The Bactericide Effects of Chitosan When Used as an Indicator of Chlorine Demand
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
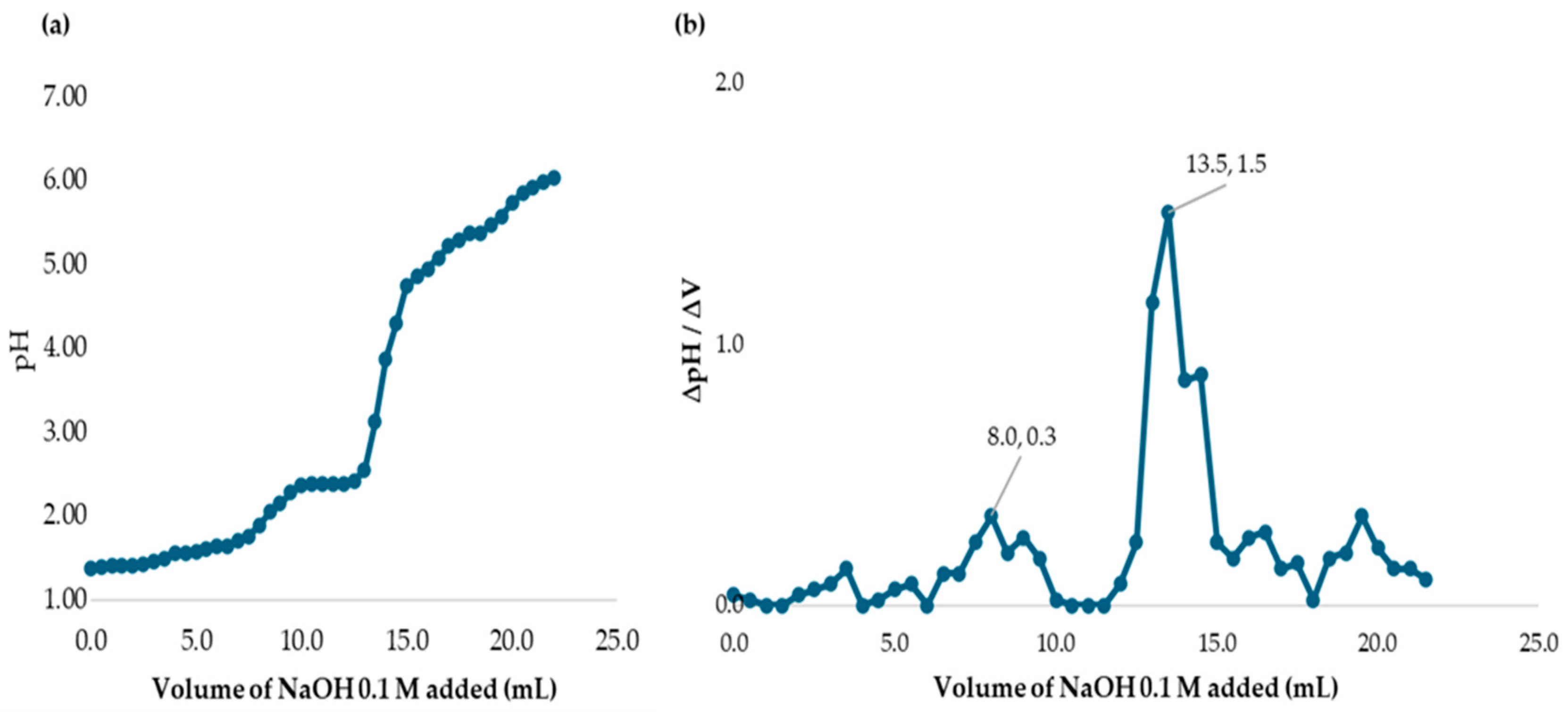
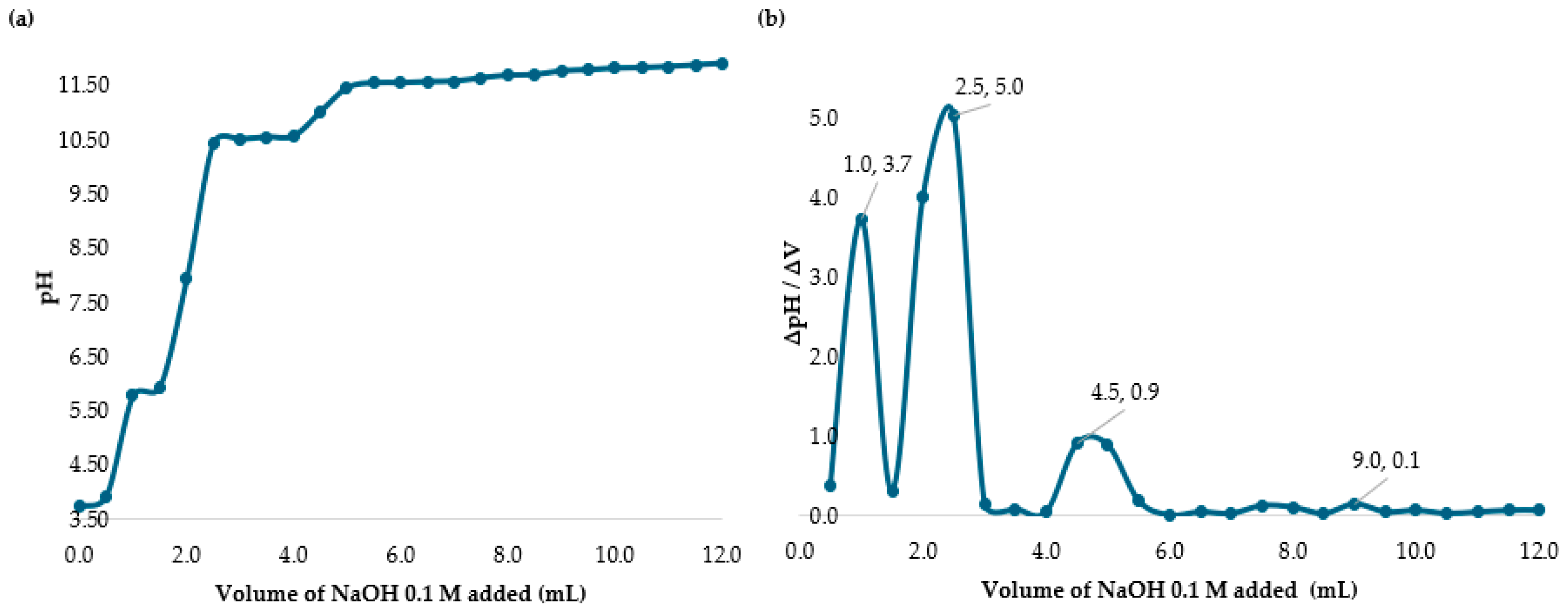
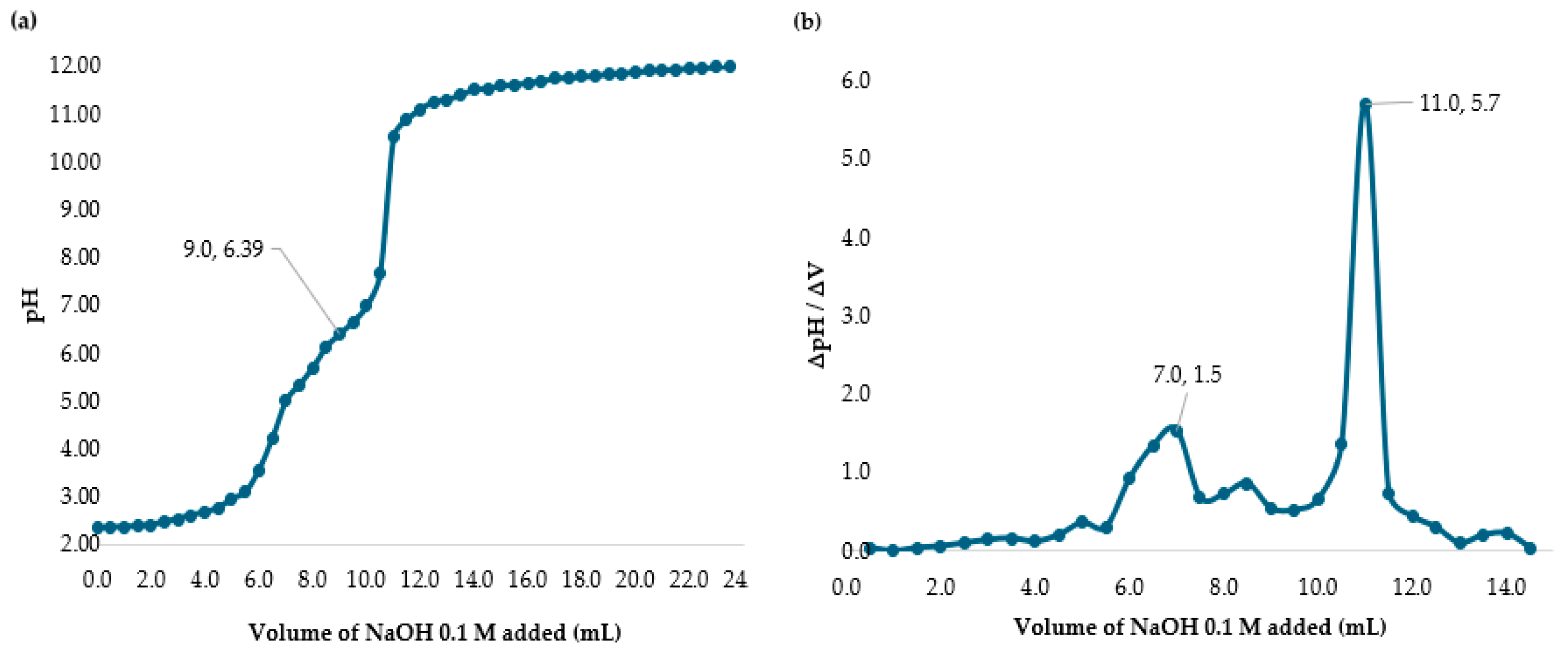
2.2. Determining the Percent of Deacetylation Degree (DD)
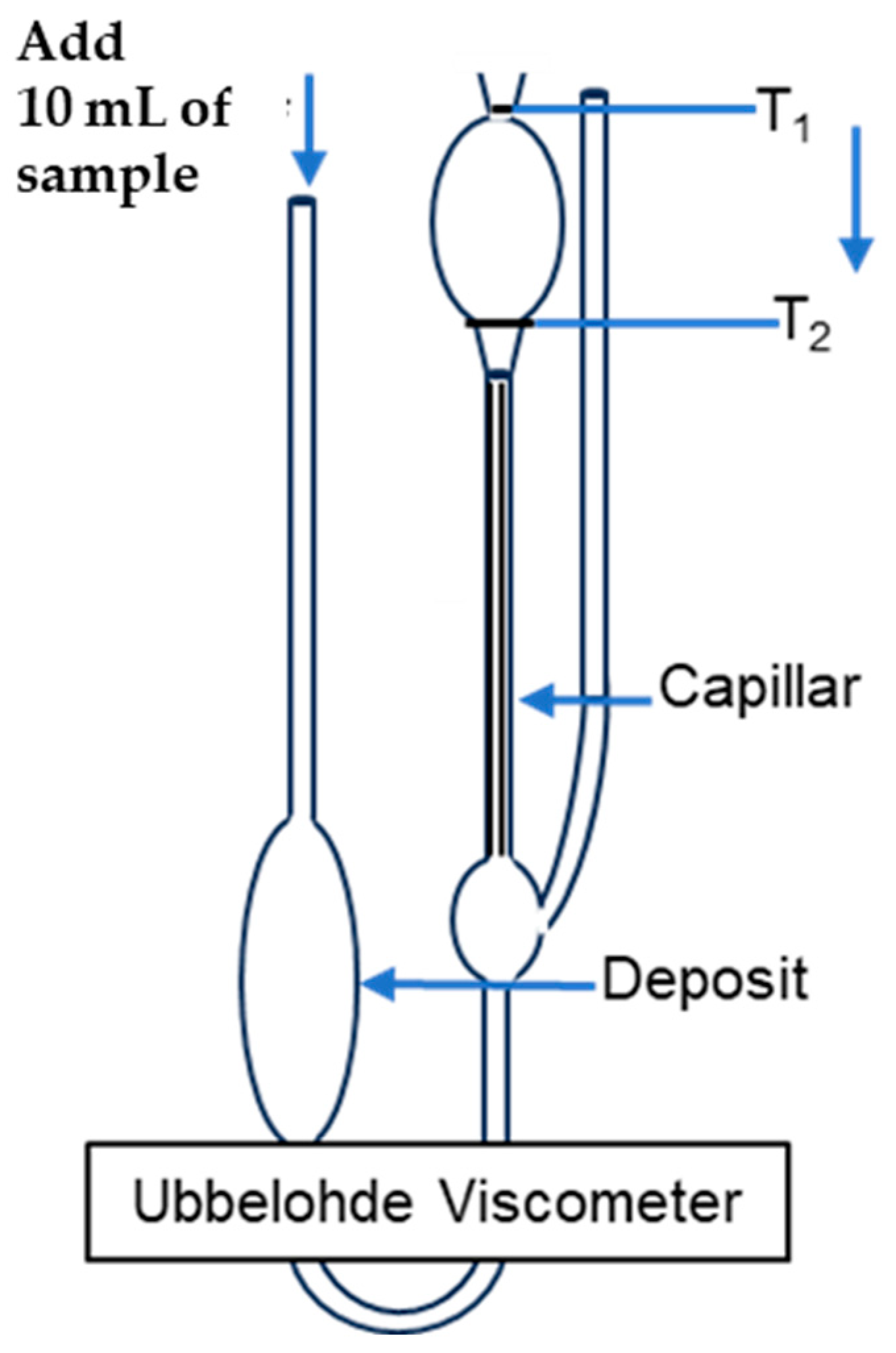
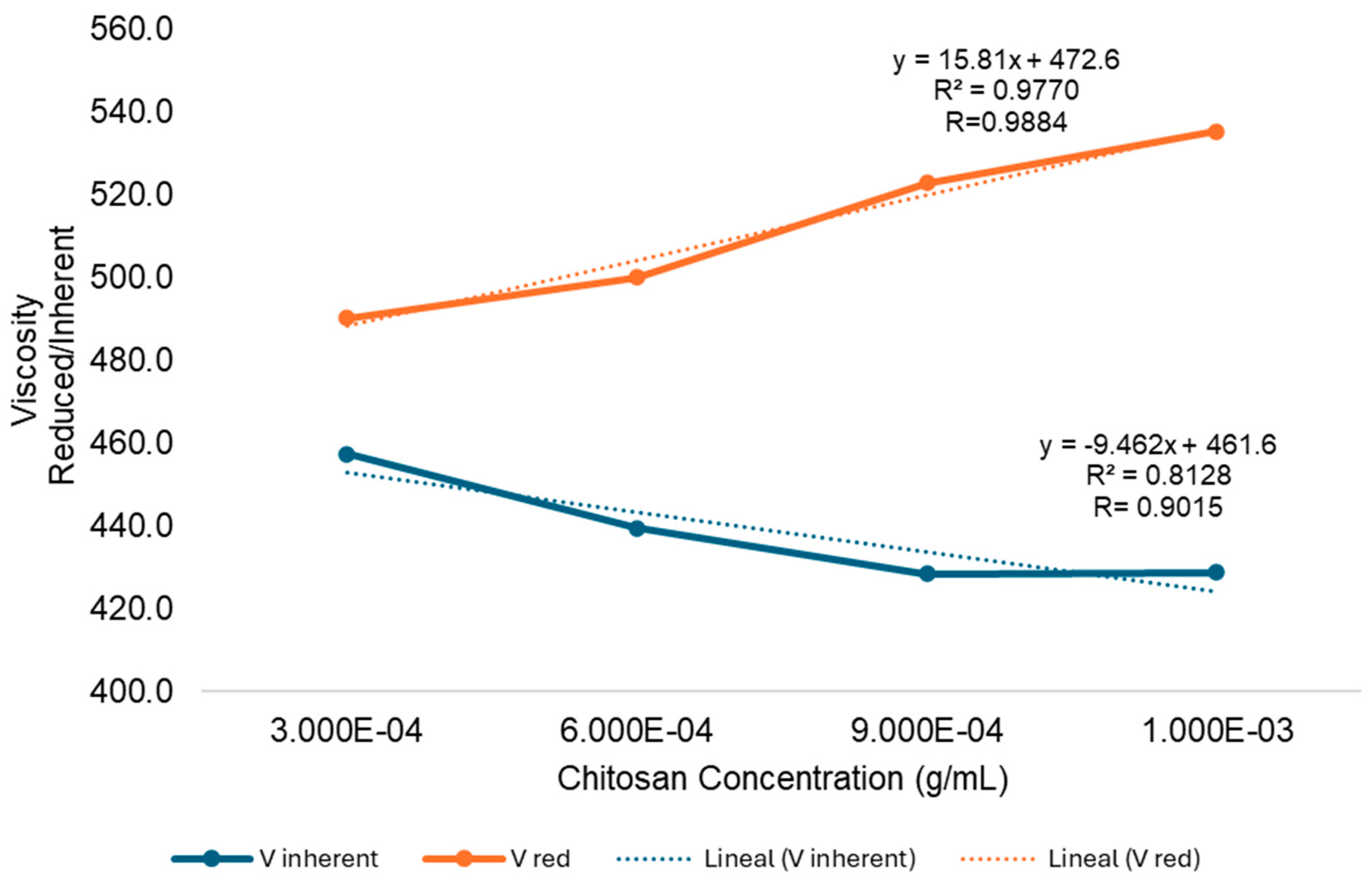
2.3. Determining the Molecular Weight (Mw)
2.4. Chitosan Stock Solutions—Preparation Procedure
2.5. Analyzing the Levels of Chlorine Doses Required for Disinfection Before and After Adding Chitosan During the MWTP Process
2.5.1. Collection and Preparation of Samples
- A consistent dose of chitosan acid solution (mg/L);
- Varying doses of sodium hypochlorite (SH) (mg/L).
2.5.2. Jar Test Analysis
- Rapid mixing: 1 min at 100 revolutions per minute (RPM).
- Slow mixing: 32 min at 30 RPM.
- Sedimentation phase: 178 min at 0 RPM.
2.5.3. Sample Analysis
- Residual chlorine measurement using the 4500-Cl(E) method. This is an EPA approved colorimeter and a standardized operational method [29].
- Temperature and pH measurement using an HQ40d Hach Meter [23].
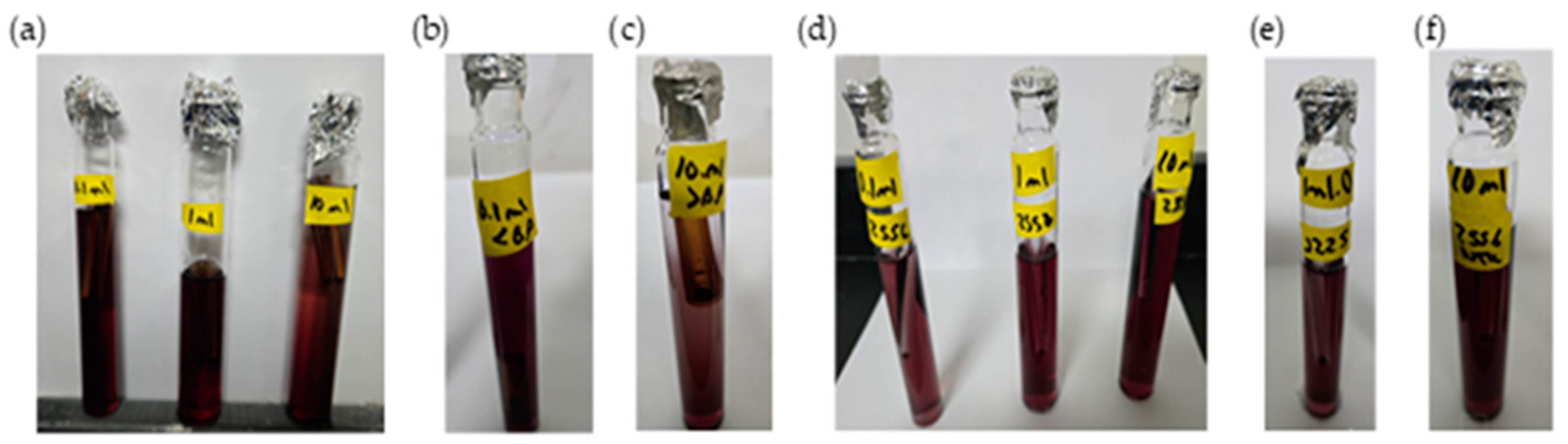
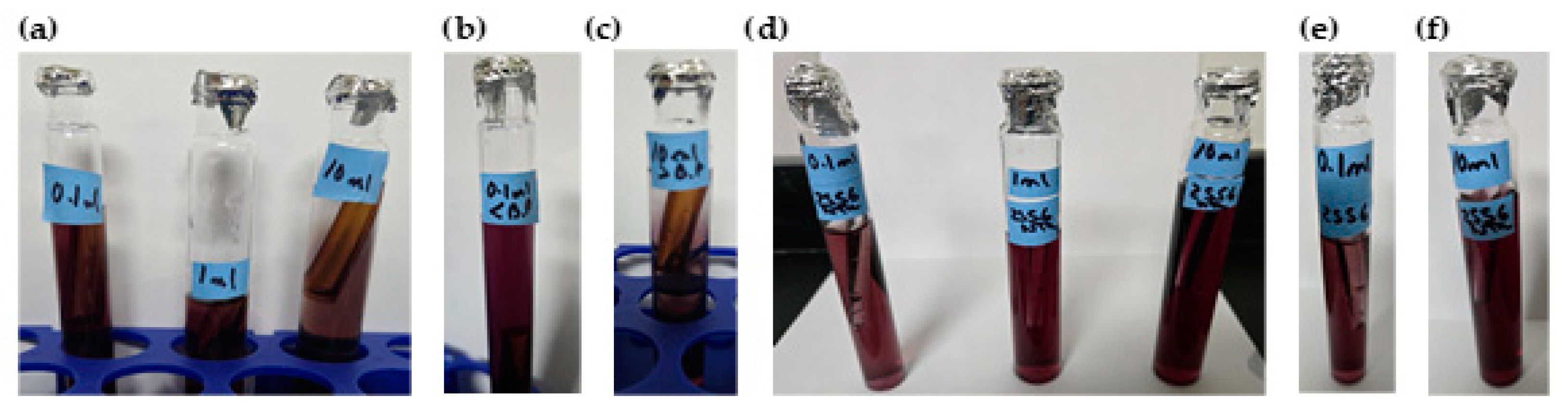
- Presence–absence (P–A) coliform test, EPA Method 9221(B) [30], was used to determine the coliform presence. For the control samples (sodium hypochlorite only), the jar analyzed corresponded to the breakpoint stage, and sample volumes of 0.1 mL, 1.0 mL, and 10 mL were added to the lactose broth. For the chitosan-treated samples, three stages were analyzed: the jar before the breakpoint (using 0.1 mL of sample), the jar at the breakpoint (using 0.1 mL, 1.0 mL, and 10 mL), and the jar after the breakpoint (using 10 mL of sample), all added to the lactose broth. Water samples were collected in sterile containers containing sodium thiosulfate to neutralize any residual chlorine. Each sample was inoculated into a P–A broth medium containing lactose (as a fermentable sugar), nutrient salts, bromocresol purple as a pH indicator, and a selective agent to inhibit non-coliform bacteria. The inoculated tubes were incubated at 35 ± 0.5 °C for 24–48 h. A positive result was indicated by a color change from purple (neutral pH) to yellow, due to acid production from lactose fermentation by coliform bacteria.
| Color | Interpretation |
| Purple | Negative—No coliforms detected |
| Yellow | Positive Coliforms present (acid production) |
- Additional microbiological verification using phenol red indicator in a lactose broth (control samples only): As a complementary analysis to the standard 9221 B Presence–Absence (P–A) test, an alternative lactose broth method using phenol red as a pH indicator was employed exclusively for control samples dosed with sodium hypochlorite (SH). This procedure served to confirm coliform presence in samples where SH was used alone, without chitosan. Six breakpoint jars were analyzed using this method, corresponding to the raw water samples with turbidities of 35.5, 236.0, and 2556 NTU (two jars per turbidity level). Each sample was inoculated into lactose broth containing phenol red and incubated at 35 ± 0.5 °C for 24–48 h. A positive result was indicated by a color change from red to yellow, due to acid production from lactose fermentation by coliform bacteria, similar to the interpretation criteria used in the 9221 Method. This colorimetric shift served as visual confirmation of bacterial activity in SH-treated samples. This complementary test was included to validate the results obtained from the standard method and to reinforce the observation of coliform presence in the SH-only control jars.
2.5.4. Repetition with Variation
3. Results and Discussions
3.1. Determining the Deacetylation Degree (DD) of Chitosan
3.2. Determining the Molecular Weight (M)
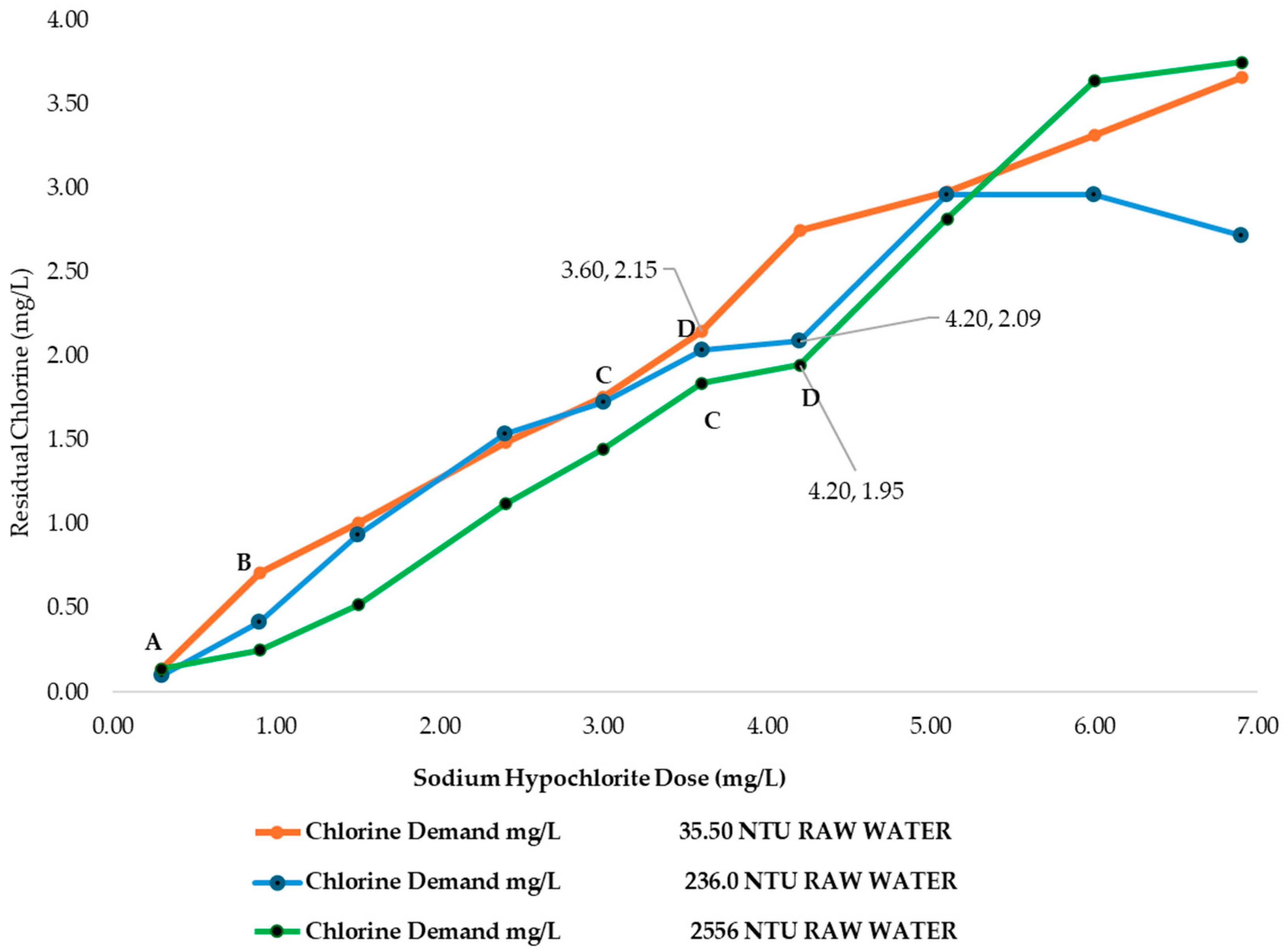
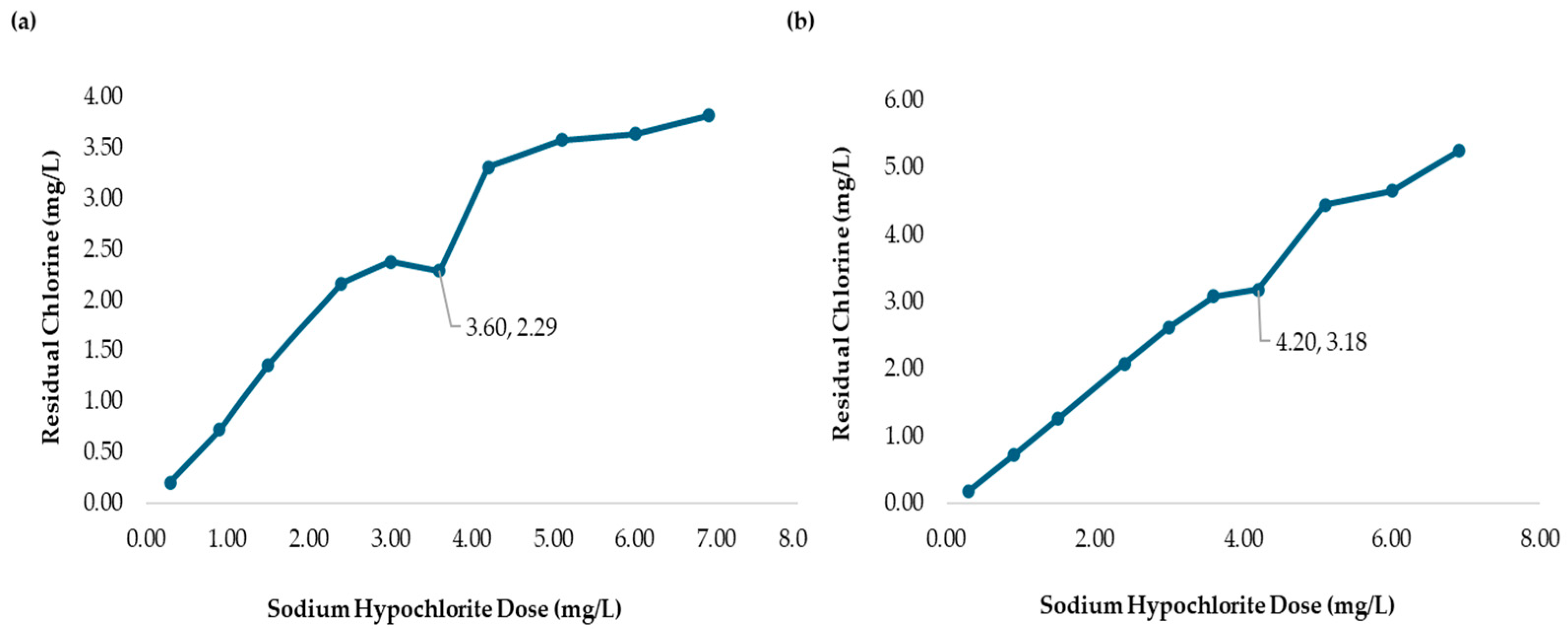
3.3. Chlorine Demand of MWTP at Different Raw Water Turbidities Without Chitosan Added
3.4. Chlorine Demand at MWTP for Different Raw Water Turbidities with the Addition of Chitosan
3.4.1. Chitosan in Acetic Acid
3.4.2. Chitosan in Citric Acid
3.4.3. Chitosan in Hydrochloric Acid
3.4.4. Chitosan in L-Ascorbic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Environmental Protection Agency. Disinfection Byproducts Rules (Stage 1 and Stage 2); Office of Water: Philadelphia, PA, USA, 1998.
- James, K.E. Chemical Disinfection. In Water of Quality & Treatment, 6th ed.; McGraw-Hill: New York, NY, USA; American Water Works Association: Denver, CO, USA, 2011; pp. 17.3–17.5. [Google Scholar]
- Raymond, D.L. Coagulation and Flocculation. In Water of Quality & Treatment, 5th ed.; McGraw-Hill: New York, NY, USA; American Water Works Association: New York, NY, USA, 1999; pp. 6.3–6.4. [Google Scholar]
- Edzwald, J.K.; Becker, W.C.; Wattier, K.L. Surrogate parameters for monitoring organic matter and THM precursors. J. AWWA 1985, 77, 122–132. [Google Scholar] [CrossRef]
- EPA Agency. Comprehensive Disinfectants and Disinfectant Byproducts Rules (Stage 1 and Stage 2): Quick Reference Guide; Office of Water: Washington, DC, USA, 2010.
- Kalita, I.; Kamilaris, A.; Havinga, P.; Reva, I. Assessing the Health Impact of Disinfection Byproducts in Drinking Water. ACS ES&T Water 2024, 4, 1564–1578. [Google Scholar]
- Valero, C. Sustancias Húmicas en Aguas para abastecimiento. Rev. Ing. Investig. 1999, 44, 63–72. [Google Scholar]
- EPA United State Environmental Agency. Total Coliform Rule Issue Paper: The Effectiveness of Disinfectant Residuals in the Distribution System; Office of Water (4601M) Office of Ground Water and Drinking Water: Washington, DC, USA, 1999; pp. 1–54.
- Mathaba, O. Effects of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane infused with Chitosan during AMD Treatment. Membranes 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.Y.; Derkach, S.R.; Konovalova, I.N.; Dolgopyatova, N.V.; Kuchina, Y.A. Mechanism of Heterogeneous Alkaline Deacetylation of Chitin: A review. Polymers 2023, 15, 1729. [Google Scholar] [CrossRef] [PubMed]
- Frederick, W.P. Chitosan as Drinking Water Treatment Coagulant. Am. J. Civ. Eng. 2016, 4, 205–215. [Google Scholar]
- Yilmaz, A.H. Antibacterial Activity of Chitosan Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Springer: Singapore, 2020; pp. 457–489. [Google Scholar]
- Chung, Y.-C.; Wang, H.L.; Chen, Y.M.; Li, S.L. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003, 3, 179. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state-of-the-art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, X.-G.; Park, H.-J.; Liu, C.-G.; Liu, C.-S.; Meng, X.-H.; Yu, L.-J. Effect of MW and concentration of Chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Malcata, F.X. Atomic force microscopy study of the antibacterial effects of chitosan on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different development stages of the bioconvertes insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef] [PubMed]
- Percival, S. Chapter Six-Escherichia coli. In Microbiology of Waterbone Diseases; Academic Press: Cambridge, MA, USA, 2014; pp. 89–117. [Google Scholar]
- Cannon Instrument Company. Cannon-Ubbelohde Manual Glass Viscometers. Available online: https://cannoninstrument.com/manual-glass-viscometers/cannon-ubbelohde-dilution-viscometer.html (accessed on 2 April 2025).
- Hach Company. DR900 Multiparameter Portable Colorimeter. 2025. Available online: https://www.hach.com/p-dr900-multiparameter-portable-colorimeter/9385100 (accessed on 2 April 2025).
- Hach Company. HQd Portable Meter. 2020. Available online: https://cdn.hach.com/7FYZVWYB/at/prbf6cn62qtkk497ckgx/DOC0225380017.pdf (accessed on 2 April 2025).
- MCR Technologies. CLM4 and CLM6 Jar Testers. Available online: https://mcrpt.com/product/clm4-and-clm6-2/ (accessed on 2 April 2025).
- Giraldo, J. Propiedades, Obtención, caracterización y aplicaciones del quitosano. Appl. Chitosan 2015, 10, 1–23. [Google Scholar]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Palacios, J.L.; Barahona, T.; Mosi-Roa, Y.; Anguita-Barrales, F.; Bustamante, M. Synthesis of New Chitosan from an Endemic Chilean Crayfish Exoskeleton (Parastacus pugnax): Physicochemical and Biological Properties. Polymers 2021, 13, 2304. [Google Scholar] [CrossRef] [PubMed]
- Xuan Jiang, L.C. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Carbohydr. Polym. 2003, 54, 457–463. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of Chitin and Chitosan from local sources. Bioresour. Technol. 2007, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H. The Mark-Houwink Saturada Equation for viscosity of linear Polyethylene. J. Phys. Chem. Ref. Data 2009, 14, 611–616. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J.; Charlet, G. Intrinsic viscosity—Molecular weight relationship for chitosan. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2591–2598. [Google Scholar] [CrossRef]
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 4500-cl chlorine (residual). In Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2023; p. 357. [Google Scholar]
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 9221-B. Presence–Absence (P–A) Coliform Test. In Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2023; p. 1140. [Google Scholar]
- Ingeniare, Universidad; Libre-Barranquilla; Rodríguez, Y. Use of a natural polymer (chitosan) as a coagulant during water treatment for consumption. Ingeniare 2020, 19, 25–32. [Google Scholar]
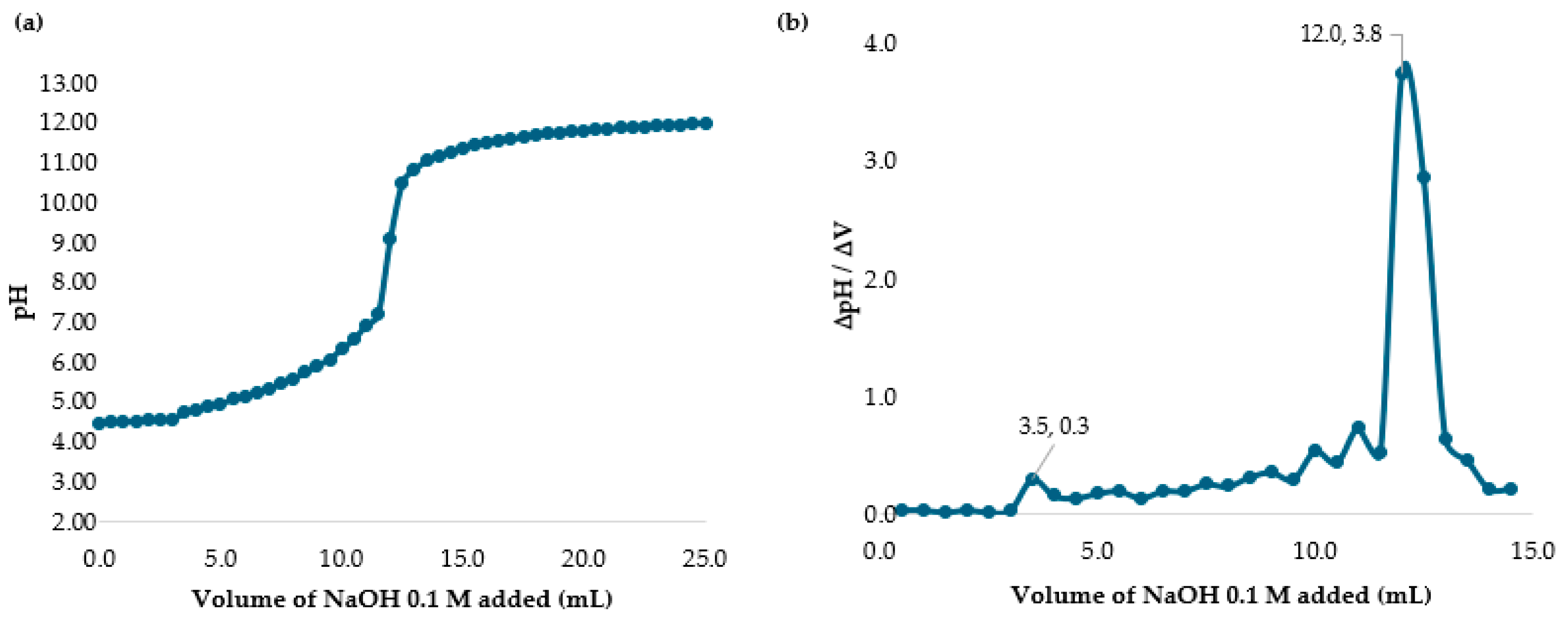





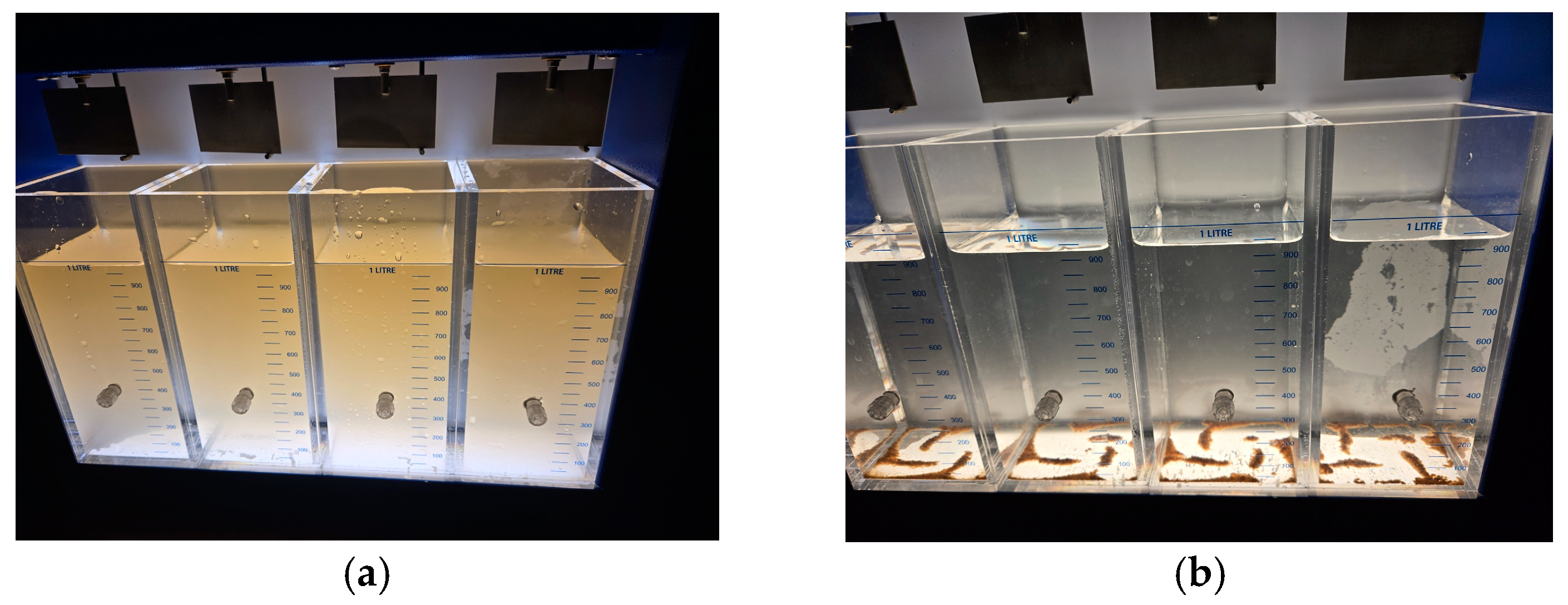
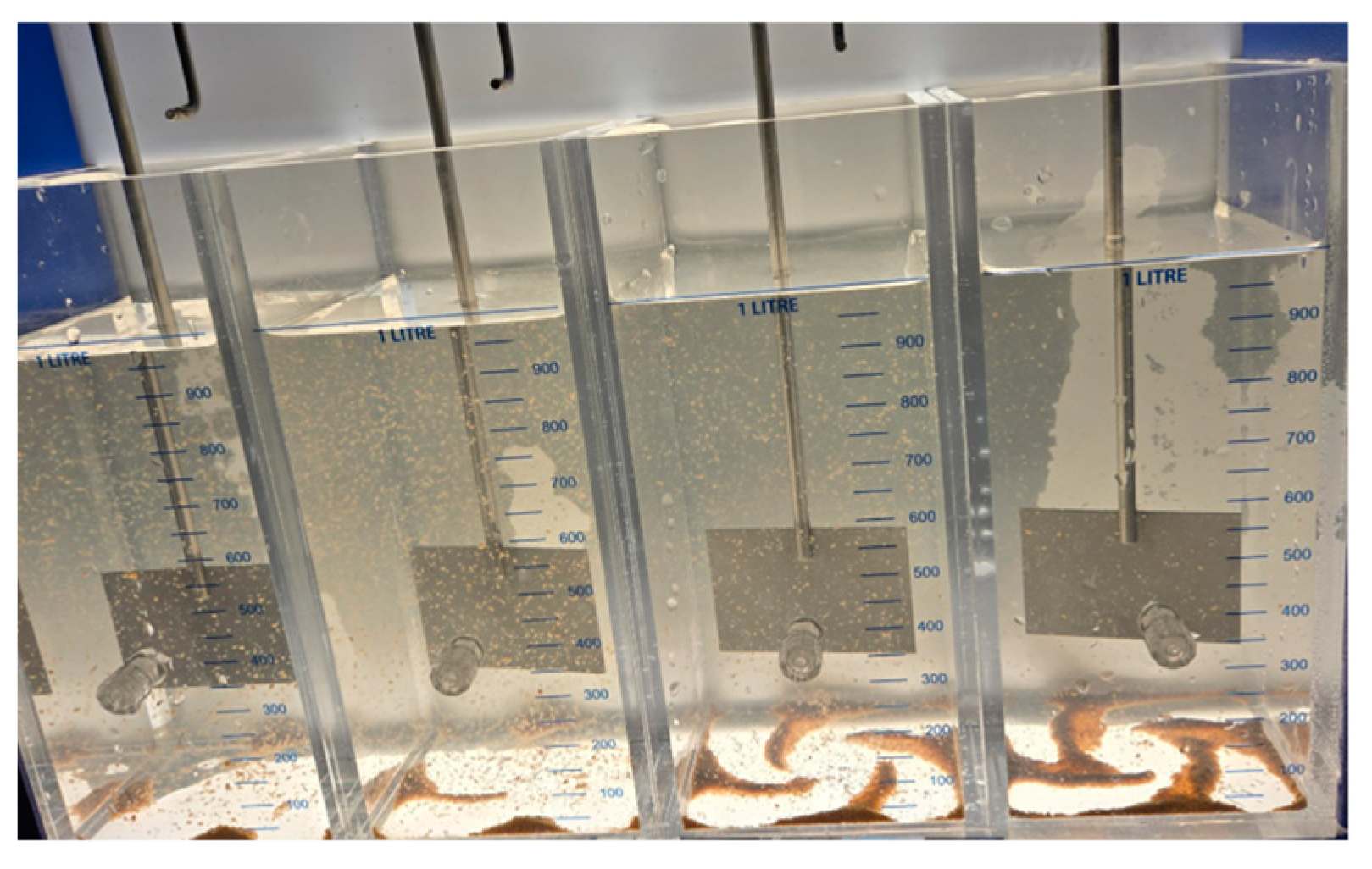
| RW Turbidity Range | RW Turbidity Range | ||
|---|---|---|---|
| 30–250 (NTU) | 250–2600 (NTU) | ||
| Jar | SH (mg/L) | Chitosan in Acidic Solution (mg/L) | Chitosan in Acidic Solution (mg/L) |
| 1 | 0.30 | 2.00 | 2.50 |
| 2 | 0.90 | 2.00 | 2.50 |
| 3 | 1.50 | 2.00 | 2.50 |
| 4 | 2.40 | 2.00 | 2.50 |
| 5 | 3.00 | 2.00 | 2.50 |
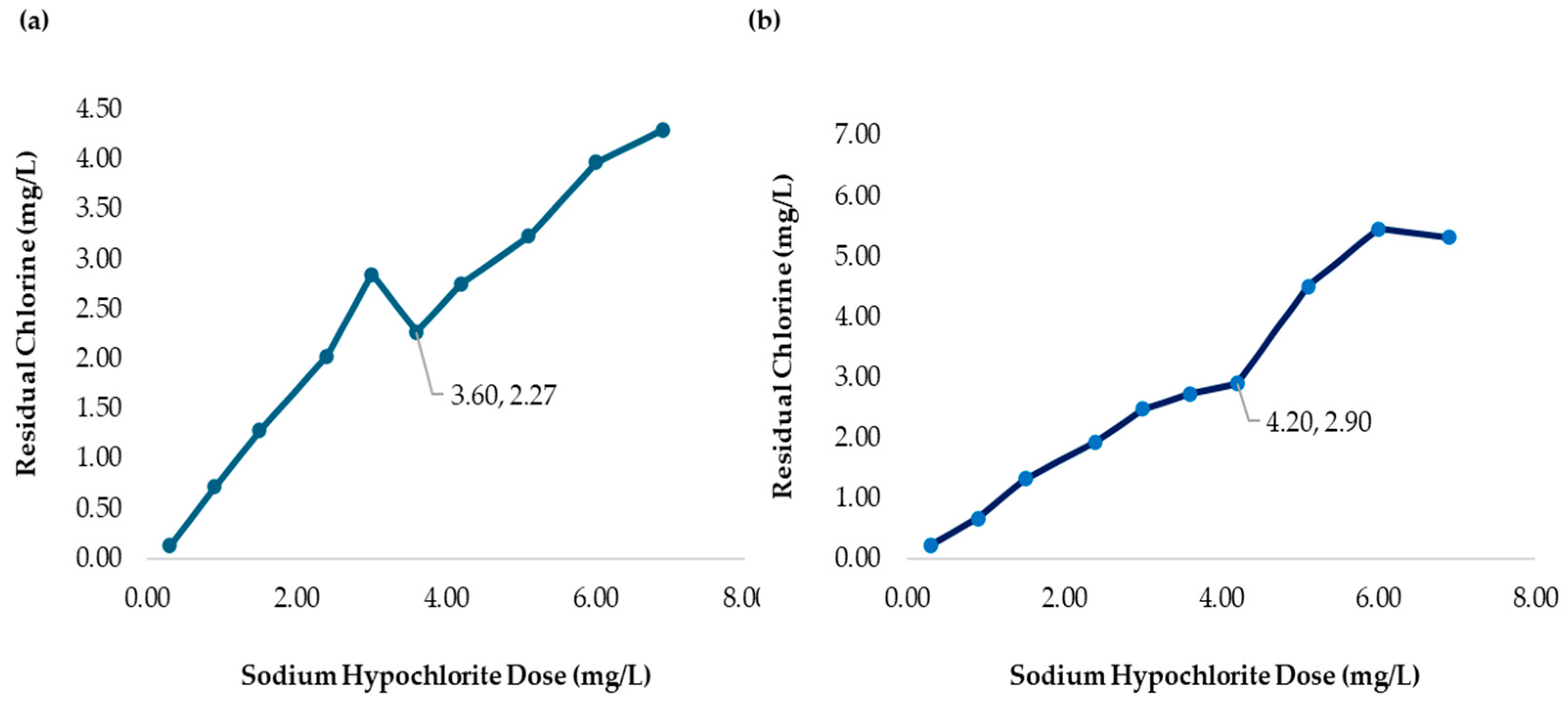
| 6 | 3.60 | 2.00 | 2.50 |
| 7 | 4.20 | 2.00 | 2.50 |
| 8 | 5.10 | 2.00 | 2.50 |
| 9 | 6.00 | 2.00 | 2.50 |
| 10 | 6.90 | 2.00 | 2.50 |
| Acetic Acid (0.1 M) | Chitosan Concentrations (g/mL) | ||||
|---|---|---|---|---|---|
| Solvent | 1.000 × 10−3 | 9.000 × 10−4 | 6.000 × 10−4 | 3.000 × 10−4 | |
| t average (min) | 1.700 ± 0.011 | 2.610 ± 0.013 | 2.500 ± 0.011 | 2.213 ± 0.017 | 1.950 ± 0.045 |
| Relative viscosity | 1.535 | 1.471 | 1.302 | 1.147 | |
| Specific viscosity | 0.535 | 0.471 | 0.302 | 0.147 | |
| Reduced viscosity | 535.3 | 522.8 | 500.0 | 490.2 | |
| Inherent viscosity | 428.7 | 428.5 | 439.5 | 457.3 | |
| 35.5 NTU | 236.0 NTU | 2556 NTU | ||||||
|---|---|---|---|---|---|---|---|---|
| Jar | SH (mg/L) | SH (µL) | RC (mg/L) | CD (mg/L) | RC (mg/L) | CD (mg/L) | RC (mg/L) | CD (mg/L) |
| 1 | 0.30 | 100 | 0.16 | 0.14 | 0.20 | 0.10 | 0.16 | 0.14 |
| 2 | 0.90 | 300 | 0.19 | 0.71 | 0.48 | 0.42 | 0.65 | 0.25 |
| 3 | 1.50 | 500 | 0.49 | 1.01 | 0.56 | 0.94 | 0.98 | 0.52 |
| 4 | 2.40 | 800 | 0.91 | 1.49 | 0.86 | 1.54 | 1.28 | 1.12 |
| 5 | 3.00 | 1000 | 1.24 | 1.76 | 1.27 | 1.73 | 1.55 | 1.45 |
| 6 | 3.60 | 1200 | 1.45 | 2.15 | 1.56 | 2.04 | 1.76 | 1.84 |
| 7 | 4.20 | 1400 | 1.45 | 2.75 | 2.11 | 2.09 | 2.25 | 1.95 |
| 8 | 5.10 | 1700 | 2.12 | 2.98 | 2.14 | 2.96 | 2.28 | 2.82 |
| 9 | 6.00 | 2000 | 2.68 | 3.32 | 3.04 | 2.96 | 2.36 | 3.64 |
| 10 | 6.90 | 2300 | 3.24 | 3.66 | 4.18 | 2.72 | 3.15 | 3.75 |
| Jar | SH (mg/L) | SH (µL) | Chitosan (mg/L) | Chitosan (µL) | RC After Flocculation (30 min, 30 RPM) | RC After Sedimentation (178 min, 0 RPM) | CD (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 100 | 2.00 | 2000 | 0.20 | 0.17 | 0.13 |
| 2 | 0.90 | 300 | 2.00 | 2000 | 0.29 | 0.18 | 0.72 |
| 3 | 1.50 | 500 | 2.00 | 2000 | 0.38 | 0.22 | 1.28 |
| 4 | 2.40 | 800 | 2.00 | 2000 | 0.67 | 0.37 | 2.03 |
| 5 | 3.00 | 1000 | 2.00 | 2000 | 0.26 | 0.15 | 2.85 |
| 6 | 3.60 | 1200 | 2.00 | 2000 | 1.73 | 1.33 | 2.27 |
| 7 | 4.20 | 1400 | 2.00 | 2000 | 1.78 | 1.45 | 2.75 |
| 8 | 5.10 | 1700 | 2.00 | 2000 | 2.62 | 1.87 | 3.23 |
| 9 | 6.00 | 2000 | 2.00 | 2000 | 2.82 | 2.03 | 3.97 |
| 10 | 6.90 | 2300 | 2.00 | 2000 | 3.44 | 2.60 | 4.30 |
| Jar | SH (mg/L) | SH (µL) | Chitosan (mg/L) | Chitosan (µL) | RC After Flocculation (30 min, 30 RPM) | RC After Sedimentation (178 min, 0 RPM) | CD (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 100 | 2.50 | 2500 | 0.15 | 0.08 | 0.22 |
| 2 | 0.90 | 300 | 2.50 | 2500 | 0.29 | 0.23 | 0.67 |
| 3 | 1.50 | 500 | 2.50 | 2500 | 0.18 | 0.18 | 1.32 |
| 4 | 2.40 | 800 | 2.50 | 2500 | 0.84 | 0.47 | 1.93 |
| 5 | 3.00 | 1000 | 2.50 | 2500 | 1.01 | 0.52 | 2.48 |
| 6 | 3.60 | 1200 | 2.50 | 2500 | 1.08 | 0.87 | 2.73 |
| 7 | 4.20 | 1400 | 2.50 | 2500 | 1.97 | 1.30 | 2.90 |
| 8 | 5.10 | 1700 | 2.50 | 2500 | 1.29 | 0.59 | 4.51 |
| 9 | 6.00 | 2000 | 2.50 | 2500 | 1.34 | 0.54 | 5.46 |
| 10 | 6.90 | 2300 | 2.50 | 2500 | 3.48 | 1.58 | 5.32 |
| Jar | SH (mg/L) | SH (µL) | Chitosan (mg/L) | Chitosan (µL) | RC After Flocculation (30 min, 30 RPM) | RC After Sedimentation (178 min, 0 RPM) | CD (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 100 | 2.00 | 2000 | 0.23 | 0.10 | 0.20 |
| 2 | 0.90 | 300 | 2.00 | 2000 | 0.25 | 0.18 | 0.72 |
| 3 | 1.50 | 500 | 2.00 | 2000 | 0.64 | 0.14 | 1.36 |
| 4 | 2.40 | 800 | 2.00 | 2000 | 1.17 | 0.24 | 2.16 |
| 5 | 3.00 | 1000 | 2.00 | 2000 | 1.60 | 0.62 | 2.38 |
| 6 | 3.60 | 1200 | 2.00 | 2000 | 2.15 | 1.31 | 2.29 |
| 7 | 4.20 | 1400 | 2.00 | 2000 | 2.58 | 0.89 | 3.31 |
| 8 | 5.10 | 1700 | 2.00 | 2000 | 3.12 | 1.52 | 3.58 |
| 9 | 6.00 | 2000 | 2.00 | 2000 | 3.62 | 2.36 | 3.64 |
| 10 | 6.90 | 2300 | 2.00 | 2000 | 3.78 | 3.08 | 3.82 |
| Jar | SH (mg/L) | SH (µL) | Chitosan (mg/L) | Chitosan (µL) | RC After Flocculation (30 min, 30 RPM) | RC After Sedimentation (178 min, 0 RPM) | CD (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 100 | 2.50 | 2500 | 0.09 | 0.12 | 0.18 |
| 2 | 0.90 | 300 | 2.50 | 2500 | 0.05 | 0.19 | 0.71 |
| 3 | 1.50 | 500 | 2.50 | 2500 | 0.11 | 0.24 | 1.26 |
| 4 | 2.40 | 800 | 2.50 | 2500 | 0.18 | 0.32 | 2.08 |
| 5 | 3.00 | 1000 | 2.50 | 2500 | 0.14 | 0.38 | 2.62 |
| 6 | 3.60 | 1200 | 2.50 | 2500 | 0.58 | 0.52 | 3.08 |
| 7 | 4.20 | 1400 | 2.50 | 2500 | 0.77 | 1.02 | 3.18 |
| 8 | 5.10 | 1700 | 2.50 | 2500 | 0.79 | 0.65 | 4.45 |
| 9 | 6.00 | 2000 | 2.50 | 2500 | 1.51 | 1.34 | 4.66 |
| 10 | 6.90 | 2300 | 2.50 | 2500 | 1.92 | 1.65 | 5.25 |
| Jar | SH (mg/L) | SH (µL) | Chitosan (mg/L) | Chitosan (µL) | RC After Flocculation (30 min, 30 RPM) | RC After Sedimentation (178 min, 0 RPM) | CD (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 100 | 2.00 | 2000 | 0.29 | 0.10 | 0.20 |
| 2 | 0.90 | 300 | 2.00 | 2000 | 0.31 | 0.12 | 0.78 |
| 3 | 1.50 | 500 | 2.00 | 2000 | 0.23 | 0.02 | 1.48 |
| 4 | 2.40 | 800 | 2.00 | 2000 | 0.55 | 0.25 | 2.15 |
| 5 | 3.00 | 1000 | 2.00 | 2000 | 0.81 | 0.43 | 2.57 |
| 6 | 3.60 | 1200 | 2.00 | 2000 | 1.45 | 1.14 | 2.46 |
| 7 | 4.20 | 1400 | 2.00 | 2000 | 1.82 | 0.86 | 3.34 |
| 8 | 5.10 | 1700 | 2.00 | 2000 | 2.94 | 1.43 | 3.67 |
| 9 | 6.00 | 2000 | 2.00 | 2000 | 3.44 | 1.97 | 4.03 |
| 10 | 6.90 | 2300 | 2.00 | 2000 | 4.80 | 2.80 | 4.10 |
| Jar | SH (mg/L) | SH (µL) | Chitosan (mg/L) | Chitosan (µL) | RC After Flocculation (30 min, 30 RPM) | RC After Sedimentation (178 min, 0 RPM) | CD (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 100 | 2.50 | 2500 | 0.15 | 0.06 | 0.24 |
| 2 | 0.90 | 300 | 2.50 | 2500 | 0.26 | 0.11 | 0.79 |
| 3 | 1.50 | 500 | 2.50 | 2500 | 0.82 | 0.18 | 1.32 |
| 4 | 2.40 | 800 | 2.50 | 2500 | 1.82 | 0.48 | 1.92 |
| 5 | 3.00 | 1000 | 2.50 | 2500 | 2.35 | 0.07 | 2.93 |
| 6 | 3.60 | 1200 | 2.50 | 2500 | 3.22 | 0.40 | 3.20 |
| 7 | 4.20 | 1400 | 2.50 | 2500 | 3.36 | 0.31 | 3.89 |
| 8 | 5.10 | 1700 | 2.50 | 2500 | 3.98 | 0.59 | 4.51 |
| 9 | 6.00 | 2000 | 2.50 | 2500 | 4.01 | 0.69 | 5.31 |
| 10 | 6.90 | 2300 | 2.50 | 2500 | 4.15 | 0.99 | 5.91 |
| Jar | SH (mg/L) | SH (µL) | Chitosan (mg/L) | Chitosan (µL) | RC After Flocculation (30 min, 30 RPM) | RC After Sedimentation (178 min, 0 RPM) | CD (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 100 | 2.00 | 2000 | 0.20 | 0.12 | 0.18 |
| 2 | 0.90 | 300 | 2.00 | 2000 | 0.34 | 0.16 | 0.74 |
| 3 | 1.50 | 500 | 2.00 | 2000 | 0.45 | 0.19 | 1.31 |
| 4 | 2.40 | 800 | 2.00 | 2000 | 0.57 | 0.23 | 2.17 |
| 5 | 3.00 | 1000 | 2.00 | 2000 | 0.79 | 0.50 | 2.50 |
| 6 | 3.60 | 1200 | 2.00 | 2000 | 1.38 | 1.22 | 2.38 |
| 7 | 4.20 | 1400 | 2.00 | 2000 | 1.74 | 0.53 | 3.67 |
| 8 | 5.10 | 1700 | 2.00 | 2000 | 2.69 | 1.40 | 3.70 |
| 9 | 6.00 | 2000 | 2.00 | 2000 | 3.28 | 2.07 | 3.93 |
| 10 | 6.90 | 2300 | 2.00 | 2000 | 3.93 | 2.84 | 4.06 |
| Jar | SH (mg/L) | SH (µL) | Chitosan (mg/L) | Chitosan (µL) | RC After Flocculation (30 min, 30 RPM) | RC After Sedimentation (178 min, 0 RPM) | CD (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 100 | 2.50 | 2500 | 0.09 | 0.06 | 0.24 |
| 2 | 0.90 | 300 | 2.50 | 2500 | 0.05 | 0.05 | 0.85 |
| 3 | 1.50 | 500 | 2.50 | 2500 | 0.11 | 0.06 | 1.44 |
| 4 | 2.40 | 800 | 2.50 | 2500 | 0.18 | 0.11 | 2.29 |
| 5 | 3.00 | 1000 | 2.50 | 2500 | 0.14 | 0.10 | 2.90 |
| 6 | 3.60 | 1200 | 2.50 | 2500 | 0.58 | 0.10 | 3.50 |
| 7 | 4.20 | 1400 | 2.50 | 2500 | 0.77 | 0.70 | 3.50 |
| 8 | 5.10 | 1700 | 2.50 | 2500 | 0.79 | 0.55 | 4.65 |
| 9 | 6.00 | 2000 | 2.50 | 2500 | 1.51 | 1.20 | 4.80 |
| 10 | 6.90 | 2300 | 2.50 | 2500 | 1.92 | 1.65 | 5.25 |
| 236.0 NTU RW | 2556 NTU RW | |||
|---|---|---|---|---|
| Acid Solution | SH Dose (mg/L) | RC (mg/L) | SH Dose (mg/L) | RC (mg/L) |
| Acetic acid | 3.60 | 1.33 | 4.20 | 1.30 |
| Citric acid | 3.60 | 1.31 | 4.20 | 1.02 |
| Hydrochloric acid | 3.60 | 0.31 | 3.60 | 0.40 |
| L-ascorbic acid | 3.60 | 1.22 | 4.20 | 0.70 |
| SH only (control) | 4.20 | 2.09 | 4.20 | 1.95 |
| Treatment Type | Typical Dosage | Estimated Cost per 1,000,000 L | Chlorine Demand Reduction | Additional Benefits |
|---|---|---|---|---|
| Sodium Hypochlorite (SH) Only | 4.20 mg/L | ~$140 (at $0.48/lb for 7000 lb/day) | None | Disinfection only; high DBP potential |
| Chitosan (as primary coagulant) [31] | 10–40 mg/L (depending on turbidity) | $100–$200 (at $10–$20/kg) | Moderate to High | Coagulation + Antimicrobial; reduces DBPs |
| Chitosan (as additive) | 2–3 mg/L | $20–$60 | High | Enhances existing coagulants; lowers chlorine demand |
| Alum (Aluminum Sulfate) | 10–50 mg/L | ~$60–$80 | None | Requires pH adjustment; potential residuals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Pinna, J.; Román-Velázquez, F.R. The Bactericide Effects of Chitosan When Used as an Indicator of Chlorine Demand. Polymers 2025, 17, 1226. https://doi.org/10.3390/polym17091226
Molina-Pinna J, Román-Velázquez FR. The Bactericide Effects of Chitosan When Used as an Indicator of Chlorine Demand. Polymers. 2025; 17(9):1226. https://doi.org/10.3390/polym17091226
Chicago/Turabian StyleMolina-Pinna, Josefine, and Félix R. Román-Velázquez. 2025. "The Bactericide Effects of Chitosan When Used as an Indicator of Chlorine Demand" Polymers 17, no. 9: 1226. https://doi.org/10.3390/polym17091226
APA StyleMolina-Pinna, J., & Román-Velázquez, F. R. (2025). The Bactericide Effects of Chitosan When Used as an Indicator of Chlorine Demand. Polymers, 17(9), 1226. https://doi.org/10.3390/polym17091226








