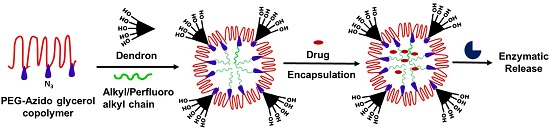
Chemo-Enzymatic Synthesis of Perfluoroalkyl-Functionalized Dendronized Polymers as Cyto-Compatible Nanocarriers for Drug Delivery Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation and Methods
2.2.1. NMR, IR Spectroscopy, and GPC Analysis
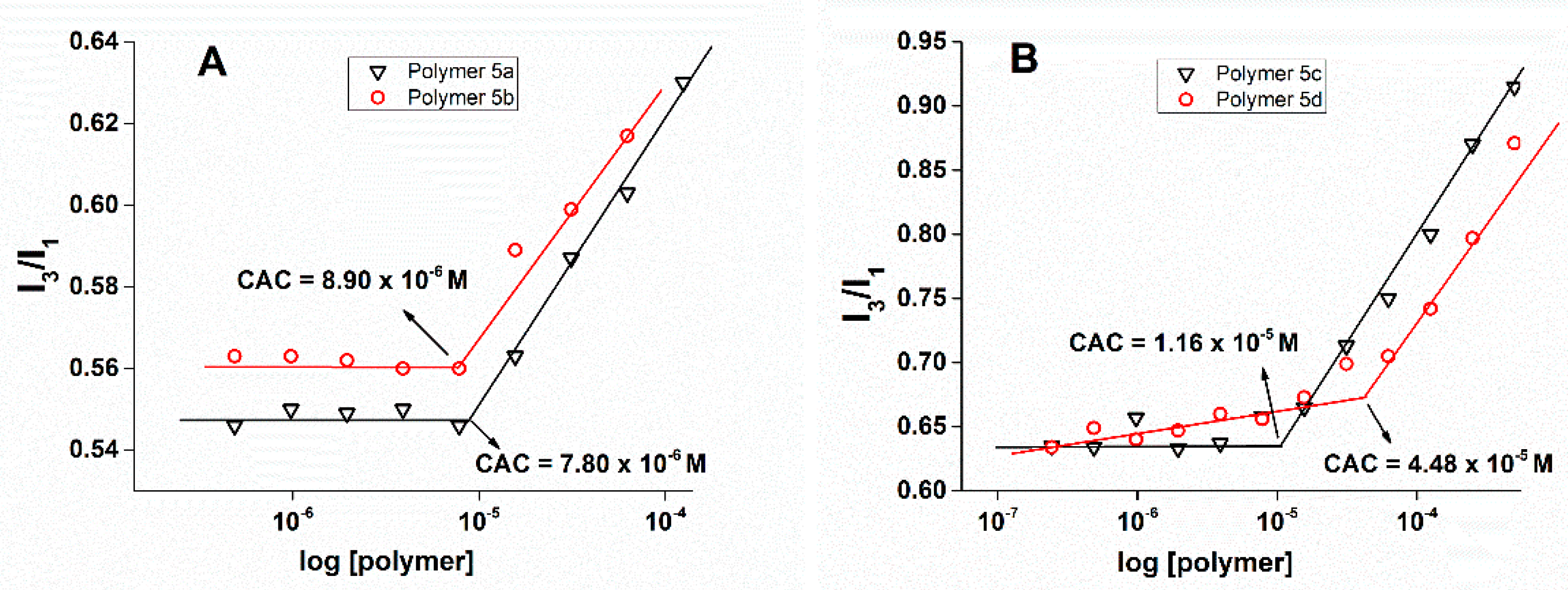
2.2.2. Critical Aggregation Concentration (CAC) Measurement
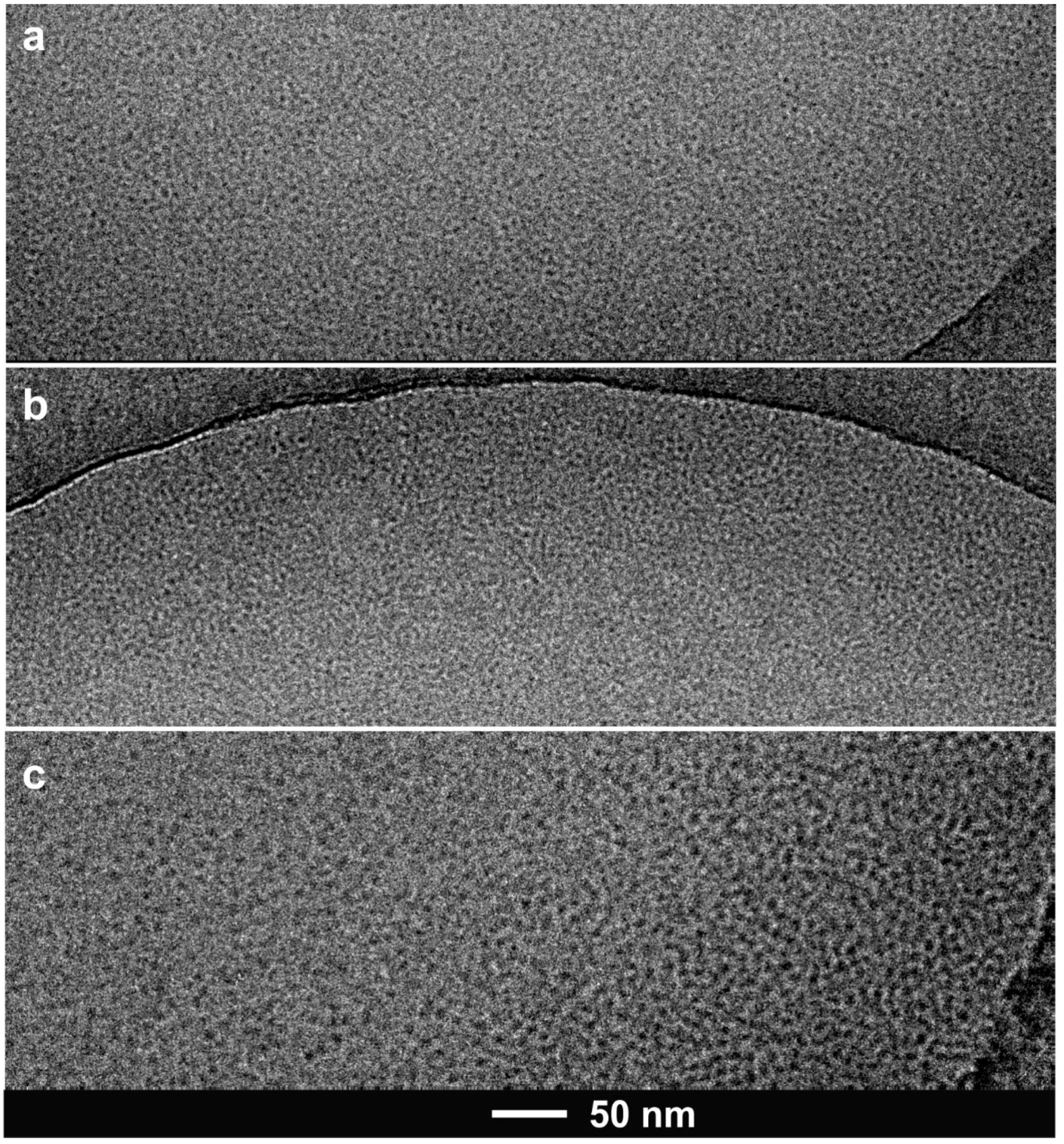
2.2.3. Dynamic Light Scattering (DLS) and Cryogenic Transmission Electron Microscopy (cryo-TEM)
2.2.4. Procedure for Curcumin Encapsulation
2.2.5. Enzyme-Triggered Release Study
2.2.6. Procedure for Dexamethasone Encapsulation
2.2.7. Cytotoxicity Studies
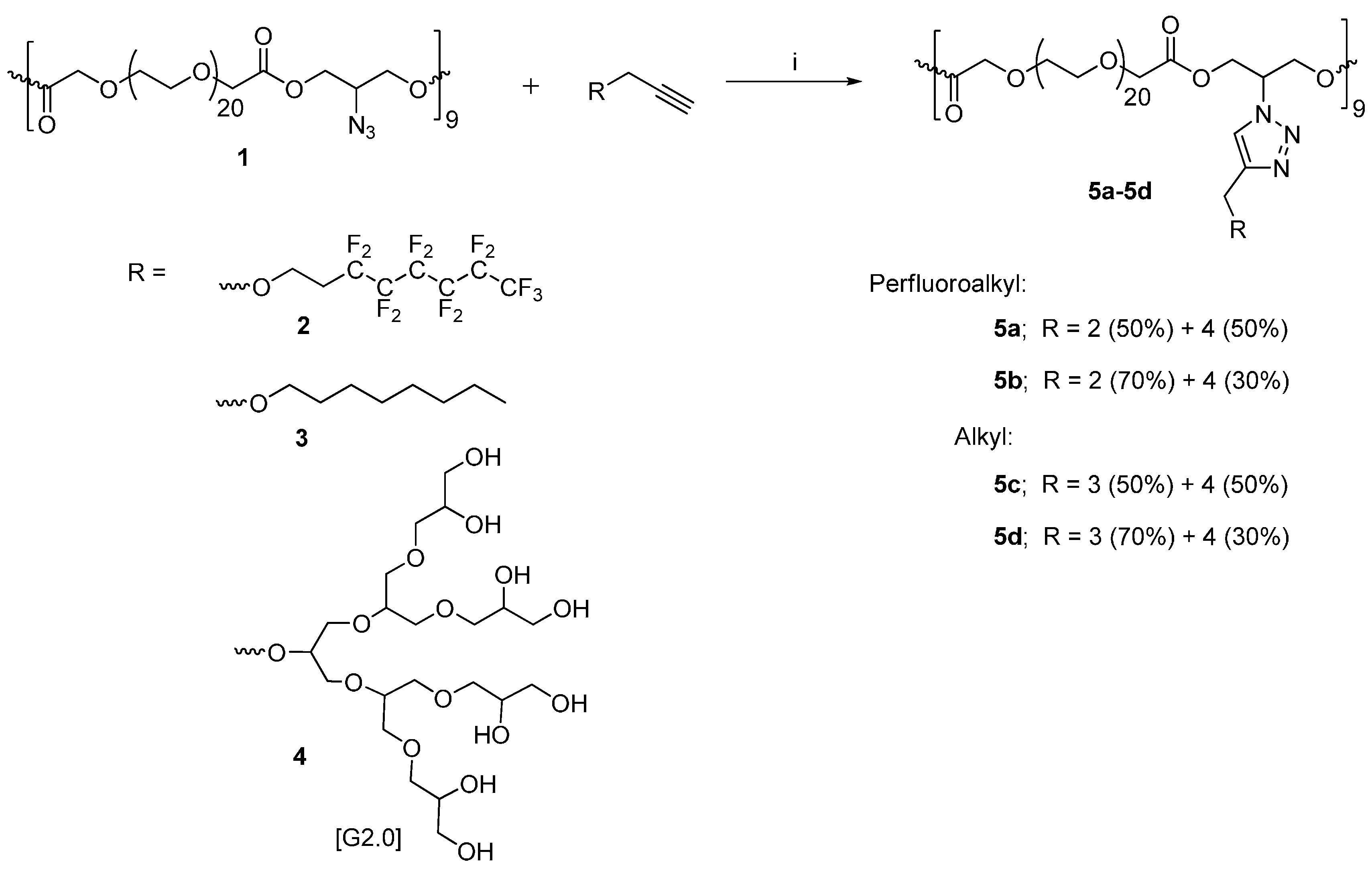
2.3. General Procedure for the Synthesis of Polymers 5a–5d
2.3.1. Polymer 5a (50% Perfluoro-alkyl Chain and 50% PG Dendron Functionalized)
2.3.2. Polymer 5b (70% Perfluoro-alkyl Chain and 30% PG Dendron Functionalized)
2.3.3. Polymer 5c (50% Alkyl Chain and 50% PG Dendron Functionalized)
2.3.4. Polymer 5d (70% Alkyl Chain and 30% PG Dendron Functionalized)
3. Results and Discussion
3.1. Synthesis and Characterization
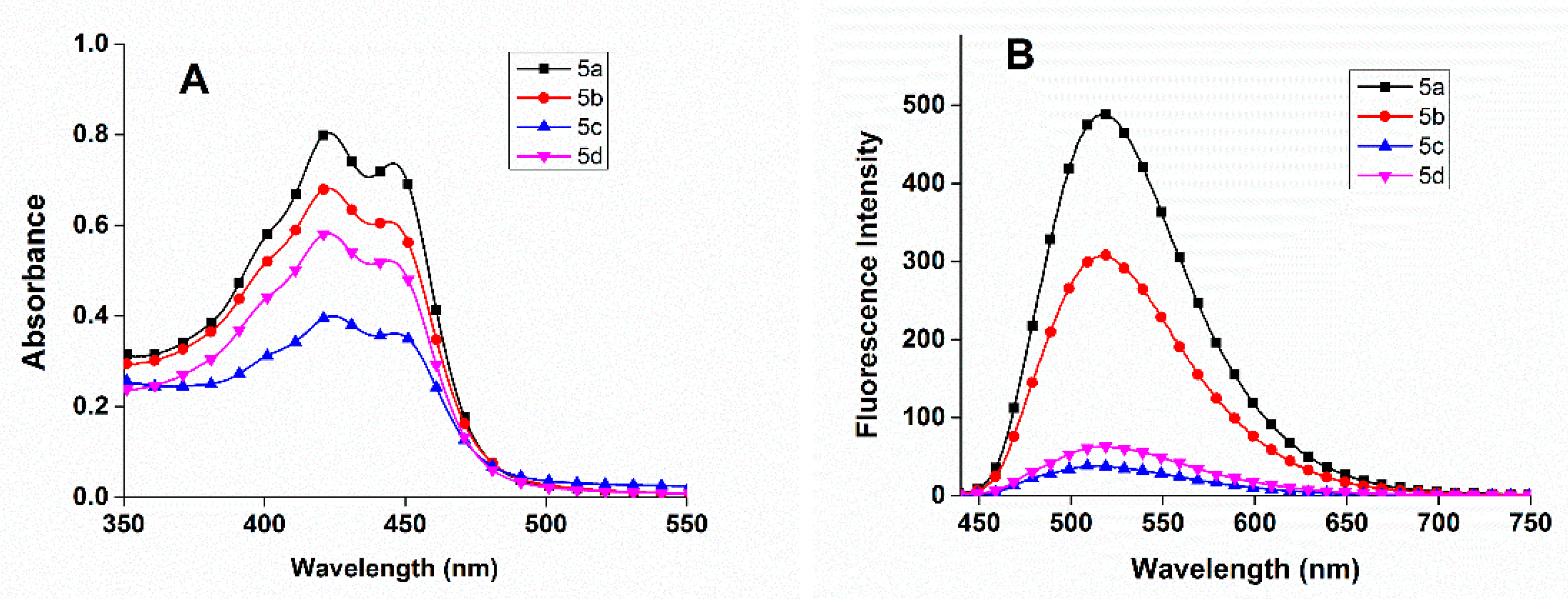
3.2. CAC Calculation Using Fluorescence Measurement
3.3. DLS and Cryo-TEM Analysis
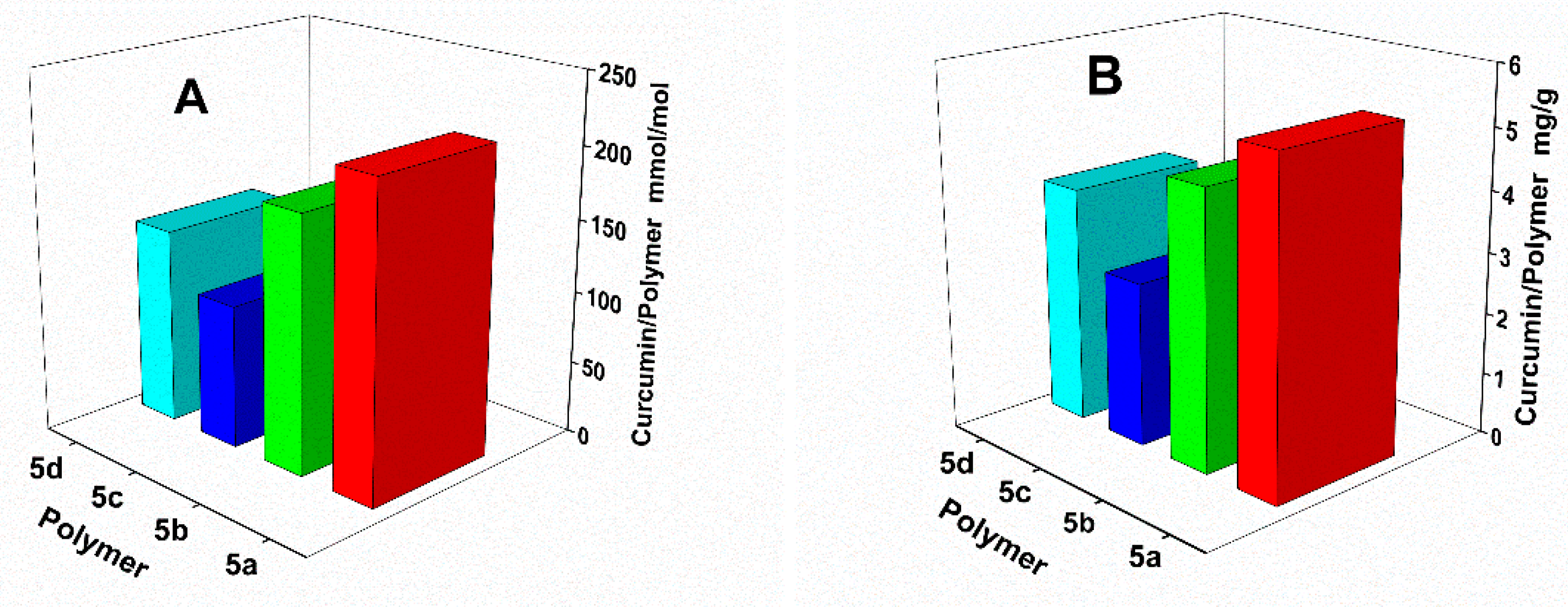
3.4. Transport Potential for Curcumin
3.5. Cytotoxicity Study
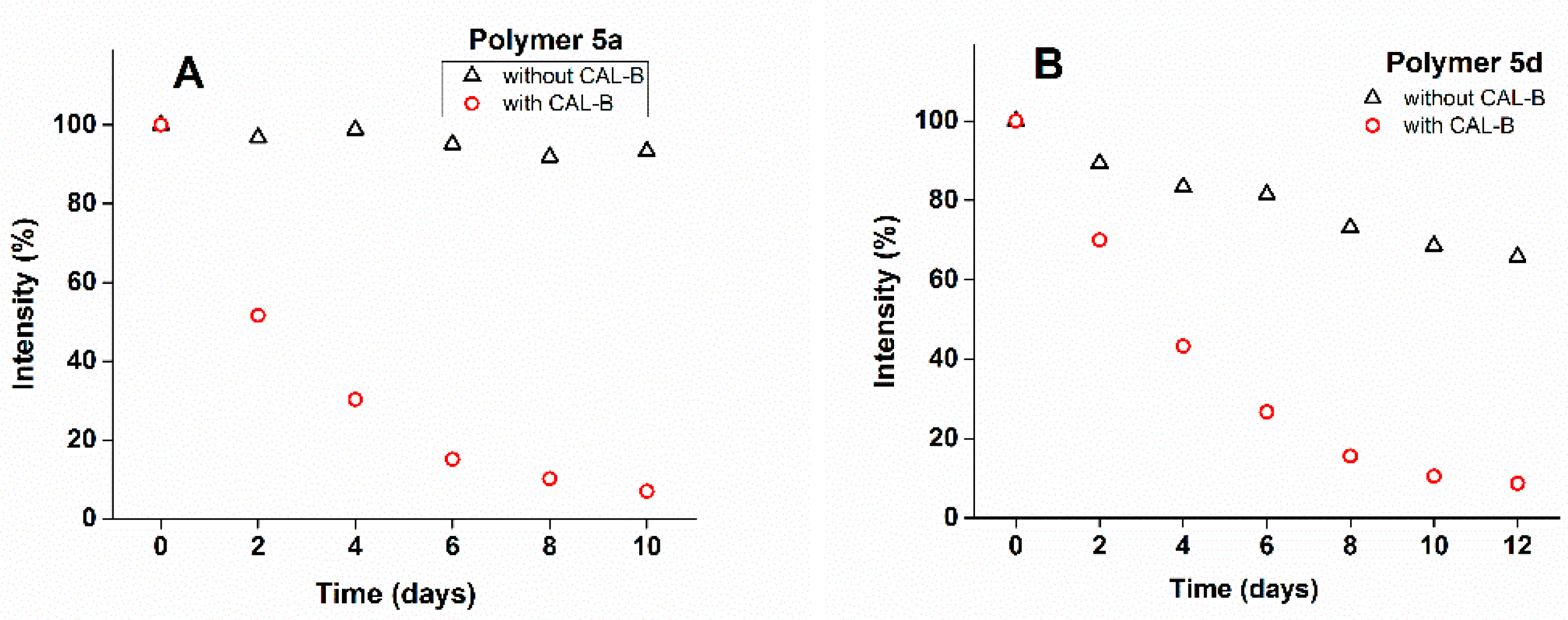
3.6. Enzyme-Triggered Release of Curcumin
3.7. Solubilization of Dexamethasone
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cai, C.; Wang, L.; Lin, J.; Zhang, X. Morphology transformation of hybrid micelles self-assembled from rod-coil block copolymer and nanoparticles. Langmuir 2012, 28, 4515–4524. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Lin, X.; Wang, F.; Liu, B.; Zhou, J.; Li, J.; Li, W. Well-defined polymeric double helices with solvent-triggered destruction from amphiphilic hairy-like nanoparticles. Macromolecules 2013, 46, 8644–8648. [Google Scholar] [CrossRef]
- Wang, X.; Guerin, G.; Wang, H.; Wang, Y.; Manners, I.; Winnik, M.A. Cylindrical block copolymer micelles and co-micelles of controlled length and architecture. Science 2007, 317, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Nishida, J.; Ookura, R.; Nishimura, S.I.; Wada, S.; Karino, T.; Shimomura, M. Honeycomb-patterned thin films of amphiphilic polymers as cell culture substrates. Mat. Sci. Eng. C 1999, 8, 495–500. [Google Scholar] [CrossRef]
- Bae, Y.; Kataoka, K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 2009, 61, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, A.; Illa, O.; Ortuño, R.M. Amphiphiles in aqueous solution: Well beyond a soap bubble. Chem. Soc. Rev. 2013, 42, 8200–8219. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Torchilin, V.P. Lipid-core micelles for targeted drug delivery. Curr. Drug Deliv. 2005, 2, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, Z.L.; Shen, Y.; Radosz, M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Amado, E.; Kressler, J. Triphilic block copolymers with perfluorocarbon moieties in aqueous systems and their biochemical perspectives. Soft Matter 2011, 7, 7144–7149. [Google Scholar] [CrossRef]
- Lehn, J.M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Ungar, G.; Peterca, M. Self-assembly in action. Science 2006, 313, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Thota, B.N.; Urner, L.H.; Haag, R. Supramolecular architectures of dendritic amphiphiles in water. Chem. Rev. 2015, 116, 2079–2102. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Mohr, A.; Mathur, D.; Parmar, V.S.; Haag, R.; Prasad, A.K. Biocatalytic route to sugar-PEG-based polymers for drug delivery applications. Biomacromolecules 2011, 12, 3487–3498. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Achazi, K.; Böttcher, C.; Licha, K.; Haag, R.; Sharma, S.K. Encapsulation and cellular internalization of cyanine dye using amphiphilic dendronized polymers. Eur. Polym. J. 2015, 69, 416–428. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.; Achazi, K.; Böttcher, C.; Khandare, J.; Sharma, S.K.; Haag, R. Dendronized multifunctional amphiphilic polymers as efficient nanocarriers for biomedical applications. Macromol. Rapid Commun. 2015, 36, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Billamboz, M.; Leonard, E.; Len, C.; Böttcher, C.; Prasad, A.K.; Haag, R.; Sharma, S.K. Self-assembly, photoresponsive behavior and transport potential of azobenzene grafted dendronized polymeric amphiphiles. RSC Adv. 2015, 5, 48301–48310. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Highly fluorinated amphiphiles and colloidal systems, and their applications in the biomedical field. A contribution. Biochimie 1998, 80, 489–514. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Selected physicochemical aspects of poly-and perfluoroalkylated substances relevant to performance, environment and sustainability-Part one. Chemosphere 2015, 129, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P. Highly fluorinated compounds induce phase separation in, and nanostructuration of liquid media. Possible impact on, and use in chemical reactivity control. J. Polym. Sci. A Polym. Chem. 2006, 44, 4251–4258. [Google Scholar] [CrossRef]
- Pandey, M.K.; Tyagi, R.; Yang, K.; Fisher, R.J.; Colton, C.K.; Kumar, J.; Parmar, V.S.; Aiazian, E.; Watterson, A.C. Design and synthesis of perfluorinated amphiphilic copolymers: Smart nanomicelles for theranostic applications. Polymer 2011, 52, 4727–4735. [Google Scholar] [CrossRef]
- Bunn, C.W.; Howells, E.R. Structures of molecules and crystals of fluorocarbons. Nature 1954, 174, 549–551. [Google Scholar] [CrossRef]
- Mecozzi, S.; Hoang, K. Encapsulation of Chemical Compounds in Fluorous-Core and Fluorous-Inner-Shell Micelles Formed from Semifluorinated-Block or Fluorinated-Block Copolymers. U.S. Patent 11/028,948, 3 January 2005. [Google Scholar]
- Greene, A.C.; Zhu, J.; Pochan, D.J.; Jia, X.; Kiick, K.L. Poly(acrylic acid-b-styrene) amphiphilic multiblock copolymers as building blocks for the assembly of discrete nanoparticles. Macromolecules 2011, 44, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.H.; Harada, T.; Dai, S.; Kee, T.W. Nanoprecipitation and spectroscopic characterization of curcumin-encapsulated polyester nanoparticles. Langmuir 2015, 31, 11419–11427. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F.; Bikádi, Z.; Simonyi, M. Molecular basis of the cotton effects induced by the binding of curcumin to human serum albumin. Tetrahedron Asymmetry 2003, 14, 2433–2444. [Google Scholar] [CrossRef]
- Francis, D.V.; Miles, D.H.; Mohammed, A.I.; Read, R.W.; Wang, X. Towards functional fluorous surfactants. Synthesis of hydrophilic fluorous 1,2,3-triazolylmethyl ethers and di(1,2,3-triazolylmethyl) ethers. J. Fluorine Chem. 2011, 132, 898–906. [Google Scholar] [CrossRef]
- Rao, M.N.A. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Brouet, I.; Ohshima, H. Curcumin, an anti-tumor promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem. Biophys. Res. Commun. 1995, 206, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Tilak, J.C.; Banerjee, M.; Mohan, H.; Devasagayam, T.P.A. Antioxidant availability of turmeric in relation to its medicinal and culinary uses. Phytother. Res. 2004, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Sethi, G.; Aggarwal, B.B. Curcumin: getting back to the roots. Ann. NY Acad. Sci. 2005, 1056, 206–217. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, M.G.; Soucy, P.A.; Chauhan, R.; Raju, M.V.R.; Patel, D.N.; Nunn, B.M.; Keynton, M.A.; Ehringer, W.D.; Nantz, M.H.; Keynton, R.S.; et al. Release-modulated antioxidant activity of a composite curcumin-chitosan polymer. Biomacromolecules 2016, 17, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, L.; Wu, Q.; Guo, W.; Li, L.; Chen, Y.; Li, Y.; Gong, C.; Qian, Z.; Wei, Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013, 443, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Die Pharmazie 2002, 57, 820–824. [Google Scholar] [PubMed]
- Baglole, K.N.; Boland, P.G.; Wagner, B.D. Fluorescence enhancement of curcumin upon inclusion into parent and modified cyclodextrins. J. Photochem. Photobiol. A Chem. 2005, 173, 230–237. [Google Scholar] [CrossRef]
- Ohori, H.; Yamakoshi, H.; Tomizawa, M.; Shibuya, M.; Kakudo, Y.; Takahashi, A.; Takahashi, S.; Kato, S.; Suzuki, T.; Ishioka, C.; et al. Synthesis and biological analysis of new curcumin analogs bearing an enhanced potential for the medicinal treatment of cancer. Mol. Cancer Ther. 2006, 5, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Zisis, T.; Freddolino, P.L.; Turunen, P.; van Teeseling, M.C.F.; Rowan, A.E.; Blank, K.G. Interfacial activation of Candida antarctica lipase B: Combined evidence from experiment and simulation. Biochemistry 2015, 54, 5969–5979. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaete, C.; Tsapis, N.; Besnard, M.; Bochot, A.; Fattal, E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int. J. Pharm. 2007, 331, 153–159. [Google Scholar] [CrossRef] [PubMed]


| Polymer | Hydrophobic unit | Hydrophilic unit | CAC (M) | DLS size (nm) | PDI | ||
|---|---|---|---|---|---|---|---|
| C8 chain | PG dendron | Intensity | Volume | Number | |||
| 5a | 50% perfluoroalkyl | 50% | 8.90 × 10−6 | 8.11 | 7.05 | 6.27 | 0.509 |
| 5b | 70% perfluoroalkyl | 30% | 7.80 × 10−6 | 10.92 | 8.33 | 6.87 | 0.486 |
| 5c | 50% alkyl | 50% | 1.16 × 10−5 | 143.5 | 110.7 | 83.15 | 0.291 |
| 5d | 70% alkyl | 30% | 4.48 × 10−5 | 124.2 | 96.24 | 75.94 | 0.269 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parshad, B.; Kumari, M.; Achazi, K.; Bӧttcher, C.; Haag, R.; Sharma, S.K. Chemo-Enzymatic Synthesis of Perfluoroalkyl-Functionalized Dendronized Polymers as Cyto-Compatible Nanocarriers for Drug Delivery Applications. Polymers 2016, 8, 311. https://doi.org/10.3390/polym8080311
Parshad B, Kumari M, Achazi K, Bӧttcher C, Haag R, Sharma SK. Chemo-Enzymatic Synthesis of Perfluoroalkyl-Functionalized Dendronized Polymers as Cyto-Compatible Nanocarriers for Drug Delivery Applications. Polymers. 2016; 8(8):311. https://doi.org/10.3390/polym8080311
Chicago/Turabian StyleParshad, Badri, Meena Kumari, Katharina Achazi, Christoph Bӧttcher, Rainer Haag, and Sunil K. Sharma. 2016. "Chemo-Enzymatic Synthesis of Perfluoroalkyl-Functionalized Dendronized Polymers as Cyto-Compatible Nanocarriers for Drug Delivery Applications" Polymers 8, no. 8: 311. https://doi.org/10.3390/polym8080311
APA StyleParshad, B., Kumari, M., Achazi, K., Bӧttcher, C., Haag, R., & Sharma, S. K. (2016). Chemo-Enzymatic Synthesis of Perfluoroalkyl-Functionalized Dendronized Polymers as Cyto-Compatible Nanocarriers for Drug Delivery Applications. Polymers, 8(8), 311. https://doi.org/10.3390/polym8080311