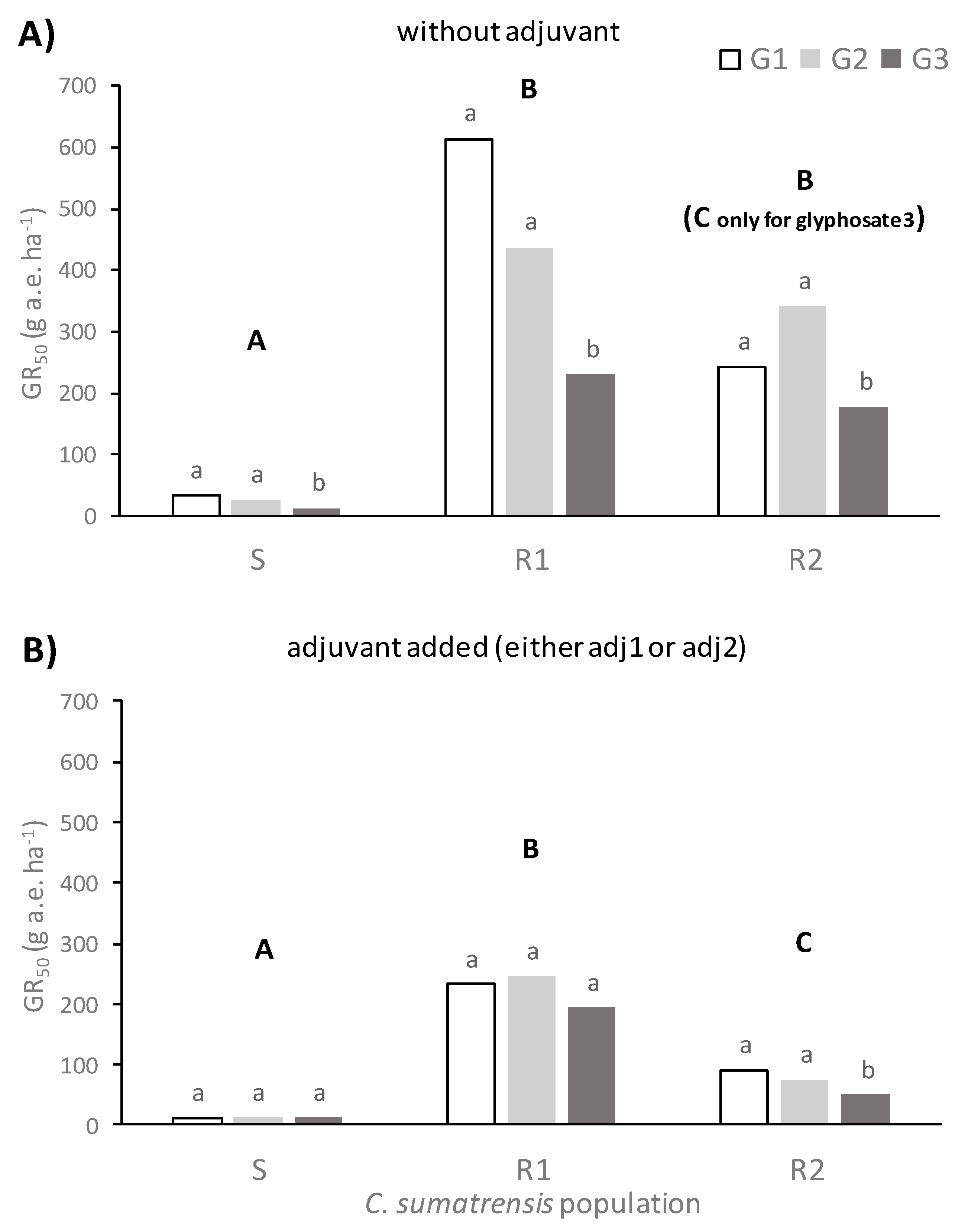
C. sumatrensis to herbicidal treatments at the rosette stage (BBCH 16–18) varied among populations and depended on the glyphosate formulation and the addition of adjuvants. In general terms, the S population exhibited significantly lower GR
50 values than the two resistant populations studied. This result was a primary indicator that there was not any TSR or NTSR mechanism on this population. On the contrary, the R1 population was approximately 14-fold and the R2 population approximately 4-fold more resistant than the S population in the presence of adjuvants and the glyphosate formulated as potassium salt in terms of GR
50 values. In the absence of adjuvants, these resistance index (RI) values were even greater (
Figure 2,
Supplementary Material).
The outcomes of this study were in line with previous research by Amaro-Blanco et al. [
2], who found that the GR
50 value of a glyphosate resistant
C. sumatrensis population from citrus orchards in Spain was 7-fold greater compared to a susceptible population when plants were sprayed with glyphosate as isopropylamine salt, a value in between the RI we have found in the studied populations in this work. Palma-Bautista et al. [
3] also revealed a RI value of about 5.5 for a resistant Sumatran fleabane population from citrus orchard following dose–response assays with glyphosate as potassium salt (Roundup Energy 450 g ae L
−1). However, a Sumatran fleabane population from vineyards was about 20-fold more resistant to glyphosate as potassium salt than a susceptible population in terms of dry weight reduction [
5]. The results found in the present study indicate that the addition of an adjuvant can significantly reduce GR
50 values in resistant
C. sumatrensis, irrespective of the glyphosate formulation. This advantage of adjuvant use demonstrates that costs of herbicide treatments can be significantly reduced, avoiding the increase in herbicide rates for the control of weeds such as those belonging to the genus
Conyza [
7].
C. sumatrensis has been reported to exhibit both TSR and NTSR mechanisms [
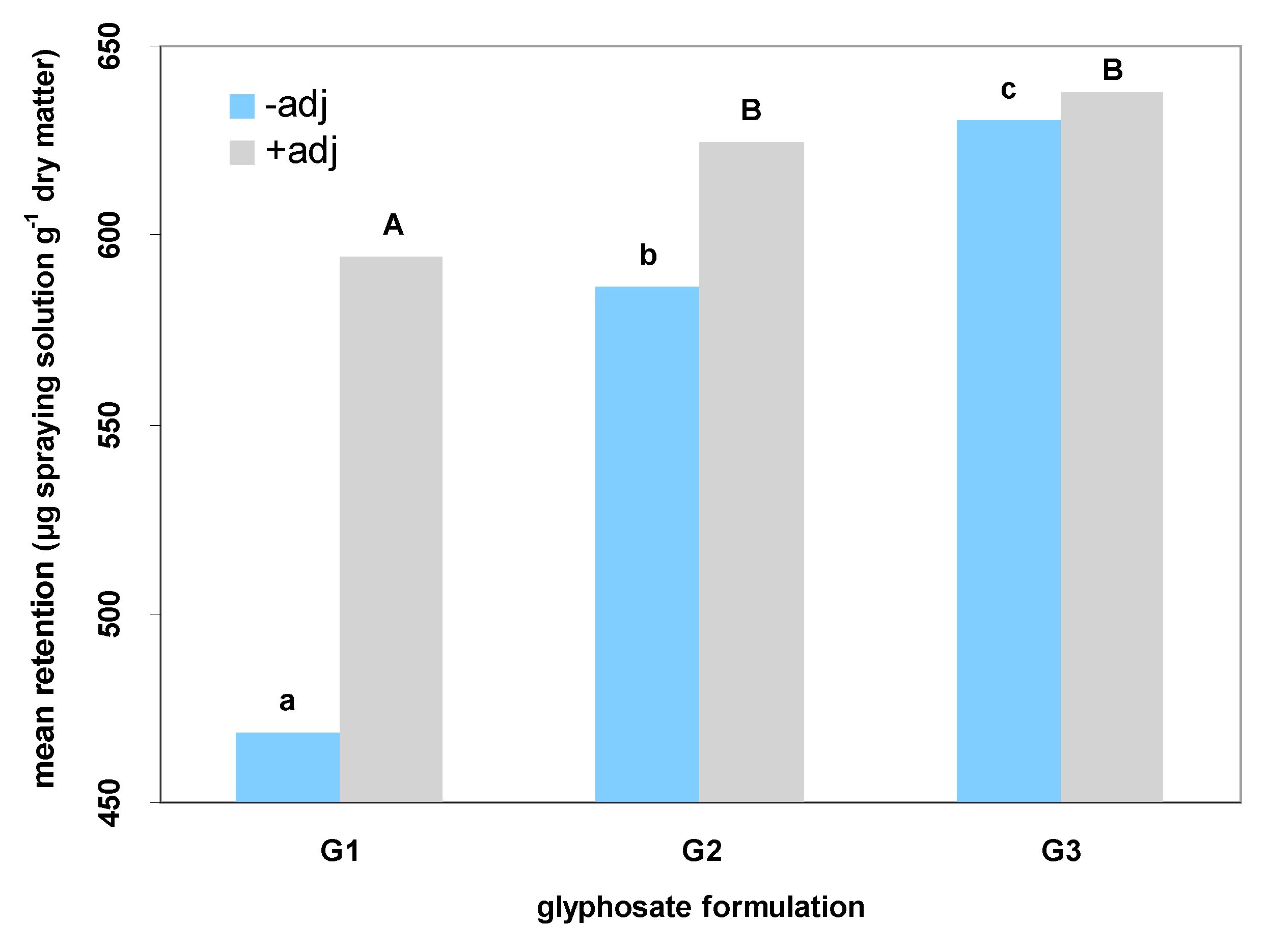
2]. In order to identify possible resistance mechanisms among the surveyed populations and quantify the effect of adjuvants in glyphosate formulations, further analyses were carried out. The herbicide efficacy can be described by spray retention, an important parameter that quantifies the amount of herbicide that penetrates the plant tissues [
22]. According to the results of the present study, glyphosate as potassium salt leads to high amounts of herbicide retained on leaf surfaces. The penetration into the tissues is strongly affected by the salt form of the formulated product [
1]. Thus, glyphosate as isopropylamine salt seems to be quite ineffective to achieve high retention of the active ingredient on
C. sumatrensis leaves. However, the addition of adjuvants may improve spray retention even if formulated products are “weak” (
Figure 3), thus constituting a useful tool for the prevention of herbicide treatment failures.
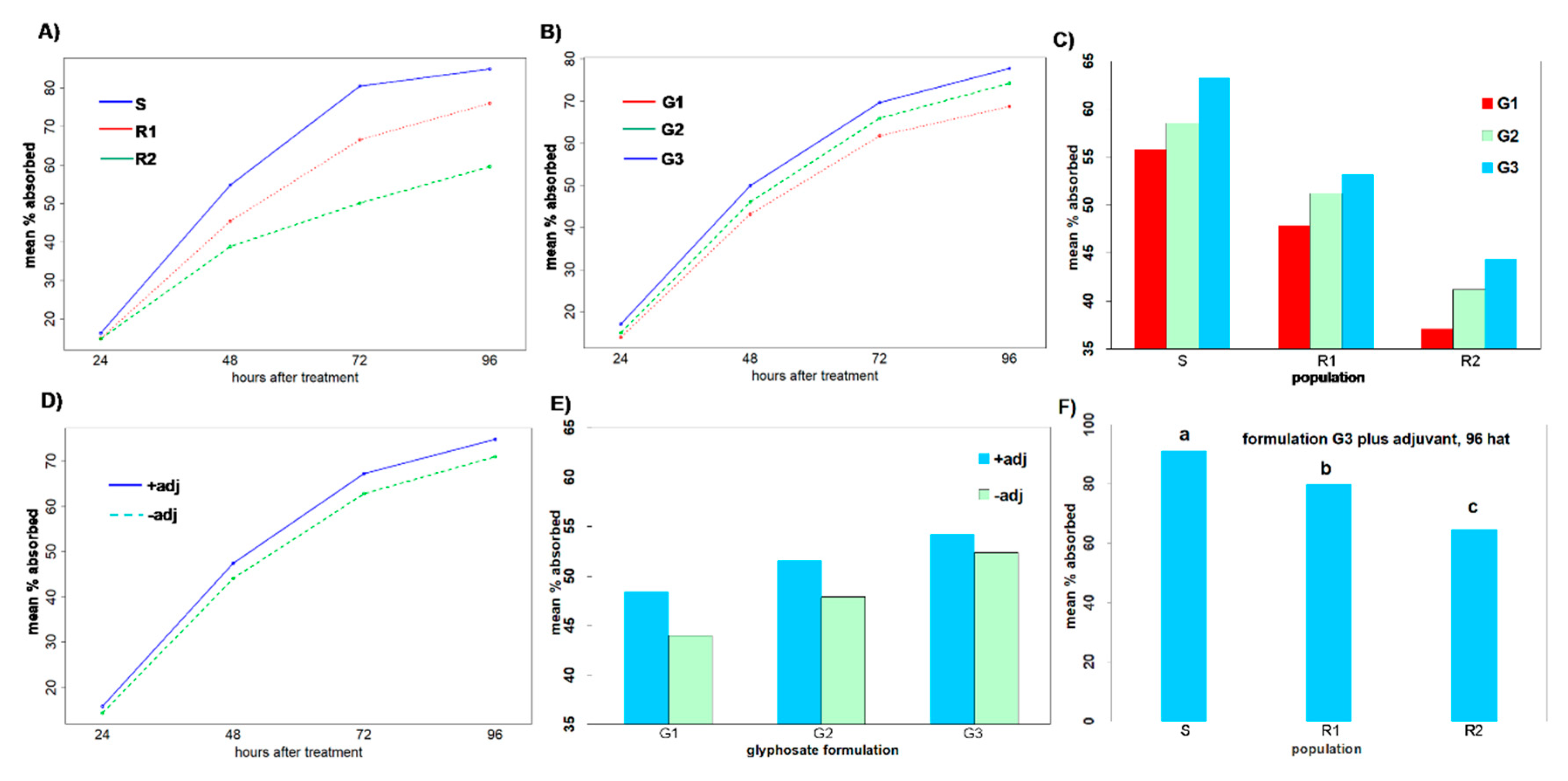
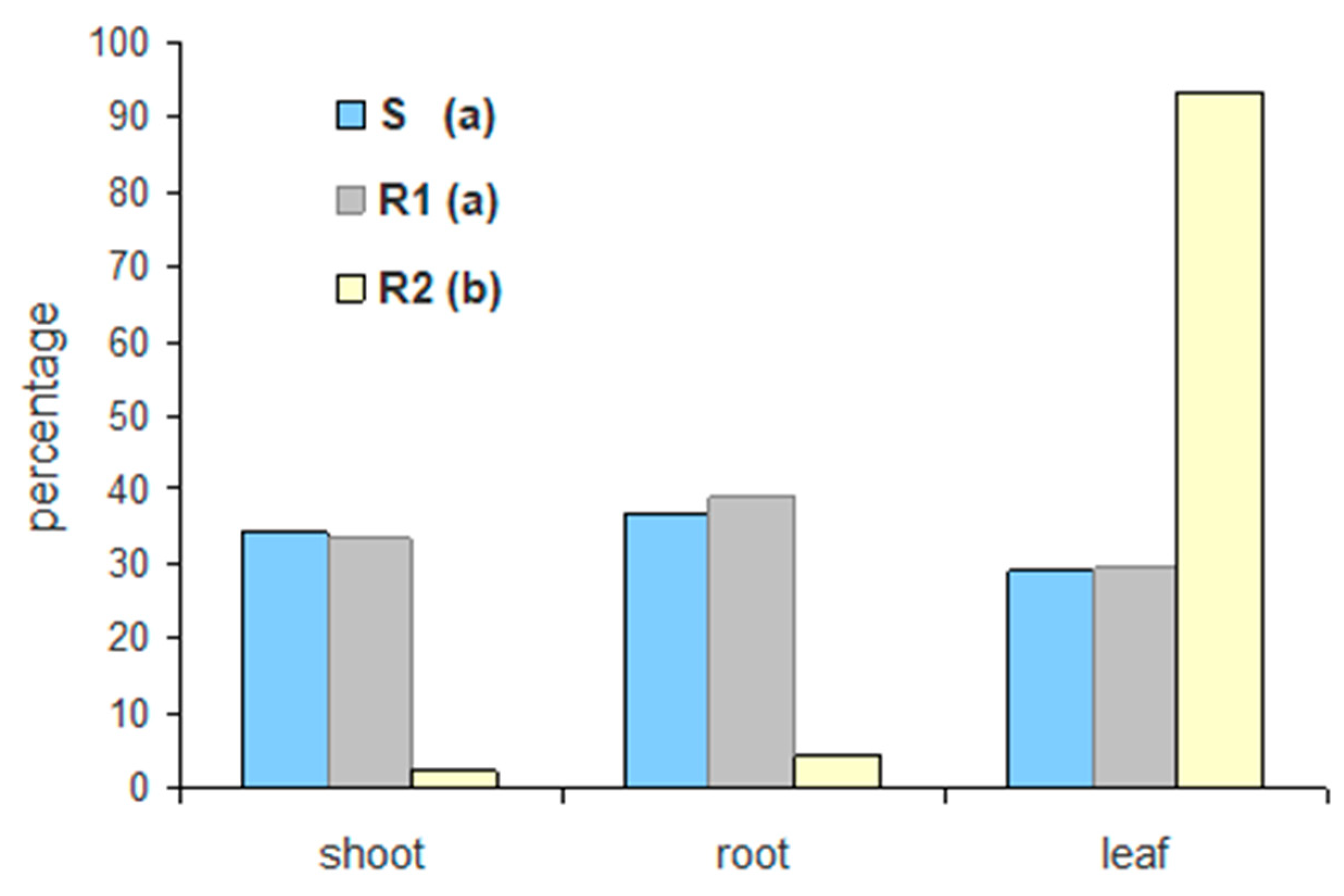
Regarding glyphosate uptake, the three populations significantly differed at all measured timings, implying that there are different resistance mechanisms. The maximum glyphosate uptake was observed at 96 HAT in all populations, with the susceptible population showing 11.7% and 42.6% greater uptake than the putative resistant populations R1 and R2, respectively (
Figure 4A). This result is in agreement with the findings by Amaro-Blanco et al. [
2], who noticed that a glyphosate susceptible Sumatran fleabane population exhibited 24% more uptake than a resistant population, which demonstrated both TSR and NTSR mechanisms 96 HAT. The R1 population had been found to exhibit a TSR mechanism to glyphosate based on an amino acid substitution Pro106Thr, the most common single mutation in the
EPSPS gene [
2], absorbing lower amounts of glyphosate and performing moderately concerning the different salt formulations of glyphosate. However, the allocation pattern of glyphosate among the treated leaf, the remaining shoots and the roots revealed that the active ingredient was highly translocated throughout the plant. On the contrary, González-Torralva et al. [
6] showed that a resistant Sumatran fleabane population exhibiting a Pro182Thr mutation retained about 61% of glyphosate in the treated leaf and only 39% was translocated in other organs. The R2 population was found to exhibit the lowest uptake and performed poorly even when glyphosate was applied on plants in the most effective formulation (G3). This result, in conjunction with the low translocation of glyphosate to shoots and roots (
Figure 5), clearly suggests that an NTSR mechanism to glyphosate exhibits in this population. The low translocation of glyphosate measured in this study is in full accordance with the results of Amaro-Blanco et al. [
2], who observed that about 86% of glyphosate remained in the treated leaf 96 HAT in a resistant population of
C. sumatrensis.
According to the results of our study, the addition of adjuvants improves the efficacy of glyphosate-based products against C. sumatrensis, especially for populations that do not exhibit NTSR or TSR mechanisms. These additives (either the non-ionic product of plant origin Retenol or the non-ionic INEX-A) increased foliar retention and glyphosate absorption in all surveyed formulations, leading to lower GR50 values for all populations of Sumatran fleabane and subsequently to higher application effectiveness.
It is widely acceptable that the efficient control of weed populations that possess non-target site resistance mechanisms to glyphosate, such as reduced translocation of the active ingredient, may be surpassed by the utilization of adjuvants. Nevertheless, the use of additives in formulated products against species exhibiting TSR mechanisms is not useful as, ultimately, there is no union with EPSPS.
Our results demonstrate that glyphosate, when appropriately formulated, remains a highly effective tool against noxious weeds such as
C. sumatrensis. However, the glyphosate resistance evolution rates should concern all stakeholders who are involved in integrated weed management schemes. The proper identification of glyphosate resistance mechanisms is imperative and acts as a driver for future research [
12]. The mitigation of herbicide resistance is a challenge for the sustainability of farming systems and should be based in innovative tank mixtures with chemical products with different modes of action [
5], novel integrated weed management tools, such as cover crops [
33], and modern cultivation techniques, such as false and stale seedbed [
34].