End-of-Life Management Strategies for Fe–Mn Nanocomposites Used in Arsenic Removal from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reusability of FMBO Nanocomposite
2.3. Immobilization Agents and PS-FMBO Preparation for Stabilization Treatment
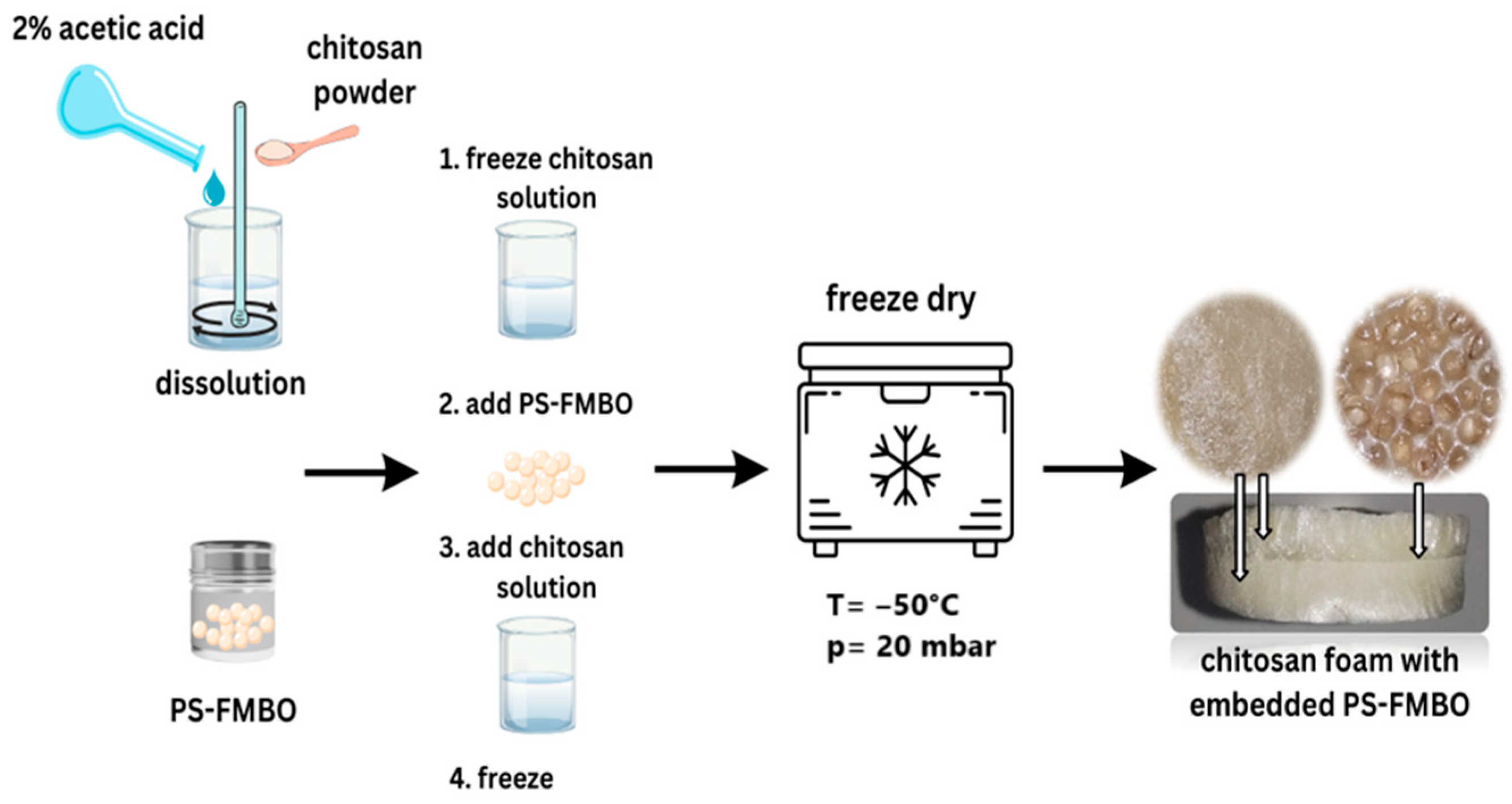
2.4. Preparation of Chitosan Foams
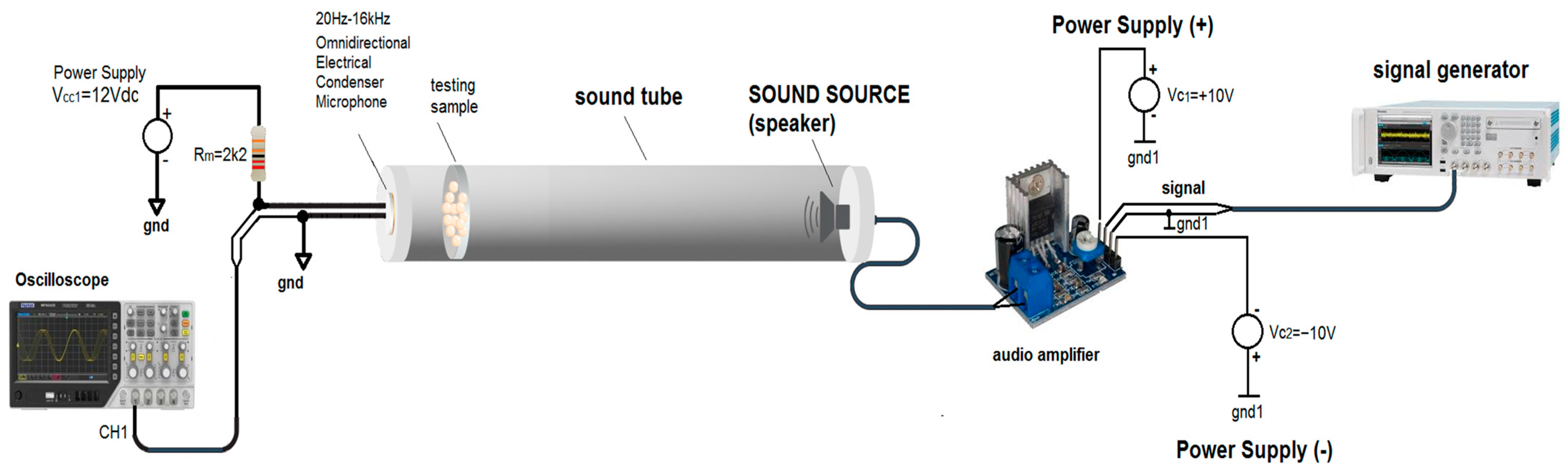
2.5. Sound Absorption Test
2.6. Methods
2.6.1. Characterization Techniques
2.6.2. Leaching Tests
2.6.3. Analytical Methods
3. Results
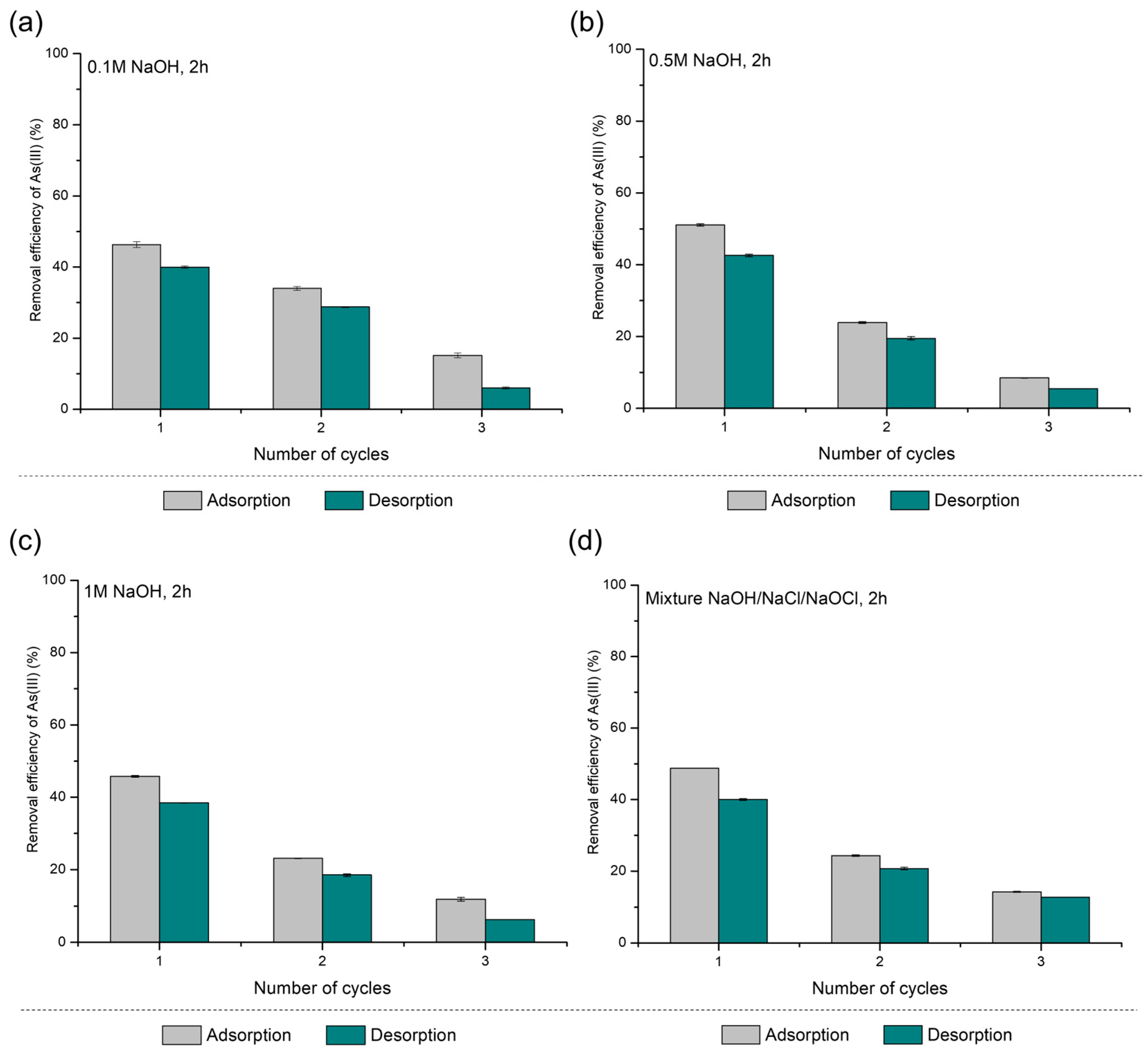
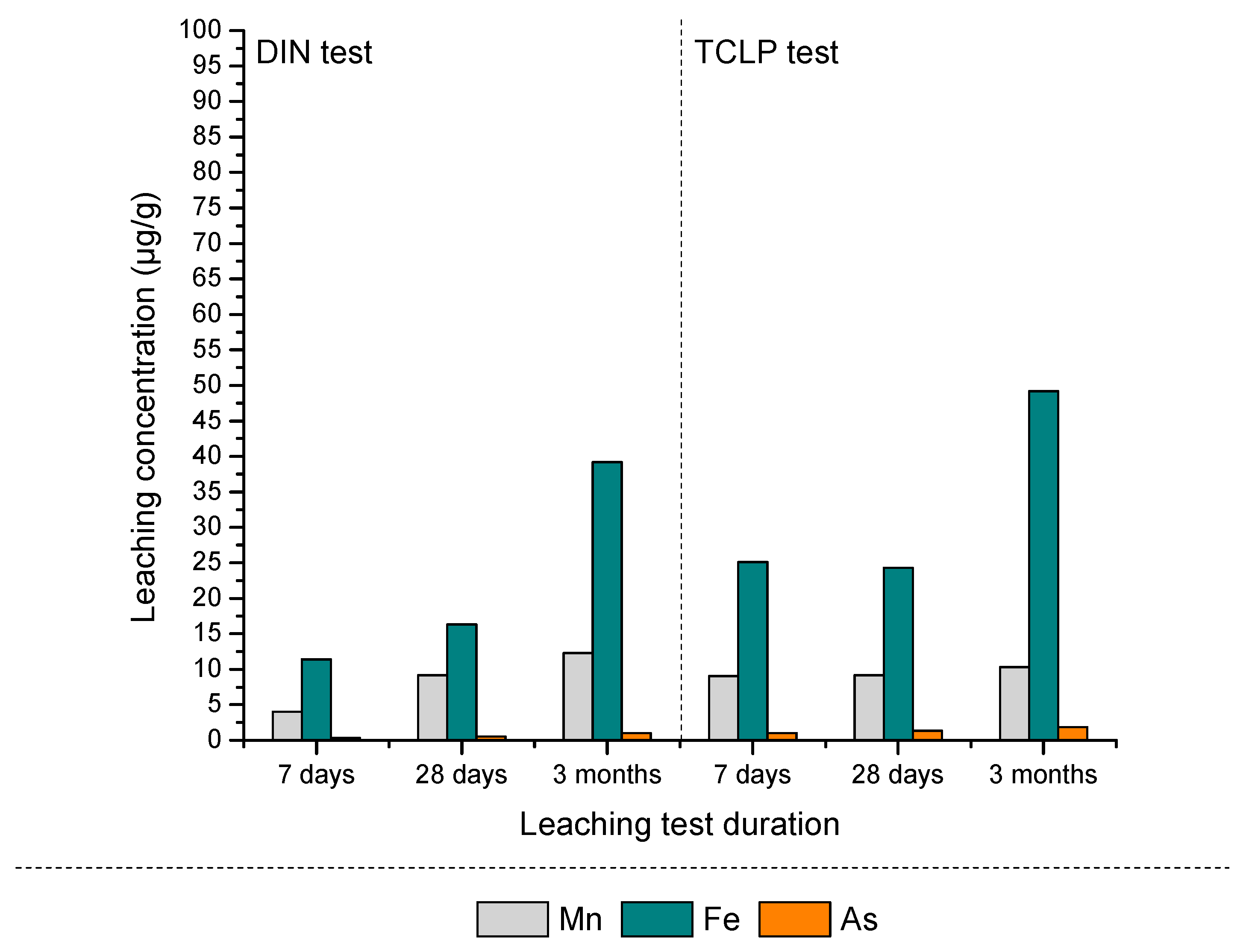
3.1. Regeneration and Reuse of PS-FMBO
3.2. Stabilization of PS-FMBO Nanocomposites
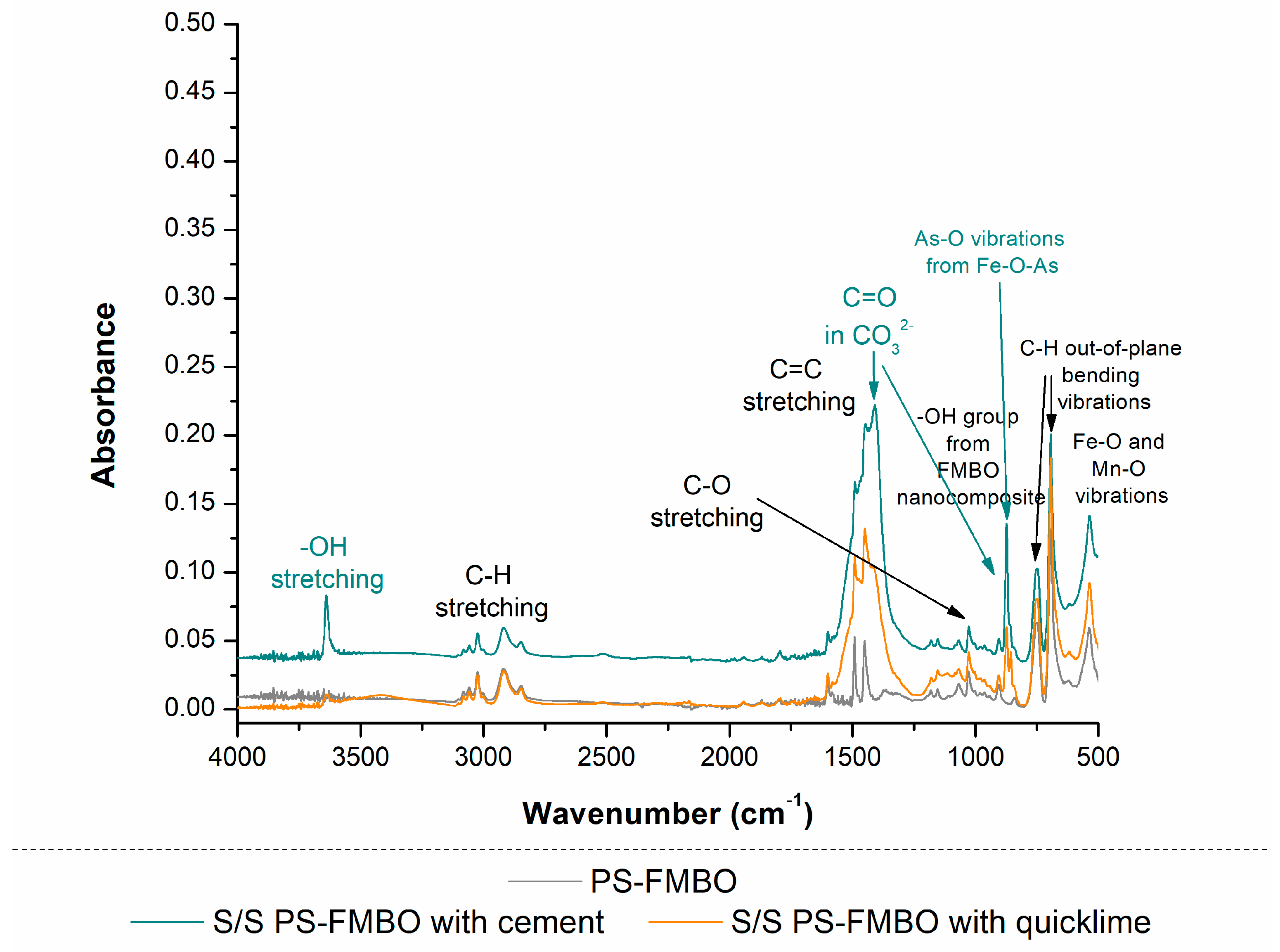
3.2.1. Physicochemical Characteristics of S/S PS-FMBO Nanocomposite
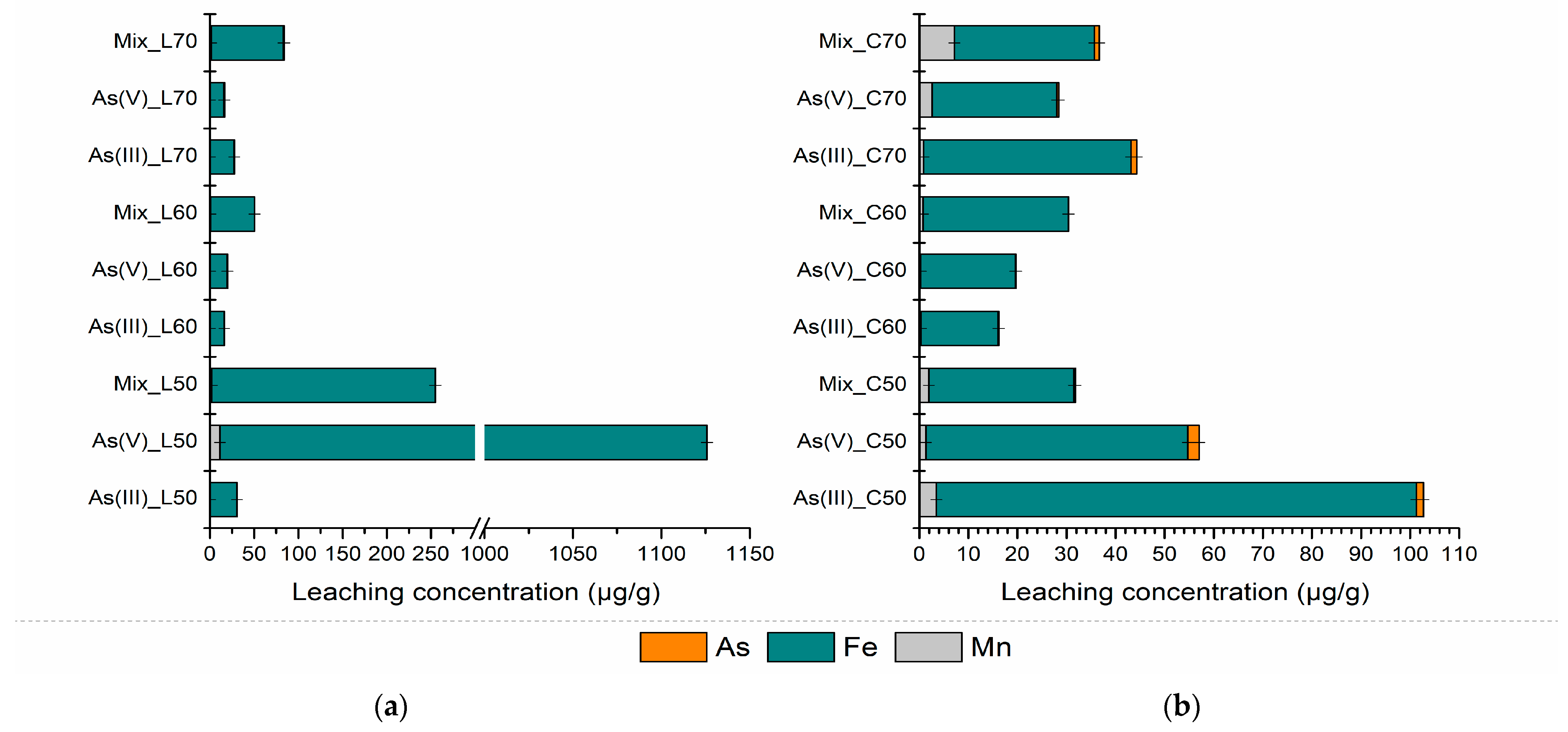
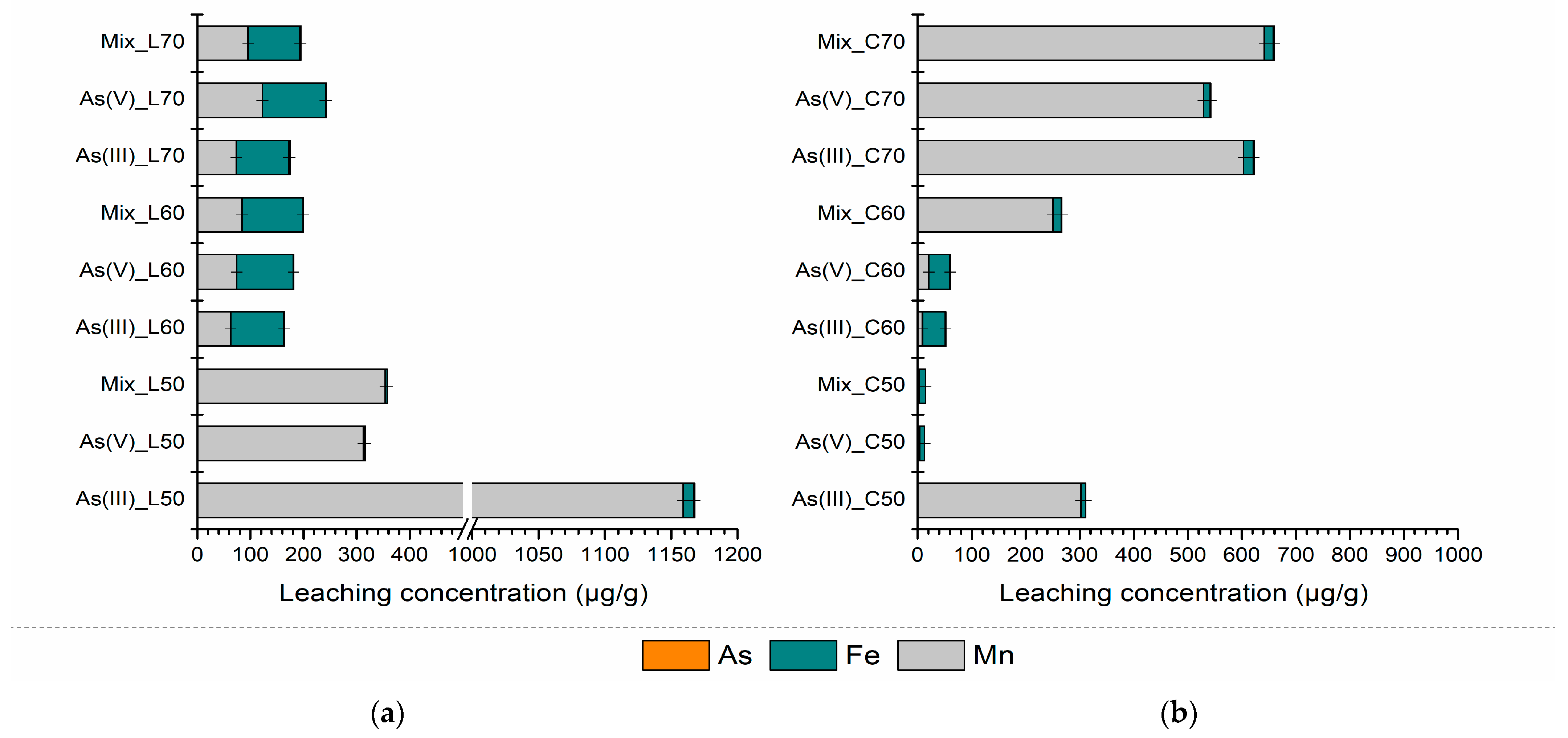
3.2.2. Leaching Characteristics and Environmental Risk Assessment of S/S PS-FMBO Nanocomposite
3.3. Stabilization of Chitosan Foam
3.3.1. Physicochemical Characteristics of Chitosan Foam
3.3.2. Sound Absorption Test
3.3.3. Chemical Stability of Chitosan Foams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tolkou, A.K.; Kyzas, G.Z.; Katsoyiannis, I.A. Arsenic(III) and Arsenic(V) Removal from Water Sources by Molecularly Imprinted Polymers (MIPs): A Mini Review of Recent Developments. Sustainability 2022, 14, 5222. [Google Scholar] [CrossRef]
- Allan, R.P.; Barlow, M.; Byrne, M.P.; Cherchi, A.; Douville, H.; Fowler, H.J.; Gan, T.Y.; Pendergrass, A.G.; Rosenfeld, D.; Swann, A.L.S.; et al. Advances in Understanding Large-Scale Responses of the Water Cycle to Climate Change. Ann. N. Y Acad. Sci. 2020, 1472, 49–75. [Google Scholar] [CrossRef] [PubMed]
- Nikić, J.; Watson, M.; Tenodi, K.Z.; Dalmacija, B.; Agbaba, J. Pilot Study on Arsenic Removal from Phosphate Rich Groundwater by In-Line Coagulation and Adsorption. J. Hazard. Mater. Adv. 2023, 10, 100280. [Google Scholar] [CrossRef]
- Agbaba, J.; Watson, M.; Kragulj Isakovski, M.; Stankov, U.; Dalmacija, B.; Tubić, A. Water Supply Systems for Settlements with Arsenic-Contaminated Groundwater—Making the Right Choice. Appl. Sci. 2023, 13, 9557. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Arsenic in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Chakraborti, D.; Rahman, M.M.; Das, B.; Nayak, B.; Pal, A.; Sengupta, M.K.; Hossain, M.A.; Ahamed, S.; Sahu, M.; Saha, K.C.; et al. Groundwater Arsenic Contamination in Ganga-Meghna-Brahmaputra Plain, Its Health Effects and an Approach for Mitigation. Environ. Earth Sci. 2013, 70, 1993–2008. [Google Scholar] [CrossRef]
- Nikić, J.; Watson, M.; Tubić, A.; Isakovski, M.K.; Maletić, S.; Mohora, E.; Agbaba, J. Arsenic Removal from Water Using a One-Pot Synthesized Low-Cost Mesoporous Fe–Mn-Modified Biosorbent. J. Serbian Chem. Soc. 2019, 84, 327–342. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2015, 13, 62. [Google Scholar] [CrossRef]
- Dixit, S.; Hering, J.G. Comparison of Arsenic(V) and Arsenic(III) Sorption onto Iron Oxide Minerals: Implications for Arsenic Mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Zhu, Z.; Zhang, L.; Zhao, N.; Fang, Y.; Zhu, Y.; Liu, G. Enhanced Arsenic Removal from Aqueous Solution by Fe/Mn-C Layered Double Hydroxide Composite. Adsorpt. Sci. Technol. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Zhang, W.; Cho, Y.; Vithanage, M.; Shaheen, S.M.; Rinklebe, J.; Alessi, D.S.; Hou, C.H.; Hashimoto, Y.; Withana, P.A.; Ok, Y.S. Arsenic Removal from Water and Soils Using Pristine and Modified Biochars. Biochar 2022, 4, 55. [Google Scholar] [CrossRef]
- Nikić, J.; Watson, M.; Jokić Govedarica, J.; Vujić, M.; Pešić, J.; Rončević, S.; Agbaba, J. Adsorption Performance of Fe–Mn Polymer Nanocomposites for Arsenic Removal: Insights from Kinetic and Isotherm Models. Materials 2024, 17, 5089. [Google Scholar] [CrossRef]
- Chang, F.; Qu, J.; Liu, H.; Liu, R.; Zhao, X. Fe-Mn Binary Oxide Incorporated into Diatomite as an Adsorbent for Arsenite Removal: Preparation and Evaluation. J. Colloid. Interface Sci. 2009, 338, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.R.; Jeon, E.K.; Yang, J.S.; Baek, K. Adsorption of As(III) and As(V) in Groundwater by Fe–Mn Binary Oxide-Impregnated Granular Activated Carbon (IMIGAC). J. Taiwan. Inst. Chem. Eng. 2017, 72, 62–69. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; Vinoth, V.; Pugazhenthiran, N.; Valdés, H.; Salvo, C.; Sepúlveda, E.; Mangalaraja, R.V. Ferrihydrite − Graphene Oxide Foams as an Efficient Adsorbent for Arsenic(III) Removal from an Aqueous Solution. Inorg. Chem. Commun. 2023, 153, 110892. [Google Scholar] [CrossRef]
- Shan, H.; Mo, H.; Liu, Y.; Zeng, C.; Peng, S.; Zhan, H. As(III) Removal by a Recyclable Granular Adsorbent through Dopping Fe-Mn Binary Oxides into Graphene Oxide Chitosan. Int. J. Biol. Macromol. 2023, 237, 124184. [Google Scholar] [CrossRef]
- Nikić, J.; Agbaba, J.; Watson, M.A.; Tubić, A.; Šolić, M.; Maletić, S.; Dalmacija, B. Arsenic Adsorption on Fe–Mn Modified Granular Activated Carbon (GAC–FeMn): Batch and Fixed-Bed Column Studies. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2019, 54, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Ociński, D. Optimization of Hybrid Polymer Preparation by Ex Situ Embedding of Waste Fe/Mn Oxides into Chitosan Matrix as an Effective As(III) and As(V) Sorbent. Environ. Sci. Pollut. Res. 2019, 26, 26026–26038. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Yacout, D.M.M.; Van Caneghem, J.; Jansson, S. Comparative Environmental Assessment of End-of-Life Carbonaceous Water Treatment Adsorbents. Bioresour. Technol. 2020, 302, 122866. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Tang, X.-F.; Ye, L.-Z.; Ma, G.-L. Assessing the Efficacy of Micro-Piles Prepared Using Polymer-Soil Mixtures for Landslide Emergency Mitigation. Constr. Build. Mater. 2024, 456, 139243. [Google Scholar] [CrossRef]
- Genua, F.; Lancellotti, I.; Leonelli, C. Geopolymer-Based Stabilization of Heavy Metals, the Role of Chemical Agents in Encapsulation and Adsorption: Review. Polymers 2025, 17, 670. [Google Scholar] [CrossRef]
- Huang, T.; Feng, Y.X.; Zhou, L.; Zhang, S. wen Enhanced Self-Cementation of Arsenic-Contaminated Soil via Activation of Non-Thermal Plasma-Irradiated Ferromanganese: A Mechanistic Investigation. Environ. Pollut. 2024, 362, 124984. [Google Scholar] [CrossRef]
- Matalkah, F.; Ababneh, A.; Aqel, R. Synthesis of Calcined Kaolin-Based Geopolymer Foam: Assessment of Mechanical Properties, Thermal Insulation, and Elevated Temperature Stability. Ceram. Int. 2023, 49, 9967–9977. [Google Scholar] [CrossRef]
- Hung Anh, L.D.; Pásztory, Z. An Overview of Factors Influencing Thermal Conductivity of Building Insulation Materials. J. Build. Eng. 2021, 44, 102604. [Google Scholar] [CrossRef]
- Li, K.; Lu, X.; Jiang, C.; Wang, D.; Zhu, J.; Xu, M.; Zhang, L.; Cheng, X. Evaluation of Leaching Characteristics of Heavy Metal Ions from Red Mud–Graphite Tailings. Toxics 2025, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, P.; Li, Z.; Chen, J.; Ke, Y.; Hu, J.; Liu, B.; Yang, J.; Liang, S.; Su, X.; et al. Iron-Calcium Reinforced Solidification of Arsenic Alkali Residue in Geopolymer Composite: Wide PH Stabilization and Its Mechanism. Chemosphere 2023, 312, 137063. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Gupta, A.K. Immobilization and Leaching Characteristics of Arsenic from Cement and/or Lime Solidified/Stabilized Spent Adsorbent Containing Arsenic. J. Hazard. Mater. 2008, 153, 434–443. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Rismanchi, B.; Ng, H.M.; Hasan, M.H.; Metselaar, H.S.C.; Muraza, O.; Aditiya, H.B. A Review on Insulation Materials for Energy Conservation in Buildings. Renew. Sustain. Energy Rev. 2017, 73, 1352–1365. [Google Scholar] [CrossRef]
- Ergun, M.E. Activated Carbon and Cellulose-Reinforced Biodegradable Chitosan Foams. Bioresources 2023, 18, 1215–1231. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, X.; Zhang, Q.; Jiang, K.; Chen, W.; Han, D. Anti-Flammability, Mechanical and Thermal Properties of Bio-Based Rigid Polyurethane Foams with the Addition of Flame Retardants. RSC Adv. 2020, 10, 32156–32161. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan Based Edible Films and Coatings: A Review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Andra, S.; Kommineni, N.; Balu, S.K.; Bulusu, R.; Boseila, A.A.; Akamo, D.O.; Ahmad, Z.; Khan, F.S.; Rahman, M.H.; et al. Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review. Materials 2022, 15, 5392. [Google Scholar] [CrossRef] [PubMed]
- Li, D.S.; Gao, G.; Huang, C.L. Chitosan Foam Reinforced by SiC Whisker for Building Insulation with High Mechanical Strength and Vapor Permeability. Sci. China Technol. Sci. 2022, 65, 2874–2882. [Google Scholar] [CrossRef]
- Tang, X.; Pikal, M.J. Design of Freeze-Drying Processes for Pharmaceuticals: Practical Advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef]
- Song, Y.; Xue, C.; Guo, W.; Bai, Y.; Shi, Y.; Zhao, Q. Foamed Geopolymer Insulation Materials: Research Progress on Insulation Performance and Durability. J. Clean. Prod. 2024, 444. [Google Scholar] [CrossRef]
- Song, W.; Xu, J.; Gao, L.; Zhang, Q.; Tong, J.; Ren, L. Preparation of Freeze-Dried Porous Chitosan Microspheres for the Removal of Hexavalent Chromium. Appl. Sci. 2021, 11, 4217. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Nowak, D. Process Parameters as Tools to Intensify the Freeze-Drying Process and Modify the Sorption Properties of the Obtained Freeze-Dried Products. Processes 2024, 12, 1932. [Google Scholar] [CrossRef]
- Li, W.L.; Lu, K.; Walz, J.Y. Freeze Casting of Porous Materials: Review of Critical Factors in Microstructure Evolution. Int. Mater. Rev. 2012, 57, 37–60. [Google Scholar] [CrossRef]
- ASTMD1557-00; Standard Test Method for Laboratory Compaction Characteristics of soil Using Modified Effort American Society for Testing Materials. ASTM: Philadelphia, PA, USA, 2000.
- U.S. Environmental Protection Agency Toxicity Characterization Leaching Procedure (TCLP), EPAMethod. EPA Method. 2002, 1311–1346.
- Carneiro, M.A.; Coelho, J.F.R.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Multi-Cycle Regeneration of Arsenic-Loaded Iron-Coated Cork Granulates for Water Treatment. J. Water Process Eng. 2022, 50, 103291. [Google Scholar] [CrossRef]
- Lv, C.; Li, Z.; Ding, J.; Huang, X.; Zhou, X.; Zheng, Z.; Cui, H.; Huang, C.; Xiang, L.; Huang, Y.; et al. Arsenate Adsorption, Desorption, and Re-Adsorption on Fe–Mn Binary Oxides: Impact of Co-Existing Ions. Desalin. Water Treat. 2024, 320, 100678. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, G.; Li, H. Efficient Removal of Arsenic from Water Using a Granular Adsorbent: Fe-Mn Binary Oxide Impregnated Chitosan Bead. Bioresour. Technol. 2015, 193, 243–249. [Google Scholar] [CrossRef]
- Simeonidis, K.; Martinez-Boubeta, C.; Rivera-Gil, P.; Ashraf, S.; Samaras, T.; Angelakeris, M.; Tresintsi, S.; Mitrakas, M.; Parak, W.J.; Monty, C.; et al. Regeneration of Arsenic Spent Adsorbents by Fe/MgO Nanoparticles. J. Chem. Technol. Biotechnol. 2017, 92, 1876–1883. [Google Scholar] [CrossRef]
- Chaudhary, B.K.; Farrell, J. Understanding Regeneration of Arsenate-Loaded Ferric Hydroxide-Based Adsorbents. Environ. Eng. Sci. 2015, 32, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Irandoust, M.; Khorshidi, F.; Feyzi, M.; Jafari, F.; Shojaeimehr, T.; Shamsipur, M. Removal of Arsenic (III) from Natural Contaminated Water Using Magnetic Nanocomposite: Kinetics and Isotherm Studies. J. Iran. Chem. Soc. 2016, 13, 1175–1188. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, Regeneration and Sustainable Management of Spent Adsorbents from Wastewater Treatment Streams: A Review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Sogut, E.; Uysal-Unalan, I.; Corredig, M. Quantification of Polystyrene Microplastics in Water, Milk, and Coffee Using Thermogravimetry Coupled with Fourier Transform Infrared Spectroscopy (TGA-FTIR). Chemosphere 2024, 368, 143777. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of Polystyrene Spheres in Different Particle Sizes and Assembly of the PS Colloidal Crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Kong, S.; Wang, Y.; Zhan, H.; Yuan, S.; Yu, M.; Liu, M. Adsorption/Oxidation of Arsenic in Groundwater by Nanoscale Fe-Mn Binary Oxides Loaded on Zeolite. Water Environ. Res. 2014, 86, 147–155. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U. Carbonation of Portland Cement Studied by Diffuse Reflection Fourier Transform Infrared Spectroscopy. Int. J. Concr. Struct. Mater. 2013, 7, 119–125. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, Z.; Zhang, X.; Chen, J. Nanostructured Iron(III)-Copper(II) Binary Oxide: A Novel Adsorbent for Enhanced Arsenic Removal from Aqueous Solutions. Water Res. 2013, 47, 4022–4031. [Google Scholar] [CrossRef]
- RS 56/2010 RS_Rulebook on Categories, Testing, Classification of Waste of 2010_56-2010_EN. 56/2010 2010.
- Council of the European Union Council Decision 2003/33/EC of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC. 2003, Volume 11, pp. 27–49.
- Rađenović, D.; Kerkez, Đ.; Pilipović, D.T.; Dubovina, M.; Grba, N.; Krčmar, D.; Dalmacija, B. Long-Term Application of Stabilization/Solidification Technique on Highly Contaminated Sediments with Environment Risk Assessment. Sci. Total Environ. 2019, 684, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Lindh, P.; Lemenkova, P. Leaching of Heavy Metals from Contaminated Soil Stabilised by Portland Cement and Slag Bremen. Ecol. Chem. Eng. S 2022, 29, 537–552. [Google Scholar] [CrossRef]
- Roy, A.; van Genuchten, C.M.; Mookherjee, I.; Debsarkar, A.; Dutta, A. Concrete Stabilization of Arsenic-Bearing Iron Sludge Generated from an Electrochemical Arsenic Remediation Plant. J. Environ. Manag. 2019, 233, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Clancy, T.M.; Snyder, K.V.; Reddy, R.; Lanzirotti, A.; Amrose, S.E.; Raskin, L.; Hayes, K.F. Evaluating the Cement Stabilization of Arsenic-Bearing Iron Wastes from Drinking Water Treatment. J. Hazard. Mater. 2015, 300, 522–529. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W. Resistance of Fly Ash Geopolymer Binders to Organic Acids. Mater. Struct./Mater. Et. Constr. 2020, 53, 115. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; De Baynast, H.; Michaud, P.; Dupont, T.; Leclaire, P. Sound Absorption Properties of a Sunflower Composite Made from Crushed Stem Particles and from Chitosan Bio-Binder. Appl. Acoust. 2016, 111, 179–187. [Google Scholar] [CrossRef]
- Wachsmann, S.B.; Ruf, M.; Prinz, C.; Oehlsen, N.; Zhou, X.; Dyballa, M.; Arweiler, C.; Leistner, P.; Steeb, H.; Garrecht, H.; et al. Chitin/Chitosan Biocomposite Foams with Chitins from Different Organisms for Sound Absorption. ACS Sustain. Chem. Eng. 2024, 12, 11879–11890. [Google Scholar] [CrossRef]
- Peng, L. Sound Absorption and Insulation Functional Composites. In Advanced High Strength Natural Fibre Composites in Construction; Woodhead Publishing: Cambridge, UK, 2017; pp. 333–373. ISBN 9780081004302. [Google Scholar]
- Lim, M.; Han, G.C.; Ahn, J.W.; You, K.S.; Kim, H.S. Leachability of Arsenic and Heavy Metals from Mine Tailings of Abandoned Metal Mines. Int. J. Environ. Res. Public Health 2009, 6, 2865–2879. [Google Scholar] [CrossRef]
- Shaumbwa, V.R.; Liu, D.; Archer, B.; Li, J.; Su, F. Preparation and Application of Magnetic Chitosan in Environmental Remediation and Other Fields: A Review. J. Appl. Polym. Sci. 2021, 138, 51241. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Q.; Qu, M.; Zhang, R.; Tang, P.; Bin, Y.; Li, S.; Zhao, W.; Wang, H. Chitosan-Based Thermal Insulation Compressible Foam Enhanced with High Performance of Piezoelectric Generation and Sensing. Carbohydr. Polym. 2022, 294, 119775. [Google Scholar] [CrossRef] [PubMed]
- Lujan, L.; Goñi, M.L.; Martini, R.E. Supporting Information Cellulose/Chitosan Biodegradable Material for Insulating Applications. ACS Sustain. Chem. Eng. 2022, 10, 12000–12008. [Google Scholar] [CrossRef]
- Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Wang, S. The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers 2022, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Dzięcioł, J.; Szlachetka, O. Waste or Raw Material? Perlite Concrete as Part of a Sustainable Materials Management Process in the Construction Sector. Sustainability 2024, 16, 6818. [Google Scholar] [CrossRef]




| Samples | Sample Name | Mass (g) | ||
|---|---|---|---|---|
| Quicklime | Cement | PS-FMBO | ||
| As(III)_50:50-PS-FMBO:L | As(III)_L50 | 10 | - | 10 |
| As(V)_50:50-PS-FMBO:L | As(V)_L50 | 10 | - | 10 |
| As(III) + As(V)_50:50-PS-FMBO:L | Mix_L50 | 10 | - | 10 |
| As(III)_40:60-PS-FMBO:L | As(III)_L60 | 12 | - | 8 |
| As(V)_40:60-PS-FMBO:L | As(V)_L60 | 12 | - | 8 |
| As(III) + As(V)_40:60-PS-FMBO:L | Mix_L60 | 12 | - | 8 |
| As(III)_30:70-PS-FMBO:L | As(III)_L70 | 14 | - | 6 |
| As(V)_30:70-PS-FMBO:L | As(V)_L70 | 14 | - | 6 |
| As(III) + As(V)_30:70-PS-FMBO:L | Mix_L70 | 14 | - | 6 |
| As(III)_50:50-PS-FMBO:C | As(III)_C50 | - | 10 | 10 |
| As(V)_50:50-PS-FMBO:C | As(V)_C50 | - | 10 | 10 |
| As(III) + As(V)_50:50-PS-FMBO:C | Mix_C50 | - | 10 | 10 |
| As(III)_40:60-PS-FMBO:C | As(III)_C60 | - | 12 | 8 |
| As(V)_40:60-PS-FMBO:C | As(V)_C60 | - | 12 | 8 |
| As(III) + As(V)_40:60-PS-FMBO:C | Mix_C60 | - | 12 | 8 |
| As(III)_30:70-PS-FMBO:C | As(III)_C70 | - | 14 | 6 |
| As(V)_30:70-PS-FMBO:C | As(V)_C70 | - | 14 | 6 |
| As(III) + As(V)_30:70-PS-FMBO:C | Mix_C70 | - | 14 | 6 |
| Immobilization Agents | Quicklime (L) | Cement (C) | |||||
|---|---|---|---|---|---|---|---|
| Samples | Leaching Concentration Metals in µg/g | Samples | Leaching Concentration Metals in µg/g | ||||
| As | Fe | Mn | As | Fe | Mn | ||
| As(III)_L50 7 d | 1.21 ± 0.016 | 25.8 ± 0.018 | 1.11 ± 0.001 | As(III)_C50 7 d | 0.47 ± 0.008 | 24.3 ± 0.010 | 0.58 ± 0.016 |
| As(III)_L50 28 d | 0.28 ± 0.010 | 29.5 ± 0.010 | 0.38 ± 0.009 | As(III)_C50 28 d | 3.26 ± 0.006 | 49.8 ± 0.012 | 4.10 ± 0.011 |
| As(V)_L50 7 d | 4.78 ± 0.009 | 364.1 ± 0.005 | 4.05 ± 0.015 | As(V)_C50 7 d | 3.38 ± 0.015 | 97.9 ± 0.005 | 84.6 ± 0.010 |
| As(V)_L50 28 d | 3.71 ± 0.010 | 758.4 ± 0.011 | 12.2 ± 0.009 | As(V)_C50 28 d | 1.33 ± 0.020 | 84.7 ± 0.019 | 1.37 ± 0.008 |
| Mix_L50 7 d | 2.78 ± 0.011 | 109.4 ± 0.018 | 10.9 ± 0.013 | Mix_C50 7 d | 0.55 ± 0.004 | 20.8 ± 0.011 | 0.47 ± 0.009 |
| Mix_L50 28 d | 1.42 ± 0.019 | 227.1 ± 0.019 | 2.16 ± 0.017 | Mix_C50 28 d | 0.61 ± 0.002 | 30.2 ± 0.005 | 1.05 ± 0.015 |
| As(III)_L60 7 d | 1.42 ± 0.019 | 53.9 ± 0.014 | 0.70 ± 0.012 | As(III)_C60 7 d | 0.74 ± 0.016 | 13.0 ± 0.018 | 38.5 ± 0.010 |
| As(III)_L60 28 d | 0.20 ± 0.007 | 15.8 ± 0.020 | 0.39 ± 0.018 | As(III)_C60 28 d | 2.34 ± 0.020 | 49.0 ± 0.014 | 5.29 ± 0.004 |
| As(V)_L60 7 d | 2.82 ± 0.007 | 67.3 ± 0.008 | 0.52 ± 0.009 | As(V)_C60 7 d | 2.06 ± 0.016 | 107.4 ± 0.019 | 0.60 ± 0.011 |
| As(V)_L60 28 d | 0.13 ± 0.011 | 19.3 ± 0.016 | 0.33 ± 0.006 | As(V)_C60 28 d | 0.68 ± 0.006 | 28.4 ± 0.009 | 0.49 ± 0.016 |
| Mix_L60 7 d | 0.25 ± 0.008 | 14.8 ± 0.003 | 2.00 ± 0.007 | Mix_C60 7 d | 0.15 ± 0.013 | 14.8 ± 0.016 | 1.38 ± 0.019 |
| Mix_L60 28 d | 0.09 ± 0.015 | 49.6 ± 0.008 | 0.82 ± 0.003 | Mix_C60 28 d | 0.55 ± 0.009 | 28.0 ± 0.001 | 1.52 ± 0.016 |
| As(III)_L70 7 d | 1.13 ± 0.004 | 23.9 ± 0.006 | 0.64 ± 0.013 | As(III)_C70 7 d | 2.68 ± 0.018 | 27.7 ± 0.003 | 0.63 ± 0.019 |
| As(III)_L70 28 d | 0.44 ± 0.016 | 22.4 ± 0.012 | 0.20 ± 0.013 | As(III)_C70 28 d | 1.31 ± 0.002 | 34.7 ± 0.009 | 1.01 ± 0.003 |
| As(V)_L70 7 d | 2.30 ± 0.016 | 56.4 ± 0.019 | 0.69 ± 0.018 | As(V)_C70 7 d | 3.43 ± 0.002 | 28.3 ± 0.008 | 7.22 ± 0.007 |
| As(V)_L70 28 d | 0.81 ± 0.003 | 11.7 ± 0.005 | 0.44 ± 0.002 | As(V)_C70 28 d | 0.44 ± 0.011 | 42.7 ± 0.015 | 1.49 ± 0.018 |
| Mix_L70 7 d | 1.91 ± 0.018 | 45.6 ± 0.014 | 0.58 ± 0.002 | Mix_C70 7 d | 1.61 ± 0.002 | 20.2 ± 0.006 | 0.32 ± 0.007 |
| Mix_L70 28 d | 1.38 ± 0.002 | 62.6 ± 0.009 | 1.53 ± 0.006 | Mix_C70 28 d | 3.48 ± 0.008 | 18.5 ± 0.009 | 8.43 ± 0.002 |
| Immobilization Agents | Quicklime (L) | Cement (C) | |||||
|---|---|---|---|---|---|---|---|
| Samples | Leaching Concentration Metals in µg/g | Samples | Leaching Concentration Metals in µg/g | ||||
| As | Fe | Mn | As | Fe | Mn | ||
| As(III)_L50 7 d | 0.48 ± 0.010 | 9.56 ± 0.008 | 1551.1 ± 0.019 | As(III)_C50 7 d | 0.35 ± 0.018 | 11.4 ± 0.016 | 4.01 ± 0.001 |
| As(III)_L50 28 d | 0.34 ± 0.007 | 9.01 ± 0.005 | 1231.7 ± 0.003 | As(III)_C50 28 d | 0.53 ± 0.016 | 10.8 ± 0.011 | 4.16 ± 0.006 |
| As(V)_L50 7 d | 4.47 ± 0.009 | 5.94 ± 0.006 | 423.0 ± 0.007 | As(V)_C50 7 d | 0.39 ± 0.010 | 11.2 ± 0.010 | 5.97 ± 0.009 |
| As(V)_L50 28 d | 0.15 ± 0.011 | 5.27 ± 0.011 | 326.9 ± 0.018 | As(V)_C50 28 d | 0.30 ± 0.003 | 10.0 ± 0.008 | 4.00 ± 0.007 |
| Mix_L50 7 d | 1.23 ± 0.005 | 5.70 ± 0.012 | 510.7 ± 0.007 | Mix_C50 7 d | 0.21 ± 0.005 | 12.7 ± 0.009 | 5.64 ± 0.015 |
| Mix_L50 28 d | 0.24 ± 0.008 | 5.12 ± 0.018 | 498.4 ± 0.002 | Mix_C50 28 d | 0.32 ± 0.008 | 12.0 ± 0.015 | 3.10 ± 0.003 |
| As(III)_L60 7 d | 2.86 ± 0.014 | 114.0 ± 0.019 | 94.6 ± 0.011 | As(III)_C60 7 d | 0.46 ± 0.011 | 47.9 ± 0.010 | 9.72 ± 0.009 |
| As(III)_L60 28 d | 0.51 ± 0.015 | 102.3 ± 0.005 | 80.75 ± 0.018 | As(III)_C60 28 d | 0.30 ± 0.006 | 42.6 ± 0.004 | 9.15 ± 0.013 |
| As(V)_L60 7 d | 1.29 ± 0.009 | 122.7 ± 0.005 | 106.5 ± 0.008 | As(V)_C60 7 d | 1.47 ± 0.018 | 43.6 ± 0.011 | 15.4 ± 0.013 |
| As(V)_L60 28 d | 0.66 ± 0.004 | 118.5 ± 0.014 | 94.1 ± 0.010 | As(V)_C60 28 d | 0.75 ± 0.012 | 39.6 ± 0.016 | 20.3 ± 0.013 |
| Mix_L60 7 d | 3.30 ± 0.001 | 123.4 ± 0.014 | 122.5 ± 0.015 | Mix_C60 7 d | 0.27 ± 0.019 | 20.0 ± 0.019 | 261.7 ± 0.017 |
| Mix_L60 28 d | 0.01 ± 0.016 | 121.5 ± 0.009 | 100.9 ± 0.005 | Mix_C60 28 d | 0.85 ± 0.019 | 18.9 ± 0.016 | 250.3 ± 0.018 |
| As(III)_L70 7 d | 1.39 ± 0.009 | 110.1 ± 0.002 | 75.8 ± 0.004 | As(III)_C70 7 d | 2.20 ± 0.014 | 25.1 ± 0.019 | 1942.6 ± 0.012 |
| As(III)_L70 28 d | 1.51 ± 0.016 | 105.3 ± 0.001 | 82.4 ± 0.011 | As(III)_C70 28 d | 1.43 ± 0.005 | 20.1 ± 0.003 | 852.1 ± 0.002 |
| As(V)_L70 7 d | 3.63 ± 0.015 | 126.7 ± 0.018 | 140.9 ± 0.016 | As(V)_C70 7 d | 1.77 ± 0.020 | 21.7 ± 0.007 | 1435.1 ± 0.018 |
| As(V)_L70 28 d | 1.36 ± 0.016 | 123.6 ± 0.009 | 142.6 ± 0.018 | As(V)_C70 28 d | 1.38 ± 0.014 | 15.4 ± 0.018 | 744.1 ± 0.002 |
| Mix_L70 7 d | 2.41 ± 0.007 | 112.9 ± 0.010 | 95.4 ± 0.016 | Mix_C70 7 d | 1.37 ± 0.008 | 27.2 ± 0.007 | 1207.4 ± 0.009 |
| Mix_L70 28 d | 1.29 ± 0.003 | 100.8 ± 0.015 | 102.3 ± 0.019 | Mix_C70 28 d | 1.44 ± 0.009 | 19.3 ± 0.002 | 826.4 ± 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujić, M.; Nikić, J.; Vijatovic Petrovic, M.; Pejin, Đ.; Watson, M.; Rončević, S.; Agbaba, J. End-of-Life Management Strategies for Fe–Mn Nanocomposites Used in Arsenic Removal from Water. Polymers 2025, 17, 1353. https://doi.org/10.3390/polym17101353
Vujić M, Nikić J, Vijatovic Petrovic M, Pejin Đ, Watson M, Rončević S, Agbaba J. End-of-Life Management Strategies for Fe–Mn Nanocomposites Used in Arsenic Removal from Water. Polymers. 2025; 17(10):1353. https://doi.org/10.3390/polym17101353
Chicago/Turabian StyleVujić, Maja, Jasmina Nikić, Mirjana Vijatovic Petrovic, Đorđe Pejin, Malcolm Watson, Srđan Rončević, and Jasmina Agbaba. 2025. "End-of-Life Management Strategies for Fe–Mn Nanocomposites Used in Arsenic Removal from Water" Polymers 17, no. 10: 1353. https://doi.org/10.3390/polym17101353
APA StyleVujić, M., Nikić, J., Vijatovic Petrovic, M., Pejin, Đ., Watson, M., Rončević, S., & Agbaba, J. (2025). End-of-Life Management Strategies for Fe–Mn Nanocomposites Used in Arsenic Removal from Water. Polymers, 17(10), 1353. https://doi.org/10.3390/polym17101353












