Effect of Repeated Application of Sulfonylurea Herbicides on Sulfosulfuron Dissipation Rate in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Types of Soils
2.2. Sulfosulfuron Residual Activity in Soil
2.3. Multi-Year Sulfonylurea Application Experiments
2.3.1. Sulfonylurea Treatments in Controlled Environment
2.3.2. Sulfonylurea Treatments in the Field
2.4. Determination of Sulfosulfuron Degradation Rates
2.5. Statistical Analysis
3. Results
3.1. Sulfosulfuron Residual Activity Following Repeated Application to Soil
3.2. Sulfosulfuron Degradation in Soil Following Multi-Year Sulfonylureas Application
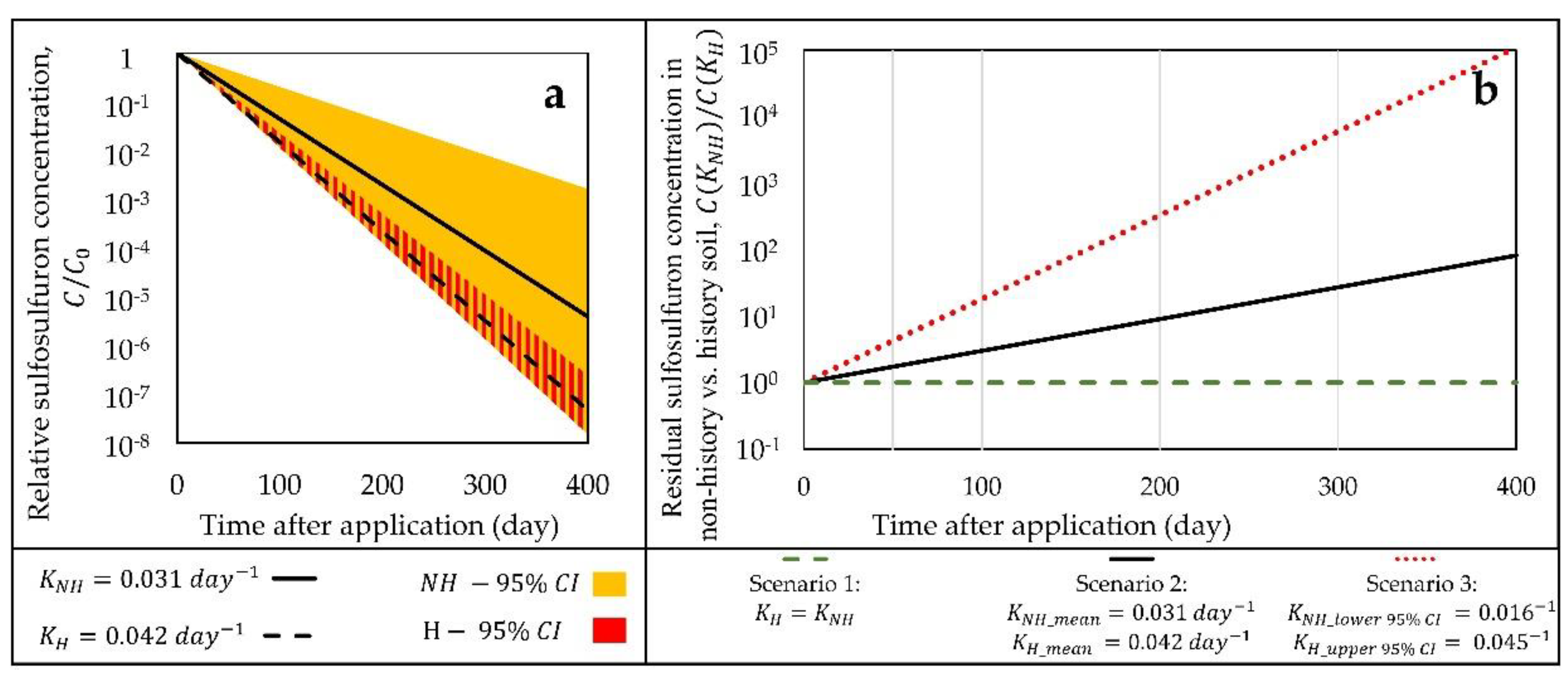
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beyer, E.M.; Duffy, M.J.; Hay, J.V.; Schlueter, D.D. Sulfonylureas. In Herbicides Chemistry, Degradation, and Mode of Action; Kearney, P.C., Kaufman, D.D., Eds.; Marcel Dekker: New York, NY, USA, 1988; pp. 117–189. [Google Scholar]
- Eizenberg, H.; Goldwasser, Y.; Achdary, G.; Hershenhorn, J. The potential of sulfosulfuron to control troublesome weeds in tomato. Weed Technol. 2003, 17, 133–137. [Google Scholar] [CrossRef]
- Geiger, P.W.; Stahlman, P.W. Dose-Responses of weeds and winter wheat (Triticum aestivum) to MON 37500. Weed Technol. 1996, 10, 870–875. [Google Scholar] [CrossRef]
- Kutzior, S.; Spitainiak, J.; Pawinska, M.; Urbanowicz, J. Sulfosulfuron use in potatoes. In 1999 Brighton Crop Protection Conference: Weeds, Proceedings of the an International Conference, Brighton, UK, 15–18 November 1999; British Crop Protection Council: Farnham, UK, 1999; pp. 349–354. [Google Scholar]
- Myhre, C.D.; Loeppky, H.A.; Stevenson, F.C. MON-37500 for weed control and alfalfa seed production. Weed Technol. 2004, 18, 810–815. [Google Scholar] [CrossRef]
- Mora, D.A.; Cheimona, N.; Palma-Bautista, C.; Rojano-Delgado, A.M.; Osuna-Ruiz, M.D.; Alcántara de la Cruz, R.; De Prado, R. Physiological, biochemical and molecular bases of resistance to tribenuron-methyl and glyphosate in Conyza canadensis from olive groves in southern Spain. Plant. Physiol. Biochem. 2019, 144, 14–21. [Google Scholar] [CrossRef]
- Kelley, J.P.; Peeper, T.F. Wheat (Triticum aestivum) and rotational crop response to MON 37500. Weed Technol. 2003, 17, 55–59. [Google Scholar] [CrossRef]
- Lyon, D.J.; Miller, S.D.; Seifert-higgins, S. MON 37500 soil residues affect rotational crops in the high plains. Weed Technol. 2003, 17, 792–798. [Google Scholar] [CrossRef]
- Paporisch, A.; Laor, Y.; Rubin, B.; Achdari, G.; Eizenberg, H. Application timing and degradation rate of sulfosulfuron in soil co-affect control efficacy of Egyptian broomrape (Phelipanche aegyptiaca) in tomato. Weed Sci. 2018, 66, 780–788. [Google Scholar] [CrossRef]
- Conclusion on the peer review of the pesticide risk assessment of the active substance sulfosulfuron. EFSA J. 2014, 12, 3764. [CrossRef]
- Eizenberg, H.; Goldwasser, Y. Control of Egyptian broomrape in processing tomato: A summary of 20 years of research and successful implementation. Plant. Dis. 2018, 102, 1477–1488. [Google Scholar] [CrossRef]
- Goldwasser, Y.; Galaz, J.C.; Eizenberg, H.; Paporisch, A.; Castro, A.; Aly, R.; Friedman, E.; Abu-Nasser, J.; Rojas, L. Phelipanche ramosa infestations and control in processing tomato in Chile. In Proceedings of the 15th World Congress on Parasitic Plants, Amsterdam, The Netherlands, 30 June–5 July 2019; p. 40. [Google Scholar]
- Brown, H.M. Mode of action, crop selectivity, and soil relations of the sulfonylurea herbicides. Pestic. Sci. 1990, 29, 263–281. [Google Scholar] [CrossRef]
- Yadav, U.; Choudhury, P.P. Biodegradation of sulfosulphuron in agricultural soil by Trichoderma sp. Lett. Appl. Microbiol. 2014, 59, 479–486. [Google Scholar] [CrossRef]
- Arya, R.; Mishra, N.K.; Sharma, A.K. Brevibacillus borstelensis and Streptomyces albogriseolus have roles to play in degradation of herbicide, sulfosulfuron. 3 Biotech. 2016, 6, 246. [Google Scholar] [CrossRef][Green Version]
- Hang, B.J.; Hong, Q.; Xie, X.T.; Huang, X.; Wang, C.H.; He, J.; Li, S.P. SulE, a sulfonylurea herbicide de-esterification esterase from Hansschlegelia zhihuaiae S113. Appl. Environ. Microbiol. 2012, 78, 1962–1968. [Google Scholar] [CrossRef]
- He, Y.H.; Shen, D.S.; Fang, C.R.; Zhu, Y.M. Rapid biodegradation of metsulfuron-methyl by a soil fungus in pure cultures and soil. World J. Microbiol. Biotechnol. 2006, 22, 1095–1104. [Google Scholar] [CrossRef]
- Yu, Y.L.; Wang, X.; Luo, Y.M.; Yang, J.F.; Yu, J.Q.; Fan, D.F. Fungal degradation of metsulfuron-methyl in pure cultures and soil. Chemosphere 2005, 60, 460–466. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, L.; Li, D.; Li, X.; Wang, C.; Zheng, M.; Pan, C.; Qiu, L. Biodegradation of thifensulfuron-methyl by Ochrobactrum sp. in liquid medium and soil. Biotechnol. Lett. 2015, 37, 1385–1392. [Google Scholar] [CrossRef]
- Valle, A.; Boschin, G.; Negri, M.; Abbruscato, P.; Sorlini, C.; D’Agostina, A.; Zanardini, E. The microbial degradation of azimsulfuron and its effect on the soil bacterial community. J. Appl. Microbiol. 2006, 101, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Xu, Y.; Qiu, L.; Pan, C. Biodegradation of pyrazosulfuron-ethyl by three strains of bacteria isolated from contaminated soils. Chemosphere 2009, 74, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.P.; Wang, Z.; Lu, P.; Wang, H.J.; Waseem Ali, S.; Li, S.P.; Huang, X. Biodegradation of the sulfonylurea herbicide chlorimuron-ethyl by the strain Pseudomonas sp. LW3. FEMS Microbiol. Lett. 2009, 296, 203–209. [Google Scholar] [CrossRef]
- Arbeli, Z.; Fuentes, C.L. Accelerated biodegradation of pesticides: An overview of the phenomenon, its basis and possible solutions; and a discussion on the tropical dimension. Crop. Prot. 2007, 26, 1733–1746. [Google Scholar] [CrossRef]
- Krutz, J.L.; Shaner, D.L.; Weaver, M.A.; Webb, R.M.T.; Zablotowicz, R.M.; Reddy, K.N.; Huang, Y.; Thomson, S.J. Agronomic and environmental implications of enhanced s-triazine degradation. Pest. Manag. Sci. 2010, 66, 461–481. [Google Scholar] [CrossRef]
- Mueller, T.C.; Parker, E.T.; Steckel, L.; Clay, S.A.; Owen, M.D.K.; Curran, W.S.; Currie, R.; Scott, R.; Sprague, C.; Stephenson, D.O.; et al. Enhanced atrazine degradation is widespread across the United States. Pest. Manag. Sci. 2017, 73, 1953–1961. [Google Scholar] [CrossRef]
- Xu, X.; Zarecki, R.; Medina, S.; Ofaim, S.; Liu, X.; Chen, C.; Hu, S.; Brom, D.; Gat, D.; Porob, S.; et al. Modeling microbial communities from atrazine contaminated soils promotes the development of biostimulation solutions. ISME J. 2019, 13, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, E.; Houot, S. Rapid mineralization of the s-triazine ring of atrazine in soils in relation to soil management. Soil Biol. Biochem. 1996, 28, 1341–1348. [Google Scholar] [CrossRef]
- Tal, A.; Rubin, B.; Katan, J. Accelerated degradation of thiocarbamate herbicides in Israeli soils following repeated use of vernolate. Pestic. Sci. 1989, 25, 343–353. [Google Scholar] [CrossRef]
- Lee, A.; Rahman, A.; Holland, P.T. Decomposition of the herbicide EPTC in soils with a history of previous EPTC applications. N. Zeal. J. Agric. Res. 1984, 27, 201–206. [Google Scholar] [CrossRef]
- Skipper, H.D.; Murdock, E.C.; Gooden, D.T.; Zublena, J.P.; Amakiri, M.A. Enhanced herbicide biodegradation in South Carolina soils previously treated with butylate. Weed Sci. 1986, 34, 558–563. [Google Scholar] [CrossRef]
- Roeth, F.W.; Wilson, R.G.; Martin, A.R.; Sea, P.J. The influence of temperature, moisture, and prior EPTC application on the degradation of EPTC in soils. Weed Sci. 1989, 3, 24–29. [Google Scholar]
- Tal, A.; Rubin, B.; Katan, J.; Aharonsons, N. Involvement of microorganisms in accelerated degradation of EPTC in soil. J. Agric. Food Chem. 1990, 38, 1100–1105. [Google Scholar] [CrossRef]
- Freund, M.; Yarden, O.; Varsano, R.; Rubin, B. Reduced fluridone efficacy in soil: A possible case for reversible microbial inactivation. Soil Biol. Biochem. 1994, 26, 689–694. [Google Scholar] [CrossRef]
- Walker, A.; Welch, S.J. Enhanced degradation of some soil-applied herbicides. Weed Res. 1991, 31, 49–57. [Google Scholar] [CrossRef]
- Rouchaud, J.; Neus, O.; Bulcke, R.; Cools, K.; Eelen, H.; Dekkers, T. Soil dissipation of diuron, chlorotoluron, simazine, propyzamide, and diflufenican herbicides after repeated applications in fruit tree orchards. Arch. Environ. Contam. Toxicol. 2000, 39, 60–65. [Google Scholar] [CrossRef] [PubMed]
- García-Jaramillo, M.; Cox, L.; Hermosín, M.C.; Cerli, C.; Kalbitz, K. Influence of green waste compost on azimsulfuron dissipation and soil functions under oxic and anoxic conditions. Sci. Total Environ. 2016, 550, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.; Walker, A.; Welch, S.J. Evidence for the accelerated degradation of isoproturon in soils. Pestic. Sci. 1996, 48, 253–260. [Google Scholar] [CrossRef]
- Ravelli, A.; Pantani, O.; Calamai, L.; Fusi, P. Rates of chlorsulfuron degradation in three Brazilian oxisols. Weed Res. 1997, 37, 51–59. [Google Scholar] [CrossRef]
- Xie, X.; Liu, W.; Abid, S. Rapid degradation of bensulfuron-methyl upon repeated application in paddy soils. J. Environ. Sci. 2004, 16, 49–52. [Google Scholar]
- García-Delgado, C.; Barba-Vicente, V.; Marín-Benito, J.M.; Mariano Igual, J.; Sánchez-Martín, M.J.; Sonia Rodríguez-Cruz, M. Influence of different agricultural management practices on soil microbial community over dissipation time of two herbicides. Sci. Total Environ. 2019, 646, 1478–1488. [Google Scholar] [CrossRef]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed management in 2050: Perspectives on the future of weed science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef]
- Peters, B.; Strek, H.J. Herbicide discovery in light of rapidly spreading resistance and ever-increasing regulatory hurdles. Pest. Manag. Sci. 2018, 74, 2211–2215. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. 2020. Available online: http://www.weedscience.org/Home.aspx (accessed on 31 May 2020).
- Matzrafi, M.; Gerson, O.; Sibony, M.; Rubin, B. Target site resistance to acetolactate synthase inhibitors in Diplotaxis erucoides and Erucaria hispanica–mechanism of resistance and response to alternative herbicides. Agronomy 2020, 10, 471. [Google Scholar] [CrossRef]
- Weiss, Y.; Rubin, B.; Shulman, A.; Ben Shir, I.; Keinan, E.; Wolf, S. Determination of plant resistance to carbamate herbicidal compounds inhibiting cell division and early growth by seed and plantlets bioassays. Nat. Protoc. 2006, 1, 2282–2287. [Google Scholar] [CrossRef]
- Chow, G.C. Test of equality between sets of coefficients in two linear regressions. Econometrica 1960, 28, 591–605. [Google Scholar] [CrossRef]
- Ramesh, A.; Maheswari, S.T. Dissipation of sulfosulfuron in soil and wheat plant under predominant cropping conditions and in a simulated model ecosystem. J. Agric. Food Chem. 2003, 51, 3396–3400. [Google Scholar] [CrossRef]
- Maheswari, S.T.; Ramesh, A. Adsorption and degradation of sulfosulfuron in soils. Environ. Monit. Assess. 2007, 127, 97–103. [Google Scholar] [CrossRef]
- Saha, S.; Kulshrestha, G. Degradation of sulfosulfuron, a sulfonylurea herbicide, as influenced by abiotic factors. J. Agric. Food Chem. 2002, 50, 4572–4575. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Kookana, R.S.; Alston, A.M. Fate and behaviour of triasulfuron, metsulfuron-methyl, and chlorsulfuron in the Australian soil environment: A review. Aust. J. Agric. Res. 1998, 49, 775–790. [Google Scholar] [CrossRef]
- Warton, B.; Matthiessen, J.N. The crucial role of calcium interacting with soil pH in enhanced biodegradation of metam-sodium. Pest. Manag. Sci. 2005, 61, 856–862. [Google Scholar] [CrossRef]
- Trabue, S.L.; Palmquist, D.E.; Lydick, T.M.; Singles, S.K. Effects of soil storage on the microbial community and degradation of metsulfuron-methyl. J. Agric. Food Chem. 2006, 54, 142–151. [Google Scholar] [CrossRef]
- Crowley, D.E.; Alvey, S.; Gilbert, E.S. Rhizosphere ecology of xenobiotic-degrading microorganisms. Am. Chem. Soc. Symp. Ser. 1997, 664, 20–36. [Google Scholar]
- Yang, C.; Yulai, W.; Wang, M.; Chen, H. Plant species influence microbial metabolic activity and butachlor biodegradation in a riparian soil from Chongming Island, China. Geoderma 2013, 193–194, 165–171. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Li, J. Plant species mediate rhizosphere microbial activity and biodegradation dynamics in a riparian soil treated with bensulfuron-methyl. Clean-Soilairwater 2011, 39, 338–344. [Google Scholar] [CrossRef]
- Krutz, L.J.; Shaner, D.L.; Accinelli, C.; Zablotowicz, R.M.; Henry, W.B. Atrazine dissipation in s-triazine-adapted and nonadapted soil from Colorado and Mississippi: Implications of enhanced degradation on atrazine fate and transport parameters. J. Environ. Qual. 2008, 37, 848–857. [Google Scholar] [CrossRef]
- Mishael, Y.; Undabeytia, T.; Rabinovitz, O.; Rubin, B.; Nir, S. Sulfosulfuron incorporated in micelles adsorbed on montmorillonite for slow release formulations. J. Agric. Food Chem. 2003, 51, 2253–2259. [Google Scholar] [CrossRef][Green Version]



| Soil Source | GPS Coordinates | pH | Organic Matter (%) | Sand (%) | Silt (%) | Clay (%) | CaCO3 (%) |
|---|---|---|---|---|---|---|---|
| H. Eden | 32°28′01.2″ N 35°29′27.6″ E | 7.8 | 1.9 | 18.8 | 31.8 | 49.5 | 41.3 |
| Kalanit | 33°10′48.0″ N 35°36′21.6″ E | 7.6 | 1.7 | 16.6 | 34.6 | 48.9 | 23.4 |
| Newe Ya’ar (Controlled environment) | 32°42′13.6″ N 35°11′05.7″ E | 7.5 | 2.0 | 15.2 | 26.3 | 58.6 | 12.5 |
| Newe Ya’ar (Field) | 32°42′30.4″ N 35°10′40.1″ E | 7.9 | 2.7 | 20.6 | 24.7 | 54.7 | 22.9 |
| Date | Herbicide | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|---|
| Application Rate (g ha−1) | ||||||||
| 5 July 2015 | Foramsulfuron | 0 | 45 | 45 | 45 | 45 | 45 | 45 |
| 7 July 2015 | Rimsulfuron | 0 | 0 | 0 | 0 | 12.5 | 12.5 | 12.5 |
| 22 May 2016 | Trifloxysulfuron | 0 | 12.2 | 12.2 | 12.2 | 22.5 | 22.5 | 22.5 |
| 13 December 2016 | Sulfosulfuron | 0 | 22.5 | 22.5 | 0 | 37.5 | 37.5 | 0 |
| 13 December 2016 | Chlorsulfuron | 0 | 0 | 0 | 12.5 | 0 | 0 | 15 |
| 22 March 2018 | Sulfosulfuron | 0 | 56.2 | 0 | 0 | 56.2 | 37.5 | 0 |
| 29 April 2018 | Sulfosulfuron | 0 | 37.5 | 0 | 0 | 37.5 | 0 | 0 |
| 29 April 2018 | Rimsulfuron | 0 | 0 | 12.5 | 12.5 | 0 | 25 | 25 |
| 20 May 2019 | Sulfosulfuron | 0 | 0 | 0 | 0 | 37.5 | 0 | 0 |
| 20 May 2019 | Rimsulfuron | 0 | 0 | 0 | 0 | 0 | 25 | 25 |
| Crop | Planting Date | Harvest Date |
|---|---|---|
| Corn | 21 June 2015 | 1 September 2015 |
| Cotton | 12 April 2016 | 2 November 2016 |
| Wheat | 30 November 2016 | 1 June 2017 |
| Tomato | 9 April 2018 | 18 July 2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paporisch, A.; Laor, Y.; Rubin, B.; Eizenberg, H. Effect of Repeated Application of Sulfonylurea Herbicides on Sulfosulfuron Dissipation Rate in Soil. Agronomy 2020, 10, 1724. https://doi.org/10.3390/agronomy10111724
Paporisch A, Laor Y, Rubin B, Eizenberg H. Effect of Repeated Application of Sulfonylurea Herbicides on Sulfosulfuron Dissipation Rate in Soil. Agronomy. 2020; 10(11):1724. https://doi.org/10.3390/agronomy10111724
Chicago/Turabian StylePaporisch, Amit, Yael Laor, Baruch Rubin, and Hanan Eizenberg. 2020. "Effect of Repeated Application of Sulfonylurea Herbicides on Sulfosulfuron Dissipation Rate in Soil" Agronomy 10, no. 11: 1724. https://doi.org/10.3390/agronomy10111724
APA StylePaporisch, A., Laor, Y., Rubin, B., & Eizenberg, H. (2020). Effect of Repeated Application of Sulfonylurea Herbicides on Sulfosulfuron Dissipation Rate in Soil. Agronomy, 10(11), 1724. https://doi.org/10.3390/agronomy10111724






