Abstract
For organ-on-a-chip (OoC) engineering, the use of biocompatible coatings and materials is not only recommended but essential. Extracellular matrix (ECM) components are commonly used as coatings due to their effects on cell orientation, protein expression, differentiation, and adhesion. Among the most frequently used coatings are collagen, fibronectin, and Matrigel, according to the specific cell type and intended OoC application. Additionally, materials such as polydimethylsiloxane (PDMS), thermoplastics, chitosan, and alginate serve as scaffolding components due to their biomechanical properties and biocompatibility. Here, we discuss some of the most employed coating techniques, including SAMs, dip coating, spin coating, microcontact printing, and 3D bioprinting, each offering advantages and drawbacks. Current challenges comprise enhancing biocompatibility, exploring novel materials, and improving scalability and reproducibility.
1. Introduction
On-chip systems integrate microtechnology, biomaterials, and cell biology to develop in vitro platforms that aim to mimic the complex interactions occurring in human tissues and living organisms [1,2,3,4]. These microfluidic devices allow for regulation of parameters, such as concentration gradients, cell patterning, mechanical stimulation, and tissue–organ interactions [1].
Organ-on-chip (OoC) and similar systems are typically constructed using materials such as polydimethylsiloxane (PDMS), glass, and other polymers due to their flexibility, transparency, and ease of fabrication [1,5,6]. Despite their widespread use, these materials often exhibit limitations in terms of biocompatibility, and their ability to promote cell adhesion and mimic the physiological microenvironment of living cells accurately [7,8]. Improving these materials is essential to enhance their compatibility with biological tissues, reduce unintended interactions, and create more accurate models of cellular environments. Such advancements could lead to more reliable simulations of human physiology and better predictive tools for drug discovery and biomedical research.
The current goals of the biomedical industry include the involvement in the search and development of accessible biocompatible materials, particularly those with tunable biodegradability and resorption [2,9,10]. To evaluate the effectiveness and safety of products made of such materials, preclinical and clinical evaluations must be carried out.
Preclinical tests using 2D cell cultures and animal modeling present plenty of limitations and concerns to ensure the safety and effectiveness of a drug, medical device, or tissue-engineered construct, such as an adequate simulation due to physiological interactions among tissues and organs, ethical issues, and replicability of results [2,11,12,13].
Moreover, integrating controlled substance-releasing materials into “on-chip” systems presents a promising avenue for advancing biomedical research and therapeutic applications [14,15]. Such materials could enable precise delivery of drugs or signaling molecules, contributing to more closely mimicking the dynamic physiological conditions of human tissues, and improved simulation of cellular responses, enhanced drug testing accuracy, and potential applications in personalized medicine. However, challenges, such as ensuring material biocompatibility, as well as maintaining or regulating release kinetics and preventing unintended interactions with the microenvironment, must be addressed [16]. Furthermore, the complexity of designing systems that can reliably replicate the intricate interplay of biological processes poses significant technical hurdles. Overcoming these obstacles would require interdisciplinary collaboration and innovative approaches in material science and bioengineering.
In recent years, several OoC platforms have been developed, mostly for the heart [11], liver [17,18,19], gut [17,20,21,22], and kidney [23]. Despite significant progress in OoC technology, challenges remain in standardizing optimized fabrication methods, ensuring reproducibility, and replicating the dynamic interactions present in human tissues [24]. While PDMS remains a widely used material due to its already mentioned optical transparency and biocompatibility, alternative materials, such as thermoplastics, extracellular matrix (ECM)-mimicking polymers, and biofunctional coatings, are being explored to improve biomimicry and physiological relevance [6,25,26,27]. Additionally, integrating microfabrication techniques with advanced imaging and analytical tools is expected to enhance the precision of multi-organ models, supporting applications in personalized medicine and drug discovery [11].
This review examines the latest advancements in scaffold materials and coatings for OoC systems, highlighting their role in engineering the cellular microenvironment and their potential to improve the physiological relevance of in vitro models. By addressing current limitations and exploring novel biomaterial approaches, OoC technology can further bridge the gap between preclinical testing and clinical applications, ultimately improving the predictive accuracy of biomedical research.
2. Organ-on-a-Chip Device Materials
The material properties play a pivotal role in the construction and functionality of organ-on-chip systems. Some synthetic polymers, for example, offer advantages, such as optical transparency, gas permeability, and ease of microfabrication, making them a popular choice for creating microfluidic channels; however, they also present limitations. Understanding and optimizing these material properties are essential to improve the physiological relevance and reliability of organ-on-chip systems.
2.1. PDMS
PDMS, a silicone-based polymer, plays a pivotal role in the field of OoC technology. It is widely used due to its desirable properties. First, its biocompatibility makes it non-toxic and suitable for 3D cell culture [5,20,23,28,29], as seen in different OoC systems. Second, its transparency allows real-time observation of cell behavior [30]. Third, PDMS is elastomeric, enabling precise molding into intricate shapes [31,32,33]. Fourth, it exhibits gas permeability, allowing oxygen and carbon dioxide diffusion [5]. Finally, its ease of fabrication via soft lithography techniques enables customizable microfluidic designs [34,35].
In OoC technology, researchers commonly use PDMS-based chips, which comprise microchannels, chambers, and semipermeable membranes, for cell culture, fluid shear stress studies, chemical gradients, and multi-organ systems, while addressing challenges such as hydrophobicity management, long-term stability, and its tendency to absorb small hydrophobic molecules, which can affect experimental outcomes [36]. As they refine designs and delve into bioprinting, PDMS continues to propel the advancement of OoC technology [5].
2.2. Polycarbonate
Polycarbonate is widely used in biomedical applications due to its biocompatibility and mechanical properties. Its chemical structure consists of bisphenol-A molecules linked by carbonate groups, and it is commonly employed in the production of food and drug packaging components, as well as medical devices [37].
In OoC technology, polycarbonate has been used in the development of membranes, including chips designed to mimic fetal membranes at the mother–child interface [38]. Additionally, its gas impermeability makes it suitable for models involving small molecule treatment studies [39].
Using polycarbonate as a gut-on-a-chip material allowed Caco2 cells to form tight junctions. This was proven to be a good model to study the anaerobic bacterium Blautia coccoides, showing that the gas-impermeable properties attributed to polycarbonate effectively prevent oxygen infiltration during chip fabrication and cell culturing [39].
Furthermore, polycarbonate is ideal for DNA heat-cycling applications due to its transparency to visible light and its high glass transition temperature (approximately 145 °C). Its potential for biological applications was further evidenced by a study on RPTEC/HUVEC cell co-culture, where cell viability was significantly higher in polycarbonate chips compared to transwell systems [40,41].
2.3. Polyurethane
Polyurethane is widely used in modern OoC systems due to its biocompatibility, high tensile strength, durability, toughness, resistance to degradation, and flexibility [42,43]. Its flexibility makes it a preferred choice over rigid thermoplastics for systems that replicate soft tissues requiring dynamic strain and stretchability, such as the lungs [44] and heart [45].
To mimic cardiac tissue, researchers have used polyurethane to create nanofibers that orient cells in alignment resembling muscle tissue [46]. A study by Kobuszewska et al. (2021) demonstrated that cell alignment closely followed the nanofiber arrangement, with a maximum deviation of 30° [47].
Another study, by Mitta et al. (2024) [44], replicated lung tissue stiffness using polyurethane membranes to model alveolar epithelial cells. These membranes supported cell proliferation and increased SFTPA1 expression compared to culturing cells on PET, indicating enhanced surfactant production and a closer resemblance to native alveolar cells. Additionally, the membranes withstood breathing-related stresses from both human and mouse models [44].
2.4. Poly-Methyl-Methacrylate (PMMA)
PMMA is a polymer widely used in cell culture, showing excellent compatibility without requiring coatings or surface treatments. This avoids the formation of cytotoxic products that can occur when thermoplastics are exposed to UV light or ozone [48]. Due to its mechanical and optical properties, PMMA serves as an ideal substrate for OoC system fabrication [49]. Another notable advantage of PMMA is its behavior during laser cutting, where it vaporizes into gaseous components. This process ensures clean cuts without burnt edges, preserving optical quality and cell viability [49].
In a study by Schneider et al. (2021) [50], PMMA was used to create a circular peristaltic on-chip pump. Stacked discs formed a channel where HUVEC cells were seeded, demonstrating good viability and CD31 expression. PMMA’s rigidity contributed to the stability and durability of the pump system [50]. However, since PMMA is impermeable to gases, OoC system channels made with this material should be short to allow adequate oxygen diffusion through the inlet and outlet [48].
2.5. Cellulose
Cellulose-based scaffolds for tissue engineering have gained attention in recent years due to their high-water retention, biocompatibility, and natural origin, offering environmentally friendly alternatives to petroleum-derived polymers [51,52].
In OoC systems, cellulose has shown promising results as a surface for cell growth, despite its limited flexibility, durability, and strength [53]. To address these limitations, cellulose membranes are often combined with other materials, such as PDMS, acetate, and PMMA [53,54].
Li et al. (2022) [54] used cellulose alongside PMMA in 3D-printed scaffolds to evaluate the viability of multiple cell types in organ-like structures. After seven days in culture, HepG2, HUVECs, AS49, and HK-2 cells demonstrated survival rates above 90% and expressed proteins related to their specific functions, as confirmed by immunofluorescence analysis [54].
Moreover, Hospodiuk-Karwowsk et al. (2022) [55] developed a pancreas-on-a-chip model using carboxymethyl bacterial cellulose to assess cell viability, insulin production, and the expression of pancreatic markers. Cells cultured with cellulose-containing hydrogels exhibited the highest insulin production, up to four times greater than other treatments [55].
2.6. Chitosan
Chitosan is a polysaccharide obtained from the complete or partial N-deacetylation of chitin, and it is particularly suitable for creating membranes and scaffolds under conditions that are not overly harsh and noncytotoxic, making it an ideal choice for OoC systems [56,57,58].
For instance, Tibbe et al. (2018) [56] developed a microfluidic chip, including a temporary chitosan-based membrane to separate astrocytes from endothelial cells but allowing direct cell–cell contact between the cell types [56]. Since chitosan is a polysaccharide that forms a gel-like solid upon deprotonation, by creating an interface between a basic buffer solution and an acidic chitosan solution, the chitosan gets deprotonated at the interface, forming the membrane, which acts as a physical barrier for cell culture and can be removed afterwards by flushing an acidic solution. The researchers added an immunofluorescent cell tracker to follow astrocyte position and morphology, and none of them were influenced by the removal of the membrane. According to the researchers, this possibility of membrane fabrication and removal can be used to create membrane-free co-cultures in a microfluidic device for OoC research.
Chitosan has also shown good results when used in a composite with alginate [58,59,60]. Upadhyay et al. (2024) [58] used a combination of chitosan and alginate biopolymers and decellularized ECM (dECM) as a bioink additive in the development of scalable OoC using a microfluidic platform. The bioink was tested with native chondrocytes and mesenchymal stem-cell-induced chondrocytes using biopolymers of alginate and chitosan composite hydrogels [58]. They used 2D and 3D biomimetic tissue construction approaches to characterize several aspects of cells and found that the bioink significantly increased both phenotypic and genotypic expression, with a statistical significance level of p < 0.05.
In another study by Chiu et al. (2012), a chitosan–alginate composite was used to make a micropattern of arrays on a chip for myocardium modeling [57]. The results showed that cardiomyocytes, cultured on gels featuring 10 μm-wide grooves, successfully formed a well-structured 3D beating tissue concerning those in the smooth surface [49]. Given the perpendicular orientation of the grooves to the electrodes, the cells were able to align with the electric field lines, thereby experiencing the maximum voltage differential.
2.7. Alginate
Alginates are polysaccharides composed of β-d-mannuronic acid (M) and α-l-guluronic acid (G), organized in homogenous blocks (-GGG- and -MMM-) or alternating blocks (-MGMG-), with a G content ranging from 30% to 70%, depending on the species and part of the marine algae of origin [61]. Their biocompatibility, non-toxicity, hydrophilicity, ease of blending, and low immunogenicity make them widely used biomaterials in tissue engineering, with applications such as wound healing, bone graft substitutes, cell therapy, and ventricular support in dilated cardiomyopathy [61,62,63].
Pangjantuk et al. (2024) reported that three-dimensional hydrogels of alginate and hyaluronic acid (AL-HA) promote cell proliferation, enabling the cultivation of cells in spheroids within the hydrogel matrix [64]. The results showed a high cell survival rate, reaching 77.36% over a 14-day cultivation period. This 3D culture approach proved effective in maintaining the viability and functionality of human mesenchymal stem cells, highlighting its potential for biomedical applications.
Once crosslinked with Ca2+, sodium alginate hydrogel exhibits high mechanical strength and convenient elasticity, making it an ideal support material for 3D cell cultures in vitro. However, to better mimic the ECM and enhance its biocompatibility, it needs to be combined with other natural polymers [65].
The incorporation of agarose, gelatin, and fibrinogen into alginate hydrogels can reduce the pore size of the matrix, providing a greater number of binding sites for cells, resulting in increased cell survival and proliferation capacity [65]. However, moderate addition is crucial, as excessive amounts may overly reduce pore size, limiting cell proliferation and migration, as well as diffusion of nutrients and waste metabolites. Optimized alginate-based composite hydrogels with an appropriate microstructure enhance cell survival and increase cell density, reduce culture costs, and create a favorable environment for cellular interactions, ultimately promoting the formation of tissues or organs [65,66,67].
3. Anchorage Dependence as a Property and the Role of the Substrate
Anchorage dependence is a property of cells implying their requirement to be attached to a surface to survive and proliferate and is also a critical factor in determining the shape, behavior, and fate of cells.
Cells of most animal tissues are attached together and to the extracellular matrix (ECM) [68]. The ECM is a dynamic scaffolding system composed of multiple macromolecules organized in a tissue-specific manner that contributes to the microenvironment and homeostasis of the cells. The components of the ECM integrate in a structurally stable composite, contributing to the mechanical properties of the tissue, which is likewise required for tissue regeneration and other biological events [69,70,71].
It has been shown that the spatial distribution of the ECM helps guide the orientation of the cell division axis, and it also plays an important role in mechanical stimuli, thanks to the activation of mechanical sensors like integrins [69,70,72].
The composition of the ECM varies according to the type of cells, tissues, and organs, but it is usually composed of complex proteins and carbohydrates, including collagens, fibronectin, laminin, and hyaluronic acid proteoglycans and glycosaminoglycans, among others [68,73]. The interaction between collagen and the cellular scaffold is essential for maintaining tissue integrity and promoting cell survival. Additionally, collagens influence cell behavior by transmitting biochemical signals that regulate cell proliferation, differentiation, endurance, and continuity [74].
In bioengineering, the ECM is exploited in several ways, mostly as scaffolds and coatings, e.g., to improve cell adhesion, to promote tissue regeneration in medical applications, and to create in vitro models for physiological study, preclinical testing, and personalized medicine [17,74,75,76,77,78].
4. Hydrogels as Biocompatibility-Enhancing Coatings
The properties of biocompatibility and biodegradability displayed by the components of the ECM, including hydrogels like the above-mentioned collagen, fibronectin, hyaluronic acid, and others, are currently exploited in a variety of applications. Hydrogels are pivotal in enhancing OoC applications due to their capacity of improving cell adhesion, proliferation, migration, and overall biocompatibility. These water-swollen polymers provide a supportive, hydrated environment that facilitates nutrient and waste exchange, critical for cell viability and function [17,26,74,75,76,77,78]. By tailoring the biochemical and physical properties of hydrogels, researchers can create specific microenvironments that promote the desired cellular behaviors, such as enhanced adhesion and proliferation [79]. Additionally, hydrogels can be engineered to include bioactive molecules, such as growth factors, which further support cell differentiation and physiology, ultimately leading to tissue formation.
Coating substrates with hydrogels can be achieved through various techniques, such as dip coating, spraying, and spin coating, allowing for the utilization of their unique properties, including lubricity, biocompatibility, and antifouling resistance. These characteristics, combined with the specific mechanical properties of the substrate, such as stiffness, toughness, and strength, provide great versatility and advantages in multiple applications [80]. In particular, the application of hydrogels in OoC systems is highly beneficial, as it enables the replication of complex, dynamic interactions, similar to those of living tissues, which is essential for accurately modeling physiological and pathological processes [81]. Additionally, recent studies have demonstrated that biocompatible hydrogel coatings can significantly enhance the antifouling and lubricious properties of materials commonly used in medical and biomedical devices, further supporting their potential for improving OoC materials [82].
5. Molecules Used to Resemble ECM
5.1. Collagen
Collagen (I to XXVIII) is a structural protein present throughout the human body, conformed by three polypeptide fibrils that form a triple helix structure, with a diameter of 10–500 nm each and a molecular weight around 285 kDa [40]. Even though there are 28 collagen types identified today, the most abundant in the human body are collagen I, II, and III [40,83,84].
In bioengineering, collagen has been widely used to mimic the ECM in scaffolds for tissue regeneration [25] and organs-on-a-chip [20,21,85] due to its mechanical strength and cellular compatibility, resembling the natural environment of tissues [2]. Epithelial cells are typically seeded and cultured on collagen coatings, in organ-on-a-chip (OoC) models, as well as in implants, tissue engineering constructs, and other scaffolds [86,87,88]. This is due to the ubiquity and abundance of collagen in human tissues, making it an ideal substrate for cell attachment and proliferation.
Jeon et al. (2020) used collagen I alongside Poly-D-Lysine in a microfluidic chip to improve cell and collagen adhesion; then, they compared the differentiation grade under static and flow conditions and looked for the establishment of a collagen-rich basal membrane separating the cell types successfully [21]. The results showed that the combination of three factors (the presence of collagen, co-culture with endothelial cells, and maintenance under flow conditions) led to a high differentiation rate and a strong barrier of CaCO2 epithelial cells. More specifically, the latter led to an increase in the length of the villi expressed by the cells, with a peak of over 35 μm in size and an impedance of 59 Ω·cm2.
In another study, collagen membranes were made for OoC engineering using ARPE-19 epithelial cells and human pluripotent stem-cell-derived endothelial cells (hiPSC-ECs) and showed expression of adhesion markers (VE-cadherin for hiPSC-ECs and ZO-1 for ARPE-19) alongside the formation of monolayers [83].
Additionally, collagen has been shown to improve stability on cell viability when used as polymers for epithelial cells, such as CaCO2, and to increase the expression of epithelial barrier markers [89]. In a study by Wang et al. (2018), they compared the viability of cells seeded in chips without collagen with transwell plates and with collagen membranes, and despite that no significant changes were seen between the three treatments regarding viability, collagen showed the most stable results [89]. In the same study, they compared tight junction protein expression, F-actin, and ezrin in cells using ZO-1, phalloidin, and ezrin staining, and for all markers, the fluorescence intensity was significantly higher when using collagen. Epithelial cells are the ones that are seeded and reproduced in collagen coatings, due to the abundance of said protein in these kinds of tissues in humans.
5.2. Matrigel
Matrigel, extracted from the Engelbreth–Holm–Swarm mouse sarcoma, is a gelatinous protein mixture used in cell culture, mainly composed of laminin, collagen IV, entactin, and heparin sulfate proteoglycan perlecan [30]. Despite not being used in a clinical way due to its tumoral origin, it is widely used in in vitro models and has shown great success in 3D culturing [90]. This coating is especially, but not restricted to, being used in tumor cell research, because it provides a supportive microenvironment that facilitates the growth, differentiation, and three-dimensional organization of tumor cells. This enables the modeling of invasive behaviors characteristic of cancer cells. For instance, Passaniti et al. (2022) [91] compiled evidence on how co-injecting tumor cells with Matrigel enhances tumor take and growth, allowing for the development of animal models that closely mimic human cancers [91].
Furthermore, Matrigel supports the formation of tumor spheroids, which are three-dimensional aggregates of tumor cells that replicate the architecture of solid tumors in vivo. The addition of Matrigel improves the growth environment of these spheroids, making them more representative of in vivo tumor conditions [92].
Examples of devices coated with Matrigel include items for a heart-on-chip, where this substance was used with hiPSC-CMs because of showing the best proliferative activity and viability, by exhibiting 94.3 ± 0.3% and 98.2 ± 0.2% living/dead ratios under static and dynamic conditions, respectively [28].
Matrigel offers biocompatibility, mimics native tissues, and finds applications in OoC technology, including microfluidic devices, drug delivery research, toxicology studies, and multi-organ systems [5,93]. Researchers appreciate its natural composition and ability to replicate under in vivo conditions.
In a study by Carvalho et al. (2019), Matrigel contributed to the endothelial invasion thanks to its vascular endothelial growth factor (VEGF), for which the cells seemed to follow a chemotactic signal toward the Matrigel source even though cell migration from the endothelium is not physically constrained in any direction by their system design [94]. Moreover, Dolega et al. (2015) showed Matrigel to promote clusters of RWPE1 prostate cancer cells within 6 to 8 days [95].
5.3. Fibrin and Blood Plasma
Fibrin is a biopolymer formed from fibrinogen when stimulated with thrombin, which cleaves fibrinopeptides from fibrinogen, producing fibrin fibers that assemble in a crosslinked mesh [96]. This mechanism proceeds by exposing a polymerization site with a binding pocket to form an association, causing double-stranded twisting fibrils [97]. Various crosslinks form, conferring mechanical and elastic properties, giving fibrin structural integrity, and stabilizing the clot [96,97]. When in soluble form as fibrinogen, it is part of the blood plasma alongside other components, such as thrombin, fibronectin, fibrinolysis inhibitors, molecules of cell adhesion [98], as well as platelets and growth factors.
Both fibrin and blood plasma have shown promising results for tissue regeneration and cell proliferation [97,99]. In a study by Yang et al. (2020) [97], cell viability was assessed using an MTT assay (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), which is a colorimetric test that measures cell viability and is also used to study cell proliferation and cytotoxicity. Stem cells were cultured on polycaprolactone (PCL)/fibrin scaffolds, in proportions varying from 0:100 to 30:70, respectively, finding that fibrin could effectively reduce the cytotoxicity of PCL [97]. In another study, rat cardiomyocytes were seeded in a PDMS chip with a fibrin/collagen hydrogel with a proportion of 85:10, in which this, alongside a specific array design of posts in the scaffold, allowed the expression of connexin 43, correct alignment of sarcomeres, optimal width and alignment of human stem-cell-derived cardiac fibers, and spontaneous contractile behavior [99].
In a study regarding blood plasma scaffolds, when put together with collagen, adipocyte stem cells showed proliferative activity, increasing by 1.23 times their number after 72 h of culture [97].
5.4. Fibronectin
Fibronectin is a glycoprotein with a size of 230 to 270 kDa consisting of monomers classified as type I, II, or III [100]. This glycoprotein can be produced either by hepatocytes, in which case it is called plasma fibronectin, or by fibroblasts, chondrocytes, myocytes, and synovial cells, where it is known as cellular fibronectin [100,101].
This molecule plays an important role in cell adhesion and migration, transmitting also mechanical and biochemical stimuli for cell morphogenesis, as it is part of the extracellular matrix, mainly on endothelial cells [102,103]. Because of this, fibronectin has been used as a coating for organ-on-a-chip engineering [19,23,104] and cell culture [105,106].
In a study by Bas Cristóbal-Menéndez et al. (2022) [23], fibronectin coating in channels where HUVECs were seeded alongside a kidney organoid increased the total area of presence of the melanoma cell adhesion module (MCAM+) and platelet endothelial cell adhesion molecule (PECAM+) immunofluorescence staining and their mutual colocalization compared to what was seen in a transwell plate [23]. Both markers are used as signals of endothelization, related directly to the main goal of the study, which was promoting vascularization on the kidney organoids. This result may not be directly related to the coating, but mostly to the contact between the organoid and the HUVEC cells. Nevertheless, fibronectin coating has been shown to improve adhesion of integrin-expressing cells [107,108], such as HUVECs [108].
Fibronectin was also used for a liver-on-a-chip platform, in which Xie et al. (2021) showed viability above 80% for both HepG2 cells and HUVECs [104]. Also, in the same study, HUVECs’ nuclei showed a homogeneous distribution while having a high expression of CD31 and VE-cadherin, which indicates the formation of a functional endothelial barrier [14,16,44,45,59].
5.5. Decellularized Extracellular Matrix
In an OoC system, decellularized ECM (dECM) is frequently paired with primary or stem cells to simulate organ-specific functions [109]. This approach allows researchers to develop models that more accurately mimic human physiological responses to drugs, materials, diseases, or other variables, surpassing the predictive capabilities of conventional 2D cell culture systems [110,111].
This coating is used to produce bioinks that recreate tissue-specific microenvironments. Combined with 3D-printing technology, they enable the precise and reproducible fabrication of platforms [112]. Bioinks involve formulations that blend decellularized matrix materials with biocompatible substances, creating a medium that supports cell growth while mimicking the native tissue environment [113,114,115]. These bioinks allow 3D printing of complex, highly accurate structures, such as vascular networks or the layered organization of organs [116].
The utility of bioprinting and the specificity and biological influence of the ECM are evident in studies like the one of Kim et al. When comparing dECM and collagen as coatings for a PDMS chip to develop a multi-OoC system to study type 2 diabetes (T2D), their dECM successfully replicated T2D pathological conditions, downregulating pIRS-1, pAMPK, and GLUT-4 in adipose tissue, and GLUT-2 in the pancreas and liver, mirroring impaired glucose signaling seen in diabetes. In contrast, collagen increased IRS-1 and AMPK phosphorylation and enhanced GLUT-2 and GLUT-4 expression, indicating a response to glucose availability rather than the metabolic dysfunction typical of diabetes [112]. (Check Figure 1 and Table 1 for more details on hydrogels used as coatings).
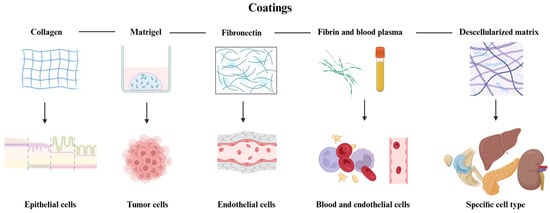
Figure 1.
Representation of the coating hydrogels reviewed in this paper. As shown, collagen is usually used for experiments with epithelia, Matrigel for tumors (due to its cancerous origin), fibronectin is used for endothelium and vascularity experiments, while fibrin and blood plasma are commonly used in studies involving blood cells and endothelium. Decellularized ECM is used to support the growth of several cell types, depending on the tissue of origin. Created in BioRender. Ramirez, G. (2025): https://BioRender.com/r96y578.

Table 1.
Evaluation of some cell scaffold and coating materials, and their influence on cell behavior.
6. Addition of Coatings on Chips
Although there are many techniques for applying coatings to biomaterials, for OoC purposes some are more commonly used than others due, for example, to the way the chips are prepared or assembled. Nevertheless, OoC represents a promising alternative to evaluate bioactive surface coatings based on stimuli-triggerable approaches, the incorporation of bioactive compounds in drug-releasing strategies, or to provide corrosive resistivity, anti-inflammatory, and antimicrobial properties to medical devices.
OoC often relies heavily on the coating using hydrogels to ensure its success, simply to allow cell attachment or even to enhance cellular behaviors and improve the biomimicry of the device [93]. The selection and application of coatings are crucial for creating a supportive environment for cells and ensuring the functionality of the OoC (see Table 2 for the main aspects of common techniques). The coating procedure must also consider the sterilization method and the stability of the molecules used.
6.1. Self-Assembled Monolayers (SAMs)
Self-assembled monolayers (SAMs) are highly organized layers formed by the adsorption of molecules onto a substrate [118]. This technique allows precise control over the chemical functionality of the surface, making it possible to tailor the surface properties to promote specific cell behaviors [119]. SAMs are typically used for their ease of application and the ability to create uniform, stable coatings that can be modified to include bioactive molecules [118,120].
Maoz et al. (2017) [121] used SAMs for the creation of the transepithelial electrical resistance with multielectrode array chips, allowing for the optimization of interactions between materials and cultured cells. SAMs were formed using APTES (3-aminopropyltriethoxysilane) and GLYMO (3-glycidoxypropyltrimethoxysilane). The process begins with an oxygen plasma treatment, promoting the activation of the surfaces of the materials used in the device, including PDMS, polyethylene terephthalate, polycarbonate, and silicon nitride [121]. According to the researchers, this step generates reactive functional groups on the surfaces, facilitating the chemical adhesion of the coating, forming stable and uniform monolayers.
SAMs derived from APTES provided reactive amino groups (-NH2), while those generated with GLYMO introduced epoxy groups on the surface, enhancing the adhesion of biomolecules and the formation of covalent bonds between the functionalized layers [122].
6.2. Layer-by-Layer (LbL) Assembly
The layer-by-layer (LbL) assembly technique involves the sequential adsorption of oppositely charged polyelectrolytes onto a substrate [123]. This method enables the fabrication of multilayered coatings with nanoscale precision with the possibility of incorporating a variety of materials, including proteins, peptides, and nanoparticles [124,125]. This technique is particularly useful for creating complex, multifunctional surfaces that can mimic the ECM [126].
Aor et al. (2020) made a chip consisting of multiple layers of chitosan and hyaluronic acid over polycarbonate, on top of which HUVECs were seeded [127]. These cells were able to initiate tubulogenesis thanks to the micropatterned platform.
6.3. Spin Coating
For this technique, a solution of the coating material is deposited onto a substrate, which is then spun at high speed to spread the material uniformly. This method is commonly used for applying thin polymer films of ECM proteins or synthetic polymers that support cell attachment and growth and is valued for its simplicity and ability to produce highly uniform coatings [128,129,130,131].
Quirós-Solano (2018) et al. spin-coated transferable porous PDMS membranes to produce OoCs [29]. This approach aimed to overcome the limitations associated with replica molding, expanding the applicability of most OoCs while improving their scalability and reproducibility. The membranes proved to be biocompatible and suitable for cell culture, as demonstrated by the successful growth of HUVECs and MDA-MB-231 (MDA) cells.
6.4. Dip Coating
Dip coating involves immersing the substrate into a solution of the coating material and then withdrawing it at a controlled speed, allowing a thin film to form as the solvent evaporates [132]. This technique is advantageous for its ability to coat substrates with complex geometries and its suitability for large-scale production [133]. The dip coating is often employed for the deposition of hydrogels and other biocompatible materials that provide a supportive matrix for cells [134].
For example, to protect sugar fibers from early dissolution during cell seeding and the hydrogel casting process, Nie et al. (2024) made a hydrophobic protective coating using this technique, which was convenient because of the complexity on the geometry of the scaffolds [135].
6.5. Microcontact Printing
Microcontact printing uses a patterned stamp to transfer molecules onto a substrate [113,136,137]. This method allows precise spatial control over the deposition of the coating material, making it possible to create micropatterned surfaces that guide cell organization and function, making this technique particularly useful for applications that require the creation of specific cellular microenvironments within the OoC.
Sun et al. (2024) made (in just 8 min) a geometric pattern in a PDMS ink spin-coated film and then tested its efficacy as a OoC platform with MCF-7 breast adenocarcinoma cells [136]. The results showed that these cells restricted their growth to the designated areas of the structure in a 3D-specific arrangement, as is usually seen in tumors. This presents microcontact patterning as an efficient technique for mass production of micro-arrangements required for OoC platforms.
6.6. 3D Bioprinting
Three-dimensional bioprinting enables the simultaneous deposition of biocompatible materials and living cells, incorporating physiologically relevant features, such as precise cellular arrangements, to replicate cellular diversity and microstructure with consistency [138]. Common techniques include extrusion-based bioprinting, micro-extrusion, inkjet bioprinting, stereolithography (SLA), and laser-induced forward transfer (LIFT) [74,113,139,140].
Extrusion-based bioprinting is particularly effective for constructing cell-laden structures in tissue engineering, ensuring fabrication precision and cell viability [139]. This method involves the layer-by-layer deposition of bioink filaments extruded continuously through a nozzle [141]. These systems typically include a material reservoir, print head, movable printing platform, and a positioning mechanism for precise movement along the x, y, and z axes [139].
Inkjet bioprinting, on the other hand, employs nozzles activated by physical stimuli to eject tiny droplets of bioink onto a substrate, forming intricate 3D tissue structures. This technique excels in high-resolution printing and precise cell placement [142,143].
The printability of biomaterials depends on their mechanical properties, including rheological behavior, bioactive components, and degradation characteristics [138]. For instance, Abudupataer et al. (2019) [144] demonstrated the use of bioprinters to deposit a bioink containing endothelial cells, smooth muscle cells, and gelatin methacryloyl in a microfluidic chip. The printed cells showed high viability and increased vascular protein expression compared to traditional 2D cell culture methods [144]. Furthermore, Upadhyay et al. (2024) demonstrated that bioink loaded with cell-containing biopolymers significantly enhanced phenotypic and genotypic expression, optimizing conditions for hyaline cartilage development in culture [58]. This advancement facilitated the study of mechanosensitive properties in microfluidic cartilage-on-a-chip systems.
7. Comparative Analysis of Coating Techniques
The choice of coating technique depends on several factors, including the desired properties of the coating, the type of substance, the sterilization method and cells being used, as well as the specific application of the OoC. Each technique offers unique advantages and limitations (Check Table 2 and Figure 2 for further details).
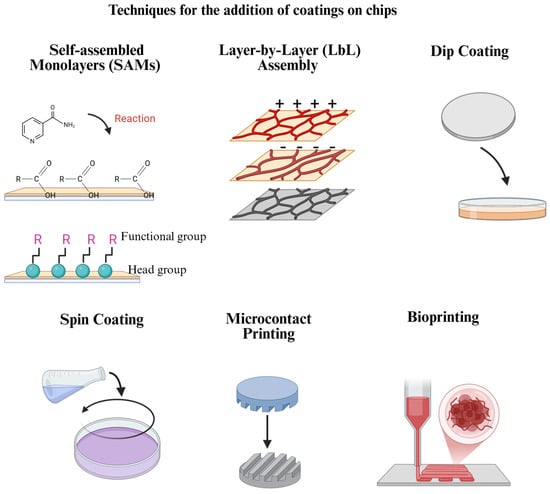
Figure 2.
Frequently used coating addition techniques for OoC platforms. Each technique has advantages and limitations in terms of costs, coating stability, reproducibility, and other aspects, which must be considered when selecting the one to use. Created in BioRender. Ramirez, G. (2025): https://BioRender.com/q32n598.

Table 2.
Techniques used for coating addition on chips.
Table 2.
Techniques used for coating addition on chips.
| Technique | Advantages | Limitations | References |
|---|---|---|---|
| Self-assembled monolayers (SAMs) | Provides precise control over surface chemistry. | Limited possibility to create thick coatings. | [118] |
| Layer-by-layer (LbL) assembly | Offers versatility and the ability to create complex, multifunctional surfaces combining several polymers. | Can be time-consuming and requires multiple steps. | [122,127] |
| Spin coating | Produces uniform thin films. | May not be suitable for substrates with complex geometries. | [11,128] |
| Dip coating | Suitable for complex geometries and large-scale production. | May result in less uniform coatings, which can, in turn, affect the results and reproducibility. | [122,132] |
| Microcontact printing | Allows for precise patterning. | Limited by the resolution of the stamp and the complexity of the patterns that can be achieved. | [136] |
| 3D Bioprinting | 3D bioprinting enables the precise arrangement of cells and biomaterials, replicating native tissue structures with high fidelity. | Requires bioinks with appropriate mechanical, rheological, and degradation properties. | [144] |
8. Challenges and Future
The main difficulties of developing an OoC platform are mostly related to the simulation of a physiological microenvironment while ensuring system functionality, reproducibility, and cost-effectiveness [145,146]. An example related to the functionality and reliability of materials used for OoC platforms is PDMS, as this material tends to absorb small hydrophobic molecules, which can interfere with drug testing, and the cells within the chip may not accurately display the same response as seen in vivo [147].
One of the primary challenges is biocompatibility, as materials must be noncytotoxic and with a low to non-immunogenic response [148,149]. Beyond compatibility, materials need to replicate the mechanical, chemical, and physical properties of native tissues, such as elasticity, porosity, and surface characteristics [150,151]. Hydrogels are promising for mimicking soft tissues but often lack the mechanical stability required for long-term applications [151,152].
The integration of sensors into OoC systems poses additional complexity, as materials must accommodate electrodes or optical tools for real-time monitoring without introducing artifacts or compromising material properties [153].
One specific challenge in coatings is achieving reproducibility and scalability in the distribution and thickness of the coating layer. In large-scale manufacturing, ensuring that each chip has a uniform coating thickness can be time-consuming and resource-intensive. Variations in coating thickness can lead to inconsistencies in product functionality, potentially resulting in higher rejection rates and increased manufacturing costs and losses.
New research regarding OoC is leading to the search for new materials and methods of fabrication that ensure a more precise simulation of the desired organ, as well as better reproducibility while reducing the costs of manufacturing [154,155,156]. One example is 3D bioprinting, such as the technique used by Conceição et al. (2022) [157], which was an innovative three-dimensional, printing-based, multi-compartment microfluidic platform that allowed both selective and dynamic multicellular paracrine signaling between sympathetic neurons, bone tropic breast cancer cells, and osteoclasts cultured together to make a metastatic bone cancer model [157].
Funding
This review article was written in the context of the INNOrganic research project: “INNovation in ORGANIC study models for preclinical testing” (CF 1510175). The project and the APC were funded by the Vice-Rectorate for Research and Outreach, Instituto Tecnológico de Costa Rica.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. OnLine 2020, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Ding, H.; Gao, B.; He, Z.; Gu, Z. Advances of Microfluidics in Biomedical Engineering. Adv. Mater. Technol. 2019, 4, 1800663. [Google Scholar] [CrossRef]
- Sung, J.H.; Shuler, M.L. A micro cell culture analog (µCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip 2009, 9, 1385–1394. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Wang, Y.; Wang, J.; Tu, Q.; Liu, R.; Wang, J. Construction of oxygen and chemical concentration gradients in a single microfluidic device for studying tumor cell–drug interactions in a dynamic hypoxia microenvironment. Lab Chip 2013, 13, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Cameron, T.C.; Randhawa, A.; Grist, S.M.; Bennet, T.; Hua, J.; Alde, L.G.; Caffrey, T.M.; Wellington, C.L.; Cheung, K.C. PDMS Organ-On-Chip Design and Fabrication: Strategies for Improving Fluidic Integration and Chip Robustness of Rapidly Prototyped Microfluidic In Vitro Models. Micromachines 2022, 13, 1573. [Google Scholar] [CrossRef]
- Schneider, S.; Brás, E.J.S.; Schneider, O.; Schlünder, K.; Loskill, P. Facile Patterning of Thermoplastic Elastomers and Robust Bonding to Glass and Thermoplastics for Microfluidic Cell Culture and Organ-on-Chip. Micromachines 2021, 12, 575. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.; Shao, Y.; Wang, C.; Xue, F.; Chu, C.; Gu, Z.; Chen, Z.; Bai, J. Next-Generation Approaches for Biomedical Materials Evaluation: Microfluidics and Organ-on-a-Chip Technologies. Adv. Healthc. Mater. 2025, 14, 2402611. [Google Scholar] [CrossRef]
- Lu, C.; Jin, A.; Gao, C.; Qiao, H.; Liu, H.; Zhang, Y.; Sun, W.; Yang, S.-M.; Liu, Y. Synergistic Approach of High-Precision 3D Printing and Low Cell Adhesion for Enhanced Self-Assembled Spheroid Formation. Biosensors 2025, 15, 7. [Google Scholar] [CrossRef]
- Menagadevi, M.; Nirmala, M.; Thiyagarajan, D.; Somasundram, D. Biomaterials and Their Applications. Biomed. Mater. Devices 2024. [Google Scholar] [CrossRef]
- Singh, R.; Bathaei, M.J.; Istif, E.; Beker, L. A Review of Bioresorbable Implantable Medical Devices: Materials, Fabrication, and Implementation. Adv. Healthc. Mater. 2020, 9, e2000790. [Google Scholar] [CrossRef]
- Abulaiti, M.; Yalikun, Y.; Murata, K.; Sato, A.; Sami, M.M.; Sasaki, Y.; Fujiwara, Y.; Minatoya, K.; Shiba, Y.; Tanaka, Y.; et al. Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function. Sci. Rep. 2020, 10, 19201. [Google Scholar] [CrossRef] [PubMed]
- Oleksy, M.; Dynarowicz, K.; Aebisher, D. Advances in Biodegradable Polymers and Biomaterials for Medical Applications—A Review. Molecules 2023, 28, 6213. [Google Scholar] [CrossRef]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Thenuwara, G.; Javed, B.; Singh, B.; Tian, F. Biosensor-Enhanced Organ-on-a-Chip Models for Investigating Glioblastoma Tumor Microenvironment Dynamics. Sensors 2024, 24, 2865. [Google Scholar] [CrossRef]
- Wang, Y.; Yung, P.; Lu, G.; Liu, Y.; Ding, C.; Mao, C.; Li, Z.A.; Tuan, R.S. Musculoskeletal Organs-on-Chips: An Emerging Platform for Studying the Nanotechnology–Biology Interface. Adv. Mater. 2025, 37, e2401334. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, T.; Huang, Q.; Wang, Y. From Organ-on-a-Chip to Human-on-a-Chip: A Review of Research Progress and Latest Applications. ACS Sens. 2024, 9, 3466–3488. [Google Scholar] [CrossRef] [PubMed]
- Choe, A.; Ha, S.K.; Choi, I.; Choi, N.; Sung, J.H. Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed. Microdevices 2017, 19, 4. [Google Scholar] [CrossRef]
- Du, K.; Li, S.; Li, C.; Li, P.; Miao, C.; Luo, T.; Qiu, B.; Ding, W. Modeling nonalcoholic fatty liver disease on a liver lobule chip with dual blood supply. Acta Biomater. 2021, 134, 228–239. [Google Scholar] [CrossRef]
- Gori, M.; Simonelli, M.C.; Giannitelli, S.M.; Businaro, L.; Trombetta, M.; Rainer, A. Investigating Nonalcoholic Fatty Liver Disease in a Liver-on-a-Chip Microfluidic Device. PLoS ONE 2016, 11, e0159729. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Z.; Su, W.; Wang, L.; Zhu, Y.; Qin, J. A Biomimetic Human Gut-on-a-Chip for Modeling Drug Metabolism in Intestine. Artif. Organs 2018, 42, 1196–1205. [Google Scholar] [CrossRef]
- Jeon, M.S.; Choi, Y.Y.; Mo, S.J.; Ha, J.H.; Lee, Y.S.; Lee, H.U.; Park, S.D.; Shim, J.-J.; Lee, J.-L.; Chung, B.G. Contributions of the microbiome to intestinal inflammation in a gut-on-a-chip. Nano Converg. 2022, 9, 8. [Google Scholar] [CrossRef]
- Boquet-Pujadas, A.; Feaugas, T.; Petracchini, A.; Grassart, A.; Mary, H.; Manich, M.; Gobaa, S.; Olivo-Marin, J.-C.; Sauvonnet, N.; Labruyère, E. 4D live imaging and computational modeling of a functional gut-on-a-chip evaluate how peristalsis facilitates enteric pathogen invasion. Sci. Adv. 2022, 8, eabo5767. [Google Scholar] [CrossRef]
- Bas-Cristóbal Menéndez, A.; Du, Z.; van den Bosch, T.P.P.; Othman, A.; Gaio, N.; Silvestri, C.; Quirós, W.; Lin, H.; Korevaar, S.; Merino, A.; et al. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci. Rep. 2022, 12, 20699. [Google Scholar] [CrossRef] [PubMed]
- Piergiovanni, M.; Leite, S.B.; Corvi, R.; Whelan, M. Standardisation needs for organ on chip devices. Lab Chip 2021, 21, 2857–2868. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Lu, L.; He, Q.; Xi, B.; Yu, H.; Luo, R.; Wang, Y.; Zhang, X. A tailored extracellular matrix (ECM)—Mimetic coating for cardiovascular stents by stepwise assembly of hyaluronic acid and recombinant human type III collagen. Biomaterials 2021, 276, 121055. [Google Scholar] [CrossRef] [PubMed]
- Akther, F.; Little, P.; Li, Z.; Nguyen, N.-T.; Ta, H.T. Hydrogels as artificial matrices for cell seeding in microfluidic devices. RSC Adv. 2020, 10, 43682–43703. [Google Scholar] [CrossRef]
- Jakus, A.E.; Laronda, M.M.; Rashedi, A.S.; Robinson, C.M.; Lee, C.; Jordan, S.W.; Orwig, K.E.; Woodruff, T.K.; Shah, R.N. “Tissue Papers” from Organ-Specific Decellularized Extracellular Matrices. Adv. Funct. Mater. 2017, 27, 1700992. [Google Scholar] [CrossRef]
- Tang, Y.; Tian, F.; Miao, X.; Wu, D.; Wang, Y.; Wang, H.; You, K.; Li, Q.; Zhao, S.; Wang, W. Heart-on-a-chip using human iPSC-derived cardiomyocytes with an integrated vascular endothelial layer based on a culture patch as a potential platform for drug evaluation. Biofabrication 2022, 15, 015010. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Solano, W.F.; Gaio, N.; Stassen, O.M.J.A.; Arik, Y.B.; Silvestri, C.; Van Engeland, N.C.A.; Van der Meer, A.; Passier, R.; Sahlgren, C.M.; Bouten, C.V.C.; et al. Microfabricated tuneable and transferable porous PDMS membranes for Organs-on-Chips. Sci. Rep. 2018, 8, 13524. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Hwang, Y.; Paydar, O.H.; Candler, R.N. 3D printed molds for non-planar PDMS microfluidic channels. Sens. Actuators Phys. 2015, 226, 137–142. [Google Scholar] [CrossRef]
- Cai, L.-H.; Kodger, T.E.; Guerra, R.E.; Pegoraro, A.F.; Rubinstein, M.; Weitz, D.A. Soft Polydimethylsiloxane Elastomers from Architecture-driven Entanglement Free Design. Adv. Mater. 2015, 27, 5132–5140. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; He, J.; Liu, W.; Wang, Y.; Huang, Z.; Pang, H.; Chen, Y. Functional PDMS Elastomers: Bulk Composites, Surface Engineering, and Precision Fabrication. Adv. Sci. 2023, 10, e2304506. [Google Scholar] [CrossRef]
- Kamei, K.; Mashimo, Y.; Koyama, Y.; Fockenberg, C.; Nakashima, M.; Nakajima, M.; Li, J.; Chen, Y. 3D printing of soft lithography mold for rapid production of polydimethylsiloxane-based microfluidic devices for cell stimulation with concentration gradients. Biomed. Microdevices 2015, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Harasek, M.; Gföhler, M. From Soft Lithography to 3D Printing: Current Status and Future of Microfluidic Device Fabrication. Polymers 2025, 17, 455. [Google Scholar] [CrossRef] [PubMed]
- Carius, P.; Weinelt, F.A.; Cantow, C.; Holstein, M.; Teitelbaum, A.M.; Cui, Y. Addressing the ADME Challenges of Compound Loss in a PDMS-Based Gut-on-Chip Microphysiological System. Pharmaceutics 2024, 16, 296. [Google Scholar] [CrossRef]
- Gómez-Gras, G.; Abad, M.D.; Pérez, M.A. Mechanical Performance of 3D-Printed Biocompatible Polycarbonate for Biomechanical Applications. Polymers 2021, 13, 3669. [Google Scholar] [CrossRef]
- Richardson, L.; Kim, S.; Menon, R.; Han, A. Organ-On-Chip Technology: The Future of Feto-Maternal Interface Research? Front. Physiol. 2020, 11, 715. [Google Scholar] [CrossRef]
- Lee, J.-B.; Kim, H.; Kim, S.; Sung, G.Y. Fabrication and Evaluation of Tubule-on-a-Chip with RPTEC/HUVEC Co-Culture Using Injection-Molded Polycarbonate Chips. Micromachines 2022, 13, 1932. [Google Scholar] [CrossRef]
- Rodríguez, M.I.A.; Barroso, L.G.R.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Brasino, D.S.; Speese, S.D.; Schilling, K.; Schutt, C.E.; Barton, M.C. A linkable, polycarbonate gut microbiome-distal tumor chip platform for interrogating cancer promoting mechanisms. Adv. Sci. 2024, 11, 2309220. [Google Scholar] [CrossRef] [PubMed]
- Kado Abdalkader, R.; Konishi, S.; Fujita, T. Development of a flexible 3D printed TPU-PVC microfluidic devices for organ-on-a-chip applications. Sci. Rep. 2025, 15, 6125. [Google Scholar] [CrossRef] [PubMed]
- Karkan, S.F.; Rahbarghazi, R.; Davaran, S.; Kaleybar, L.S.; Khoshfetrat, A.B.; Heidarzadeh, M.; Zolali, E.; Akbarzadeh, A. Electrospun polyurethane/poly (ɛ-caprolactone) nanofibers promoted the attachment and growth of human endothelial cells in static and dynamic culture conditions. Microvasc. Res. 2021, 133, 104073. [Google Scholar] [CrossRef]
- Mitta, E.; Gilmore, A.P.; Malliri, A.; Cartmell, S.H. A stretchable and biomimetic polyurethane membrane for lung alveolar in vitro modelling. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kobuszewska, A.; Kolodziejek, D.; Wojasinski, M.; Ciach, T.; Brzozka, Z.; Jastrzebska, E. Study of Stem Cells Influence on Cardiac Cells Cultured with a Cyanide-P-Trifluoromethoxyphenylhydrazone in Organ-on-a-Chip System. Biosensors 2021, 11, 131. [Google Scholar] [CrossRef]
- Iwoń, Z.; Krogulec, E.; Kierlańczyk, A.; Baranowska, P.; Łopianiak, I.; Wojasiński, M.; Jastrzębska, E. Improving rodents and humans cardiac cell maturity in vitro through polycaprolactone and polyurethane nanofibers. Biomed. Mater. 2024, 19, 025031. [Google Scholar] [CrossRef] [PubMed]
- Kobuszewska, A.; Kolodziejek, D.; Wojasinski, M.; Jastrzebska, E.; Ciach, T.; Brzozka, Z. Lab-on-a-chip system integrated with nanofiber mats used as a potential tool to study cardiovascular diseases (CVDs). Sens. Actuators B Chem. 2021, 330, 129291. [Google Scholar] [CrossRef]
- Persson, H.; Park, S.; Mohan, M.; Cheung, K.K.; Simmons, C.A.; Young, E.W.K. Rapid assembly of PMMA microfluidic devices with PETE membranes for studying the endothelium. Sens. Actuators B Chem. 2022, 356, 131342. [Google Scholar] [CrossRef]
- Busek, M.; Nøvik, S.; Aizenshtadt, A.; Amirola-Martinez, M.; Combriat, T.; Grünzner, S.; Krauss, S. Thermoplastic Elastomer (TPE)–Poly(Methyl Methacrylate) (PMMA) Hybrid Devices for Active Pumping PDMS-Free Organ-on-a-Chip Systems. Biosensors 2021, 11, 162. [Google Scholar] [CrossRef]
- Schneider, S.; Bubeck, M.; Rogal, J.; Weener, H.J.; Rojas, C.; Weiss, M.; Heymann, M.; van der Meer, A.D.; Loskill, P. Peristaltic on-chip pump for tunable media circulation and whole blood perfusion in PDMS-free organ-on-chip and Organ-Disc systems. Lab Chip 2021, 21, 3963–3978. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Cellulose-Based Composites as Scaffolds for Tissue Engineering: Recent Advances. Molecules 2022, 27, 8830. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Cellulose/calcium phosphate hybrids: New materials for biomedical and environmental applications. Int. J. Biol. Macromol. 2019, 127, 606–617. [Google Scholar] [CrossRef]
- Sathe, T.; Bodas, D. Development and characterization of a polydimethylsiloxane-cellulose acetate hybrid membrane for application in organ-on-a-chip. Mater. Sci. Eng. B 2023, 291, 116366. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Wang, Z.; Li, W.; Antonio Robledo-Lara, J.; Shrike Zhang, Y. 3D-printed, configurable, paper-based, and autonomous multi-organ-on-paper platforms. Mol. Syst. Des. Eng. 2022, 7, 1538–1548. [Google Scholar] [CrossRef]
- Hospodiuk-Karwowski, M.; Chi, K.; Pritchard, J.; Catchmark, J.M. Vascularized pancreas-on-a-chip device produced using a printable simulated extracellular matrix. Biomed. Mater. 2022, 17, 065006. [Google Scholar] [CrossRef]
- Tibbe, M.P.; Leferink, A.M.; van den Berg, A.; Eijkel, J.C.T.; Segerink, L.I. Microfluidic Gel Patterning Method by Use of a Temporary Membrane for Organ-On-Chip Applications. Adv. Mater. Technol. 2018, 3, 1700200. [Google Scholar] [CrossRef]
- Chiu, L.L.; Janic, K.; Radisic, M. Engineering of Oriented Myocardium on Three-Dimensional Micropatterned Collagen-Chitosan Hydrogel. Int. J. Artif. Organs 2012, 35, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, U.; Kolla, S.; Maredupaka, S.; Priya, S.; Srinivasulu, K.; Chelluri, L.K. Development of an alginate–chitosan biopolymer composite with dECM bioink additive for organ-on-a-chip articular cartilage. Sci. Rep. 2024, 14, 11765. [Google Scholar] [CrossRef]
- Saberian, M.; Roudsari, R.S.; Haghshenas, N.; Rousta, A.; Alizadeh, S. How the combination of alginate and chitosan can fabricate a hydrogel with favorable properties for wound healing. Heliyon 2024, 10, e32040. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, D.; Lv, Y.; Guo, X.; Lou, R.; Wang, S.; Wang, X.; Yu, W.; Ma, X. Microfabrication of a tunable collagen/alginate-chitosan hydrogel membrane for controlling cell–cell interactions. Carbohydr. Polym. 2016, 153, 652–662. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lam, M.T. Alginate Application for Heart and Cardiovascular Diseases. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 185–212. ISBN 978-981-10-6910-9. [Google Scholar]
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob. Cardiol. Sci. Pract. 2016, 2016, e201604. [Google Scholar] [CrossRef] [PubMed]
- Pangjantuk, A.; Kaokaen, P.; Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. 3D culture of alginate-hyaluronic acid hydrogel supports the stemness of human mesenchymal stem cells. Sci. Rep. 2024, 14, 4436. [Google Scholar] [CrossRef]
- Jiao, W.; Li, X.; Shan, J.; Wang, X. Study of Several Alginate-Based Hydrogels for In Vitro 3D Cell Cultures. Gels 2022, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, M.; Cristiano, M.C.; Fresta, M.; Paolino, D.; Rafaniello, C. Alginate-Based Composites for Corneal Regeneration: The Optimization of a Biomaterial to Overcome Its Limits. Gels 2022, 8, 431. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Liang, C.; Liao, L.; Tian, W. Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration. Biomolecules 2023, 13, 673. [Google Scholar] [CrossRef]
- Padhi, A.; Nain, A.S. ECM in Differentiation: A Review of Matrix Structure, Composition and Mechanical Properties. Ann. Biomed. Eng. 2019, 48, 1071–1089. [Google Scholar] [CrossRef]
- Khanna, A.; Zamani, M.; Huang, N.F. Extracellular Matrix-Based Biomaterials for Cardiovascular Tissue Engineering. J. Cardiovasc. Dev. Dis. 2021, 8, 137. [Google Scholar] [CrossRef]
- Libby, J.R.; Royce, H.; Walker, S.R.; Li, L. The role of extracellular matrix in angiogenesis: Beyond adhesion and structure. Biomater. Biosyst. 2024, 15, 100097. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.-J. The Role of the Extracellular Matrix in Cancer Stemness. Front. Cell Dev. Biol. 2019, 7, 86. [Google Scholar] [CrossRef]
- Sohail, M.R.; Esquer Garrigos, Z.; Elayi, C.S.; Xiang, K.; Catanzaro, J.N. Preclinical evaluation of efficacy and pharmacokinetics of gentamicin containing extracellular-matrix envelope. Pacing Clin. Electrophysiol. 2020, 43, 341–349. [Google Scholar] [CrossRef]
- Ertekin, Ö.; Monavari, M.; Krüger, R.; Fuentes-Chandía, M.; Parma, B.; Letort, G.; Tripal, P.; Boccaccini, A.R.; Bosserhoff, A.K.; Ceppi, P.; et al. 3D hydrogel-based microcapsules as an in vitro model to study tumorigenicity, cell migration and drug resistance. Acta Biomater. 2022, 142, 208–220. [Google Scholar] [CrossRef]
- González-Díaz, E.C.; Varghese, S. Hydrogels as Extracellular Matrix Analogs. Gels 2016, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xue, B.; Zhou, Y.; Qin, M.; Wang, W.; Cao, Y. Spray-Painted Hydrogel Coating for Marine Antifouling. Adv. Mater. Technol. 2021, 6, 2000911. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Z.; Zeng, W.; Zhang, Y.; Zhang, F.; Wu, D.; Wang, Y. Biocompatible Hydrogel Coating on Silicone Rubber with Improved Antifouling and Durable Lubricious Properties. Gels 2024, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Arık, Y.B.; de sa Vivas, A.; Laarveld, D.; van Laar, N.; Gemser, J.; Visscher, T.; van den Berg, A.; Passier, R.; van der Meer, A.D. Collagen I Based Enzymatically Degradable Membranes for Organ-on-a-Chip Barrier Models. ACS Biomater. Sci. Eng. 2021, 7, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Z.; Zou, Y.; Lu, G.; Ronca, A.; D’Amora, U.; Liang, J.; Fan, Y.; Zhang, X.; Sun, Y. Advanced application of collagen-based biomaterials in tissue repair and restoration. J. Leather Sci. Eng. 2022, 4, 30. [Google Scholar] [CrossRef]
- Polidoro, M.A.; Ferrari, E.; Soldani, C.; Franceschini, B.; Saladino, G.; Rosina, A.; Mainardi, A.; D’Autilia, F.; Pugliese, N.; Costa, G.; et al. Cholangiocarcinoma-on-a-chip: A human 3D platform for personalised medicine. JHEP Rep. 2024, 6, 100910. [Google Scholar] [CrossRef]
- Dabaghi, M.; Shahriari, S.; Saraei, N.; Da, K.; Chandiramohan, A.; Selvaganapathy, P.R.; Hirota, J.A. Surface Modification of PDMS-Based Microfluidic Devices with Collagen Using Polydopamine as a Spacer to Enhance Primary Human Bronchial Epithelial Cell Adhesion. Micromachines 2021, 12, 132. [Google Scholar] [CrossRef]
- Van den Borre, C.E.; Zigterman, B.G.R.; Mommaerts, M.Y.; Braem, A. How surface coatings on titanium implants affect keratinized tissue: A systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1713–1723. [Google Scholar] [CrossRef]
- Pongkorpsakol, P.; Satianrapapong, W.; Wongkrasant, P.; Steinhagen, P.R.; Tuangkijkul, N.; Pathomthongtaweechai, N.; Muanprasat, C. Establishment of Intestinal Epithelial Cell Monolayers and Their Use in Calcium Switch Assay for Assessment of Intestinal Tight Junction Assembly. In Permeability Barrier: Methods and Protocols; Turksen, K., Ed.; Springer: New York, NY, USA, 2021; pp. 273–290. ISBN 978-1-07-161673-4. [Google Scholar]
- Wang, C.; Tanataweethum, N.; Karnik, S.; Bhushan, A. Novel Microfluidic Colon with an Extracellular Matrix Membrane. ACS Biomater. Sci. Eng. 2018, 4, 1377–1385. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Passaniti, A.; Kleinman, H.K.; Martin, G.R. Matrigel: History/background, uses, and future applications. J. Cell Commun. Signal. 2022, 16, 621–626. [Google Scholar] [CrossRef]
- Badea, M.A.; Balas, M.; Hermenean, A.; Ciceu, A.; Herman, H.; Ionita, D.; Dinischiotu, A. Influence of Matrigel on Single- and Multiple-Spheroid Cultures in Breast Cancer Research. SLAS Discov. 2019, 24, 563–578. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Cui, K.; Guo, Y.; Zhang, X.; Qin, J. Advances in Hydrogels in Organoids and Organs-on-a-Chip. Adv. Mater. 2019, 31, 1902042. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Barata, D.; Teixeira, L.M.; Giselbrecht, S.; Reis, R.L.; Oliveira, J.M.; Truckenmüller, R.; Habibovic, P. Colorectal tumor-on-a-chip system: A 3D tool for precision onco-nanomedicine. Sci. Adv. 2019, 5, eaaw1317. [Google Scholar] [CrossRef] [PubMed]
- Dolega, M.E.; Abeille, F.; Picollet-D’hahan, N.; Gidrol, X. Controlled 3D culture in Matrigel microbeads to analyze clonal acinar development. Biomaterials 2015, 52, 347–357. [Google Scholar] [CrossRef]
- Rojas-Murillo, J.A.; Simental-Mendía, M.A.; Moncada-Saucedo, N.K.; Delgado-Gonzalez, P.; Islas, J.F.; Roacho-Pérez, J.A.; Garza-Treviño, E.N. Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair. Int. J. Mol. Sci. 2022, 23, 9879. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Wang, D.; Mu, S.; Lv, W.; Hao, Y.; Lu, X.; Zhang, G.; Nan, W.; Chen, H.; et al. Improved mechanical properties by modifying fibrin scaffold with PCL and its biocompatibility evaluation. J. Biomater. Sci. Polym. Ed. 2020, 31, 658–678. [Google Scholar] [CrossRef]
- Pieters, M.; Wolberg, A.S. Fibrinogen and fibrin: An illustrated review. Res. Pract. Thromb. Haemost. 2019, 3, 161–172. [Google Scholar] [CrossRef]
- Veldhuizen, J.; Cutts, J.; Brafman, D.A.; Migrino, R.Q.; Nikkhah, M. Engineering anisotropic human stem cell-derived three-dimensional cardiac tissue on-a-chip. Biomaterials 2020, 256, 120195. [Google Scholar] [CrossRef]
- Dalton, C.J.; Lemmon, C.A. Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling. Cells 2021, 10, 2443. [Google Scholar] [CrossRef]
- Xu, J.; Mosher, D. Fibronectin and Other Adhesive Glycoproteins. In The Extracellular Matrix: An Overview; Mecham, R.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 41–75. ISBN 978-3-642-16555-9. [Google Scholar]
- Parisi, L.; Toffoli, A.; Ghezzi, B.; Mozzoni, B.; Lumetti, S.; Macaluso, G.M. A glance on the role of fibronectin in controlling cell response at biomaterial interface. Jpn. Dent. Sci. Rev. 2020, 56, 50–55. [Google Scholar] [CrossRef]
- Daum, R.; Mrsic, I.; Hutterer, J.; Junginger, A.; Hinderer, S.; Meixner, A.J.; Gauglitz, G.; Chassé, T.; Schenke-Layland, K. Fibronectin adsorption on oxygen plasma-treated polyurethane surfaces modulates endothelial cell response. J. Mater. Chem. B 2021, 9, 1647–1660. [Google Scholar] [CrossRef]
- Xie, X.; Maharjan, S.; Kelly, C.; Liu, T.; Lang, R.J.; Alperin, R.; Sebastian, S.; Bonilla, D.; Gandolfo, S.; Boukataya, Y.; et al. Customizable Microfluidic Origami Liver-on-a-Chip (oLOC). Adv. Mater. Technol. 2022, 7, 2100677. [Google Scholar] [CrossRef]
- Lee, E.-J.; Ahmad, K.; Pathak, S.; Lee, S.; Baig, M.H.; Jeong, J.-H.; Doh, K.-O.; Lee, D.-M.; Choi, I. Identification of Novel FNIN2 and FNIN3 Fibronectin-Derived Peptides That Promote Cell Adhesion, Proliferation and Differentiation in Primary Cells and Stem Cells. Int. J. Mol. Sci. 2021, 22, 3042. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, S.; Gonzalez-Garcia, C.; Rico, P.; Reid, A.; Windmill, J.; Dalby, M.J.; Salmeron-Sanchez, M. Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials 2020, 252, 120104. [Google Scholar] [CrossRef]
- Arredondo, R.; Poggioli, F.; Martínez-Díaz, S.; Piera-Trilla, M.; Torres-Claramunt, R.; Tío, L.; Monllau, J.C. Fibronectin-coating enhances attachment and proliferation of mesenchymal stem cells on a polyurethane meniscal scaffold. Regen. Ther. 2021, 18, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Casali, B.C.; Gozzer, L.T.; Baptista, M.P.; Altei, W.F.; Selistre-de-Araújo, H.S. The Effects of αvβ3 Integrin Blockage in Breast Tumor and Endothelial Cells under Hypoxia In Vitro. Int. J. Mol. Sci. 2022, 23, 1745. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.W.; Tong, W.Y.; Pang, S.W.; Voelcker, N.H.; Lam, Y.W. Deconstructing, Replicating, and Engineering Tissue Microenvironment for Stem Cell Differentiation. Tissue Eng. Part B Rev. 2020, 26, 540–554. [Google Scholar] [CrossRef]
- Cavero, I.; Guillon, J.M.; Holzgrefe, H.H. Human organotypic bioconstructs from organ-on-chip devices for human-predictive biological insights on drug candidates. Expert Opin. Drug Saf. 2019, 18, 651–677. [Google Scholar] [CrossRef]
- Pun, S.; Haney, L.C.; Barrile, R. Modelling Human Physiology on-Chip: Historical Perspectives and Future Directions. Micromachines 2021, 12, 1250. [Google Scholar] [CrossRef]
- Kim, J.J.; Park, J.Y.; Nguyen, V.V.; Bae, M.; Kim, M.; Jang, J.; Won, J.Y.; Cho, D. Pathophysiological Reconstruction of a Tissue-Specific Multiple-Organ On-A-Chip for Type 2 Diabetes Emulation using 3D Cell Printing. Adv. Funct. Mater. 2023, 33, 2213649. [Google Scholar] [CrossRef]
- Rothbauer, M.; Eilenberger, C.; Spitz, S.; Bachmann, B.E.M.; Kratz, S.R.A.; Reihs, E.I.; Windhager, R.; Toegel, S.; Ertl, P. Recent Advances in Additive Manufacturing and 3D Bioprinting for Organs-On-A-Chip and Microphysiological Systems. Front. Bioeng. Biotechnol. 2022, 10, 837087. [Google Scholar] [CrossRef]
- Abaci, A.; Guvendiren, M. Designing Decellularized Extracellular Matrix-Based Bioinks for 3D Bioprinting. Adv. Healthc. Mater. 2020, 9, 2000734. [Google Scholar] [CrossRef]
- Bera, A.K.; Sriya, Y.; Pati, F. Formulation of Dermal Tissue Matrix Bioink by a Facile Decellularization Method and Process Optimization for 3D Bioprinting toward Translation Research. Macromol. Biosci. 2022, 22, 2200109. [Google Scholar] [CrossRef] [PubMed]
- Loukelis, K.; Koutsomarkos, N.; Mikos, A.G.; Chatzinikolaidou, M. Advances in 3D bioprinting for regenerative medicine applications. Regen. Biomater. 2024, 11, rbae033. [Google Scholar] [CrossRef]
- Weiß, F.; Holthaus, D.; Kraft, M.; Klotz, C.; Schneemann, M.; Schulzke, J.D.; Krug, S.M. Human duodenal organoid-derived monolayers serve as a suitable barrier model for duodenal tissue. Ann. N. Y. Acad. Sci. 2022, 1515, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bodón, J.; Andrade del Olmo, J.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2022, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Patel, T.; Huang, J.; Krukiewicz, K. Multifunctional organic monolayer-based coatings for implantable biosensors and bioelectronic devices: Review and perspectives. Biosens. Bioelectron. X 2023, 14, 100349. [Google Scholar] [CrossRef]
- Maoz, B.M.; Herland, A.; Henry, O.Y.F.; Leineweber, W.D.; Yadid, M.; Doyle, J.; Mannix, R.; Kujala, V.J.; FitzGerald, E.A.; Parker, K.K.; et al. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 2017, 17, 2294–2302. [Google Scholar] [CrossRef]
- van der Meer, A.D.; van den Berg, A. Organs-on-chips: Breaking the in vitro impasse. Integr. Biol. 2012, 4, 461–470. [Google Scholar] [CrossRef]
- Abdul-Jabbar, S.; Martin, G.P.; Martini, L.G.; Lawrence, J.; Royall, P.G. Polyelectrolyte Multi-Layered Griseofulvin Nanoparticles: Conventional versus Continuous In-Situ Layer-by-Layer Fabrication. J. Nanosci. Nanotechnol. 2021, 21, 5611–5621. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Rahdar, A. LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications. Nanomaterials 2022, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Lvov, Y.; Decher, G. There is still plenty of room for layer-by-layer assembly for constructing nanoarchitectonics-based materials and devices. Phys. Chem. Chem. Phys. 2022, 24, 4097–4115. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.; Zeng, J.; Liu, X.Q.; Chang, H.; Monge, C.; Garot, C.; Ren, K.; Machillot, P.; Vrana, N.E.; Lavalle, P.; et al. Recent Developments in Layer-by-Layer Assembly for Drug Delivery and Tissue Engineering Applications. Adv. Healthc. Mater. 2024, 13, e2302713. [Google Scholar] [CrossRef]
- Aor, B.; Khan, I.; Glinel, K.; Jonas, A.M.; Demoustier-Champagne, S.; Durrieu, M.-C. Microchannel Molding Combined with Layer-by-Layer Approach for the Formation of Three-Dimensional Tube-like Structures by Endothelial Cells. ACS Appl. Bio Mater. 2020, 3, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Vale, A.C.; Alves, N.M. Spin-coated freestanding films for biomedical applications. J. Mater. Chem. B 2021, 9, 3778–3799. [Google Scholar] [CrossRef]
- Otomo, T.; Noh, H.; Matsubara, T.; Kim, D.-H.; Ikeuchi, M.; Yoshida, K.; Kim, J. Fabrication of Biomimetic Cell Culture Membranes Using Robust and Reusable Nickel Micropillar Molds. BioChip J. 2025, 19, 91–98. [Google Scholar] [CrossRef]
- Kasi, D.G.; de Graaf, M.N.S.; Motreuil-Ragot, P.A.; Frimat, J.-P.M.S.; Ferrari, M.D.; Sarro, P.M.; Mastrangeli, M.; van den Maagdenberg, A.M.J.M.; Mummery, C.L.; Orlova, V.V. Rapid Prototyping of Organ-on-a-Chip Devices Using Maskless Photolithography. Micromachines 2022, 13, 49. [Google Scholar] [CrossRef]
- Keshtiban, M.M.; Zand, M.M.; Ebadi, A.; Azizi, Z. PDMS-based porous membrane for medical applications: Design, development, and fabrication. Biomed. Mater. 2023, 18, 045012. [Google Scholar] [CrossRef]
- Nguyen, H.M. Thin Films of Self-Assembled Materials by Dip-Coating Technique. Doctoral Thesis, Aalto University, Espoo, Finland, 2024. [Google Scholar]
- Butt, M.A. Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Bauer, M.; Duerkop, A.; Baeumner, A.J. Critical review of polymer and hydrogel deposition methods for optical and electrochemical bioanalytical sensors correlated to the sensor’s applicability in real samples. Anal. Bioanal. Chem. 2023, 415, 83–95. [Google Scholar] [CrossRef]
- Nie, J.; Lou, S.; Pollet, A.M.A.O.; van Vegchel, M.; Bouten, C.V.C.; den Toonder, J.M.J. A Cell Pre-Wrapping Seeding Technique for Hydrogel-Based Tubular Organ-On-A-Chip. Adv. Sci. 2024, 11, 2400970. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, J.; Xuanyuan, T.; Liu, X.; Liu, W. Facile and Rapid Microcontact Printing of Additive-Free Polydimethylsiloxane for Biological Patterning Diversity. ACS Appl. Mater. Interfaces 2024, 16, 20132–20142. [Google Scholar] [CrossRef]
- Lee, H.; Cho, D.-W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016, 16, 2618–2625. [Google Scholar] [CrossRef]
- Jian, H.; Wang, M.; Wang, S.; Wang, A.; Bai, S. 3D bioprinting for cell culture and tissue fabrication. Bio-Design Manuf. 2018, 1, 45–61. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Melchels, F.P.W.; Ferreira, M.J.S.; Moxon, S.R.; Potjewyd, G.; Dargaville, T.R.; Kimber, S.J.; Domingos, M. Emulating Human Tissues and Organs: A Bioprinting Perspective Toward Personalized Medicine. Chem. Rev. 2020, 120, 11093–11139. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Bioprinting of Cells, Organoids and Organs-on-a-Chip Together with Hydrogels Improves Structural and Mechanical Cues. Cells 2024, 13, 1638. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.J.; Lee, J.M.; Lutolf, M.; McAlpine, M.C.; Yeong, W.Y.; Zenobi-Wong, M. 3D extrusion bioprinting. Nat. Rev. Methods Primers 2021, 1, 75. [Google Scholar] [CrossRef]
- Zhang, P.; Abate, A.R. High-Definition Single-Cell Printing: Cell-by-Cell Fabrication of Biological Structures. Adv. Mater. 2020, 32, e2005346. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, R.; Shao, N.; Zhao, Y. Developing 3D bioprinting for organs-on-chips. Lab Chip 2025, 25, 1081–1096. [Google Scholar] [CrossRef]
- Abudupataer, M.; Chen, N.; Yan, S.; Alam, F.; Shi, Y.; Wang, L.; Lai, H.; Li, J.; Zhu, K.; Wang, C. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomed. Microdevices 2019, 22, 10. [Google Scholar] [CrossRef]
- Nejati, B.; Shahhosseini, R.; Hajiabbasi, M.; Ardabili, N.S.; Baktash, K.B.; Alivirdiloo, V.; Moradi, S.; Rad, M.F.; Rahimi, F.; Farani, M.R.; et al. Cancer-on-chip: A breakthrough organ-on-a-chip technology in cancer cell modeling. Med. Biol. Eng. Comput. 2024, 63, 321–337. [Google Scholar] [CrossRef]
- Liu, X.; Su, Q.; Zhang, X.; Yang, W.; Ning, J.; Jia, K.; Xin, J.; Li, H.; Yu, L.; Liao, Y.; et al. Recent Advances of Organ-on-a-Chip in Cancer Modeling Research. Biosensors 2022, 12, 1045. [Google Scholar] [CrossRef]
- Grant, J.; Özkan, A.; Oh, C.; Mahajan, G.; Prantil-Baun, R.; Ingber, D.E. Simulating drug concentrations in PDMS microfluidic organ chips. Lab Chip 2021, 21, 3509–3519. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.A.; Rhee, E.K.; Alaeddine, M.; Nikkhah, M. Advances in Biomaterials for Promoting Vascularization. Curr. Stem Cell Rep. 2022, 8, 184–196. [Google Scholar] [CrossRef]
- Adel, I.M.; ElMeligy, M.F.; Elkasabgy, N.A. Conventional and Recent Trends of Scaffolds Fabrication: A Superior Mode for Tissue Engineering. Pharmaceutics 2022, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: New biosynthesis methods, recent advances, and emerging applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Fuchs, S.; Johansson, S.; Tjell, A.Ø.; Werr, G.; Mayr, T.; Tenje, M. In-Line Analysis of Organ-on-Chip Systems with Sensors: Integration, Fabrication, Challenges, and Potential. ACS Biomater. Sci. Eng. 2021, 7, 2926–2948. [Google Scholar] [CrossRef]
- Avula, L.R.; Grodzinski, P. How organ-on-a-chip is advancing cancer research and oncology—A cancer hallmarks’ perspective. Front. Lab Chip Technol. 2024, 3, 1487377. [Google Scholar] [CrossRef]
- Tajeddin, A.; Mustafaoglu, N. Design and Fabrication of Organ-on-Chips: Promises and Challenges. Micromachines 2021, 12, 1443. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzade, S.; Hooshiar, M.H.; Akbari, H.; Tajer, M.H.M.; Sahneh, K.K.; Ziaei, S.Y.; Jalali, F.; Akouchakian, E. Recent advances in Organ-on-a-Chip models: How precision engineering integrates cutting edge technologies in fabrication and characterization. Appl. Mater. Today 2024, 38, 102231. [Google Scholar] [CrossRef]
- Conceição, F.; Sousa, D.M.; Loessberg-Zahl, J.; Vollertsen, A.R.; Neto, E.; Søe, K.; Paredes, J.; Leferink, A.; Lamghari, M. A metastasis-on-a-chip approach to explore the sympathetic modulation of breast cancer bone metastasis. Mater. Today Bio 2022, 13, 100219. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).