Microbe-Plant Growing Media Interactions Modulate the Effectiveness of Bacterial Amendments on Lettuce Performance Inside a Plant Factory with Artificial Lighting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Root-Associated Bacterial Communities
2.2. Plant Growing Media Composition
2.3. Plant Growth and Inoculation
2.4. Plant Sample Analysis
2.4.1. Plant Sample Processing
2.4.2. Total Phenolic Content
2.4.3. Nitrate Content
2.4.4. Chlorophylls and Carotenoids
2.5. Statistical Analysis
3. Results
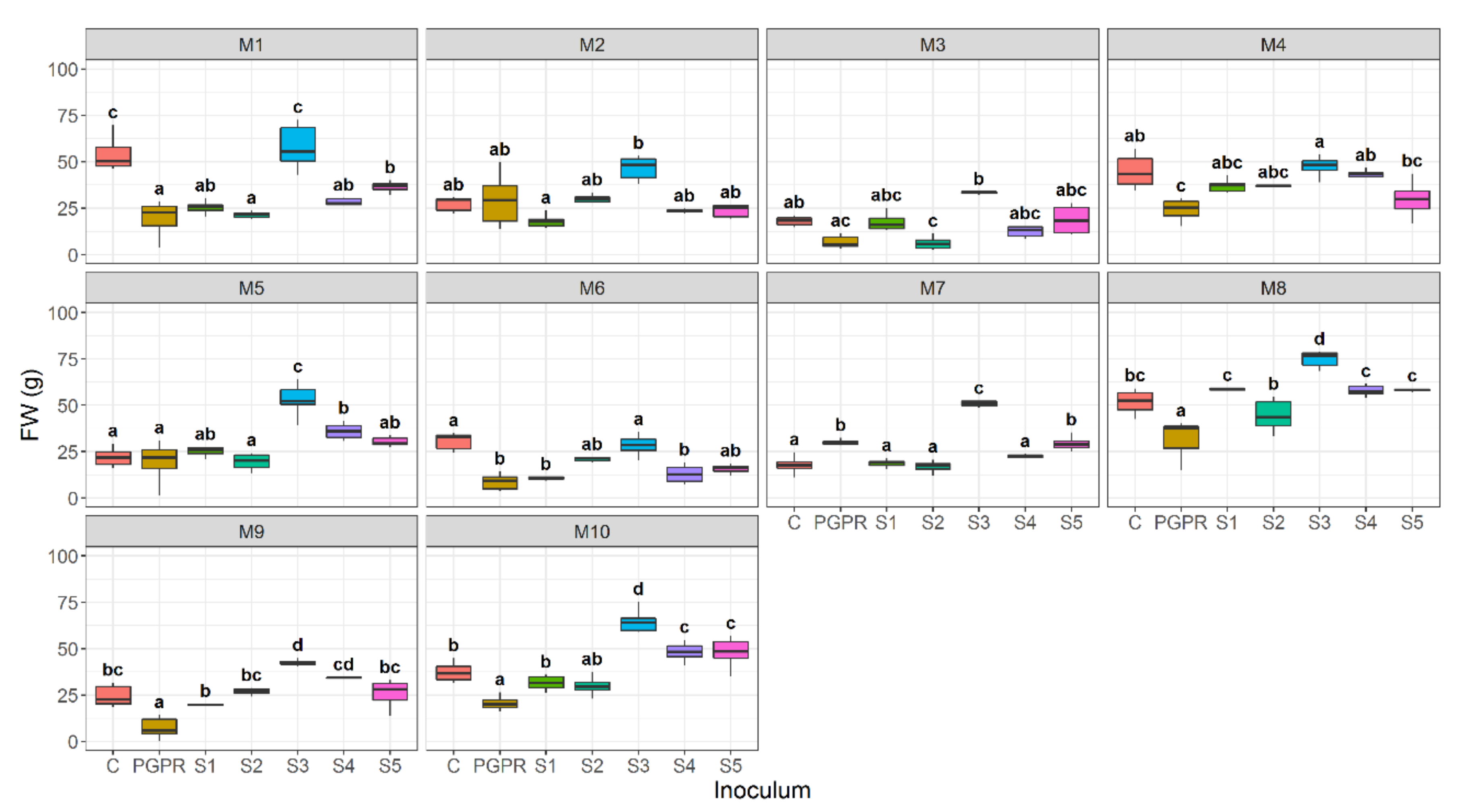
3.1. Effect of Bacterial Community Inoculum and Plant Growing Medium on Shoot Fresh Weight
3.2. Effect of Bacterial Community Inoculum and Plant Growing Medium on Lettuce Head Area
3.3. Effect of Bacterial Community Inoculum and Plant Growing Medium on Root Fresh Weight
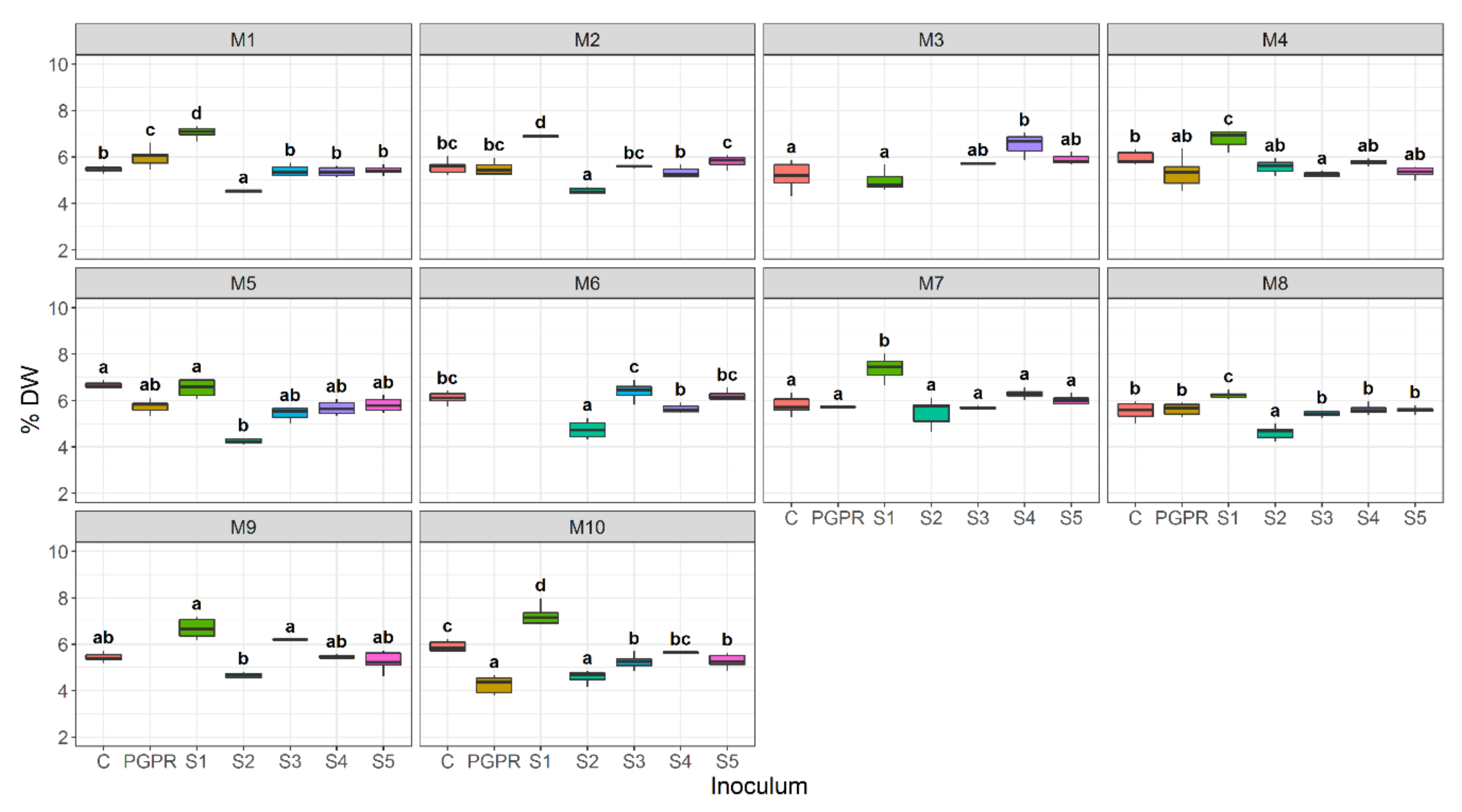
3.4. Effect of Bacterial Community Inoculum and Plant Growing Medium on Shoot Dry Weight
3.5. Effect of Bacterial Community Inoculum and Plant Growing Medium on Total Phenolic Content
3.6. Effect of Bacterial Community Inoculum and Plant Growing Medium on NO3-Content
3.7. Effect of Bacterial Community Inoculum and Plant Growing Medium on Leaf Pigments
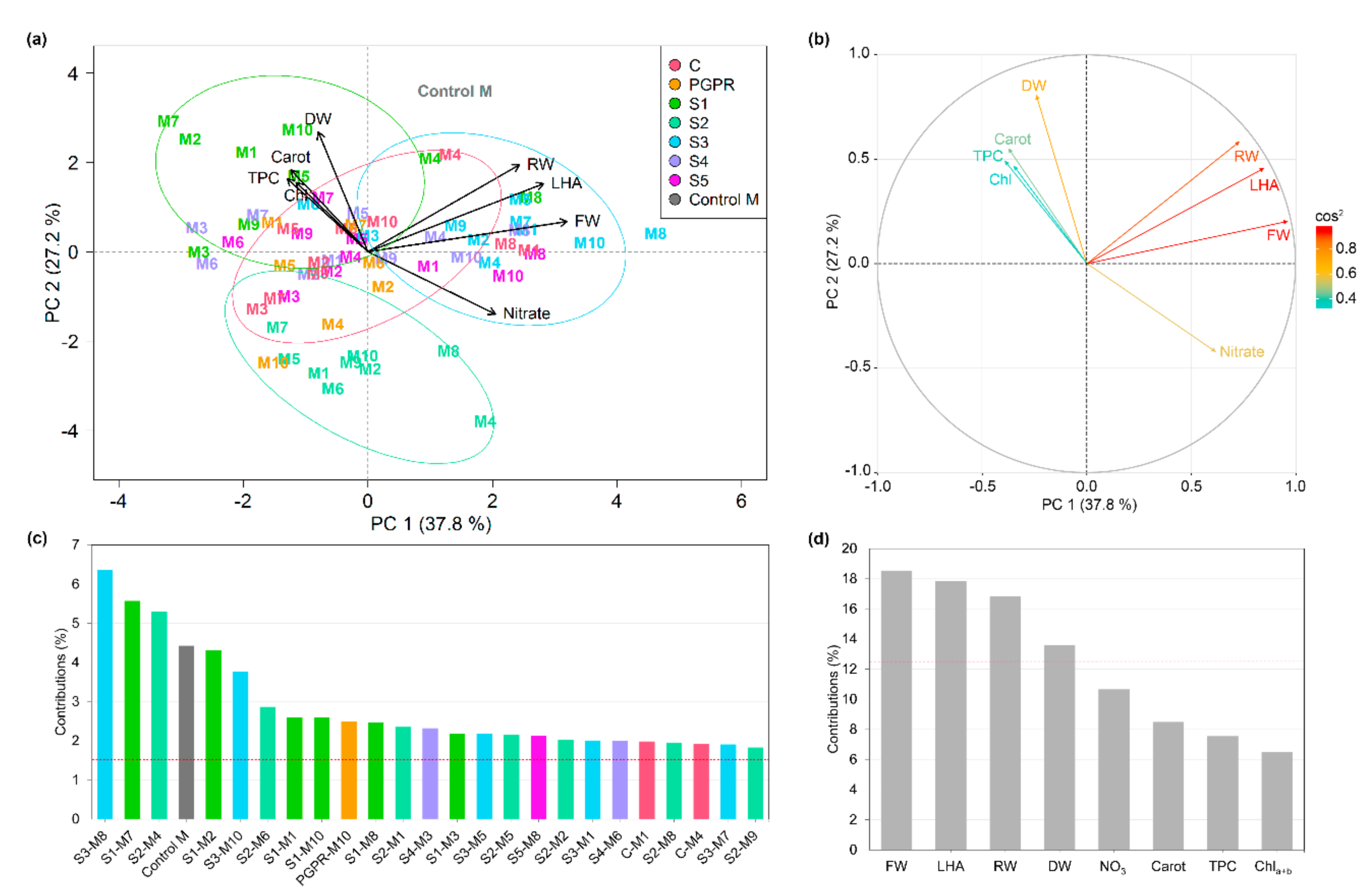
3.8. Principal Component Analysis
4. Discussion
4.1. Plant Growing Medium Constituents Have Differing Effects on Lettuce Performance
4.2. Microbe-Plant Growing Medium Interactions and the Bacterial Source Determine Plant Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Controlled-environment agriculture | CEA |
| Plant factory with artificial lighting | PFAL |
| Plant growth-promoting rhizobacteria | PGPR |
| Tryptic soy broth | TSB |
| Bacterial community inoculum | BCI S1–5 |
| Experimental plant growing media | M1–10 |
| Peat | PT |
| Black peat | BP |
| White peat | WP |
| Other organics | OO |
| Coir pith | CP |
| Wood fiber | WF |
| Composted materials | CM |
| Composted bark | CB |
| Green waste compost | GC |
| Inorganic materials | IM |
| Perlite | P |
| Sand | S |
| Arabic gum | AG |
| Shoot fresh weight | FW |
| Lettuce head area | LHA |
| Root fresh weight | RW |
| Shoot dry weight | DW |
| Total phenolic content | TPC |
| Gallic acid equivalents | GAE |
| Chlorophyll a+b | Chla+b |
| Design of experiments | DOE |
| Principal component analysis | PCA |
References
- UN World Population Prospects, the 2019 Revision. Available online: https://esa.un.org/unpd/wpp/ (accessed on 8 June 2020).
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture (SOLAW)—Managing Systems at Risk; Food and Agriculture Organization of the United Nations: Rome, Italy; Earthscan: London, UK, 2011; ISBN 9789251066140. [Google Scholar]
- Kozai, T.; Niu, G. Plant factory as a resource-efficient closed plant production system. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 93–115. ISBN 9780128166918. [Google Scholar]
- Kozai, T.; Niu, G. Role of the plant factory with artificial lighting (PFAL) in urban areas. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–34. ISBN 9780128166918. [Google Scholar]
- Goto, E. Plant production in a closed plant factory with artificial lighting. In Proceedings of the VII International Symposium on Light in Horticultural Systems, Wageningen, The Netherlands, 15–18 October 2012; Volume 956, pp. 37–49. [Google Scholar]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Schmilewski, G. The role of peat in assuring the quality of growing media. Mires Peat 2008, 3, 1–8. [Google Scholar]
- Carlile, W.R.; Cattivello, C.; Zaccheo, P. Organic growing media: Constituents and properties. Vadose Zone J. 2015, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Alexander, P.D.; Bragg, N.C.; Meade, R.; Padelopoulos, G.; Watts, O. Peat in horticulture and conservation: The UK response to a changing world. Mires Peat 2008, 3, 1–10. [Google Scholar]
- Cleary, J.; Roulet, N.T.; Moore, T.R. Greenhouse gas emissions from Canadian peat extraction, 1990–2000: A life-cycle analysis. AMBIO J. Hum. Environ. 2005, 34, 456–461. [Google Scholar] [CrossRef]
- Carlile, B.; Coules, A. Towards sustainability in growing media. In Proceedings of the International Symposium on Growing Media, Composting and Substrate Analysis, Barcelona, Spain, 17–21 October 2011; Volume 1013, pp. 341–349. [Google Scholar]
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic use of peat and charred material in growing media—An option to reduce the pressure on peatlands? J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type, ratio, and nutrient levels in growing media affects seedling production and plant performance. Agronomy 2020, 10, 1421. [Google Scholar] [CrossRef]
- Tüzel, Y.; Ekinci, K.; Öztekin, G.B.; Erdal, İ.; Varol, N.; Merken, Ö. Utilization of olive oil processing waste composts in organic tomato seedling production. Agronomy 2020, 10, 797. [Google Scholar] [CrossRef]
- Gohardoust, M.R.; Bar-Tal, A.; Effati, M.; Tuller, M. Characterization of Physicochemical and hydraulic properties of organic and mineral soilless culture substrates and mixtures. Agronomy 2020, 10, 1403. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M.; Yu, P.; Zhou, C.; Liu, X. Biochar and vermicompost amendments affect substrate properties and plant growth of basil and tomato. Agronomy 2020, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Blok, C. Growing media for food and quality of life in the period 2020–2050. Acta Hortic. 2020. in press. Available online: https://peatlands.org/document/growing-media-blok-2018/ (accessed on 18 June 2020).
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant. Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [Green Version]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [Green Version]
- Venturi, V.; Keel, C. Signaling in the rhizosphere. Trends Plant. Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, X.F.; Chaparro, J.M.; Badri, D.V.; Manter, D.K.; Vivanco, J.M.; Guo, J. Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant Soil 2016, 401, 259–272. [Google Scholar] [CrossRef]
- Rosier, A.; Medeiros, F.H.V.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Rybakova, D.; Grube, M.; Köberl, M. The plant microbiome explored: Implications for experimental botany. J. Exp. Bot. 2016, 67, 995–1002. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Nuzzo, A.; Satpute, A.; Albrecht, U.; Strauss, S.L. Impact of soil microbial amendments on tomato rhizosphere microbiome and plant growth in field soil. Microb. Ecol. 2020, 80, 398–409. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant. Sci. 2018, 871, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x ananassa var. Selva) in conditions of reduced fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225. [Google Scholar] [CrossRef]
- Lee, S.; An, R.; Grewal, P.; Yu, Z.; Borherova, Z.; Lee, J. High-performing windowfarm hydroponic system: Transcriptomes of fresh produce and microbial communities in response to beneficial bacterial treatment. Mol. Plant Microbe Interact. 2016, 29, 965–976. [Google Scholar] [CrossRef]
- Sundaramoorthy, S.; Raguchander, T.; Ragupathi, N.; Samiyappan, R. Combinatorial effect of endophytic and plant growth promoting rhizobacteria against wilt disease of Capsicum annum L. caused by Fusarium solani. Biol. Control. 2012, 60, 59–67. [Google Scholar] [CrossRef]
- Yergeau, E.; Bell, T.H.; Champagne, J.; Maynard, C.; Tardif, S.; Tremblay, J.; Greer, C.W. Transplanting soil microbiomes leads to lasting effects on willow growth, but not on the rhizosphere microbiome. Front. Microbiol. 2015, 6, 1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Hale, L.; Crowley, D. Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol. Fertil. Soils 2016, 52, 515–522. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Imran, M.; Naveed, M.; Khan, M.Y.; Ahmad, M.; Zahir, Z.A.; Crowley, D.E. Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J. Sci. Food Agric. 2017, 97, 5139–5145. [Google Scholar] [CrossRef] [PubMed]
- Grunert, O.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Perneel, M.; Van Labeke, M.C.; Reheul, D.; Boon, N. Mineral and organic growing media have distinct community structure, stability and functionality in soilless culture systems. Sci. Rep. 2016, 6, 18837. [Google Scholar] [CrossRef] [Green Version]
- Grunert, O.; Hernandez-Sanabria, E.; Perneel, M.; Van Labeke, M.C.; Reheul, D.; Boon, N. High-throughput sequencing analysis provides a comprehensive insight into the complex bacterial relationships in horticultural growing substrates. Acta Hortic. 2017, 1168, 19–26. [Google Scholar] [CrossRef]
- Castaño, R.; Borrero, C.; Avilés, M. Organic matter fractions by SP-MAS 13C NMR and microbial communities involved in the suppression of Fusarium wilt in organic growth media. Biol. Control. 2011, 58, 286–293. [Google Scholar] [CrossRef]
- De Corato, U.; Viola, E.; Arcieri, G.; Valerio, V.; Zimbardi, F. Use of composted agro-energy co-products and agricultural residues against soil-borne pathogens in horticultural soil-less systems. Sci. Hortic. 2016, 210, 166–179. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, Y.; Gao, Y.; Li, J. Microbial diversity in compost is critical in suppressing plant fungal pathogen survival and enhancing cucumber seedling growth. Compost Sci. Util. 2018, 26, 189–200. [Google Scholar] [CrossRef]
- Borrero, C.; Ordovás, J.; Trillas, M.I.; Avilés, M. Tomato Fusarium wilt suppressiveness. The relationship between the organic plant growth media and their microbial communities as characterised by Biolog®. Soil Biol. Biochem. 2006, 38, 1631–1637. [Google Scholar] [CrossRef]
- Nieto, A.; Gascó, G.; Paz-Ferreiro, J.; Fernández, J.M.; Plaza, C.; Méndez, A. The effect of pruning waste and biochar addition on brown peat based growing media properties. Sci. Hortic. 2016, 199, 142–148. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Cayuela, M.L.; Sánchez-García, M.; Vandecasteele, B.; D’Hose, T.; López, G.; Martínez-Gaitán, C.; Kuikman, P.J.; Sinicco, T.; Mondini, C. Agronomic evaluation of biochar, compost and biochar-blended compost across different cropping systems: Perspective from the European project FERTIPLUS. Agronomy 2019, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Blok, C.; van der Salm, C.; Hofland-Zijlstra, J.; Streminska, M.; Eveleens, B.; Regelink, I.; Fryda, L.; Visser, R. Biochar for horticultural rooting media improvement: Evaluation of biochar from gasification and slow pyrolysis. Agronomy 2017, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Méndez, A.; Paz-Ferreiro, J.; Gil, E.; Gascó, G. The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci. Hortic. 2015, 193, 225–230. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Paudel, I.; Graber, E.R.; Cytryn, E.; Frenkel, O. Linking the belowground microbial composition, diversity and activity to soilborne disease suppression and growth promotion of tomato amended with biochar. Sci. Rep. 2017, 7, 44382. [Google Scholar] [CrossRef]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Beneficial bacteria and fungi in hydroponic systems: Types and characteristics of hydroponic food production methods. Sci. Hortic. 2015, 195, 206–215. [Google Scholar] [CrossRef]
- Lee, S.; Ge, C.; Bohrerova, Z.; Grewal, P.S.; Lee, J. Enhancing plant productivity while suppressing biofilm growth in a windowfarm system using beneficial bacteria and ultraviolet irradiation. Can. J. Microbiol. 2015, 61, 457–466. [Google Scholar] [CrossRef]
- Gül, A.; Kidoglu, F.; Tüzel, Y.; Tüzel, I.H. Effects of nutrition and Bacillus amyloliquefaciens on tomato (Solanum lycopersicum L.) growing in perlite. Span. J. Agric. Res. 2008, 6, 422–429. [Google Scholar]
- García, J.A.L.; Probanza, A.; Ramos, B.; Palomino, M.; Mañero, F.J.G. Effect of inoculation of Bacillus lichenformis on tomato and pepper. Agronomie 2004, 24, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Vecstaudza, D.; Senkovs, M.; Nikolajeva, V.; Kasparinskis, R.; Muter, O. Wooden biochar as a carrier for endophytic isolates. Rhizosphere 2017, 3, 126–127. [Google Scholar] [CrossRef]
- Saxena, J.; Rana, G.; Pandey, M. Impact of addition of biochar along with Bacillus sp. on growth and yield of French beans. Sci. Hortic. 2013, 162, 351–356. [Google Scholar] [CrossRef]
- Barillot, C.D.C.; Sarde, C.O.; Bert, V.; Tarnaud, E.; Cochet, N. A standardized method for the sampling of rhizosphere and rhizoplan soil bacteria associated to a herbaceous root system. Ann. Microbiol. 2013, 63, 471–476. [Google Scholar] [CrossRef]
- Van Nevel, S.; Koetzsch, S.; Weilenmann, H.U.; Boon, N.; Hammes, F. Routine bacterial analysis with automated flow cytometry. J. Microbiol. Methods 2013, 94, 73–76. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Ameloot, N.; Navarrete, O.; Vandecruys, M.; Perneel, M.; Boon, N.; Geelen, D. Microbial activity in peat-reduced plant growing media: Identifying influential growing medium constituents and physicochemical properties using fractional factorial design of experiments. J. Clean. Prod. 2020, 256, 120323. [Google Scholar] [CrossRef]
- Verdonck, O.; Gabriels, R. Reference method for the determination of physical and chemical properties of plant substrates. Acta Hortic. 1992, 169–180. [Google Scholar] [CrossRef]
- Gabriels, D.; Hartmann, R.; Verplancke, H.; Cornelis, W.; Verschoore, P. Werkwijzen Voor Grondanalyses.; University Gent: Gent, Belgium, 1998; p. 90. [Google Scholar]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT Food Sci. Technol. 2007; 40, 552–557. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant. Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Bingham, D.; Sitter, R.R. Fractional factorial split-plot designs for robust parameter experiments. Technometrics 2003, 45, 80–89. [Google Scholar] [CrossRef]
- Mathers, H.M.; Lowe, S.B.; Scagel, C.; Struve, D.K.; Case, L.T. Abiotic factors influencing root growth of woody nursery plants in containers. Horttechnology 2007, 17, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Brückner, U. Physical properties of different potting media and substrate mixtures—Especially air-and water capacity. Acta Hortic. 1997, 450, 263–270. [Google Scholar]
- Kozai, T.; Niu, G. Challenges for the Next-Generation PFALs; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128166918. [Google Scholar]
- Evans, M.R.; Gachukia, M.M. Physical properties of sphagnum peat-based root substrates amended with perlite or parboiled fresh rice hulls. Horttechnology 2007, 17, 312–315. [Google Scholar] [CrossRef] [Green Version]
- Verhagen, J.B.G.M.; Zevenhoven, M.A. Growing Media A. Available online: https://www.rhp.nl/en/home (accessed on 2 May 2018).
- Spiers, T.; Fietje, G. Green waste compost as a component in soilless growing media. Compos. Sci. Util. 2000, 8, 19–23. [Google Scholar] [CrossRef]
- Gruda, N. Sustainable peat alternative growing media. Acta Hortic. 2012, 927, 973–979. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. Influence of wood fiber substrates and N application rates on the growth of tomato transplants. Adv. Hortic. Sci. 1999, 13, 20–24. [Google Scholar]
- Vandecasteele, B.; Muylle, H.; De Windt, I.; Van Acker, J.; Ameloot, N.; Moreaux, K.; Coucke, P.; Debode, J. Plant fibers for renewable growing media: Potential of defibration, acidification or inoculation with biocontrol fungi to reduce the N drawdown and plant pathogens. J. Clean. Prod. 2018, 203, 1143–1154. [Google Scholar] [CrossRef]
- Reeve, J.R.; Hoagland, L.A.; Villalba, J.J.; Carr, P.M.; Atucha, A.; Cambardella, C.; Davis, D.R.; Delate, K. Organic farming, soil health, and food quality: Considering possible links. In Advances in Agronomy; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 137, pp. 319–367. ISBN 9780128046920. [Google Scholar]
- Roesti, D.; Gaur, R.; Johri, B.N.; Imfeld, G.; Sharma, S.; Kawaljeet, K.; Aragno, M. Plant growth stage, fertiliser management and bio-inoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria affect the rhizobacterial community structure in rain-fed wheat fields. Soil Biol. Biochem. 2006, 38, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Pii, Y.; Borruso, L.; Brusetti, L.; Crecchio, C.; Cesco, S.; Mimmo, T. The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant. Physiol. Biochem. 2016, 99, 39–48. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant. Soil 2009, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Yang, S.; Tang, F.; Zhu, H. Symbiosis specificity in the legume—Rhizobial mutualism. Cell. Microbiol. 2012, 14, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Zebelo, S.; McNear, D.; Kloepper, J.; Fadamiro, H. Plant growth-promoting rhizobacteria induce changes in Arabidopsis thaliana gene expression of nitrate and ammonium uptake genes. J. Plant. Interact. 2019, 14, 224–231. [Google Scholar] [CrossRef]
- Kováčik, J.; Bačkor, M. Changes of phenolic metabolism and oxidative status in nitrogen-deficient Matricaria chamomilla plants. Plant. Soil 2007, 297, 255–265. [Google Scholar] [CrossRef]
- Muthukumar, A.; Eswaran, A.; Sangeetha, G. Induction of systemic resistance by mixtures of fungal and endophytic bacterial isolates against Pythium aphanidermatum. Acta Physiol. Plant. 2011, 33, 1933–1944. [Google Scholar] [CrossRef]
- Thangavelu, R.; Palaniswami, A.; Doraiswamy, S.; Velazhahan, R. The effect of Pseudomonas fluorescens and Fusarium oxysporum f.sp. cubense on induction of defense enzymes and phenolics in banana. Biol. Plant. 2003, 46, 107–112. [Google Scholar] [CrossRef]
- Domenech, J.; Reddy, M.S.; Kloepper, J.W.; Ramos, B.; Gutierrez-Mañero, J. Combined application of the biological product LS213 with Bacillus, Pseudomonas or Chryseobacterium for growth promotion and biological control of soil-borne diseases in pepper and tomato. BioControl 2006, 51, 245–258. [Google Scholar] [CrossRef]

| Sample | Collection Date | Location | Crop | Cultivation Method | Plant Growing Medium |
|---|---|---|---|---|---|
| S1 | 3 October 2017 | Wachtebeke, Belgium | Lactuca sativa var. crispa (oakleaf) | Organic open field | Sand |
| S2 | 17 October 2017 | Moerbeke-Waas, Belgium | Lactuca sativa var. crispa (oakleaf) | Organic open field | Loamy sand |
| S3 | 21 November 2017 | Onze-Lieve-Vrouw-Waver, Belgium | Lactuca sativa var. crispa (lollo bionda) | Soilless | Black peat |
| S4 | 12 December 2017 | Ardooie, Belgium | Lactuca sativa var. capitata (butterhead) | Soilless | Black peat |
| S5 | 5 June 2018 | Lochristi, Belgium | Lactuca sativa var. crispa (lollo bionda) | Organic open field | Sand |
| Plant Growing Medium | Peat (60% v/v) | Other Organics (20% v/v) | Composted Materials (10% v/v) | Inorganic Materials (10% v/v) | Arabic Gum (kg·m−3) |
|---|---|---|---|---|---|
| M1 | WP | CP | CB | P | 1 |
| M2 | WP | WF | CB | P | 5 |
| M3 | BP | CP | CB | S | 5 |
| M4 | WP | CP | GC | P | 5 |
| M5 | WP | WF | CB | S | 1 |
| M6 | WP | CP | CB | S | 5 |
| M7 | BP | WF | CB | P | 5 |
| M8 | BP | CP | GC | S | 1 |
| M9 | WP | WF | GC | S | 5 |
| M10 | BP | WF | GC | P | 1 |
| PC 1 (37.8%) | PC 2 (27.2%) | |||
|---|---|---|---|---|
| Correlation | P Value | Correlation | P Value | |
| FW | 0.961 | 1.71 × 10−37 | / | n.s. |
| LHA | 0.848 | 2.62 × 10−19 | 0.457 | 1.13 × 10−4 |
| RW | 0.731 | 3.15 × 10−12 | 0.585 | 2.53 × 10−7 |
| DW | / | n.s. | 0.806 | 3.41 × 10−16 |
| TPC | −0.390 | 1.21 × 10−3 | 0.491 | 2.90 × 10−5 |
| NO3 | 0.615 | 3.96 × 10−8 | −0.420 | 4.46 × 10−4 |
| Chla+b | −0.347 | 4.28 × 10−3 | 0.466 | 8.15 × 10−5 |
| Carotenoids | −0.373 | 2.07 × 10−3 | 0.550 | 1.68 × 10−6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Gerrewey, T.; Vandecruys, M.; Ameloot, N.; Perneel, M.; Van Labeke, M.-C.; Boon, N.; Geelen, D. Microbe-Plant Growing Media Interactions Modulate the Effectiveness of Bacterial Amendments on Lettuce Performance Inside a Plant Factory with Artificial Lighting. Agronomy 2020, 10, 1456. https://doi.org/10.3390/agronomy10101456
Van Gerrewey T, Vandecruys M, Ameloot N, Perneel M, Van Labeke M-C, Boon N, Geelen D. Microbe-Plant Growing Media Interactions Modulate the Effectiveness of Bacterial Amendments on Lettuce Performance Inside a Plant Factory with Artificial Lighting. Agronomy. 2020; 10(10):1456. https://doi.org/10.3390/agronomy10101456
Chicago/Turabian StyleVan Gerrewey, Thijs, Maarten Vandecruys, Nele Ameloot, Maaike Perneel, Marie-Christine Van Labeke, Nico Boon, and Danny Geelen. 2020. "Microbe-Plant Growing Media Interactions Modulate the Effectiveness of Bacterial Amendments on Lettuce Performance Inside a Plant Factory with Artificial Lighting" Agronomy 10, no. 10: 1456. https://doi.org/10.3390/agronomy10101456
APA StyleVan Gerrewey, T., Vandecruys, M., Ameloot, N., Perneel, M., Van Labeke, M.-C., Boon, N., & Geelen, D. (2020). Microbe-Plant Growing Media Interactions Modulate the Effectiveness of Bacterial Amendments on Lettuce Performance Inside a Plant Factory with Artificial Lighting. Agronomy, 10(10), 1456. https://doi.org/10.3390/agronomy10101456





