Effect of 5-Aminolevulinic Acid (ALA) on the Biochemical and Physiological Parameters of Postharvest Quality of Polygonatum multiflorum L. All. ‘Variegatum’ Cut Foliage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Vase Life
2.3. Membrane Permeability
2.4. Chlorophyll Content
2.5. Free Proline Assay
2.6. Enzyme Assay
2.7. Statistical Analysis
3. Results
3.1. Vase Life
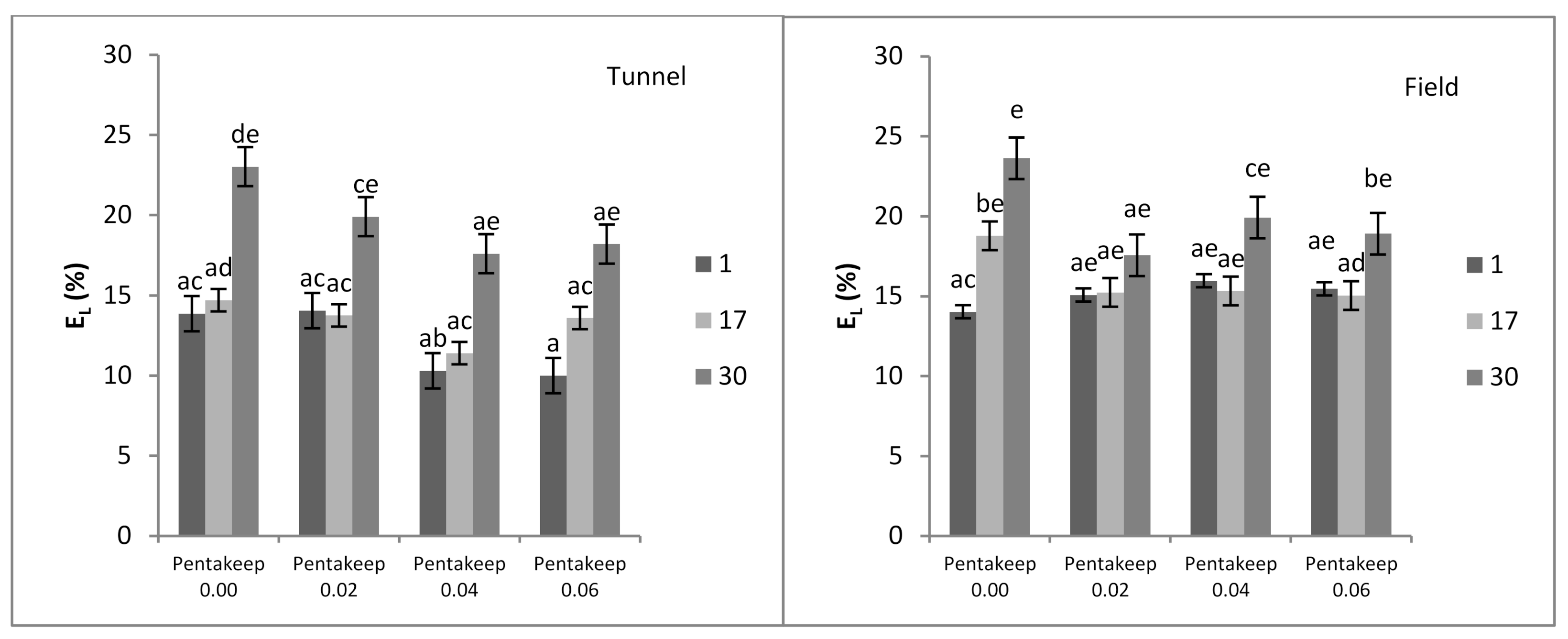
3.2. Membrane Permeability
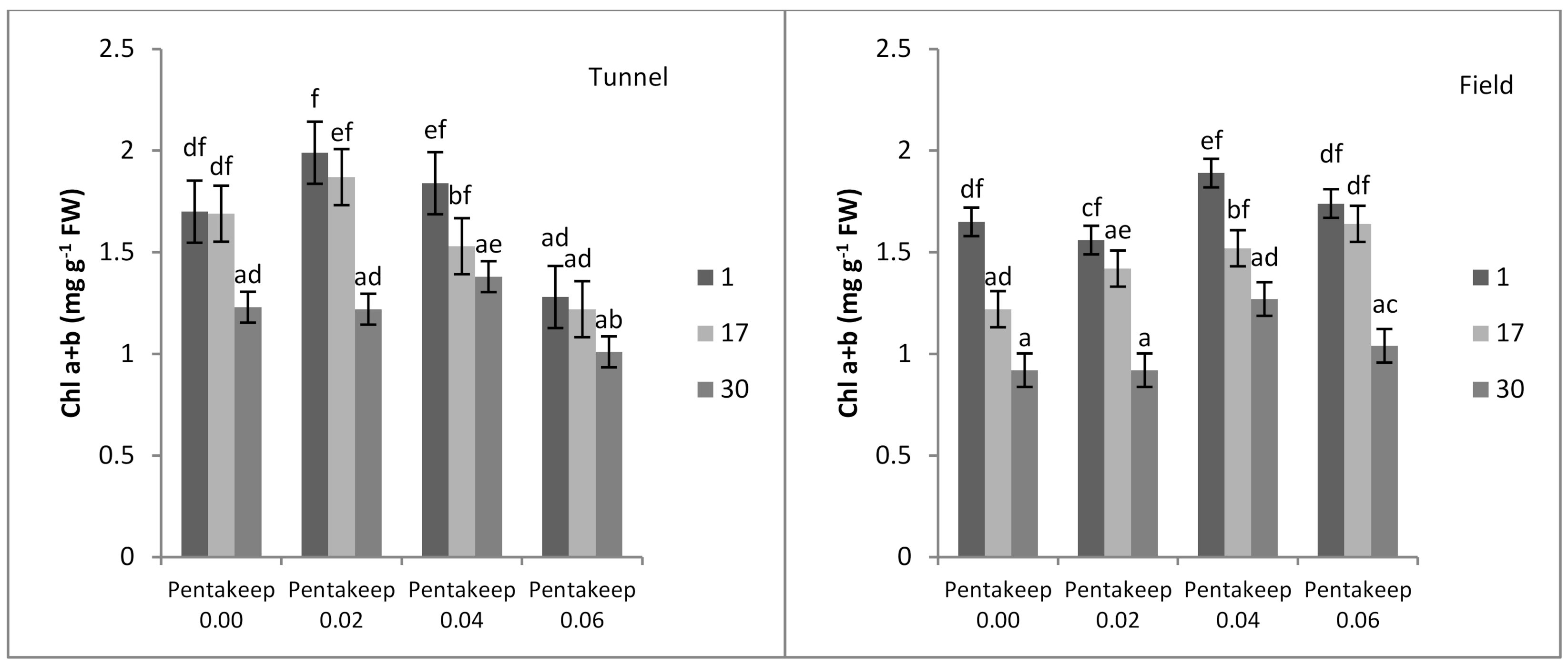
3.3. Chlorophyll a + b Content
3.4. Free Proline Content
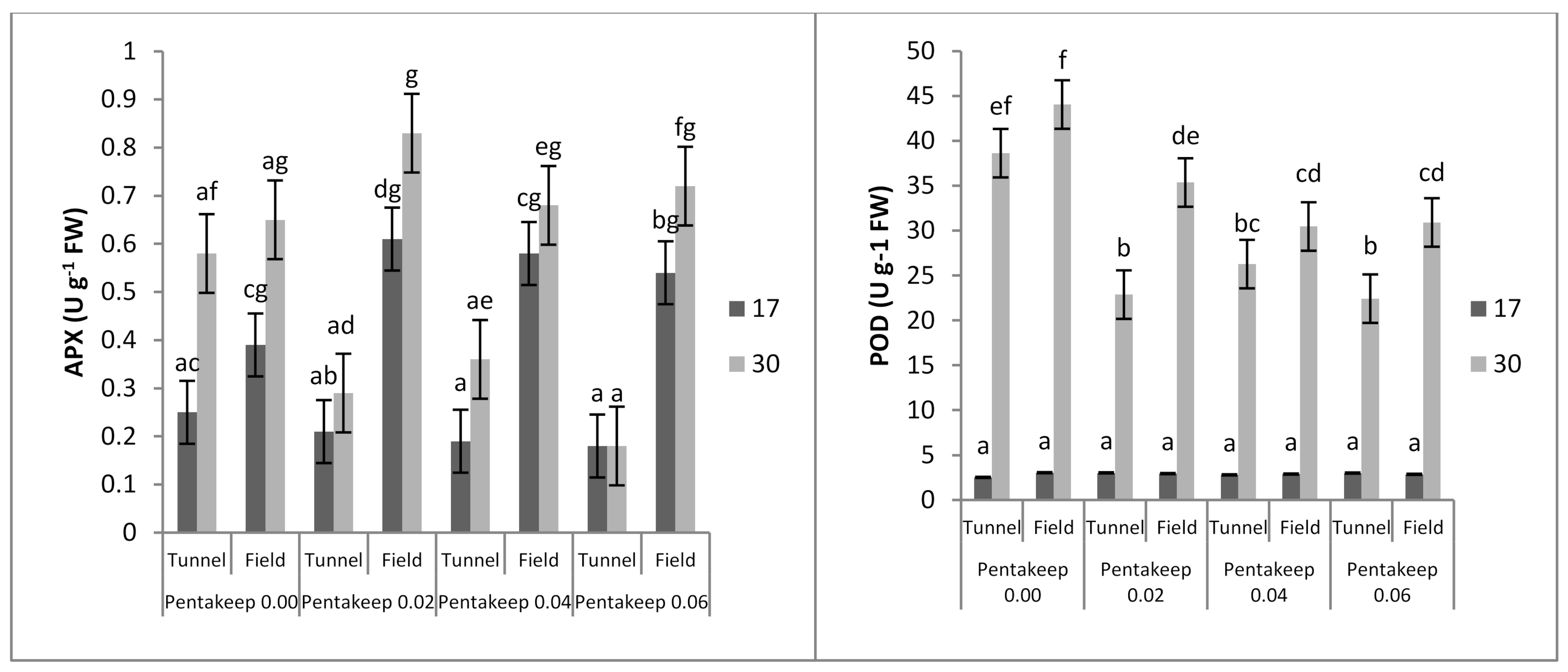
3.5. Enzyme Activity
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Naveena, N.; Thamaraiselvi, S.P.; Rajadurai, K.R.; Sivakumar, R. Effect of colored shade nets on physiology and quality of cut foliage plants. J. Pharmacogn. Phytochem. 2019, 8, 1141–1144. [Google Scholar]
- Nooden, L.D.; Guiamet, J.J.; John, I. Senescence mechanism. Physiol. Plant. 1997, 101, 746–753. [Google Scholar] [CrossRef]
- Bartosz, G. Oxidative stress in plants. Acta Physiol. Plant. 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Borochov, A.; Mayak, S.; Broun, R. The involvement of water stress and ethylene in senescence of cut carnation flowers. J. Exp. Bot. 1982, 33, 1202–1209. [Google Scholar] [CrossRef]
- Sylvestre, I.; Droillard, M.J.; Bureau, J.M.; Paulin, A. Effects of the ethylene rise on the peroxidation of membrane lipids during the senescence of cut ccarnation. Plant Physiol. Biochem. 1989, 27, 407–413. [Google Scholar]
- Rubinstein, B. Regulation of cel death in flower petals. Plant Mol. Biol. 2000, 44, 303–318. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide and glutathione-associated mechanism of acclimatory stress tolerance and signaling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Ikehara, N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997, 115, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef]
- Chen, J.C.; Jiang, C.Z.; Gookin, T.E. Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Mol. Biol. 2004, 55, 521–530. [Google Scholar] [CrossRef]
- van Meeteren, U.; van Gelder, H.; van Ieperen, W. Effects of growth conditions on post harvest rehydration ability of cut chrysanthemum flowers. Acta Hortic. 2005, 669, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Fanourakis, D.; Carvalho, D.R.A.; Almeida, D.P.F.; van Kooten, O.; van Doorn, W.G.; Heuvelink, E. Postharvest water relations in cut rose cultivars with contrasting sensitivity to high relative air humidity during growth. Postharvest Biol. Technol. 2012, 64, 64–73. [Google Scholar] [CrossRef]
- Fanourakis, D.; Carvalho, D.R.A.; Gitonga, V.W.; van Heusden, A.W.; Almeida, D.P.F.; Heuvelink, E. Breeding cut roses for better keeping quality: First steps. Acta Hortic. 2012, 937, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Łukaszewska, A.; Skutnik, E. Przewodnik Florysty, Materiał Roślinny, Akcesoria, Kompozycje, Przedłużanie Trwałości; Wyd. SGGW: Warszawa, Poland, 2003. [Google Scholar]
- Wien, H.C. Floral crop production in high tunnels. HortTechnology 2009, 19, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Wien, H.C.; Pritts, M.P. Use of high tunnels in the northern USA: Adaptation to cold climates. Acta Hortic. 2009, 807, 55–59. [Google Scholar] [CrossRef]
- Smoleń, S.; Sady, W. Effects of plant biostimulation with Pentakeep V fertilizer and nitrogen fertilization on the content of macro- and micronutrients in spinach. J. Elementol. 2010, 15, 343–353. [Google Scholar] [CrossRef]
- Korkmaz, A. Effects of exogenous application of 5-aminolevulinic acid in crop plants. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M.N.V., Eds.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Liu, L.; El-Shemy, A.; Saneoka, H. Effects of 5-aminolevulinic acid on water uptake, ionic toxicity, and antioxidant capacity of Swiss chard (Beta vulgaris L.) under sodic- alkaline conditions. J. Plant Nutr. Sci. 2017, 180, 535–543. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Niu, J.; Wang, R.; Song, J.; Lv, J.; Zong, X.; Wang, S. Interaction of drought and 5-aminolevulinic acid on growth and drought resistance of Leymus chinensis seedlings. Acta Ecol. Sin. 2016, 36, 180–188. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, F.L.; Wang, Y.K. Effects of 5-aminolevulinic acid on the growth, the yield and quality of compact Jujube in mountainous region. J. Northwest For. Univ. 2010, 25, 93–96. [Google Scholar]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic; and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, R.; Yasmeen, H.; Hussain, I.; Iqbal, M.; Ashraw, M.A.; Parveen, A. Exogenously applied 5-aminolevulinic acid modulates growth, secondary metabolism and oxidative defense in sunflower under water deficit stress. Physiol. Mol. Biol. Plants 2020, 26, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Averina, N.G.; Gritskevich, E.R.; Vershilovskaya, I.V.; Usatov, A.V.; Yaronkaya, E.B. Mechanisms of salt stress tolerance development in barley plants under the influence of 5-aminolevulinic acid. Russ. J. Plant Physiol. 2010, 57, 792–798. [Google Scholar] [CrossRef]
- Kisvarga, S.; Honfi, P.; Tilly-Mándy, A. Effect of Pentakeep-V on Begonia x tuberhybrida ‘Nonstop’ line. Bull. UASVM Hortic. 2015, 72, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Tilly-Mándy, A.; Honfi, P.; Stefanovits-Bányai, É.; Mosonyi, I.D.; Köbli, V.; Hrotkó, K. The effect of 5aminolevulinic acid (ALA) on the development of Saintpaulia ionantha. Int. J. Hortic. Sci. 2010, 16, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Awad, M.A. Promotive effects of a 5-aminolevulinic acid-based fertilizer on growth of tissue culture-derived date palm plants (Phoenix dactylifera L.) during acclimatization. Sci. Hortic. 2008, 118, 48–52. [Google Scholar] [CrossRef]
- Tulik, M.; Karczewski, J.; Szeliga, N.; Jura-Morawiec, J.; Jarzyna, I. Morphological characteristics and allometric relationship of shoot in two undergrowth plants: Polygonatum odoratum and Polygonatum multiflorum. Forests 2018, 9, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Pogroszewska, E.; Szot, P.; Rubinowska, K.; Konopińska-Mamej, A.; Świstowska, A.; Zdybel, A.; Parzymies, M.; Durlak, W. The effect of silicon on morphological traits and mechanical properties of Polygonatum multiflorum L. All. ‘Variegatum’ cut shoots. Acta Sci. Pol. Hortorum Cultus 2018, 17, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Kościelniak, J. Wpływ następczy temperatur chłodowych w termoperiodyzmie dobowym na produktywność fotosyntetyczną kukurydzy (Zea mays L.). Zesz. Nauk. AR Kraków 1993, 174. [Google Scholar]
- Heath, R.L.; Packer, L. Effect of light on lipid peroxidation in chloroplasts. Biochem. Biophys. Res. Commun. 1968, 19, 716–720. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.R.; Teare, I.D. Rapid determination of free proline on water -stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Małolepsza, U.; Urbanek, H.; Polit, J. Some biochemical of strawberry plants to infection with Botrytis cinerea and salicilic acid treatment. Acta Agrobot. 1994, 47, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate–specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Owen, W.G.; Hilligoss, A.; Lopez, R.G. Late-season high tunnel planting of specialty cut flowers in the Midwestern United States influences yield and stem quality. HortTechnology 2016, 26, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Antioxidant phytochemicals in lettuce grown in high tunnels and open field. Hortic. Environ. Biotechnol. 2011, 52, 133–139. [Google Scholar] [CrossRef]
- Oritz, M.A.; Hyrczyk, K.; Lopez, R.G. Comparizon of high tunnel and field production of specialty cut flowers in the Midwest. HortScience 2012, 47, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- Salachna, P.; Byczyńska, A. Effect of nitric oxide and production location on vase life of cut Eucomis ‘Sparkling Burgundy’ flowers. World Sci. News 2017, 83, 229–234. [Google Scholar]
- Balestrasse, K.B.; Tomaro, M.L.; Batlle, A.; Noriega, G.O. The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 2010, 71, 2038–2045. [Google Scholar] [CrossRef]
- Korkmaz, A.; Korkmaz, Y.; Demirkiran, A.R. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010, 67, 495–501. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.-J.; Gao, Y.; Yu, B.; Xia, C.-X.; Bai, J.-G. Pretreatment with 5-aminolevilinic acid mitigates heat stress of cucumber leaves. Biol. Planta 2012, 56, 780–784. [Google Scholar] [CrossRef]
- Pic, E.; de la Serve, B.T.; Tardieu, F.; Turc, O. Leaf senescence induced by mild water deficit follows the same subsequence of macroscopic, biochemical, and molecular events as monocarpic senescence in pea. Plant Physiol. 2002, 128, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Skutnik, E.; Łukaszewska, A. Regulacja pozbiorczej trwałości gatunków uprawianych na zieleń ciętą. Postęp. Nauk Rol. 2001, 48, 111–124. [Google Scholar]
- Rubinowska, K.; Michałek, W.; Pogroszewska, E. The effect of chemical substances on senescence of Weigela florida (Bunge) A. DC. ‘Variegata Nana’ cut stems. Acta Sci. Pol. Hortorum Cultus 2012, 11, 17–28. [Google Scholar]
- Rubinowska, K.; Pogroszewska, E.; Michałek, W. The effect of polyamines on physiological parameters of post-harvest quality of cut stems of Rosa ‘Red Berlin’. Acta Sci. Pol. Hortorum Cultus 2012, 6, 81–94. [Google Scholar]
- Rubinowska, K.; Pogroszewska, E.; Laskowska, H.; Szot, P.; Zdybel, A.; Stasiak, D.; Kozak, D. The subsequent effect of silicon on physiological and biochemical parameters of Polygonatum multiflorum L. All. ‘Variegatum’ cut shoots. Acta Sci. Pol. Hortorum Cultus 2014, 13, 167–178. [Google Scholar]
- Hotta, Y.; Tanaka, T.; Takaoka, H.; Takeuchi, Y.; Konnai, M. New physiological effect of 5-aminolevulinic acid in plants: The increase of photosynthesis, chlorophyll content and plant growth. Biosic. Biotechnol. Biochem. 1997, 61, 2025–2028. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Zhang, Z.P.; Lee, M.R.; Sun, Y.P.; Wang, L.J. Effect of 5-aminolevulinic acid on leaf senescence and nitrogen metabolism of pakchoi under different nitrate levels. J. Plant Nutr. 2012, 35, 49–63. [Google Scholar] [CrossRef]
- Ördögh, M.; Beregi, Z.S.; Tillyné Mándy, A. The effect of different biostimulators on morphological and biochemical parameters of micropropagated Hosta ‘Gold Drop’. Int. J. Hortic. Sci. 2019, 25, 22–29. [Google Scholar] [CrossRef]
- Sacała, E. Role of silicon in plant resistance to water stress. J. Elementol. 2009, 14, 619–630. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Li, H.Z.; Zhou, W.J.; Takeuchi, Y.; Yoneyama, K. Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul. 2006, 49, 27–34. [Google Scholar] [CrossRef]
- Shan, C.; Zhao, X. Lanthanum delays the senescence of Lilium longiflorum cut flowers by improving antioxidant defense system and water retaining capacity. Sci. Hortic. 2015, 197, 516–520. [Google Scholar] [CrossRef]
- Lin, J.N.; Kao, C.H. Effect of oxidative stress caused by hydrogen peroxide on senescence of rice leaves. Bot. Bull. Acad. Sin. 1998, 39, 161–165. [Google Scholar]
- Aroca, R.; Amodeo, G.; Fernandez-Illescas, S.; Herman, E.M.; Chaumont, F.; Chrispeels, M.J. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol. 2005, 137, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Pei, Z.F.; Naeem, M.S.; Ming, D.F.; Liu, H.B.; Khan, F.; Zhou, W.J. 5-Aminolevulinic acid activates antioxidant defense system and seedling growth in Brassica napus L. under water-deficit stress. J. Agron. Crop. Sci. 2011, 197, 284–295. [Google Scholar] [CrossRef]
- Li, D.M.; Zhang, J.; Sun, W.J.; Li, Q.; Dai, A.H.; Bai, J.G. 5-Aminolevulinic acid pretreatment mitigates drought stress of cucumber leaves through altering antioxidant enzyme activity. Sci. Hortic. 2011, 130, 820–828. [Google Scholar] [CrossRef]
- Xu, W.; Shi, W.; Ueda, A.; Takabe, T. Mechanisms of salt tolerance in transgenic Arabidopsis thaliana carrying a peroxisomal ascorbate peroxidase gene from barley. Pedosphere 2008, 18, 486–495. [Google Scholar] [CrossRef]
- Jiménez, A.; Hernández, J.A.; Pastori, G.; del Rio, L.A.; Sevilla, F. Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea-leaves. Plant Physiol. 1998, 118, 1327–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Vivancos, P.; Rubio, M.; Mesonero, V.; Periago, P.M.; Ros Barcelo, A.; Martinez-Gomez, P.; Hernandez, J.A. The apoplastic antioxidant system in Prunus: Response to long—term plum pox virus infection. J. Exp. Bot. 2006, 57, 3813–3824. [Google Scholar] [CrossRef] [Green Version]


| Cultivation Site | Pentakeep V Concentration (%) | Vase Life (Days) | Mean for the Cultivation Site |
|---|---|---|---|
| Tunnel | 0.00 | 28.00 bd 1 | 28.75 b |
| 0.02 | 30.00 d | ||
| 0.04 | 29.00 cd | ||
| 0.06 | 28.00 bd | ||
| Field | 0.00 | 23.00 a | 23.25 a |
| 0.02 | 24.00 ab | ||
| 0.04 | 25.00 ac | ||
| 0.06 | 21.00 a |
| Cultivation Site | EL | TBARS | Chl. a + b | Proline | APX | POD |
|---|---|---|---|---|---|---|
| Tunnel | 15.00 a 1 | 36.32 a | 1.50 b | 109.17 a | 0.26 a | 15.20 a |
| Field | 17.08 b | 39.03 b | 1.40 a | 138.22 b | 0.65 b | 19.07 b |
| Pentakeep V Concentration (%) | EL | TBARS | Chl. a + b | Proline | APX | POD |
|---|---|---|---|---|---|---|
| 0.00 | 18.01 b 1 | 43.74 b | 1.40 ab | 178.89 c | 0.47 a | 22.07 b |
| 0.02 | 15.87 a | 43.29 b | 1.51 bc | 120.42 b | 0.48 a | 16.05 a |
| 0.04 | 15.09 a | 31.00 a | 1.57 c | 99.03 ab | 0.44 a | 15.61 a |
| 0.06 | 15.20 a | 32.68 a | 1.32 a | 96.27 a | 0.40 a | 14.87 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubinowska, K.; Pogroszewska, E.; Szot, P. Effect of 5-Aminolevulinic Acid (ALA) on the Biochemical and Physiological Parameters of Postharvest Quality of Polygonatum multiflorum L. All. ‘Variegatum’ Cut Foliage. Agronomy 2020, 10, 1502. https://doi.org/10.3390/agronomy10101502
Rubinowska K, Pogroszewska E, Szot P. Effect of 5-Aminolevulinic Acid (ALA) on the Biochemical and Physiological Parameters of Postharvest Quality of Polygonatum multiflorum L. All. ‘Variegatum’ Cut Foliage. Agronomy. 2020; 10(10):1502. https://doi.org/10.3390/agronomy10101502
Chicago/Turabian StyleRubinowska, Katarzyna, Elżbieta Pogroszewska, and Paweł Szot. 2020. "Effect of 5-Aminolevulinic Acid (ALA) on the Biochemical and Physiological Parameters of Postharvest Quality of Polygonatum multiflorum L. All. ‘Variegatum’ Cut Foliage" Agronomy 10, no. 10: 1502. https://doi.org/10.3390/agronomy10101502
APA StyleRubinowska, K., Pogroszewska, E., & Szot, P. (2020). Effect of 5-Aminolevulinic Acid (ALA) on the Biochemical and Physiological Parameters of Postharvest Quality of Polygonatum multiflorum L. All. ‘Variegatum’ Cut Foliage. Agronomy, 10(10), 1502. https://doi.org/10.3390/agronomy10101502







