Review of Biopolymer Polyhydroxybutyrate (PHB) and Blends: Modification of Thermal and Mechanical Properties via Additive Manufacturing Processing
Abstract
1. Introduction
- Evaluate a rational design of bioplastics’ properties for real use (e.g., packaging).
- Discuss PHB and bio-blends/plasticizers and their potential to enhance the mechanical properties of bio-composites.
- Investigate PHB and blends using the commercially scalable processes, including hot-processing, chemical melt extrusion, and additive manufacturing techniques.
- Compare the carbon footprint and environmental impacts of PHB bioplastics with varying recycling strategies.
2. Background
2.1. General Aspects About Bioplastics
2.2. Structure and Properties of PHB and Its Copolymers
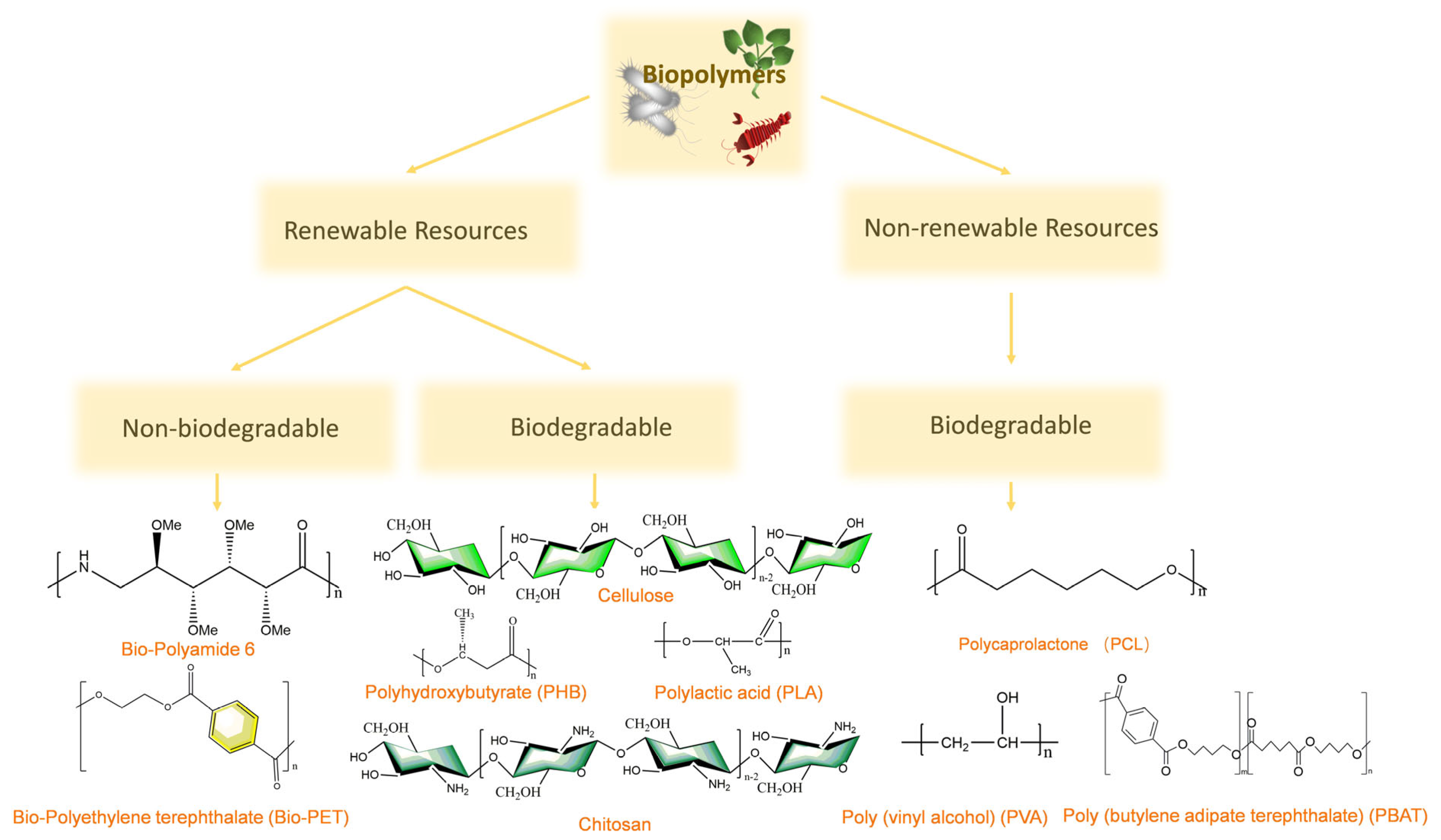
2.2.1. Biopolymers
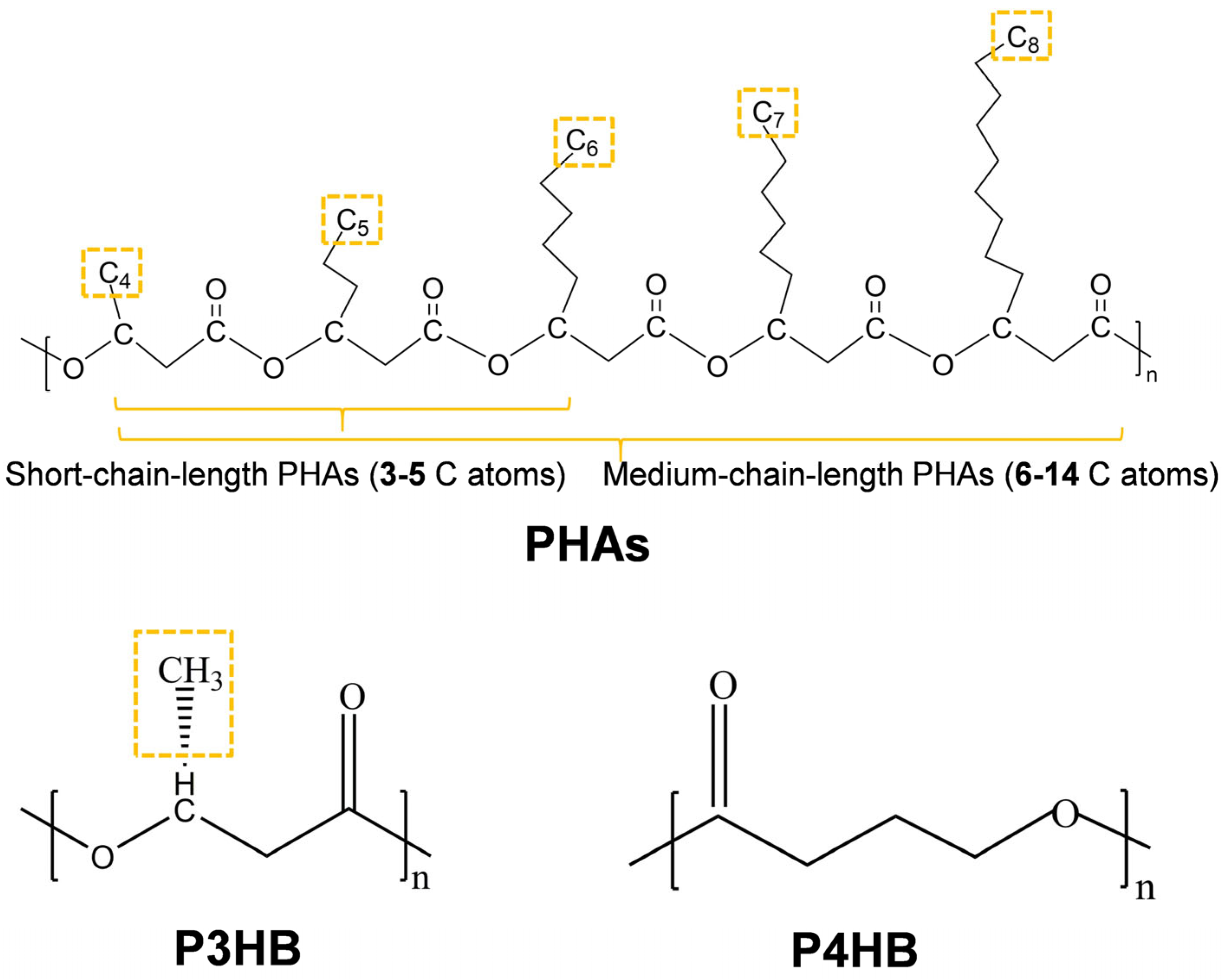
2.2.2. Polyhydroxybutytrate (PHB)
PHB Production
PHB Properties
3. Predictive Models and Future Perspectives on PHB Degradation
3.1. Microbial and Enzymatic Degradation
3.2. Key Degrading Microorganisms and Their Mechanisms
- Molecular-level analysis of degradation mechanisms: in-depth study of the structure and function of PHA depolymerases to elucidate their interaction mechanisms with PHB surfaces.
- Effects of environmental factors: systematic investigation of the influence of temperature, humidity, pH, salinity, and other environmental parameters on PHB degradation.
- Regulation of microbial communities: exploring methods to enhance PHB degradation efficiency by modulating microbial community structures.
- Optimization of predictive models: integrating big data and machine learning technologies to improve the accuracy and applicability of PHB degradation prediction models.
4. Current Industry Processing Approaches
4.1. Additive Manufacturing Processing
4.1.1. Fused Deposition Modeling (FDM)
4.1.2. Powder/Gel Extrusion
4.1.3. Stereolithography (SLA)
4.1.4. Electrospinning
5. PHB Composites
5.1. Blends Using Biopolymers
5.1.1. PCL and PBAT
5.1.2. Nanoclay-Based
5.1.3. Natural Fibers
5.1.4. Natural Rubber
5.2. Plasticizers
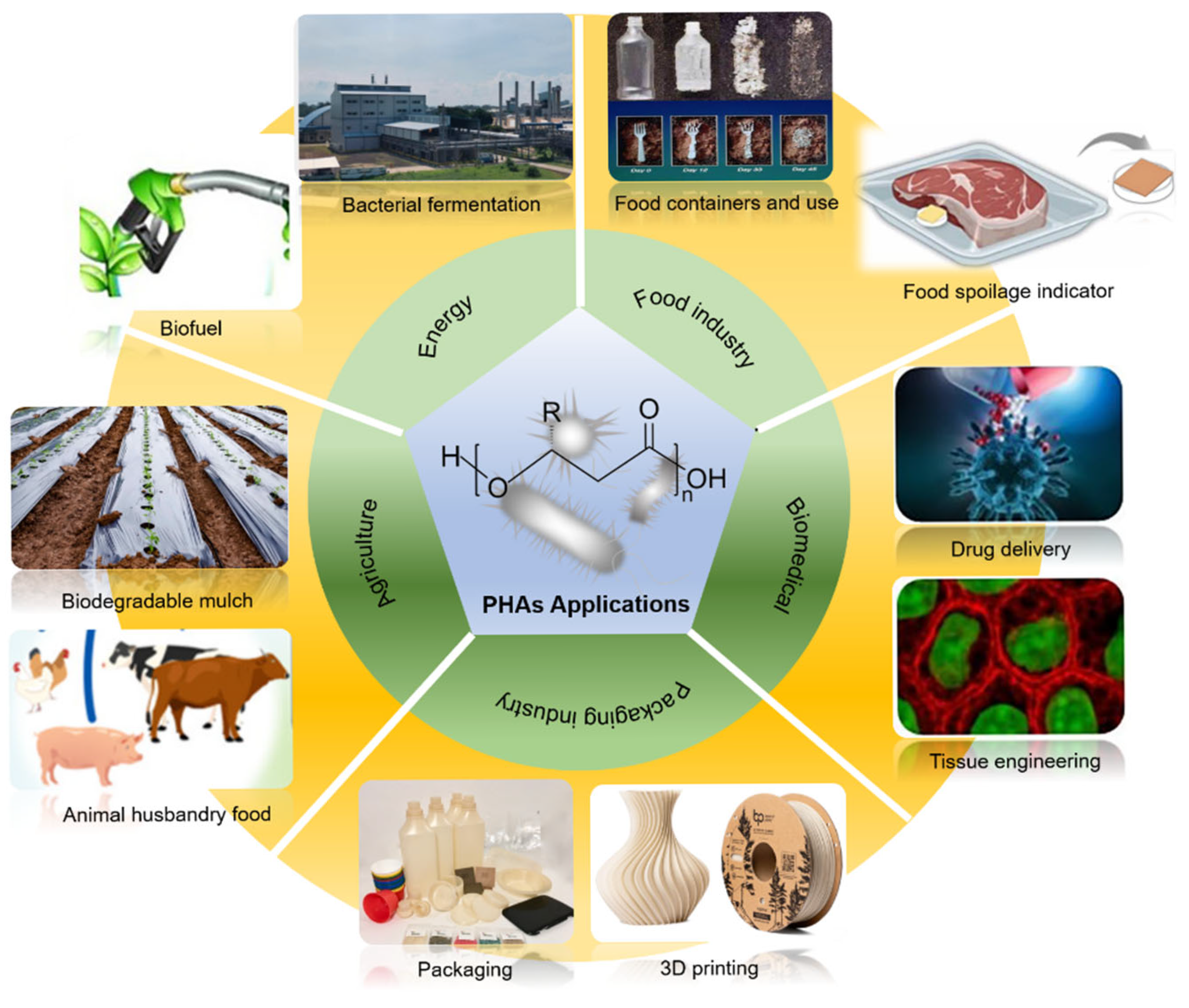
6. Applications
6.1. Traditional Use-Food Packaging
6.2. Emerging Applications
7. Discussion
- Cost Competitiveness: PHA production costs are currently higher than those of traditional plastics. Research efforts will focus on improving fermentation processes, exploring alternative feedstocks, and scaling up production to reduce costs and increase competitiveness.
- Performance Optimization: Enhancing the mechanical, thermal, and barrier properties of PHA-based materials through advanced processing techniques and innovative additives remains a critical area of research.
- Regulatory Frameworks: Developing supportive regulatory frameworks and standards for PHAs, particularly regarding biodegradability claims, actual on-site GWP values data, and end-of-life management, will be essential to ensure market acceptance and environmental impact assessment consistency.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Wang, Y.; Chen, X.; Yu, X.; Li, W.; Zhang, S.; Meng, X.; Zhao, Z.M.; Dong, T.; Anderson, A.; et al. Sustainable bioplastics derived from renewable natural resources for food packaging. Matter 2023, 6, 97–127. [Google Scholar] [CrossRef]
- Abu Bakar, A.A.; Zainuddin, M.Z.; Abdullah, S.M.; Tamchek, N.; Mohd Noor, I.S.; Alauddin, M.S.; Alforidi, A.; Ghazali, M.I.M. The 3D Printability and Mechanical Properties of Polyhydroxybutyrate (PHB) as Additives in Urethane Dimethacrylate (UDMA) Blends Polymer for Medical Application. Polymers 2022, 14, 4518. [Google Scholar] [CrossRef]
- Morris, M.R.; Stanton, A.; Blomberg, T.; Hicks, A. Human behavior outcomes at point of disposal of a biodegradable plastic cup at a U.S.-based university campus. Resour. Conserv. Recycl. 2024, 203, 107412. [Google Scholar] [CrossRef]
- Benavides, P.T.; Dunn, J.B.; Han, J.; Biddy, M.; Markham, J. Exploring Comparative Energy and Environmental Benefits of Virgin, Recycled, and Bio-Derived PET Bottles. ACS Sustain. Chem. Eng. 2018, 6, 9725–9733. [Google Scholar] [CrossRef]
- Müller Carneiro, J.; Figueirêdo, M.C.B.; Rodrigues, C.; Azeredo, H.M.C.; Freire, F. Ex-ante life cycle assessment framework and application to a nano-reinforced biopolymer film based on mango kernel. Resour. Conserv. Recycl. 2023, 188, 106637. [Google Scholar] [CrossRef]
- Lee, J.G.; Raj, R.R.; Day, N.B.; Shields, C.W.I. Microrobots for Biomedicine: Unsolved Challenges and Opportunities for Translation. ACS Nano 2023, 17, 14196–14204. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Takkellapati, S.; Riegerix, R.C. Recycling of Plastics in the United States: Plastic Material Flows and Polyethylene Terephthalate (PET) Recycling Processes. ACS Sustain. Chem. Eng. 2022, 10, 2084–2096. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy-Towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Fu, X.; Xu, H.; Zhang, Q.; Xi, J.; Zhang, H.; Zheng, M.; Xi, B.; Hou, L. A review on polyhydroxyalkanoates production from various organic waste streams: Feedstocks, strains, and production strategy, Resources. Conserv. Recycl. 2023, 198, 107166. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Ojumu, T.V.; Yu, J.; Solomon, B.O. Production of polyhydroxyalkanoates, a bacterial biodegradable polymers. Afr. J. Biotechnol. 2004, 3, 18–24. [Google Scholar] [CrossRef]
- Utsunomia, C.; Ren, Q.; Zinn, M. Poly(4-Hydroxybutyrate): Current State and Perspectives. Front. Bioeng. Biotechnol. 2020, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.A.D.; Oliveira-Filho, E.R.; Piccoli, R.A.M.; Gomez, J.G.C.; Silva, L.F. Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] biotechnological production: Challenges and opportunities. Biomass Convers. Biorefin. 2024, 14, 26631–26650. [Google Scholar] [CrossRef]
- Mitra, R.; Xiang, H.; Han, J. Current Advances towards 4-Hydroxybutyrate Containing Polyhydroxyalkanoates Production for Biomedical Applications. Molecules 2021, 26, 7244. [Google Scholar] [CrossRef]
- Hou, X.A.; Sun Wen Liu, Z.B.; Liu, S.Q.; Chee, J.; Yeo, C.; Lu, X.H.; He, C.B. Tailoring crystalline morphology via entropy-driven miscibility: Toward ultratough, biodegradable, and durable polyhydroxybutyrate. Macromolecules 2022, 55, 5527–5534. [Google Scholar] [CrossRef]
- Ding, Y.; Li, M.; Dong, W.; Kan, Z.; Li, Z.B. Favorable compatibility efficiency and thermal stability of PLA/P4HB/PGMA blends contributed by phase interface-located chain expansion reaction. Polym. Degrad. Stab. 2025, 232, 111159. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-food wastes for bioplastics: European prospective on possible applications in their second life for a circular economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef]
- Zhang, Z.; Gowda, R.R.; Chen, Y.X. Chemosynthetic P4HB: A Ten-Year Journey from a Non-Polymerizable Monomer to a High-Performance Biomaterial. Acc. Mater. Res. 2024, 5, 1340–1352. [Google Scholar] [CrossRef]
- Lemoigne, M. Produits de déshydratation et de polymérisation de l’acide β-oxobutyrique. Bull. Société Chim. Biol. 1927, 9, 456–467. [Google Scholar]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.Q.; Pandey, A.; Gnansounou, E.; Lin, K.Y.A.; Daniel, C.W.; Tsang Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects—ScienceDirect. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Roblero, M.A. Evaluation of Fed-Batch Fermentation for Production of Polyhydroxybutyrate with a Banana Pulp Juice Substrate from an Agro Industrial By-Product. Front. Sustain. Food Syst. 2021, 5, 9. [Google Scholar] [CrossRef]
- Wang, J.F.; Huang, J.Q.; Liu, S.J. The production, recovery, and valorization of polyhydroxybutyrate (PHB) based on circular bioeconomy. Biotechnol. Adv. 2024, 72, 108340. [Google Scholar] [CrossRef] [PubMed]
- Zytner, P.; Kumar, D.; Elsayed, A.; Mohanty, A.; Ramaraoc, B.V.; Misra, M. A review on polyhydroxyalkanoate (PHA) production through the use of lignocellulosic biomass. RSC Sustain. 2023, 1, 2120–2134. [Google Scholar] [CrossRef]
- Nath, D.; Misra, M.; Al-Daoud, F.; Mohanty, A.K. Studies on poly (butylene succinate) and poly (butylene succinate-co-adipate)-based biodegradable plastics for sustainable flexible packaging and agricultural applications: A comprehensive review. RSC Sustain. 2025, 3, 1267–1302. [Google Scholar] [CrossRef]
- Kanabenja, W.; Passornraprasit, N.; Aumnate, C.; Tim, A.; Osswald, T.A.; Aht-Ong, D.; Potiyaraj, P. Enhancing 3D printability of polyhydroxybutyrate (PHB) and poly (3-hydroxybutyrate-co-3-hydroxy valerate) (PHBV) based blends through melt extrusion based-3D printing. Addit. Manuf. 2024, 86, 104205. [Google Scholar] [CrossRef]
- Modi, S.; Koelling, K.; Vodovotz, Y. Assessment of PHB with varying hydroxyvalerate content for potential packaging applications. Eur. Polym. J. 2011, 47, 179–186. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, Z.; Sun, X.; Turng, L.S.; Peng, X.f. Morphology and properties of injection molded solid and microcellular polylactic acid/polyhydroxybutyrate-valerate (PLA/PHBV) blends. Ind. Eng. Chem. Res. 2013, 52, 2569–2581. [Google Scholar] [CrossRef]
- Choi, S.Y.; Cho, I.J.; Lee, Y.; Kim, Y.J.; Kim, K.J.; Lee, S.Y. Microbial polyhydroxyalkanoates and nonnatural polyesters. Adv. Mater. 2020, 32, 1907138. [Google Scholar] [CrossRef]
- Martin, D.P.; Williams, S.F. Medical applications of poly-4-hydroxybutyrate: A strong flexible absorbable biomaterial. Biochem. Eng. J. 2003, 16, 97–105. [Google Scholar] [CrossRef]
- Yang, X.Y.; Luo, H.Q.; Zhou, R.Y.; Wei, C.Y.; Deng, J.; Luo, J.H.; Yan, X.F.; Yu, K.; Yuan, S.; Zhou, W. Miscibility, crystallization and morphology in the novel polylactide/poly(4-hydroxybutyrate) blends. Addit. Manuf. 2024, 309, 104205. [Google Scholar] [CrossRef]
- Williams, S.F.; Rizk, S.; Martin, D.P. Poly-4-hydroxybutyrate (P4HB): A new generation of resorbable medical devices for tissue repair and regeneration. Biomed. Technol. 2013, 58, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.F.; Martin, D.P.; Moses, A.C. The history of GalaFLEX P4HB scaffold. Aesthetic Surg. J. 2016, 36, S33–S42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Zelzer, M.; Ulijn, R.V. 6—Enzyme-responsive polymers: Properties, synthesis and applications. In Smart Polymers and Their Applications; Aguilar, M.R., Román, J.S., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 166–203. [Google Scholar]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Kinetics and mechanism of the sonolytic conversion of the aqueous perfluorinated surfactants, perfluorooctanoate (PFOA), and perfluorooctane sulfonate (PFOS) into inorganic products. J. Phys. Chem. A 2008, 112, 4261–4270. [Google Scholar] [CrossRef]
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; Connor, K. A review on biological synthesis of the biodegradable polymers polyhydroxyalkanoates and the development of multiple applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Koller, M.; Heeney, D.; Mukherjee, A. Biodegradability of polyhydroxyalkanoate (PHA) biopolyesters in nature: A review. Biodegradation 2025, 36, 76. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Lee, J.K. Exploiting Polyhydroxyalkanoates for Biomedical Applications. Polymers 2023, 15, 20734360. [Google Scholar] [CrossRef]
- Keridou, I.; Franco, L.; Valle, L.J.D.; Juan, C.; Funk, L.; Turon, P.; Puiggalí, J. Hydrolytic and enzymatic degradation of biobased poly(4-hydroxybutyrate) films. Selective etching of spherulites. Polym. Degrad. Stab. 2021, 183, 109451. [Google Scholar] [CrossRef]
- Fernandes Cavaleiro, M.Â. Biodegradation of PHA/PBAT Packaging Materials by Soil Microorganisms. Ph.D. Thesis, Universidade do Minho, Braga, Portugal, 2023. [Google Scholar]
- Tran, D.H.; Rubarth, C.; Leeds, S.G.; Fair, L.; Gowan, T.M.; Ramakrishnan, S.; Shabbir, R.; Ogola, G.; Ward, M.A.; Aladegbami, B. The use of poly-4-hydroxybutyrate (P4HB, Phasix™) mesh in ventral hernia repair: A systematic review and meta-analysis. Hernia 2024, 28, 989–1004. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2016, 37, 24–38. [Google Scholar] [CrossRef]
- Lenz, R.W.; Marchessault, R.H. Bacterial Polyesters: Biosynthesis, Biodegradable Plastics and Biotechnology | Biomacromolecules. Biomacromolecules 2005, 6, 1–8. [Google Scholar] [CrossRef]
- D6691-17; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment by a Defined Microbial Consortium or Natural Sea Water Inoculum. ASTM International: West Conshohocken, PA, USA, 2017.
- D7473/D7473M-21; Standard Test Method for Weight Attrition of Non-Floating Plastic Materials by Open System Aquarium Incubations. ASTM International: West Conshohocken, PA, USA, 2021.
- D7991-15; Standard Test Method for Determining Aerobic Biodegradation of Plastics Buried in Sandy Marine Sediment under Controlled Laboratory Conditions. ASTM International: West Conshohocken, PA, USA, 2015.
- D5338-15; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials under Controlled Composting Conditions, Incorporating Thermophilic Temperatures. ASTM International: West Conshohocken, PA, USA, 2015.
- Dintcheva, N. Overview of polymers and biopolymers degradation and stabilization towards sustainability and materials circularity. Polymer 2024, 306, 127136. [Google Scholar] [CrossRef]
- Uefuji, M.; Kasuya, K.-I.; Doi, Y. Enzymatic degradation of poly[(R)-3-hydroxybutyrate]: Secretion and properties of PHB depolymerase from Pseudomonas stutzeri. Polym. Degrad. Stab. 1997, 58, 275–281. [Google Scholar] [CrossRef]
- Yoshie, N.; Oike, Y.; Kasuya, K.-I.; Doi, Y.; Inoue, Y. Change of Surface Structure of Poly(3-hydroxybutyrate) Film upon Enzymatic Hydrolysis by PHB Depolymerase. Biomacromolecules 2002, 3, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, S.; Singh, D.; Mittal, N.; Srivastava, G.; Siddiqui, S.; Faridi, S.A.; Siddiqui, M.H. Polyhydroxybutyrate biosynthesis from different waste materials, degradation, and analytic methods: A short review. Polym. Bull. 2023, 80, 5965–5997. [Google Scholar] [CrossRef]
- Kwon, S. A Review of Biodegradation Mechanisms and Evaluation Methods for Biobased Materials: Polysaccharides, Lignin, and Biopolyesters. J. Korea TAPPI 2025, 57, 5–19. [Google Scholar] [CrossRef]
- Zhang, X.; Fevre, M.; Jones, O.J.; Waymouth, R.M. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 2018, 118, 839–885. [Google Scholar] [CrossRef]
- Lott, C.; Eich, A.; Makarow, D.; Unger, B.; Eekert, M.V.; Schuman, E.; Reinach, M.S.; Lasut, M.T.; Weber, M. Half-life of biodegradable plastics in the marine environment depends on material, habitat, and climate zone. Front. Mar. Sci. 2021, 8, 662074. [Google Scholar] [CrossRef]
- Jendrossek, D.; Frisse, A.; Behrends, A.; Kratzin, H.D.; Stanislawski, T.; Schlegel, H.G. Biochemical and molecular characterization of the Pseudomonas lemoignei polyhydroxyalkanoate depolymerase system. J. Bacteriol. 1995, 177, 596–607. [Google Scholar] [CrossRef]
- Zhang, L.; Tsui, T.H.; Fu, J.; Dai, Y.J.; Tong, Y.W. Valorization of poly-β-hydroxybutyrate (PHB)-based bioplastic waste in anaerobic digesters of food waste for bioenergy generation: Reactor performance, microbial community analysis, and bioplastic biodegradation. Carbon Neutrality 2022, 1, 8. [Google Scholar] [CrossRef]
- Hernandez, M.M.; Gupta, N.S.; Lee, K.S.; Pital, A.C.; Marrone, B.L.; Iverson, C.N.; Dumont, J.H. Characterization of Polyhydroxybutyrate-Based Composites Prepared by Injection Molding. Polymers 2021, 13, 4444. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.; Fernandes, B.; Covas, J.A.; Vicente, A.A.; Hilliou, L. Film blowing of PHBV blends and PHBV-based multilayers for the production of biodegradable packages. J. Appl. Polym. Sci. 2016, 133, 42165. [Google Scholar] [CrossRef]
- Teixeira, P.F.; Covas, J.A.; Suarez, M.J.; Angulo, I.; Hilliou, L. Film Blowing of PHB-Based Systems for Home Compostable Food Packaging. Int. Polym. Process. 2020, 35, 440–447. [Google Scholar] [CrossRef]
- Zambaux, M.F.; Bonneaux, F.; Gref, R.; Maincent, P.; Dellacherie, E.; Alonso, M.J.; Labrude, P.; Vigneron, C. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. J. Control. Release 1998, 50, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Marcello, E.; Nigmatullin, R.; Basnett, P.; Maqbool, M.; Prieto, A.; Knowles, J.C.; Boccaccini, A.R.; Roy, I. 3D Melt-Extrusion Printing of Medium Chain Length Polyhydroxyalkanoates and Their Application as Antibiotic-Free Antibacterial Scaffolds for Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 18. [Google Scholar] [CrossRef]
- Bossu, J.; Le Moigne, N.; Dieudonne-George, P.; Dumazert, L.; Guillard, V.; Coussy, H.A. Impact of the processing temperature on the crystallization behavior and mechanical properties of poly [R-3-hydroxybutyrate-co-(R-3-hydroxyvalerate)]. Polym. Int. J. Sci. Technol. Polym. 2021, 229, 123987. [Google Scholar] [CrossRef]
- Bucci, D.; Tavares, L.; Sell, I. PHB packaging for the storage of food products. Polym. Test. 2005, 24, 564–571. [Google Scholar] [CrossRef]
- Getova Vasilena, E.; Pascual, A.; Dijkstra, R.; Castilla-Casado, C.; Van Bochove, B.; Van Osch, G.J.V.M.; Bernsen, M.R.; Moreira Teixeira, L.S. Multilayered Tissue Assemblies Through Tuneable Biodegradable Polyhydroxyalkanoate Polymer (Mesh)-Reinforced Organ-Derived Extracellular Matrix Hydrogels. Gels 2025, 11, 539. [Google Scholar] [CrossRef]
- Ďurfina, M.; Babaei, N.; Vanovčanová, Z.; Křivská, B.; Kucharczyk, P.; Sedlařík, V.; Hodan, J.; Kučka, J.; Sochorová, M.; Rychtera, M.; et al. Bio-Based Polyhydroxyalkanoate (PHA) Blends for 3D Printing: Rheological, Mechanical, Biocompatibility, and Biodegradation Properties. Polymers 2025, 17, 1477. [Google Scholar] [CrossRef]
- Buyuksoy-Fekraoui, K.; Mallet, K.; Benguigui, L.; Soulestin, J.; Lacrampe, M.-F.; Krawczak, P. Characterization of optimized ternary PLA/PHB/organoclay composites processed through fused filament fabrication and injection molding. Materials 2022, 15, 3398. [Google Scholar] [CrossRef]
- Strangis, G.; Labardi, M.; Gallone, G.; Milazzo, M.; Capaccioli, S.; Forli, F.; Cinelli, P.; Berrettini, S.; Seggiani, M.; Danti, S.; et al. 3D Printed Piezoelectric BaTiO3/Polyhydroxybutyrate Nanocomposite Scaffolds for Bone Tissue Engineering. Bioengineering 2024, 11, 193. [Google Scholar] [CrossRef]
- Kanabenja, W.; Passarapark, K.; Subchokpool, T.; Nawaaukkaratharnant, N.; Román, A.J.; Osswald, T.A.; Aumnate, C.; Potiyaraj, P. 3D printing filaments from plasticized Polyhydroxybutyrate/Polylactic acid blends reinforced with hydroxyapatite. Addit. Manuf. 2022, 59, 103130. [Google Scholar] [CrossRef]
- Carvalho, D.; Gomes, J.; Zanini, N.C.; Claro, A.M.; Amaral, D.; Cavichiolli, N.; Barud, H.S.; Mulinari, D.R. Composite filaments OF PHBV reinforced with ZrO2·nH2O particles for 3D printing. Polym. Bull. 2022, 79, 2113–2132. [Google Scholar] [CrossRef]
- D’arienzo, L.; Acierno, S.; Patti, A.; Di Maio, L. Cellulose/Polyhydroxybutyrate (PHB) Composites as a Sustainable Bio-Based Feedstock to 3D-Printing Applications. Materials 2024, 17, 916. [Google Scholar] [CrossRef]
- Şahin, G.; Özyıldırım, H.; Şahin, A. Investigation of mechanical and printing properties of poly (lactic acid) and its composite filaments used in 3D printing. Iran. Polym. J. 2024, 33, 79–91. [Google Scholar] [CrossRef]
- Dan, L.; Zhang, Y.; Zhang, L.; Liu, Y.; Li, Y.; Chen, Y. Three-dimensional printed and biocompatible conductive composites comprised of polyhydroxybutyrate and multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2021, 60, 885–897. [Google Scholar] [CrossRef]
- Zainuddin, M.Z.; Abu Bakar, A.A.; Adam, A.N.; Abdullah, S.M.; Tamchek, N.; Alauddin, M.S.; Mahat, M.M.; Wiwatcharagoses, N.; Alforidi, A.; Ghazali, M.I.M. Mechanical and Structural Properties of Polyhydroxybutyrate as Additive in Blend Material in Additive Manufacturing for Medical Applications. Polymers 2023, 15, 1849. [Google Scholar] [CrossRef] [PubMed]
- Menčík, P.; Přikryl, R.; Krobot, Š.; Melčová, V.; Kontárová, S.; Plavec, R.; Bočkaj, J.; Horváth, V.; Alexy, P. Evaluation of the Properties of PHB Composite Filled with Kaolin Particles for 3D Printing Applications Using the Design of Experiment. Int. J. Mol. Sci. 2022, 23, 14409. [Google Scholar] [CrossRef] [PubMed]
- Moroni, S.; Khorshid, S.; Aluigi, A.; Tiboni, M.; Casettari, L. Poly(3-hydroxybutyrate): A potential biodegradable excipient for direct 3D printing of pharmaceuticals. Int. J. Pharm. 2022, 623, 121960. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Kridiotis, P.; Vergel, A.J.; Medved, Z.; Barbosa, R.; Werker, A. Direct melt extrusion of polyhydroxyalkanoate solvent-rich gels after polymer extraction and melt processing with integrated solvent recovery. J. Clean. Prod. 2025, 517, 8. [Google Scholar] [CrossRef]
- Wei, L.Q.; Armando, G.; Nicole MStark, M. Grafting of Bacterial Polyhydroxybutyrate (PHB) onto Cellulose via In Situ Reactive Extrusion with Dicumyl Peroxide. Biomacromolecules 2015, 16, 1040–1049. [Google Scholar] [CrossRef]
- Aguilar-De-Leyva, N.; Casas, M.; Ferrero, C.; Muñoz-Rubio, A.; Velasco, D.; Santoveña, B. 3D Printing Direct Powder Extrusion in the Production of Drug Delivery Systems: State of the Art and Future Perspectives. Pharmaceutics 2024, 16, 437. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Raza, Z.A.; Naeem, A.R.; Shafi, R.; Abid, S. Chitosan-incorporated poly(hydroxybutyrate) porous electrospun scaffold for potential biomedical applications. Polym. Bull. 2024, 81, 1691–1705. [Google Scholar] [CrossRef]
- Zhao, X.; Niu, Y.; Mi, C.; Gong, H.; Yang, X.; Cheng, J.; Zhou, Z.; Liu, J.; Peng, X.; Wei, D. Electrospinning nanofibers of microbial polyhydroxyalkanoates for applications in medical tissue engineering. J. Polym. Sci. 2021, 59, 1994–2013. [Google Scholar] [CrossRef]
- Chiesa, E.; Clerici, F.; Bucci, R.; Anastasi, F.; Bottiglieri, M.; Patrini, M.; Genta, I.; Bittner, A.M.; Gelmi, M.L. Smart Electrospun Nanofibers from Short Peptidomimetics Based on Pyrrolo-pyrazole Scaffold. Biomacromolecules 2024, 25, 2378–2389. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly(hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; Incarnato, L. Study on 3D printability of PLA/PBAT/PHBV biodegradable blends for packaging applications. Polym. Test. 2025, 145, 108748. [Google Scholar] [CrossRef]
- Yuan, H.; Tang, L.; Yang, Z.W.; Zhang, Y.; Liu, Y.; Zhao, Z.; Li, J. PHB/PBAT blended melt-blown micro-nano fiber carbon source for efficient solid-phase denitrification: Insights into low-carbon treatment and enhanced mechanism. Chem. Eng. J. 2025, 519, 165128. [Google Scholar] [CrossRef]
- Suttiwijitpukdee, N.; Sato, H.; Unger, M.; Ozaki, Y. Effects of Hydrogen Bond Intermolecular Interactions on the Crystal Spherulite of Poly(3-hydroxybutyrate) and Cellulose Acetate Butyrate Blends: Studied by FT-IR and FT-NIR Imaging Spectroscopy. Macromolecules 2012, 45, 2738–2748. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Villano, M.; Oliveira, C.; Albuquerque, M.G.; Majone, M.; Reis, M.; Lopez-Rubio, A.; Lagaron, J.M. Characterization of polyhydroxyalkanoates synthesized from microbial mixed cultures and of their nanobiocomposites with bacterial cellulose nanowhiskers. New Biotechnol. 2014, 31, 364–376. [Google Scholar] [CrossRef]
- Abdalkarim, S.Y.H.; Yu, H.-Y.; Wang, C.; Yang, L.; Guan, Y.; Huang, L.; Yao, J. Sheet-like cellulose nanocrystal-ZnO nanohybrids as multifunctional reinforcing agents in biopolyester composite nanofibers with ultrahigh UV-shielding and antibacterial performances. ACS Appl. Bio Mater. 2018, 1, 714–727. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T.; Tsou, C.Y.; Chen, S.C. Processing and characterization of solid and microcellular PHBV/PBAT blend and its RWF/nanoclay composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 979–987. [Google Scholar]
- Berthet, M.A.; Angellier-Coussy, H.; Chea, V.; Guillard, V.; Gastaldi, E.; Gontard, N. Sustainable food packaging: Valorising wheat straw fibres for tuning PHBV-based composites properties. Compos. Part A Appl. Sci. Manuf. 2015, 72, 139–147. [Google Scholar] [CrossRef]
- Asare, E.; Azimi, B.; Vasili, E.; Caporalini, S.; Azimi, B.; Zergat, S.; Ansari Chaharsoughi, M.; Maleki, H.; Batoni, G.; Danti, S. Saverio Caporalini, Bahareh Azimi, Samir Zergat, Mahdi Ansari Chaharsoughi, Homa Maleki, Giovanna Batoni, Serena Danti, Electrospinning Enables Opportunity for Green and Effective Antibacterial Coatings of Medical Devices. J. Funct. Biomater. 2025, 16, 249. [Google Scholar]
- Tomano, N.; Ikuhara, T.; Hiraishi, T.; Taguchi, S.; Kasuya, K.; Abe, H. Enhancing impact resistance and biodegradability of PHBV by melt blending with ENR. Sci. Rep. 2022, 12, 22633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal mechanical properties of biodegradable natural rubber-toughened PHBV bioplastics intended for food packaging applications. Food Packag. Shelf Life 2019, 21, 100348. [Google Scholar] [CrossRef]
- Jaffur, B.N.; Kumar, G.; Khadoo, P. Production and functionalization strategies for superior polyhydroxybutyrate blend performance. Int. J. Biol. Macromol. 2024, 278 Pt 3, 134907. [Google Scholar] [CrossRef]
- Erceg, M.; Kovacic, T.; Klaric, I. Thermal degradation of poly(3-hydroxybutyrate) plasticized with acetyl tributyl citrate. Polym. Degrad. Stab. 2005, 90, 313–318. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W.; Wang, X.; Chen, X.; Chen, G.Q.; Xu, K. Processability modifications of poly(3-hydroxybutyrate) by plasticizing, blending, and stabilizing. J. Appl. Polym. Sci. 2008, 107, 166–173. [Google Scholar] [CrossRef]
- Nosal, H.; Moser, K.; Warzała, M.; Klim, M.; Zimniewska, M.; Rydzkowski, T.; Milewska, A.; Rydz, J. Selected fatty acids esters as potential PHB-V bioplasticizers: Effect on mechanical properties of the polymer. J. Polym. Environ. 2021, 29, 38–53. [Google Scholar] [CrossRef]
- Das, S.K.; Eshkalak, S.K.; Chinnappan, A.; Bhat, A.H.; AlAliAlMaadeed, M.A.; Karim, A. Plastic recycling of polyethylene terephthalate (PET) and polyhydroxybutyrate (PHB)-A comprehensive review. Mater. Circ. Econ. 2021, 3, 9. [Google Scholar] [CrossRef]
- Gong, J.; Qiang, Z.; Ren, J. In situ grafting approach for preparing PLA/PHBV degradable blends with improved mechanical properties. Polym. Bull. 2022, 79, 9543–9562. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ariffin, H.; Hassan, M.A.; Ibrahim, N.A.; Nishida, H. Performance evaluation and chemical recyclability of a polyethylene/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) blend for sustainable packaging. RSC Adv. 2013, 3, 24378–24388. [Google Scholar] [CrossRef]
- Shahdan, D.; Rosli, N.A.; Chen, R.S.; Ahmad, S.; Gan, S. Strategies for strengthening toughened poly(lactic acid) blend via natural reinforcement with enhanced biodegradability: A review. Int. J. Biol. Macromol. 2023, 251, 126214. [Google Scholar] [CrossRef]
- Chikh, A.; Benhamida, A.; Kaci, M.; Bourmaud, A.; Bruzaud, S. Recyclability assessment of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(butylene succinate) blends: Combined influence of sepiolite and compatibilizer. Polym. Degrad. Stab. 2017, 142, 234–243. [Google Scholar] [CrossRef]
- Thanh, N.H.; Olekhnovich, R.; Sitnikova, V.; Kremleva, A.; Snetkov, P.; Uspenskaya, M. PHB/PEG Nanofiber Mat Obtained by Electrospinning and Their Performances. Technologies 2023, 11, 48. [Google Scholar] [CrossRef]
- Aliotta, L.; Gigante, V.; Lazzeri, A. Analytical Modeling of Stress Relaxation and Evaluation of the Activation Volume Variation: Effect of Temperature and Plasticizer Content for Poly (3-hydroxybutyrate-3-hydroxyvalerate). ACS Omega 2022, 7, 23662–23672. [Google Scholar] [CrossRef]
- Das, S.K.; Eshkalak, S.K.; Chinnappan, A.; Bhat, A.H.; AlAliAlMaadeed, M.A.; Karim, A. Atomization of Microfibrillated Cellulose and Its Incorporation into Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Reactive Extrusion. Appl. Sci. 2022, 12, 2111. [Google Scholar]
- Sun, S.; Liu, P.; Ji, N.; Hou, H.; Dong, H. Effects of low polyhydroxyalkanoate content on the properties of films based on modified starch acquired by extrusion blowing. Food Hydrocoll. 2017, 72, 81–89. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T.; Cai, Y.X. Characterisation, biodegradability and application of palm fibre-reinforced polyhydroxyalkanoate composites. Polym. Degrad. Stab. 2017, 140, 55–63. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, S.; Zhang, W.; Yin, F.; Cao, Q.; Lian, T.; Dong, H. Polyhydroxyalkanoates production from lactic acid fermentation broth of agricultural waste without extra purification: The effect of concentrations. Environ. Technol. Innov. 2023, 32, 103311. [Google Scholar] [CrossRef]
- Ogura, T.; Shinzawa, H.; Nishida, M.; Kanematsu, W. Tensile properties of polyhydroxyalkanoate/polycaprolactone blends studied by rheo-optical near-infrared (NIR) spectroscopy. J. Mol. Struct. 2016, 1124, 92–97. [Google Scholar]
- Sun, S.; Liu, P.; Ji, N.; Hou, H.; Dong, H. Effects of various cross-linking agents on the physicochemical properties of starch/PHA composite films produced by extrusion blowing. Food Hydrocoll. 2018, 77, 964–975. [Google Scholar] [CrossRef]
- Wu, C.S. Characterization, functionality and application of siliceous sponge spicules additive-based manufacturing biopolymer composites. Addit. Manuf. 2018, 22, 13–20. [Google Scholar] [CrossRef]
- Mármol, G.; Gauss, C.; Fangueiro, R. Potential of Cellulose Microfibers for PHA and PLA Biopolymers Reinforcement. Molecules 2020, 25, 4653. [Google Scholar] [CrossRef]
- Christian, S.J.; Billington, S.L. Mechanical response of PHB- and cellulose acetate natural fiber-reinforced composites for construction applications. Compos. Part B Eng. 2011, 42, 1920–1928. [Google Scholar] [CrossRef]
- Gigante, V.; Cinelli, P.; Seggiani, M.; Lazzeri, A. Processing and thermomechanical properties of PHA. In The Handbook of Polyhydroxyalkanoates; CRC Press: Boca Raton, FL, USA, 2020; pp. 91–118. [Google Scholar]
- Nigmatullin, R.; Taylor, C.S.; Basnett, P.; Pina, S.; Chaloupka, K.; Knowles, J.C.; Roy, I. Medium chain length polyhydroxyalkanoates as potential matrix materials for peripheral nerve regeneration. Regen. Biomater. 2023, 10, rbad063. [Google Scholar] [CrossRef]
- Haraźna, K.; Fricker, A.T.; Konefał, R.; Medaj, A.; Zimowska, M.; Leszczyński, B.; Wróbel, A.; Bojarski, A.J.; Roy, I.; Guzik, M. Physicochemical, structural and biological characterisation of poly(3-hydroxyoctanoate) supplemented with diclofenac acid conjugates—Harnessing the potential in the construction of materials for skin regeneration processes. Int. J. Biol. Macromol. 2024, 268, 131476. [Google Scholar] [CrossRef]
- Chuenchart, W.; Surendra, K.C.; Khanal, S.K. Understanding Anaerobic Co-digestion of Organic Wastes through Meta-Analysis. ACS ES&T Eng. 2024, 4, 1177–1192. [Google Scholar] [CrossRef]
- Cvek, M.; Paul, U.C.; Zia, J.; Mancini, G.; Sedlarik, V.; Athanassiou, A. Biodegradable Films of PLA/PPC and Curcumin as Packaging Materials and Smart Indicators of Food Spoilage. ACS Appl. Mater. Interfaces 2022, 14, 14654–14667. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, I.; Seo, D.; Kim, H.; Joo, G.; Lee, S.; Park, K. Life Cycle Assessment of aPHA Production. ACS Sustain. Chem. Eng. 2024, 12, 72–84. [Google Scholar] [CrossRef]
- Yousuf, R.G. Novel Polyhydroxybutyrate (PHB) Production Using a Waste Date Seed Feedstock. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2018. [Google Scholar]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Morphology and barrier properties of nanobiocomposites of poly (3-hydroxybutyrate) and layered silicates. J. Appl. Polym. Sci. 2008, 108, 2787–2801. [Google Scholar] [CrossRef]
- Chen, J.; Gong, C. Preparation of polyhydroxyalkanoate nanocomposites for biomedical applications. Polym. Int. 2025, 74, 405–414. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Zhang, P. Polyhydroxyalkanoates (PHAs) as Biomaterials for the Regeneration of Bone Tissue. In Polyhydroxyalkanoates: Sustainable Production and Biotechnological Applications III: Biomedical Sector; Springer Nature: Singapore, 2025; pp. 51–80. [Google Scholar]
- Mohamed, S.M.D.S.; Tuffin, J.; Watson, J.; Anderson, C.; Claeyssens, F.; Miller, C.A.; Rook, T.; Owen, R.; Irvine, S.; Locke, I.C.; et al. Biosynthesis, characterisation and biocompatibility of a unique and elastomeric medium chain-length polyhydroxyalkanoates for kidney glomerular tissue engineering. Mater. Today Bio 2025, 33, 101932. [Google Scholar] [CrossRef]
- Maciá Torregrosa, M.E.; Camacho Diez, J. Shaping a Sustainable Future: Use of Biodegradable Plastics in Ephemeral Constructions//Innovations in Energy Efficient Construction Through Sustainable Materials. IGI Glob. 2025, 33, 325–370. [Google Scholar]
- Jayalath, S.U.; Alwis, A.P.D. the Greenest Plastic So Far: Advancing Microbial Synthesis. Recovery, and Sustainable Applications for Circularity. ACS Omega 2025, 10, 32564–32586. [Google Scholar] [CrossRef]
- Jaisri, J.; Balaji, S. Biodegradable Elegance: Assessing Luxury Textile Biodegradability. In Crafting Sustainability in Luxury Textiles for a Zero-Waste Future; Springer Nature: Cham, Switzerland, 2025; pp. 43–55. [Google Scholar]
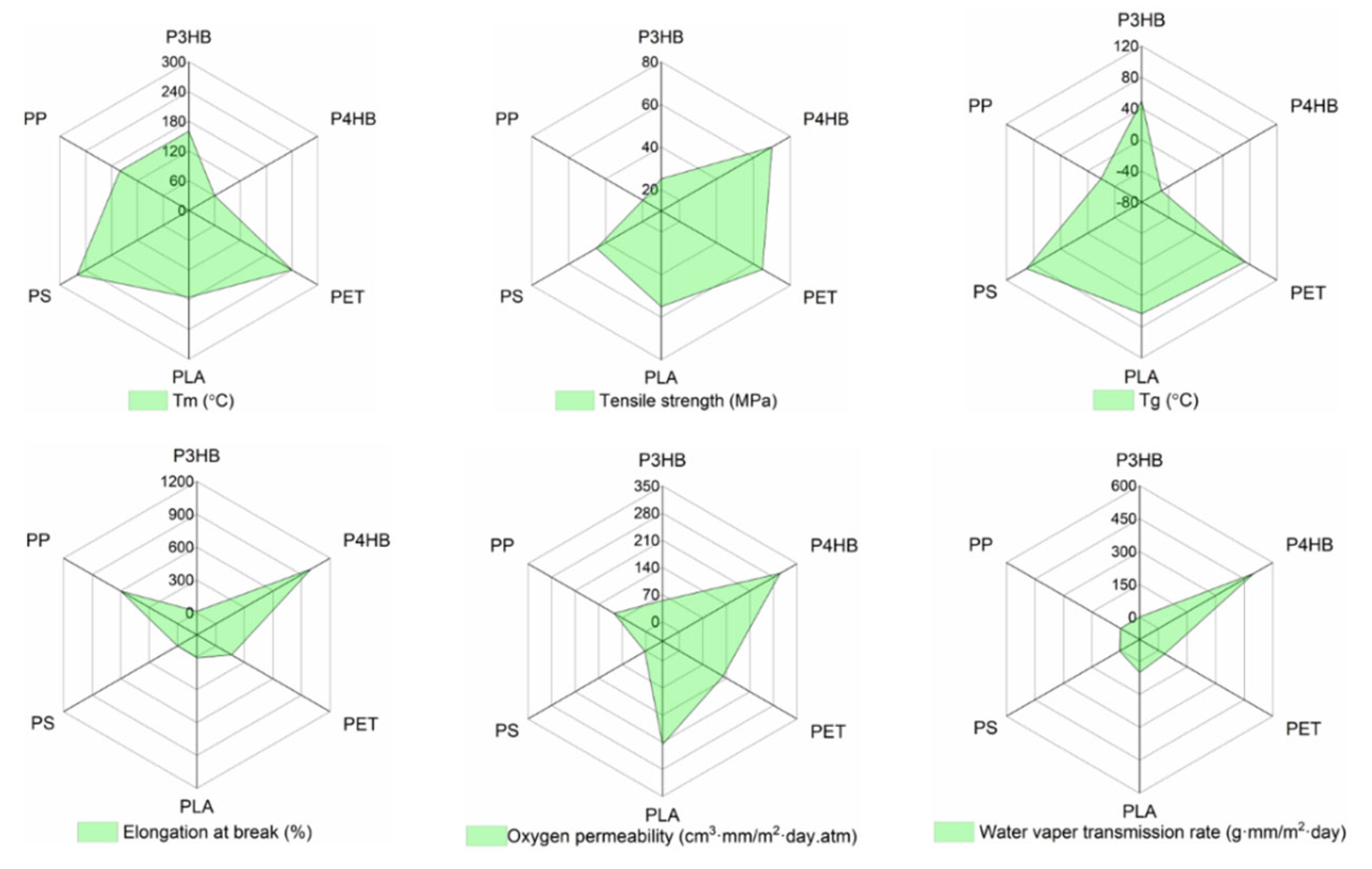

| P(3HB) | P(4HB) | |
|---|---|---|
| Melting Temperature, Tm (°C) | 175 | 60 |
| Glass Transition Temperature, Tg (°C) | 4–10 | −51 |
| Density, p (g/cm3) | 1.18–1.26 | 1.17–1.22 |
| Crystallinity (Xc, %) | 65–80 | 20–35 |
| Young’s Modulus, E (GPa) | 1.4–3.5 | 0.07 |
| Ultimate Tensile Strength (MPa) | 15–40 | 50–70 |
| Elongation at Break (%) | 4–10 | 1000 |
| Material | Processing Method | Structure Produced | Application | References |
|---|---|---|---|---|
| PLA/PHB-Organoclay composite | FDM | - | - | [67] |
| PHB-BaTiO3 Nanocomposite | FDM | porous cubic scaffold | vascularized bone tissue engineering | [68] |
| PHB/PLA-hydroxyapatite composite | FDM | - | - | [26,69] |
| PHBV-ZrO2 composite | FDM | porous scaffold | regenerative medicine | [70] |
| PHB–cellulose composite | FDM | - | - | [71] |
| PHB-graphite composite, PHB/PLA blend | FDM | dog-bone specimen | - | [72] |
| PHB-MWCNTs composite | FDM | scaffold, conductive traces | Tissue regeneration | [73] |
| PLA/PHB blend | FDM | scaffold | Medical applications | [74] |
| PHB/PUA blend | FDM | finger splint cast | medical devices | [74] |
| PHB/PLA-Kaolin composite | FDM | - | - | [75] |
| PHB/acetaminophen | DPE | cubic structure | pharmaceutical forms | [76] |
| Urethane Dimethacrylate/PHB | SLA | fracture bone cast | temporary medical devices | [2] |
| Blends | Youngs’ Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Use | Source |
|---|---|---|---|---|---|
| PLA/PHBV (80/20)/0.3 wt% 2,5-dimethyl-2,5-di(tert-butylperoxy)hexane (DBPH) | - | 15.95 | - | [100] | |
| PE/PHBV (80/20, 70/30) | 348.39 + 12.5 299.7 + 8.67 | 25.93 + 0.72 17.5 + 0.3 | - | Packaging, oxygen transmission rate (~1200 cm3/cm2 per day) | [101,102] |
| PHBV/PBS (50/50) | 1900 | 36 | - | - | [103] |
| PHB/PEG (9:1) | 430.97 ± 31.09 | 12.57 ± 1.09 | 3.34 ± 0.93 | antimicrobial packaging | [104] |
| PHB/PEG (8/1) | 254.4 ± 26.7 | 3.4 ± 0.3 | 24 ± 6 | air filtration or water filtration | [104] |
| PHB-5% TABC | - | 14.8 | 6.3 | [105] | |
| PHBV-5%MFC/epoxidized soybean oil | 2670.2 ± 21.7 | 27.3 ± 1.2 | 1.27 ± 0.11 | packaging | [106] |
| starch/12%PHA | - | 3.75 | 72.4 | packaging | [107] |
| (maleic anhydride-grafted polyhydroxyalkanoate) PHA-g-MA/(palm fiber) TPF | 338 | 12.9 | - | imitation wood, and in medical and conductive filaments | [108] |
| PHA-g-MA/TPF | 424 | 23.7 | - | - | [109] |
| PHA/50% (polycaprolactone) PCL | 280 | 6.4 | 51.9 | plastic | [110] |
| boric acid cross-linked starch/PHA (cross-linking agent: boric acid) | - | 8.55 | 38.6 | packaging | [111] |
| PHA/(siliceous sponge spicules) 2% SSS | 342 | 15 | 518 | biomedical material | [112] |
| PHA-g-(Acrylic acid) AA/2% SSS | 372 | 22 | 565 | - | [112] |
| 1% (Cellulose nanocrystals) CNC/PHA | 720 ± 20 | 22.5 ± 0.02 | 10.43 ± 0.23 | paper | [113] |
| PHA/20% (cellulose microfibers) MF | 940 ± 0.14 | 24.9 ± 0.05 | 3.78 ± 0.11% | paper | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yang, Y.; Liu, R.; Wu, Y.; Guo, F. Review of Biopolymer Polyhydroxybutyrate (PHB) and Blends: Modification of Thermal and Mechanical Properties via Additive Manufacturing Processing. Polymers 2025, 17, 3083. https://doi.org/10.3390/polym17223083
Li D, Yang Y, Liu R, Wu Y, Guo F. Review of Biopolymer Polyhydroxybutyrate (PHB) and Blends: Modification of Thermal and Mechanical Properties via Additive Manufacturing Processing. Polymers. 2025; 17(22):3083. https://doi.org/10.3390/polym17223083
Chicago/Turabian StyleLi, Dan, Yunxia Yang, Ruochen Liu, Yufeng Wu, and Fu Guo. 2025. "Review of Biopolymer Polyhydroxybutyrate (PHB) and Blends: Modification of Thermal and Mechanical Properties via Additive Manufacturing Processing" Polymers 17, no. 22: 3083. https://doi.org/10.3390/polym17223083
APA StyleLi, D., Yang, Y., Liu, R., Wu, Y., & Guo, F. (2025). Review of Biopolymer Polyhydroxybutyrate (PHB) and Blends: Modification of Thermal and Mechanical Properties via Additive Manufacturing Processing. Polymers, 17(22), 3083. https://doi.org/10.3390/polym17223083